Summary
Infection with hepatitis C virus (HCV) may suppress co-infection with HBV during acute or chronic HBV infection. We examined relationships between HBV infection, HCV infection and other factors among injection drug users (IDUs) with antibodies to both viruses. Participants enrolled in a cross-sectional study during 1998–2000 were considered to have been infected with HBV if they had core antibody, to be chronically infected if they had HBV surface antigen (HBsAg), to have been infected with HCV if they had HCV antibody, and to be chronically infected if they had HCV RNA. Among 1,694 participants with antibody to both viruses, HBsAg prevalence decreased with increasing age among those positive for HCV RNA [from 4.55% in those 18–29 years to 1.03% in those ≥ 50 years old (ptrend=0.02)], but not among those who were negative for HCV RNA. Chronic HBV infection was less common overall among those with chronic HCV infection (odds ratio [OR], 0.25; p<0.0001), but this inverse relationship was much stronger in the oldest (> 50 years; OR= 0.15) than the youngest (18–29 years; OR=0.81) participants (ptrend=0.03). Similar results were obtained when duration of injection drug use was substituted for age (ptrend= 0.05). Among IDUs who have acquired both HBV and HCV, chronic HBV infection is much less common among those with chronic HCV infection, but this inverse relationship increases markedly with increasing years of age and injection drug use. Co-infection with HCV may enhance the resolution of HBsAg during the chronic phases of these infections.
Keywords: epidemiology, hepatitis B virus, hepatitis C virus, injection drug use, United States, viral suppression
Injection drug users (IDUs) are at high risk of acquiring blood-borne infections, and in the United States, most have been infected with both hepatitis B virus (HBV) (1–4) and hepatitis C virus (HCV) (2, 4–8). Chronic infection with HBV, as measured by the presence of hepatitis B surface antigen (HBsAg), is relatively infrequent among IDUs, (9, 10) while chronic HCV infection, as measured by HCV RNA, is common (9, 10). Questions regarding co-infection with HBV and HCV are important because chronic infection with either HBV or HCV increases the risk of cirrhosis, end-stage liver disease, and hepatocellular carcinoma, and this risk may be greatest in individuals who are chronically infected with both viruses (11–19).
Previous epidemiological studies of IDUs and persons with hemophilia have shown an inverse relationship between chronic HBV infection and chronic HCV infection (9, 15, 16, 20–24). This relationship could reflect enhanced clearance of HCV infection in the presence of HBV or enhanced clearance of HBV in the presence of HCV (25), but the evidence appears to be stronger for the latter explanation (26). Viral suppression of HBV by HCV could prevent chronic HBV infection from being established or enhance resolution of chronic HBV subsequently. Interference between the viruses in co-infected patients could occur during the acute phase of either infection or the chronic phases of both infections, but the relative importance or frequency of viral suppression in each of these circumstances is unknown (25, 26)
The Urban Health Study (UHS) was an epidemiological and prevention research study of IDUs in the San Francisco Bay area from 1986 to 2005 (27, 28). In a previous paper (4), we examined the prevalence and predictors of antibody to HCV and HBV among UHS participants during 1998–2000 to assess risk factors for having acquired these infections. The present analysis examines the distribution and determinants of chronic infection with HBV or HCV among the study participants who had antibodies to these viruses, in order to assess risk factors for chronicity once infection has already been acquired. To examine the possibility that the viruses suppress each other during the chronic phase of infection, we focused on the relationship between chronic HBV infection and chronic HCV infection, and how that relationship varied over time with increasing age and duration of injection drug use.
SUBJECTS AND METHODS
Subjects and Data Collection
The study subjects in the present analysis participated in UHS between 1998 and 2000 (4). Every month UHS investigators recruited IDUs from street settings in one of six inner-city San Francisco Bay area neighborhoods that were visited in rotation (27). All individuals 18 years of age or older who had injected illicit drugs within the past 30 days or who had previously participated in UHS were eligible for enrollment. Study participants received modest monetary compensation. Although some participants had received hepatitis B vaccine (4), few, if any, were treated for HBV or HCV infection.
Trained staff obtained informed consent, interviewed participants using a standardized instrument, counseled them on reducing infection risks, and referred them to appropriate medical and social services. Participants were asked about sociodemographic factors and their injection drug history, including age at first injection. Blood samples were collected by a trained and certified phlebotomist. Further details about UHS are provided elsewhere (27, 28). The study was approved by the Committee on Human Subjects Research at the University of California, San Francisco and an Institutional Review Board of the National Cancer Institute.
We assessed possible repeat enrollment by comparing demographic information, including gender, birth date, race and site of enrollment. Enrollees who appeared very similar demographically were evaluated by DNA testing as described below. Among 2,351 potential subjects with complete data available, we excluded 55 duplicates and the remaining 2,296 subjects are included in the present analysis.
