Abstract
Objective
To compare drug discontinuation risk between adalimumab (ADA) and etanercept (ETN) treatment among anti-tumor necrosis factor (anti-TNF)-naïve rheumatoid arthritis (RA) patients, in particular the influence of concomitant dose of methotrexate (MTX).
Methods
This retrospective nationwide population-based cohort study identified 4,592 anti-TNF-naïve RA patients in whom ETN (n=2,609) or ADA (n=1,983) was initiated using National Health Insurance claims data. After adjustment for prior medication, concomitant medication, and baseline demographic data, the relative risk of drug discontinuation in ADA users compared with ETN users was quantified by calculating adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) using Cox proportional hazard regression analyses, stratified by the follow-up time (≤1 year, >1 year) and/or concomitant MTX dose (≤10 mg/wk, >10 mg/wk).
Results
ADA users had a higher risk of drug discontinuation compared with ETN users during the first year of follow-up (aHR, 1.13; 95% CI, 1.01–1.27), but not during all treatment periods (aHR, 1.06; 95% CI, 0.98–1.16) or after 1 year (aHR, 0.99; 95% CI, 0.87–1.13). However, ADA users had a significantly higher risk of drug discontinuation compared with ETN users among patients on concomitant MTX >10 mg/wk during all treatment periods (aHR, 1.27; 95% CI, 1.10–1.47), during the first year of follow-up (aHR, 1.48; 95% CI, 1.22–1.78), or after 1 year (aHR, 1.42; 95% CI, 1.06–1.90), but not among patients on concomitant MTX 0–10 mg/wk.
Conclusion
This population-based cohort study demonstrated a modification effect of concomitant MTX dose on the relative risk of anti-TNF discontinuation for ADA compared with ETN among anti-TNF-naïve RA patients. However, the lack of exact cause of anti-TNF discontinuation limited causal inference of such a concomitant MTX dose-related modification effect.
Keywords: adalimumab, etanercept, methotrexate, rheumatoid arthritis, treatment discontinuation
Introduction
Rheumatoid arthritis (RA) is a common chronic inflammatory rheumatic disease in Taiwan,1 with an annual incidence rate of 15.8 per 100,000 persons and a prevalence rate of 57.7–99.6 per 100,000 persons.2 Since tumor necrosis factor (TNF) plays a pivotal role in accelerating RA progression, anti-TNF-α agents such as etanercept (ETN), adalimumab (ADA), and infliximab (IFB) have been successfully developed, and their safety and efficacy have been proven by randomized clinical trials and long-term observational studies in a majority of RA patients.3–5 Anti-TNF therapy has been recommended as therapy for patients intolerant to at least two disease-modifying antirheumatic drugs (DMARDs) including methotrexate (MTX) and is more effective when combined with MTX.3,4,6–10 The National Health Insurance Administration (NHIA) in Taiwan, which manages the National Health Insurance (NHI), approved the use of ETN in 2003 and ADA in 2007. Other anti-TNF agents were not available before 2012 in Taiwan. In Taiwan, the indication of initiating anti-TNF therapy for RA patients follows the criterion proposed by the British Society for Rheumatology, using a 28-joint disease activity score (DAS28) of above 5.1 after standard therapy failure with at least two DMARDs, where one failed or where one intolerant therapy must be MTX. According to the regulations, the criteria for discontinuation of anti-TNF therapy are indicated by the failure of DAS28 >1.2, DAS ≤3.2 after 12 weeks, or adverse events such as infection, toxicity, or pregnancy.11
Drug survival has been used as a useful surrogate marker of effectiveness and safety in real life.12 Significant predictors of anti-TNF drug survival include age, previous response to treatment with a TNF antagonist, concomitant treatment with MTX, the presence of comorbidities, and the category of TNF antagonist used.13–15 ETN is a soluble TNF-α receptor that fuses the TNF-α receptor to the constant end of the IgG1 antibody, whereas ADA is an anti-TNF-α human monoclonal antibody. Some studies using registry data have found ADA to have a higher discontinuation rate than ETN.13,14,16–19 The differences in drug survival between ADA and ETN may be attributed to the differences in immunogenicity, molecular structure, and mechanism of action, resulting in different infection risk and sustained effectiveness.