Viral Serology and other Laboratory Tests
We used serologic testing to classify each participant’s HBV infection status. All subjects were screened for antibody to hepatitis B core antigen (anti-HBc; HBc ELISA Test System, Ortho-Clinical Diagnostics, Raritan, NJ), and hepatitis B surface antigen (HBsAg; Genetic Systems HBsAg EIA version 3.0, Bio-Rad Laboratories, Redmond, WA). Subjects who tested positive for anti-HBc were defined as having been infected with HBV. We considered an HBV-infected subject to have resolved HBV infection if they were negative for HBsAg. To differentiate chronic infection from acute infection among the subjects who were positive for HBsAg, we tested specimens for IgM anti-HBc (ETI-Core-IgMK plus, DiaSorin, Stillwater, MN) at the Mayo Clinic Laboratory. Participants with a negative anti-HBc-IgM result were considered to have chronic HBV infection and those with a positive result were considered to have acute infection. For the subjects who were not classified as HBV-infected, we tested a specimen for antibody to HBsAg (ETI-AB-AUK plus for anti-HBs, DiaSorin, Stillwater, MN). Those who were negative for anti-HBc, but positive for anti-HBs were defined as vaccinated.
To define HCV infection status, we first tested for HCV antibody by HCV version 3.0 ELISA Test System (Ortho-Clinical Diagnostics, Raritan, NJ). Participants who were positive by HCV EIA were considered to have been infected with HCV and were tested for HCV viremia using a branched-chain DNA assay [VERSANT® HCV RNA 3.0 Assay (bDNA), Bayer-Diagnostics, Tarrytown, NY; analytical sensitivity, 2.5×103 copies/ml (n=2073)] or an HCV RNA TaqMan assay (n=19; (29)). Those positive for HCV RNA were considered to have chronic HCV infection and those with a negative result were considered to have resolved HCV infection.
Plasma from each participant was tested for antibodies to human immunodeficiency virus type 1 (HIV-1) by Genetic Systems™ rLAV EIA (Bio-Rad Laboratories, Redmond, WA) and reactive samples were confirmed by HIV-1 Western Blot. We performed DNA fingerprinting to exclude duplicate participants using the AmpFLSTR Profiler Plus® PCR amplification kit (Applied Biosystems, Foster City, CA) to type nine tetranucleotide short tandem repeat loci and the Amelogenin locus from DNA extracted from peripheral blood mononuclear cells.
Statistical Analyses
Analyses were performed using SAS program version 8.2 (SAS Institute, Cary, NC). We determined the prevalence of chronic infection with HBV and HCV overall, and among subgroups defined by demographic, behavioral or viral variables. The analyses of chronic HBV and HCV infections were restricted to the subset of the participants with antibody to the respective viruses. To compare prevalence among subgroups, we calculated an odds ratio (OR), a 95% confidence interval (95% CI) and a two-sided p-value. We used the chi-square test to calculate the p-value unless an expected count was <5, in which case we used Fishers’ exact test. In the multivariate analysis, logistic regression was performed to calculate the OR and 95% CIs, as well as to evaluate statistical interaction (30).
RESULTS
Study Population
A total of 2,296 subjects were included in the current study. The median age at enrollment was 45 years, the median age at which the subjects first injected drugs was 19 years and the median time from the first use of injection drugs to enrollment was 24 years (Table 1). Most participants (71.0%) were men. Almost half (49.5%) of the participants considered themselves African American, 37.8% white (non-Hispanic), and 7.1% Latino. As previously reported (4), the seroprevalence of HBV infection was 80.5% (after excluding 106 participants who had serological evidence of hepatitis B vaccination) and the prevalence of HCV antibody was 91.1%. Antibody to HIV-1 was present in 11.9% of the participants.
Table 1.
Characteristics of the 2,296 injection drug users screend from the San Francisco Bay area, between 1998–2000.
| Characteristic (No. subjects with missing data) | Median | IQRa |
|---|---|---|
| Age at enrollment | 45 | [38–49] |
| Years of injection drug use (59) | 24 | [15–31] |
| No. | % | |
| Gender (31) | ||
| Male | 1608 | 71.0% |
| Female | 657 | 29.0% |
| Race | ||
| White | 869 | 37.8% |
| African American | 1136 | 49.5% |
| Latino | 164 | 7.1% |
| Others | 127 | 5.5% |
| Hepatitis B virus infectionb | ||
| Anti-HBc positive (ever infected) | 1764 | 80.5% |
| HBsAg positive (currently infected) | 73 | |
| Anti-HBc IgM positive (acutely infected) | 19 | |
| Anti-HBc IgM negative (chronically infected) | 54 | |
| HBsAg negative (resolved infection) | 1691 | |
| Anti-HBc and anti-HBs negative (never infected) | 426 | 19.5% |
| Hepatitis C virus infection | ||
| Anti-HCV positive (ever infected) | 2092 | 91.1% |
| HCV RNA positive (chronically infected) | 1717 | |
| HCV RNA negative (resolved infection) | 375 | |
| Anti-HCV negative (never infected) | 204 | 8.9% |
| HIV-1 infection (3) | 273 | 11.9% |
IQR: inter-quartile range
Subjects with serologic evidence of vaccination for HBV (n=106), defined by the presence of anti- HBs and the absence of anti-HBc, were excluded.
Prevalence and Predictors of Chronic HBV Infection
After 19 IDUs with evidence of acute HBV infection and 106 with evidence of hepatitis B vaccination were excluded, 1,745 participants had HBV core antibody (Table 1). The results presented in Table 2 are limited to these participants, of whom 54 (3.1%) were chronically infected. Chronic HBV infection was more prevalent at younger ages, ranging from 4.9% among participants who were 18–29 years of age to 1.8% among those ≥50 years old (p=0.03, test for linear trend), but was not significantly different with increasing length of injection drug use. Chronic HBV infection was most strongly associated with resolved HCV infection; 8.0% of subjects with resolved HCV infection and 2.1% of participants with chronic HCV infection were chronically infected with HBV (OR=0.25, 95% CI, 0.14–0.43; p<0.0001). Among the 51 participants who had antibodies for HBV, but not HCV, one was positive for HBsAg (Table 2). Although this proportion is low (2.0%), it is based on very sparse data and it does not differ significantly from that of the resolved group.
Table 2.
Chronic HBV infection (HBsAg positivity) among injection drug users who had antibodies to HBVa in the San Francisco Bay area between 1998–2000
| Characteristic | No. | % HBsAg positive |
OR (95% CI) | p-valueb |
|---|---|---|---|---|
| Overall | 1745 | 3.1% | ||
| Age | ||||
| 18–29 years | 81 | 4.9% | 1.00 (referent) | 0.03 c |
| 30–39 years | 302 | 4.3% | 0.87 (0.27–2.73) | |
| 40–49 years | 906 | 3.2% | 0.64 (0.22–1.86) | |
| ≥50 years | 456 | 1.8% | 0.34 (0.10–1.17) | |
| Duration injection drug use | ||||
| ≤9 years | 137 | 2.2% | 1.00 (referent) | 0.57 c |
| 10–19 years | 299 | 4.0% | 1.87 (0.52–6.73) | |
| 20–29 years | 632 | 3.6% | 1.69 (0.50–5.70) | |
| ≥30 years | 632 | 2.5% | 1.16 (0.33–4.04) | |
| Gender | ||||
| Male | 1241 | 3.4% | 1.00 (referent) | |
| Female | 478 | 2.5% | 0.74 (0.38–1.41) | 0.35 |
| Race | ||||
| African American | 929 | 2.7% | 1.00 (referent) | |
| White | 589 | 3.7% | 1.40 (0.78–2.51) | 0.25 |
| Latino | 127 | 3.1% | 1.18 (0.40–3.44) | 0.77 |
| Others | 100 | 3.0% | 1.12 (0.33–3.77) | 0.86 |
| HCV infection | ||||
| Resolved | 301 | 8.0% | 1.00 (referent) | |
| Chronic | 1393 | 2.1% | 0.25 (0.14–0.43) | <0.0001 |
| Never infected | 51 | 2.0% | 0.23 (0.01–1.49) | 0.15 |
| HIV-1 | ||||
| Uninfected | 1506 | 2.8% | 1.00 (referent) | |
| Infected | 236 | 4.7% | 1.70 (0.86–3.36) | 0.12 |
Infection with HBV was defined by anti-HBc. Nineteen subjects with acute HBV infection, defined by the presence of HBc-IgM, were excluded.
P value from chi-square test unless indicated otherwise
P value for linear trend
Prevalence and Predictors of Chronic HCV Infection
Of 2,092 subjects with antibody to HCV, 1,717 (82.1%) were chronically infected (Table 3). Chronic infection with HCV was associated with older age, with prevalence ranging from 69.8% among 18–29 year olds to 87.4% among those ≥50 years (p<0.0001, test for linear trend) and with longer duration of drug injection (p=0.001, test for linear trend). Chronic HCV infection was more common in men (83.8%) than women (77.8%; p=0.001) and in African Americans (88.5%) compared to members of other ethnic groups (p<0.0001 for all comparisons). Chronic HCV infection was much less prevalent in participants with chronic HBV infection (54.7%) than those with resolved HBV infection (83.1%; p<0.0001), but more prevalent in participants who were infected with HIV-1 (90.8%) than those who were not (80.8%; p<0.0001).
Table 3.