ADA has been associated with a higher risk of infection, especially tuberculosis bacteria (TB) infection, than ETN.20–22 ADA binds to transmembrane TNF on activated macrophages/monocytes with more avidity than ETN, leading to higher complement-mediated cytotoxicity and more apoptosis, which may have biological implications for serious infections.23–25 All biological therapies can induce an unwanted immune response, but the immunogenicity of a TNF antagonist is dependent on its structural properties.26,27 The immunogenic region (Fc fragment) of ETN is nonneutralizing and directs antibodies from an immune response to the fusion region.28 On the contrary, the immunogenic regions of ADA or IFB are neutralizing, which trigger immune responses through the hypervariable complementarity-determining regions. Bartelds et al29 demonstrated that the development of anti-ADA antibodies was associated with lower ADA concentration and worse treatment effect in RA patients. Compared with ETN, Krieckaert et al30 found that the long-term effectiveness of ADA for RA treatment was better in the subgroup without anti-ADA antibodies, but worse in those with anti-ADA antibodies. In addition, Krieckaert et al31 also showed that MTX may reduce the production of anti-ADA antibodies in a dose-dependent manner.
Although concomitant MTX administration may exert differential effects in reducing the immunogenicity induced by anti-TNF agents and different synergistic effects and infection risk when treated with ADA or ETN, the potential modification effects caused by the dosage of concomitant MTX on the relative risk (RR) of anti-TNF therapy discontinuation compared between ADA and ETN users has not been evaluated. The Taiwanese National Health Insurance Research Database (NHIRD) has facilitated population-based longitudinal studies that could minimize selection bias. We, therefore, used the NHIRD to investigate these issues.
Materials and methods
Study design
This is a retrospective, population-based cohort study.
Data source
Taiwan implemented the NHI program on March 1, 1995. The NHI program provides mandatory universal health insurance and has covered over 98% of the Taiwanese population. This study was initiated using 1999–2011 claims data retrieved from Taiwan’s NHIRD, provided by the Bureau of NHI but maintained by the National Health Research Institute (NHRI). Data for inclusion into the database are encrypted before being sent to NHRI. Sensitive data that may enable the identification of individuals are further removed before it is released to researchers.
Patients with major or catastrophic illnesses, including malignancies and some rheumatic diseases (such as RA and systemic lupus erythematous), were registered in the NHI system. Those who had a catastrophic illness certificate are free from copayment. An RA catastrophic illness certificate can only be issued after thorough medical records examination by two qualified rheumatologists. This study utilized the data of NHI catastrophic illness files, including encrypted outpatient/inpatient claims files and enrollment files. The outpatient/inpatient files offer information regarding drug prescription, diagnosis, date of visit/hospitalization, and medical costs. The enrollment files provide information on demography and enrollment. This study was approved by the Ethics Committee for Clinical Research at Taichung Veterans General Hospital. As all personal information was anonymized before analysis, patient consent was not deemed necessary by the Ethics Committee.
Patients
The study was initiated with the identification of 4,592 anti-TNF-naïve RA patients (aged ≥16 years at diagnosis) treated with ETN (n=2,609) or ADA (n=1,983). The patients identified for the study have catastrophic illness files, and they were concurrently on DMARDs and corticosteroids. The patients in Taiwan have been validated for RA based on the 1987 American College of Rheumatology criteria.32
Outcome variable
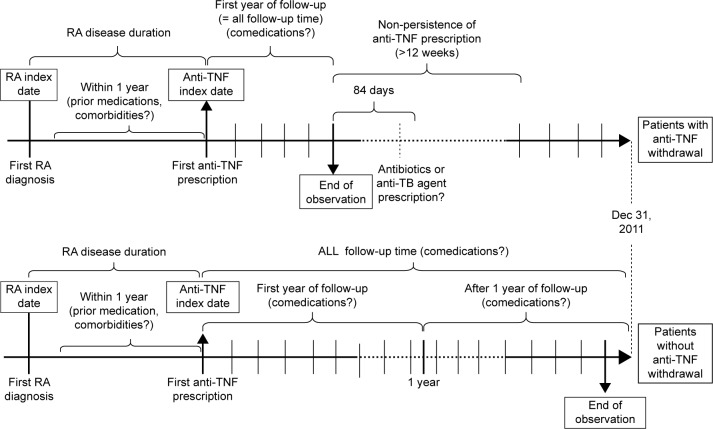
The outcome measured was the time to anti-TNF drug discontinuation. Drug discontinuation was defined as nonpersistence of a prescription for more than 12 weeks. Figure 1 illustrates the drug discontinuation (and other relevant) dynamics. For patients who discontinued treatment, the observation period was from the first date of anti-TNF prescription (anti-TNF index date) to the date of last anti-TNF prescription before the first episode of drug discontinuation. For patients who continued treatment, the observation period was from the anti-TNF index date to the date of last anti-TNF prescription. The incidence of drug discontinuation was defined as the number of events divided by a thousand person-years of follow-up duration between ETN and ADA users. We further identified drug discontinuation events followed by prescriptions of antibiotics or anti-TB drugs. Anti-TB drug prescription was defined as the simultaneous prescription of at least two antituberculosis agents, including isoniazid, rifampin, ethambutol, pyrazinamide, streptomycin, amikin, kanamycin, thioridazine, clarithromycin, prothionamide, ofloxacin, ciprofloxacin, levofloxacin, and moxifloxacin.