Chronic HCV infection (HCV RNA positivity) among injection drug users who had antibodies to HCVa, in the San Francisco Bay area between 1998–2000
| Characteristic | No. | % HCV RNA positive |
OR (95% CI) | p-valueb |
|---|---|---|---|---|
| Overall | 2092 | 82.1% | ||
| Age | ||||
| 18–29 years | 129 | 69.8% | 1.00 (referent) | <0.0001 c |
| 30–39 years | 415 | 77.3% | 1.48 (0.95–2.30) | |
| 40–49 years | 1048 | 82.9% | 2.10 (1.40–3.17) | |
| ≥50 years | 500 | 87.4% | 3.01 (1.90–4.76) | |
| Duration injection drug use | ||||
| ≤9 years | 235 | 74.5% | 1.00 (referent) | 0.001 c |
| 10–19 years | 390 | 81.5% | 1.51 (1.03–2.23) | |
| 20–29 years | 733 | 82.3% | 1.59 (1.12–2.25) | |
| ≥30 years | 677 | 84.8% | 1.91 (1.33–2.74) | |
| Gender | ||||
| Male | 1461 | 83.8% | 1.00 (referent) | |
| Female | 600 | 77.8% | 0.68 (0.54–0.86) | 0.001 |
| Race | ||||
| African American | 1061 | 88.5% | 1.00 (referent) | |
| White | 759 | 77.5% | 0.45 (0.35–0.58) | <0.0001 |
| Latino | 155 | 71.0% | 0.32 (0.21–0.47) | <0.0001 |
| Others | 117 | 68.4% | 0.28 (0.18–0.43) | <0.0001 |
| HBV infection | ||||
| Resolved | 1641 | 83.1% | 1.00 (referent) | |
| Chronic | 53 | 54.7% | 0.25 (0.14–0.43) | <0.0001 |
| Never infected | 302 | 81.8% | 0.91(0.66–1.26) | 0.57 |
| HIV-1 | ||||
| Uninfected | 1827 | 80.8% | 1.00 (referent) | |
| Infected | 262 | 90.8% | 2.36 (1.53–3.65) | <0.0001 |
Infection with HCV was defined by HCV antibody. Chronic HCV infection was defined by HCV RNA.
P value from chi-square test unless indicated otherwise
P value for linear trend
Relationship between Chronic HCV and Chronic HBV Infections
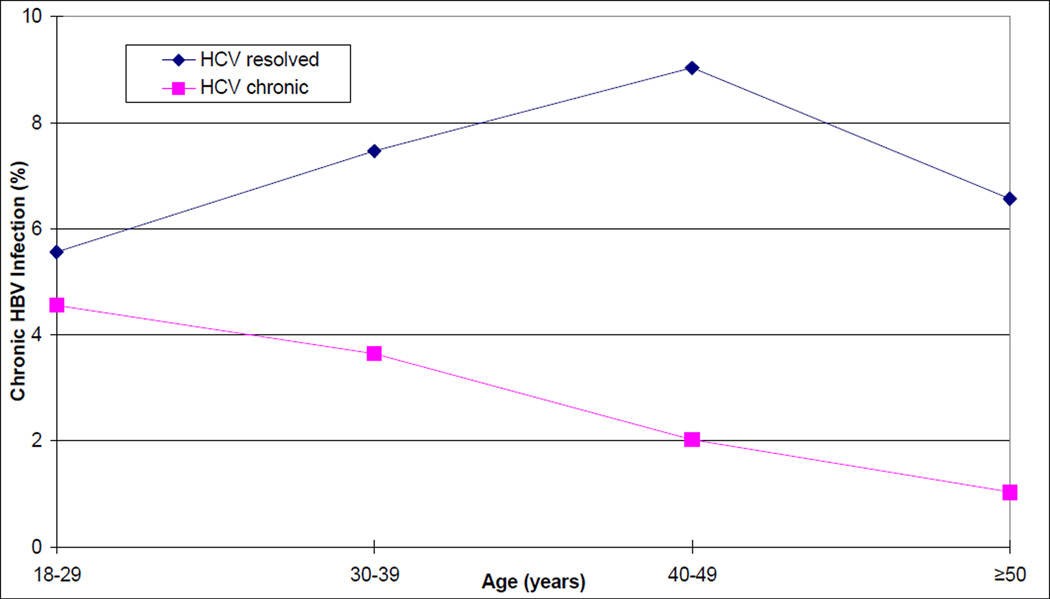
To examine the relationship between chronic HBV infection and chronic HCV infection further, we performed analyses that were limited to the 1,694 subjects who had antibody to both viruses. Among subjects with chronic HCV infection, the frequency of chronic HBV infection decreased with increasing age, from 4.55% in those aged 18–29 years to 1.03% in those ≥ 50 years old (p=0.02, chi-square test for linear trend), whereas this trend was not observed among participants with resolved HCV infection (Figure 1; Table 4). As a result, the overall inverse association that we observed between chronic infection with HBV and HCV (OR, 0.25) varied with age such that this OR was lower with each successive older age group. While no relationship between chronic HCV infection and chronic HBV infection was detectable among participants 18–29 years of age (OR, 0.81; 95% CI, 0.07–9.53), the relationship was extremely strong among participants 40–49 years of age (OR, 0.21; 95% CI, 0.10–0.44) and >50 years (OR, 0.15; 95% CI, 0.04–0.61). We fitted a logistic regression model to the likelihood of chronic HBV infection that included age (as a continuous variable), potential confounding variables (gender, ethnicity, and HIV-1 infection status), chronic HCV infection, and a term for an interaction between age and HCV. In this model, a significant interaction term would indicate that the relationship between chronic HBV infection and chronic HCV infection differed significantly by age, and thereby serve as a test for trend. The association between chronic HCV infection and a lower prevalence of HBsAg in this model grew stronger with increasing age (p=0.03, test for trend by interaction). We also found that HBsAg was more common in participants who were infected with HIV-1 (adjusted OR=2.03; 95% CI=1.00–4.13; p=0.05) in this model.