Figure 1.
Anti-TNF drug discontinuation (and other relevant) dynamics.
Abbreviations: RA, rheumatoid arthritis; TNF, tumor necrosis factor; anti-TB, antituberculosis.
Covariates
Covariates used included categories of anti-TNF (ETN and ADA); the starting age of anti-TNF administration; sex; RA disease duration; average daily corticosteroid dose, nonsteroid anti-inflammatory drug (NSAID), MTX, leflunomide, salazopyrin (SSZ), and hydroxychloroquine (HCQ) for RA within 1 year before anti-TNF treatment; concomitant use of MTX, leflunomide, SSZ, HCQ, NSAID, and average daily corticosteroid dose; and the Charlson comorbidity index (CCI), as adapted by Deyo et al33 within 1 year before anti-TNF initiation. The use of MTX was further categorized into two groups based on the average weekly dose: ≤10 and >10 mg/wk. This study used the average daily systemic corticosteroid use (prednisolone equivalent dose >5 mg/d versus ≤5 mg/d), NSAID use, and DMARDs use within 1 year before anti-TNF initiation as a proxy of RA disease activity because the NHIRD lacked the data of composite measures of RA disease activity, such as DAS28. RA disease duration was defined as the time from RA index date (first date of RA diagnosis) to anti-TNF index date.
Statistical analysis
After adjusting for age at anti-TNF use, sex, RA disease duration, CCI, other RA-related drugs within 1 year before anti-TNF use and during anti-TNF treatment period, the adjusted relative risks (RRs) and hazard ratios (HRs) with 95% confidence interval (CI) of discontinuation (ADA versus ETN) was calculated using Cox proportional hazard regression analysis, as stratified by the follow-up time (≤1 year, >1 year) and MTX dose (≤10 mg/wk, >10 mg/wk). The adjusted HR was tabulated from the crude HR and compared between two different regimens of concomitant MTX for ADA versus ETN users during different treatment periods. Comparisons between groups were made using the t-test, where a two-tailed P-value of <0.05 was considered statistically significant. The significance of modification effect by each covariate on anti-TNF choice, (ETN or ADA)-associated drug discontinuation risk, was investigated by calculating the P-value of the coefficient associated with the product of each indicator of the covariate and the indicator of anti-TNF choice using the Wald test. The calculation of statistical values was performed using SPSS version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Table 1 compares the baseline characteristics and clinical data of patients treated with ETN and ADA. The mean duration of RA in patients before ETN treatment was shorter than that in patients before ADA treatment (6.0±3.7 versus 6.3±3.7 years, P=0.005), and the proportion of patients with RA for more than 3 years before ETN treatment was also lower than that before ADA treatment (70.2% versus 73.0%, P=0.038). One year before anti-TNF treatment, the proportion of ETN versus ADA users with CCI ≥2 was lower (46.2% versus 50.0%, P=0.011).
Table 1.
Baseline characteristics of RA patients treated with etanercept and adalimumab
| Variables | Etanercept (n=2,609) | Adalimumab (n=1,983) | P-value |
|---|---|---|---|
| Sex | 0.170 | ||
| Male | 474 (18.2) | 392 (19.8) | |
| Female | 2,135 (81.8) | 1,591 (80.2) | |
| Age at RA onset, yearsa | 49.2±12.8 | 49.5±12.5 | 0.485 |
| Age at anti-TNF initiation, yearsa | 55.1±13.1 | 55.7±12.6 | 0.143 |
| Age at anti-TNF initiation, ≥65 years | 647 (24.8) | 488 (24.6) | 0.883 |
| RA duration, yearsa | 6.0±3.7 | 6.3±3.7 | 0.005 |
| RA duration, >3 years | 1,831 (70.2) | 1,447 (73.0) | 0.038 |
| History within 1 year before anti-TNF treatment | |||
| Charlson comorbidity index ≥2 | 1,205 (46.2) | 991 (50.0) | 0.011 |
| Methotrexate, >10 mg/wk | 1,460 (56.0) | 1,074 (54.2) | 0.224 |
| Leflunomide | 873 (33.5) | 798 (40.2) | <0.001 |
| Salazopyrin | 1,970 (75.5) | 1,449 (73.1) | 0.061 |
| Hydroxychloroquine | 2,098 (80.4) | 1,544 (77.9) | 0.034 |
| NSAID | 2,587 (99.2) | 1,958 (98.7) | 0.164 |
| Pd equivalent, >5 mg/d | 1,347 (51.6) | 1,039 (52.4) | 0.607 |
Notes: Results are shown as number (%), unless otherwise indicated. The median (IQ range) methotrexate dose during 1 year before anti-TNF treatment was 10.6 (7.3, 13.3) mg/wk.