Figure 1.
Prevalence of chronic HBV infection by HCV infection status and age at enrollment in IDUs from the San Francisco Bay area, between 1998–2000.
Table 4.
Prevalence of HBsAg among 1,694 injection drug users who had antibodies to both HBVa and HCV, by HCV infection status and age or duration of drug use, in the San Francisco Bay area between 1998–2000.
| Chronic HCV Infection (HCV RNA positive) |
Resolved HCV Infection (HCV RNA negative) |
Association between HBsAg and HCV RNAb |
||||
|---|---|---|---|---|---|---|
| Age | HBsAg(+)/ Total | (%) | HBsAg(+)/Total | (%) | ORc | 95% CI |
| 18–29 years | 2/44 | 4.55% | 1/18 | 5.56% | 0.81 | 0.07–9.53 |
| 30–39 years | 8/220 | 3.64% | 5/67 | 7.46% | 0.47 | 0.15–1.48 |
| 40–49 years | 15/742 | 2.02% | 14/155 | 9.03% | 0.21 | 0.10–0.44 |
| ≥50 years | 4/387 | 1.03% | 4/61 | 6.56% | 0.15 | 0.04–0.61 |
| Duration of Injection Drug Use | ||||||
| ≤9 years: | 2/79 | 2.53% | 0/30 | 0.00% | undefined | 0.23-∞ |
| 10–19 years | 7/231 | 3.03% | 5/55 | 9.09% | 0.31 | 0.10–1.03 |
| 20–29 years | 14/514 | 2.72% | 9/111 | 8.11% | 0.32 | 0.13–0.75 |
| ≥30 years | 6/530 | 1.13% | 10/99 | 10.10% | 0.10 | 0.04–0.29 |
Nineteen subjects with acute HBV infection were excluded.
The association between HBsAg and HCV RNA varied by age (p=0.03) and duration of injection drug use (p=0.05) in logistic regression models that controlled for sex, ethnicity, and HIV infection status.
Similar results were obtained when duration of injection drug use was substituted for age (Table 4). Among participants who had injected drugs for 30 or more years, those with chronic HCV infection were ten-fold less likely than those who did not to have chronic HBV infection also (OR, 0.10; 95% CI, 0.04–0.29). The inverse relationship between chronic HCV infection and chronic HBV infection increased with increasing duration of injection drug use (p=0.05, test for trend by interaction) and HBsAg was again associated with HIV-1 infection (adjusted OR=2.18; 95% CI=1.07–4.43; p=0.03).
We also used logistic regression to examine predictors of chronic HCV infection. Male gender (adjusted OR=1.51; 95% CI=1.14–2.00; p=0.004)], African American ethnicity (adjusted OR=2.34; 95% CI, 1.77–3.09; p<0.0001) and infection with HIV-1 (adjusted OR=2.59; 95% CI=1.59–4.24; p=0.0001) were independently associated with chronic HCV infection (in a model that included age, chronic HBV infection and an interaction between chronic HBV infection and age).
The data were too sparse to examine the relationship between HBsAg and age in participants who had been infected with HBV, but not HCV. Of the 51 participants who were positive for anti-HBV but anti-HCV negative, only one was positive for HBsAg (Table 2).
DISCUSSION
In this large sample of IDUs with antibody to both HBV and HCV, participants with chronic HCV infection (detectable HCV RNA) were much less likely to also have chronic HBV infection (detectable HBsAg). Similar findings have been reported in previous studies (9, 15, 16, 20–24), but we explored this relationship in more depth and discovered that the inverse association between chronic HCV infection and chronic HBV infection varied with age at enrollment and the number of years of injection drug use. Although the numbers of young IDUs (<30 years of age) and recent initiates to injection drug use (<10 years) in this study were too small to provide a precise estimate, there was no evidence of an inverse relationship between chronic HBV infection and chronic HCV infection in these groups. Thus, we found no evidence for mutual suppression or interference between the viruses during the acute phases of the infections. Among the IDUs who were oldest and had injected for the longest amounts of time, however, chronic HBV infection was seven- to ten-fold less common among those with chronic HCV infection than among those who had cleared HCV infection—inverse associations of enormous magnitude. These results suggest that the strong inverse association between chronic HCV infection and chronic HBV observed in this study overall likely resulted from ongoing resolution of chronic HBV infection among those chronically infected with HCV, rather than events occurring during the acute phases of either infection.
Because our study is cross-sectional, its analysis cannot distinguish suppression of HBV by HCV from suppression of HCV by HBV. We believe that the evidence from in vitro studies and previous epidemiological studies suggest that the former explanation is more likely. In vitro studies showed that expression of the HCV core protein resulted in decreased levels of HBV transcripts and particles by affecting two steps in the HBV life cycle: gene expression and virion formation (31, 32). The limits of in vitro systems for HCV (33) make it challenging to assess whether HBV has a similar antagonistic effect on HCV. In epidemiologic studies among populations in which HCV infection is rare or absent, HBV carriers were observed to clear HBsAg over time (34), whereas spontaneous clearance of chronic HCV appears to be very rare (35), although it has been reported in Alaska Natives (36). Finally, among people who were infected with HBV as children, HBsAg clearance appears to have been enhanced by the presence of HCV (37, 38). Therefore, the most likely explanation for our results is enhancement of natural clearance of HBsAg by the presence of HCV.