Presented as mean ± SD.
Abbreviations: RA, rheumatoid arthritis; TNF, tumor necrosis factor; NSAID, nonsteroid anti-inflammatory drug; Pd, prednisolone; SD, standard deviation; IQ, interquartile.
Table 2 compares the comedication and incidence rate of drug discontinuation among RA patients treated with ETN and ADA according to the follow-up time. During the first year, the number of cases who discontinued their anti-TNF treatment was 682 out of 2,609 ETN users and 562 out of 1,983 ADA users, after 2,094 and 1,507 person-years of follow-up, with incidence rates of drug discontinuation of 326 and 373 cases per 1,000 person-years. After the first year, 616 out of 1,482 ETN users and 376 out of 969 ADA users discontinued anti-TNF treatment after 1,846 and 1,012 person-years of follow–up, with the corresponding incidence rates of drug discontinuation of 334 and 372 cases per 1,000 person-years, respectively. During all follow-up times, there were more ETN users who were on concomitant SSZ, HCQ, and NSAID treatments compared to ADA users who were on leflunomide (P<0.05).
Table 2.
Comedication and incidence rate of drug discontinuation of RA patients treated with etanercept and adalimumab according to the follow-up time
| Variables | Etanercept | Adalimumab | P-value |
|---|---|---|---|
| All follow-up periods | n=2,609 | n=1,983 | |
| Comedication | |||
| Methotrexate, >10 mg/wka | 957 (36.7) | 774 (39.0) | 0.103 |
| Leflunomide | 546 (20.9) | 562 (28.3) | <0.001 |
| Salazopyrin | 1,477 (56.6) | 1,045 (52.7) | 0.008 |
| Hydroxychloroquine | 1,627 (62.4) | 1,080 (54.5) | <0.001 |
| NSAID | 2,519 (96.6) | 1,887 (95.2) | 0.018 |
| Pd equivalent, >5 mg/da | 565 (21.7) | 489 (24.7) | 0.782 |
| Person-years | 3,939 | 2,520 | |
| Anti-TNF discontinuation | 1,298 (49.8) | 938 (47.3) | 0.100 |
| Incidence of discontinuation, /103 person-years | 330 | 372 | 0.008 |
| First year of follow-up | n=2,609 | n=1,983 | |
| Comedication | |||
| Methotrexate, >10 mg/wka | 1,170 (44.8) | 886 (44.7) | 0.911 |
| Leflunomide | 523 (20.0) | 543 (27.4) | <0.001 |
| Salazopyrin | 1,452 (55.7) | 1,020 (51.4) | 0.005 |
| Hydroxychloroquine | 1,592 (61.0) | 1,056 (53.3) | <0.001 |
| NSAID | 2,504 (96.0) | 1,874 (94.5) | 0.019 |
| Pd equivalent, >5 mg/da | 832 (31.9) | 640 (32.3) | 0.782 |
| Person-years | 2,094 | 1,507 | |
| Anti-TNF discontinuation | 682 (26.1) | 562 (28.3) | 0.097 |
| Incidence of discontinuation, /103 years | 326 | 373 | 0.008 |
| After 1 year of follow-up | n=1,482 | n=969 | |
| Comedication | |||
| Methotrexate, >10 mg/wka | 369 (24.9) | 237 (24.5) | 0.805 |
| Leflunomide | 199 (13.4) | 183 (18.9) | <0.001 |
| Salazopyrin | 604 (40.8) | 313 (32.3) | <0.001 |
| Hydroxychloroquine | 641 (43.3) | 353 (36.4) | 0.001 |
| NSAID | 1,272 (85.8) | 795 (82.0) | 0.012 |
| Pd equivalent, >5 mg/da | 157 (10.6) | 120 (12.4) | 0.171 |
| Person-years of follow-up | 1,846 | 1,012 | |
| Anti-TNF discontinuation | 616 (41.6) | 376 (38.8) | 0.173 |
| Incidence of discontinuation, /103 years | 334 | 372 | 0.288 |
Notes: Results are shown as number (%) unless otherwise indicated. The median (IQ range) concomitant methotrexate doses were 8.3 (4.1, 12.1) mg/wk for all treatment periods, 9.1 (4.7, 13.0) mg/wk for the first year, and 6.3 (0.0, 9.9) mg/wk after 1 year.