We also considered survival bias as an explanation for the inverse association between chronic HBV infection and chronic HCV infection observed here and in previous studies. Compared to IDUs who are chronically infected with a single virus, those who are chronically infected with both HBV and HCV are at higher risk of morbidity and mortality (11, 19) that would have precluded study enrollment. Substantial excess mortality in co-infected persons, beyond the combined mortality expected from each infection alone, could thus cause an observed inverse association between the two infections that would increase over time, but be unrelated to suppression of one virus by the other. To account for the inverse association we observed between chronic infection with the two viruses, however, 90 excess deaths among HBV/HCV coinfected persons would have been required. This excess mortality among coinfected persons — 90 deaths beyond the sum of those expected to have died from each virus alone — is more than 3 times the number of surviving coinfected persons we observed in the cohort after a median of 24 years of injection drug use (29 persons). This implausibly high case-fatality rate from HBV/HCV coinfection makes survival bias an unlikely explanation for our findings.
Some other findings from our study should be noted. Older age and more years of injection drug use were strongly associated with the presence of chronic HCV infection among IDUs with HCV antibody. A relationship between age and chronic infection was not observed in a cross-sectional study of HCV seropositive blood donors (39), but findings consistent with ours have been reported among other IDUs (9) and persons with hemophilia (29). The explanation for this relationship is unknown, but IDUs who initially clear HCV but continue to inject drugs may eventually be reinfected with HCV strains that cause chronic HCV infection. Although people who clear one HCV infection are more likely than never-infected people to clear another, they are not completely immune from acquiring chronic HCV infection (40, 41). Another potential explanation is that some patients with undetectable HCV continue to harbor the virus (42, 43) and that immunological control of the virus wanes over time due to immunosenescence.
The high proportion of HCV antibody positive participants with chronic HCV infection (82.1%) is consistent with previous studies, and after considering the complex relationships noted above, some other factors were also associated with chronic HCV infection. Consistent with previous studies (5, 9), HCV-seropositive IDUs that were male, African American or co-infected with HIV-1 more often had chronic HCV infection and had higher HCV RNA levels when chronic infection was present. The association with ethnicity supports a possible genetic component to HCV control. Chronic HBV infection was more common among participants who were co-infected with HIV-1.
The strengths and limitations of our study should be considered. The present study is larger than most previous studies of the determinants of chronic HCV infection, and a large population was needed to examine the relationship between chronic HCV infection, chronic HBV infection and the duration of drug use. We have noted that the cross-sectional design is a limitation of this study. We cannot determine the timing of HCV, HBV and HIV infections for individuals. We also cannot differentiate the effect of duration of infection (as estimated by number of years of injection drug) from the effect of age because these factors are highly correlated. We would have liked to have examined the relationship between age and HBsAg among IDUs who had been infected with HBV, but not HCV; however, the data were too sparse to perform such an analysis. PCR based assays have greater sensitivity for detection of HBV and HCV than the assays that we employed in this study. Our results, therefore, must be interpreted within the context of the sensitivity of the HBsAg and HCV bDNA assays. Specifically we could not identify occult HBV infection, which is defined as detectable HBV DNA in the absence of HBsAg (26).
Nonetheless, our study documented a strong inverse relationship between chronic infections with HBV and HCV that appears to confirm reports of interference between these two viruses. This interference appeared and intensified over decades, suggesting that it was due primarily to interference during the chronic phases of the infections.
Acknowledgments
We would like to thank: Dr. Karen Seal, Ms.Jennifer Lorvick and the entire UHS staff for conducting the field operations; Dr. Leslie H. Tobler, Ms. Christine Gamache, Mrs. Georgina Mbisa and Mr. Wendell J. Miley for performing the viral testing; and the UHS participants, who made this study possible.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics, and by Federal funds from National Cancer Institute contracts # NO1-CO-12400 and # N02-CP-91027. This work was also supported by National Institutes of Health grants #R01-DA09532, R01-DA12109, R01-DA13245 and R01-DA16159 (NIH); Substance Abuse and Mental Health Services Administration grant #H79-TI12103 (Center for Substance Abuse Treatment); and the City and County of San Francisco Department of Public Health.
Footnotes
Conflicts of interest - None
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Preliminary results from this study were presented at Digestive Disease Week 2005, May 14 – 19, 2005, Chicago, Illinois.