The classifications were based on average dose during the corresponding period.
Abbreviations: RA, rheumatoid arthritis; NSAID, nonsteroid anti-inflammatory drug; Pd, prednisolone; TNF, tumor necrosis factor; IQ, interquartile.
Within 1 year, the incidence rates of ETN versus ADA users on concomitant MTX 0–10 mg/d who discontinued anti-TNF therapy and subsequently were prescribed new antibiotics/anti-TB drugs within 84 days were 148.0/3.7 and 166.5/14.1 after 1,072 person-years and 781 person-years of follow-up (Table 3). For ETN versus ADA users on concomitant MTX >10 mg/d who discontinued anti-TNF therapies and were subsequently prescribed new antibiotics/anti-TB drugs within 84 days, the incidence rates were 69.2/2.0 and 136.5/19.3 after 1,021 and 726 person-years of follow-up.
Table 3.
Drug discontinuation case number and IRs and those with subsequent newly prescribed antibiotics/anti-TB drugs within 84 days after discontinuation
| Drugs discontinued | Concomitant MTX 0–10 mg/d
|
Concomitant MTX >10 mg/d
|
||||||
|---|---|---|---|---|---|---|---|---|
| ETN
|
ADA
|
ETN
|
ADA
|
|||||
| Case | IRa | Case | IRa | Case | IRa | Case | IRa | |
| All periodsb | ||||||||
| All discon’t | 869 | 365.5 | 564 | 380.1 | 429 | 274.7 | 374 | 361.0 |
| Antibiotics | 352 | 148.0 | 255 | 171.9 | 160 | 102.5 | 163 | 157.3 |
| Anti-TB | 5 | 2.1 | 15 | 10.1 | 7 | 4.5 | 18 | 17.4 |
| Within 1 yearc | ||||||||
| All discon’t | 461 | 430.0 | 337 | 431.5 | 221 | 216.5 | 225 | 309.9 |
| Antibiotics | 184 | 148.0 | 142 | 166.5 | 61 | 69.2 | 90 | 136.5 |
| Anti-TB | 4 | 3.7 | 11 | 14.1 | 2 | 2.0 | 14 | 19.3 |
| After 1 yeard | ||||||||
| All discon’t | 503 | 398.6 | 283 | 409.0 | 113 | 193.8 | 93 | 290.6 |
| Antibiotics | 161 | 127.6 | 106 | 153.2 | 36 | 61.7 | 31 | 96.9 |
| Anti-TB | 3 | 2.4 | 6 | 8.7 | 3 | 5.1 | 2 | 6.3 |
Notes:
Per 103 years.
Concomitant MTX 0–10 mg/d – ETN: n=1,652, 2,378 person-years; ADA: n=1,209 and 1,484 person-years follow-up; concomitant MTX >10 mg/d – ETN: n=957 and 1,562 person-years follow-up; ADA: n=774 and 1,036 person-years follow-up.
Concomitant MTX 0–10 mg/d – ETN: n=1,439 and 1,072 person-years follow-up; ADA: n=1,097 and 781 person-years follow-up; concomitant MTX >10 mg/d – ETN: n=1,170 and 1,021 person-years follow-up; ADA: n=886 and 726 person-years follow-up.
Concomitant MTX 0–10 mg/d – ETN: n=1,113 and 1,262 person-years follow-up; ADA: n=732 and 692 person-years follow-up; concomitant MTX >10 mg/d – ETN: n=369 and 583 person-years follow-up; ADA: n=237 and 320 person-years follow-up.
Abbreviations: IRs, incidence rates; ETN, etanercept; ADA, adalimumab; MTX, methotrexate; discon’t, discontinuation; anti-TB, antituberculosis.
Analysis using Cox proportional hazard regression analysis showed that the adjusted HRs with 95% CI for drug discontinuation in ADA versus ETN users within and after 1 year follow-up were 1.13 (1.01–1.27) and 0.99 (0.87–1.13) (Table 4), respectively. The crude and adjusted HRs with 95% CIs of drug discontinuation for ADA compared with ETN users on concomitant MTX 0–10 mg/wk within 1 year follow-up were 1.01 (0.88–1.17) and 0.98 (0.85–1.13), respectively. During the same period, the crude and adjusted HRs with 95% CIs of drug discontinuation for those on concomitant MTX >10 mg/wk were 1.48 (1.23–1.78) and 1.48 (1.22–1.78), respectively. Beyond 1 year of follow-up, the crude and adjusted HRs with 95% CIs of drug discontinuation for ADA compared with ETN users on concomitant MTX 0–10 mg/wk were 0.98 (0.85–1.13) and 0.91 (0.79–1.06), and for those on concomitant MTX >10 mg/wk, the crude and adjusted HRs were 1.50 (1.14–1.98) and 1.42 (1.06–1.90), respectively. The results showed that ADA, compared with ETN, users on concomitant MTX of over 10 mg/wk were at significantly higher risk of discontinuing from anti-TNF treatment regardless of follow-up time (P for interactions <0.05). Table S1 reveals subgroup analyses for drug discontinuation risk of ADA compared with ETN based on other covariates, with the significance of their modification effects among patients with average concomitent MTX dose over 10 mg/wk. During all treatment periods, prior MTX dose and prior HCQ use were significant effect modifiers. After 1 year, CCI and prior MTX dose were significant effect modifiers. Concomitant medications other than MTX were not significant effect modifiers.
Table 4.
The crude and adjusted HRs with 95% confidence intervals of drug discontinuation for adalimumab compared with etanercept, stratified by concomitant MTX dose
| Follow-up time | All patients | Concomitant MTX 0–10 mg/wk | Concomitant MTX >10 mg/wk | P for modification |
|---|---|---|---|---|
| All treatment periods | n=4,592 | n=2,861 | n=1,731 | |
| Crude HR | 1.12 (1.03–1.22) | 1.03 (0.93–1.14) | 1.32 (1.15–1.52) | 0.009 |
| Adjusted HR | 1.06 (0.98–1.16) | 0.97 (0.87–1.09) | 1.27 (1.10–1.47) | 0.013 |
| Within 1 year | n=4,592 | n=2,536 | n=2,056 | |
| Crude HR | 1.16 (1.04–1.30) | 1.01 (0.88–1.17) | 1.48 (1.23–1.78) | 0.002 |
| Adjusted HR | 1.13 (1.01–1.27) | 0.98 (0.85–1.13) | 1.48 (1.22–1.78) | 0.002 |
| After 1 year | n=2,451 | n=1,845 | n=606 | |
| Crude HR | 1.07 (0.94–1.22) | 0.98 (0.85–1.13) | 1.50 (1.14–1.98) | 0.017 |
| Adjusted HR | 0.99 (0.87–1.13) | 0.91 (0.79–1.06) | 1.42 (1.06–1.90) | 0.036 |
Notes: Cox proportional hazard regression analyses were conducted to calculate crude HRs, and adjusted HRs after adjusting for sex, age at anti-TNF initiation, RA duration, Charlson comorbidity index (<2, ≥2) within 1 year before anti-TNF use, use of leflunomide, salazopyrin, nonsteroid anti-inflammatory drugs, MTX (0–10 mg/wk, >10 mg/wk) and corticosteroid (prednisolone equivalent ≤5 mg/d, >5 mg/d) before and after starting anti-TNF use during all treatment periods.
Abbreviations: HRs, hazard ratios; MTX, methotrexate; TNF, tumor necrosis factor; RA, rheumatoid arthritis.