References
- 1.Levine OS, Vlahov D, Koehler J, Cohn S, Spronk AM, Nelson KE. Seroepidemiology of hepatitis B virus in a population of injecting drug users. Association with drug injection patterns. Am J Epidemiol. 1995;142:331–341. doi: 10.1093/oxfordjournals.aje.a117639. [DOI] [PubMed] [Google Scholar]
- 2.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Zetina J, Kerndt P, Ford W, Woerhle T, Weber M. Prevalence of HIV and hepatitis B and self-reported injection risk behavior during detention among street-recruited injection drug users in Los Angeles County, 1994–1996. Addiction. 2001;96:589–595. doi: 10.1080/09652140020031638. [DOI] [PubMed] [Google Scholar]
- 4.Tseng FC, O'Brien TR, Zhang M, Kral AH, Ortiz-Conde BA, Lorvick J, Busch MP, et al. Seroprevalence of hepatitis C virus and hepatitis B virus among San Francisco injection drug users, 1998 to 2000. Hepatology. 2007;46:666–671. doi: 10.1002/hep.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DL, Vlahov D, Solomon L, Cohn S, Taylor E, Garfein R, Nelson KE. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore) 1995;74:212–220. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hagan H, McGough JP, Thiede H, Weiss NS, Hopkins S, Alexander ER. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol. 1999;149:203–213. doi: 10.1093/oxfordjournals.aje.a009792. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe LE, Ouellet LJ, Levy JR, Williams IT, Monterroso ER. Hepatitis C virus infection: prevalence, risk factors, and prevention opportunities among young injection drug users in Chicago, 1997–1999. J Infect Dis. 2000;182:1588–1594. doi: 10.1086/317607. [DOI] [PubMed] [Google Scholar]
- 8.Lorvick J, Kral AH, Seal K, Gee L, Edlin BR. Prevalence and duration of hepatitis C among injection drug users in San Francisco, Calif. Am J Public Health. 2001;91:46–47. doi: 10.2105/ajph.91.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. Jama. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 10.Torbenson M, Kannangai R, Astemborski J, Strathdee SA, Vlahov D, Thomas DL. High prevalence of occult hepatitis B in Baltimore injection drug users. Hepatology. 2004;39:51–57. doi: 10.1002/hep.20025. [DOI] [PubMed] [Google Scholar]
- 11.Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347–354. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Goedert JJ, Eyster ME, Lederman MM, Mandalaki T, De Moerloose P, White GC, 2nd, Angiolillo AL, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood. 2002;100:1584–1589. [PubMed] [Google Scholar]
- 13.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22–26. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 14.Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, Tran van Nhieu J, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 15.Mathurin P, Thibault V, Kadidja K, Ganne-Carrie N, Moussalli J, El Younsi M, Di Martino V, et al. Replication status and histological features of patients with triple (B, C, D) and dual (B, C) hepatic infections. J Viral Hepat. 2000;7:15–22. doi: 10.1046/j.1365-2893.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- 16.Sagnelli E, Coppola N, Scolastico C, Filippini P, Santantonio T, Stroffolini T, Piccinino F. Virologic and clinical expressions of reciprocal inhibitory effect of hepatitis B, C, and delta viruses in patients with chronic hepatitis. Hepatology. 2000;32:1106–1110. doi: 10.1053/jhep.2000.19288. [DOI] [PubMed] [Google Scholar]
- 17.Sagnelli E, Pasquale G, Coppola N, Scarano F, Marrocco C, Scolastico C, Santantonio T, et al. Influence of chronic coinfection with hepatitis B and C virus on liver histology. Infection. 2004;32:144–148. doi: 10.1007/s15010-004-3080-6. [DOI] [PubMed] [Google Scholar]
- 18.Liaw YF, Chen YC, Sheen IS, Chien RN, Yeh CT, Chu CM. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology. 2004;126:1024–1029. doi: 10.1053/j.gastro.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, Hainaut P, et al. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004;39:211–219. doi: 10.1002/hep.20027. [DOI] [PubMed] [Google Scholar]
- 20.Thomas DL, Astemborski J, Vlahov D, Strathdee SA, Ray SC, Nelson KE, Galai N, et al. Determinants of the quantity of hepatitis C virus RNA. J Infect Dis. 2000;181:844–851. doi: 10.1086/315314. [DOI] [PubMed] [Google Scholar]
- 21.Liaw YF. Concurrent hepatitis B and C virus infection: Is hepatitis C virus stronger? J Gastroenterol Hepatol. 2001;16:597–598. doi: 10.1046/j.1440-1746.2001.02523.x. [DOI] [PubMed] [Google Scholar]
- 22.Liaw YF. Hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. J Gastroenterol. 2002;37(Suppl 13):65–68. doi: 10.1007/BF02990102. [DOI] [PubMed] [Google Scholar]
- 23.Jardi R, Rodriguez F, Buti M, Costa X, Cotrina M, Galimany R, Esteban R, et al. Role of hepatitis B, C, and D viruses in dual and triple infection: influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology. 2001;34:404–410. doi: 10.1053/jhep.2001.26511. [DOI] [PubMed] [Google Scholar]