Table 5 lists the crude and adjusted HRs with 95% CIs of drug discontinuation followed by newly prescribed antibiotics for ADA versus ETN users, stratified by concomitant MTX dose. The crude and adjusted HRs with 95% CIs of drug discontinuation followed by newly prescribed antibiotics for ADA compared with ETN for those on concomitant MTX 0–10 mg/wk within 1 year follow-up were 1.06 (0.86–1.33) and 1.00 (0.80–1.25), and for those on concomitant MTX >10 mg/wk were 2.12 (1.53–2.94) and 2.11 (1.51–2.93), respectively. After 1 year, the crude and adjusted HRs with 95% CIs of drug discontinuation followed by newly prescribed antibiotics for ADA compared with ETN for those on concomitant MTX 0–10 mg/wk were 1.14 (0.89–1.46) and 1.00 (0.78–1.28) and for those on concomitant MTX >10 mg/wk were 1.56 (0.97–2.53) and 1.33 (0.80–2.23), respectively. The results showed that ADA, compared with ETN, users on concomitant MTX of over 10 mg/wk were at significantly higher risk of discontinuing from anti-TNF treatment during all treatment periods and within 1 year of follow-up (P for modifications <0.05). Table S2 shows the adjusted HRs with 95% CI for drug discontinuation followed by antibiotics prescription in ADA versus ETN users on concomitant MTX >10 mg/wk, stratified by other covariates with the significance of their modification effects. During all treatment periods and after 1 year, prior corticosteroid dose was a significant effect modifier. Concomitant medications other than MTX were not significant effect modifiers.
Table 5.
The crude and adjusted HRs with 95% confidence intervals of drug discontinuation followed by newly prescribed antibiotics for adalimumab compared with etanercept, stratified by concomitant MTX dose
| Follow-up time | All patients | Concomitant MTX 0–10 mg/wk | Concomitant MTX >10 mg/wk | P for modification |
|---|---|---|---|---|
| All treatment periods | n=4,592 | n=2,861 | n=1,731 | |
| Crude HR | 1.19 (1.03–1.36) | 1.15 (0.98–1.35) | 1.58 (1.27–1.97) | 0.057 |
| Adjusted HR | 1.06 (0.98–1.16) | 1.07 (0.91–1.26) | 1.50 (1.19–1.87) | 0.013 |
| Within 1 year | n=4,592 | n=2,536 | n=2,056 | |
| Crude HR | 1.33 (1.11–1.59) | 1.06 (0.86–1.33) | 2.12 (1.53–2.94) | 0.001 |
| Adjusted HR | 1.26 (1.05–1.51) | 1.00 (0.80–1.25) | 2.11 (1.51–2.93) | 0.001 |
| After 1 year | n=2,451 | n=1,845 | n=606 | |
| Crude HR | 1.28 (0.98–1.51) | 1.14 (0.89–1.46) | 1.56 (0.97–2.53) | 0.325 |
| Adjusted HR | 1.08 (0.86–1.34) | 1.00 (0.78–1.28) | 1.33 (0.80–2.23) | 0.294 |
Notes: Cox proportional hazard regression analyses were conducted to calculate crude HRs and adjusted HRs after adjusting for sex, age at anti-TNF initiation (<65 years, ≥65 years), RA duration (≤3 years, >3 years), Charlson comorbidity index (<2, ≥2) within 1 year before anti-TNF use, and use of leflunomide, salazopyrin, nonsteroid anti-inflammatory drugs, MTX (0–10 mg/wk, >10 mg/wk), and corticosteroid (prednisolone equivalent ≤5 mg/d, >5 mg/d) before and after starting anti-TNF use during all treatment periods.
Abbreviations: HRs, hazard ratios; MTX, methotrexate; TNF, tumor necrosis factor; RA, rheumatoid arthritis.
As shown in Table 6, other risk factors included a higher dose of concomitant corticosteroid use, and prior use of leflunomide, SSZ, HCQ, and NSAID. Protective factors included higher concomitant MTX dose, and concomitant use of leflunomide, SSZ, HCQ, and NSAID. However, concomitant MTX over 10 mg/wk had protective effects on drug discontinuation in ETN users but not in ADA users (P for interaction <0.05).
Table 6.