- 24.Kao JH, Chen PJ, Lai MY, Chen DS. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol. 2002;40:4068–4071. doi: 10.1128/JCM.40.11.4068-4071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L, Verslype C, van Pelt JF, van Ranst M, Fevery J. Viral interaction and clinical implications of coinfection of hepatitis C virus with other hepatitis viruses. Eur J Gastroenterol Hepatol. 2006;18:1311–1319. doi: 10.1097/01.meg.0000243881.09820.09. [DOI] [PubMed] [Google Scholar]
- 26.Crockett SD, Keeffe EB. Natural history and treatment of hepatitis B virus and hepatitis C virus coinfection. Ann Clin Microbiol Antimicrob. 2005;4:13. doi: 10.1186/1476-0711-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson J, Edlin BR, Engels EA, Kral AH, Seal K, Gamache CJ, Whitby D, et al. Seroprevalence of human herpesvirus 8 among injection drug users in San Francisco. J Infect Dis. 2003;187:974–981. doi: 10.1086/368332. [DOI] [PubMed] [Google Scholar]
- 28.Kral AH, Bluthenthal RN, Lorvick J, Gee L, Bacchetti P, Edlin BR. Sexual transmission of HIV-1 among injection drug users in San Francisco, USA: risk-factor analysis. Lancet. 2001;357:1397–1401. doi: 10.1016/S0140-6736(00)04562-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Rosenberg PS, Brown DL, Preiss L, Konkle BA, Eyster ME, Goedert JJ. Correlates of spontaneous clearance of hepatitis C virus among people with hemophilia. Blood. 2006;107:892–897. doi: 10.1182/blood-2005-07-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breslow NE, Day NE, Gart JJ. Statistical methods in cancer research. Lyon: International Agency for Research on Cancer; 1980. International Agency for Research on Cancer. v. [Google Scholar]
- 31.Chen SY, Kao CF, Chen CM, Shih CM, Hsu MJ, Chao CH, Wang SH, et al. Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein. J Biol Chem. 2003;278:591–607. doi: 10.1074/jbc.M204241200. [DOI] [PubMed] [Google Scholar]
- 32.Shih CM, Lo SJ, Miyamura T, Chen SY, Lee YH. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J Virol. 1993;67:5823–5832. doi: 10.1128/jvi.67.10.5823-5832.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bukh J, Purcell RH. A milestone for hepatitis C virus research: a virus generated in cell culture is fully viable in vivo. Proc Natl Acad Sci U S A. 2006;103:3500–3501. doi: 10.1073/pnas.0600551103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo KJ, Tong MJ, Chien MC, Tsai YT, Liaw YF, Yang KC, Chian H, et al. The natural course of hepatitis B surface antigen-positive chronic active hepatitis in Taiwan. J Infect Dis. 1982;146:205–210. doi: 10.1093/infdis/146.2.205. [DOI] [PubMed] [Google Scholar]
- 35.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 36.Scott JD, McMahon BJ, Bruden D, Sullivan D, Homan C, Christensen C, Gretch DR. High rate of spontaneous negativity for hepatitis C virus RNA after establishment of chronic infection in Alaska Natives. Clin Infect Dis. 2006;42:945–952. doi: 10.1086/500938. [DOI] [PubMed] [Google Scholar]
- 37.Sheen IS, Liaw YF, Lin DY, Chu CM. Role of hepatitis C and delta viruses in the termination of chronic hepatitis B surface antigen carrier state: a multivariate analysis in a longitudinal follow-up study. J Infect Dis. 1994;170:358–361. doi: 10.1093/infdis/170.2.358. [DOI] [PubMed] [Google Scholar]
- 38.Utili R, Zampino R, Bellopede P, Marracino M, Ragone E, Adinolfi LE, Ruggiero G, et al. Dual or single hepatitis B and C virus infections in childhood cancer survivors: long-term follow-up and effect of interferon treatment. Blood. 1999;94:4046–4052. [PubMed] [Google Scholar]
- 39.Busch MP, Glynn SA, Stramer SL, Orland J, Murphy EL, Wright DJ, Kleinman S. Correlates of hepatitis C virus (HCV) RNA negativity among HCV-seropositive blood donors. Transfusion. 2006;46:469–475. doi: 10.1111/j.1537-2995.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 40.Mehta SH, Cox A, Hoover DR, Wang XH, Mao Q, Ray S, Strathdee SA, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 41.Proust B, Dubois F, Bacq Y, Le Pogam S, Rogez S, Levillain R, Goudeau A. Two successive hepatitis C virus infections in an intravenous drug user. J Clin Microbiol. 2000;38:3125–3127. doi: 10.1128/jcm.38.8.3125-3127.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carreno V, Pardo M, Lopez-Alcorocho JM, Rodriguez-Inigo E, Bartolome J, Castillo I. Detection of hepatitis C virus (HCV) RNA in the liver of healthy, anti-HCV antibody-positive, serum HCV RNA-negative patients with normal alanine aminotransferase levels. J Infect Dis. 2006;194:53–60. doi: 10.1086/504692. [DOI] [PubMed] [Google Scholar]
- 43.Quiroga JA, Llorente S, Castillo I, Rodriguez-Inigo E, Lopez-Alcorocho JM, Pardo M, Carreno V. Virus-specific T-cell responses associated with hepatitis C virus (HCV) persistence in the liver after apparent recovery from HCV infection. J Med Virol. 2006;78:1190–1197. doi: 10.1002/jmv.20680. [DOI] [PubMed] [Google Scholar]