The adjusted hazard ratios with 95% confidence intervals of drug discontinuation associated with variables in ETN and ADA users during all treatment periods
| Variable | Model 1
|
Model 2
|
Model 3
|
|---|---|---|---|
| All (n=4,592) | ETN (n=2,609) | ADA (n=1,983) | |
| ADA vs ETN | 1.06 (0.98–1.16) | – | – |
| Female | 0.93 (0.83–1.03) | 1.06 (0.92–1.23) | 1.06 (0.90–1.25) |
| RA disease duration, >3 years | 1.01 (0.92–1.11) | 0.95 (0.84–1.07) | 1.07 (0.92–1.24) |
| Age at drug initiation, ≥65 years | 1.20 (1.09–1.32) | 1.143 (1.006–1.299) | 1.30 (1.12–1.50) |
| Within 1 year before anti-TNF use | |||
| CCI, ≥2 | 1.01 (0.93–1.10) | 0.97 (0.86–1.08) | 1.08 (0.94–1.23) |
| MTX, >10 mg/wk | 0.90 (0.83–0.99) | 0.89 (0.80–1.08) | 0.90 (0.78–1.04) |
| Leflunomide | 1.15 (1.03–1.28) | 1.06 (0.92–1.23) | 1.25 (1.07–1.48) |
| Salazopyrin | 1.24 (1.10–1.40) | 1.28 (1.09–1.51) | 1.21 (1.01–1.45) |
| Hydroxychloroquine | 1.26 (1.10–1.43) | 1.54 (1.28–1.84) | 0.98 (0.81–1.18) |
| NSAID | 2.46 (1.55–3.90) | 1.60 (0.88–2.92) | 3.41 (1.64–7.08) |
| Pd, >5 mg/d | 0.88 (0.80–0.97) | 0.82 (0.72–0.92) | 0.99 (0.85–1.15) |
| Comedication | |||
| MTX, >10 mg/wk | 0.83 (0.76–0.91) | 0.75 (0.66–0.85) | 0.95 (0.83–1.10) |
| Leflunomide | 0.85 (0.76–0.96) | 0.86 (0.73–1.02) | 0.86 (0.72–1.02) |
| Salazopyrin | 0.82 (0.74–0.92) | 0.86 (0.75–0.99) | 0.77 (0.66–0.91) |
| Hydroxychloroquine | 0.88 (0.81–0.99) | 0.85 (0.74–0.97) | 0.93 (0.80–1.09) |
| NSAID | 0.22 (0.18–0.27) | 0.16 (0.12–0.20) | 0.30 (0.22–0.42) |
| Pd, >5 mg/d | 1.86 (1.68–2.05) | 1.85 (1.61–2.11) | 1.86 (1.60–2.16) |
Abbreviations: ADA, adalimumab; ETN, etanercept; RA, rheumatoid arthritis; TNF, tumor necrosis factor; CCI, Charlson comorbidity index; MTX, methotrexate; NSAID, nonsteroid anti-inflammatory drug; Pd, prednisolone.
Discussion
To our knowledge, this study is not only the first to compare drug survival between ADA and ETN in anti-TNF-naïve RA patients in Taiwan, but also the first to explore a possible modification effect of concomitant MTX dose in anti-TNF-naïve RA patients using administrative data. This study showed that ADA users had a higher risk of drug discontinuation than ETN users only when the concomitant MTX dose was more than 10 mg/wk. The results were different from the findings of previous studies that showed better drug survival in ETN users regardless of concomitant MTX dose.13,14,16–19 There are two possible explanations for this finding. First, ADA had lower sustained effectiveness due to a higher risk of antidrug antibody production compared with ETN.34 If this is the primary cause, the difference in discontinuation risk between ADA and ETN should be greater after the first year of follow-up and when concomitant MTX dose was lower (given that a higher dose of MTX was associated with lower risk of anti-ADA antibody production).31 However, this study did not show such associations. The second possible explanation is that compared with ETN, ADA use had a higher risk of adverse effect when the concomitant MTX dose was more than 10 mg/wk. Recently, the randomized CONCERTO trial showed that in biologic and MTX-naïve RA patients initiating ADA and MTX combination treatment, the efficacy of 10 and 20 mg/wk MTX appeared similar.35 In addition, the risk of adverse events was not increased with increasing MTX dose in combination with ADA, except infection and abnormal hair loss.35 Some studies have shown that ADA had a higher risk of infections, especially TB infection, than ETN.20–22 In this study, although the risk of TB was higher in ADA users compared with ETN users, the incidence rate ratio (IRR) was not higher in patients on MTX >10 mg/wk (IRR =3.9) compared with those on MTX 0–10 mg/wk (IRR =4.8). In our study, when using the time to drug discontinuation followed by antibiotics prescription as the outcome, the magnitude of HR in ADA users compared with ETN users was increased. Therefore, we may infer that infections other than TB may more likely be the cause of the different discontinuation risk between ADA and ETN in patients on MTX more than 10 mg/wk.
This study also found that concomitant usage of MTX >10 mg/wk had protective effects on drug discontinuation in ETN users but not in ADA users. Concomitant MTX use has synergic effects in RA patients treated with anti-TNF agents, but the CONCERTO trial found that the efficacy of 10 and 20 mg/wk MTX appeared similar.35 Our data supported the authors’ suggestion of using no more than 10 mg/wk MTX in RA patients treated with ADA.35
The results of this observational study are not without limitations. First, although ETN and ADA users can be directly compared, the study was not randomized, and the data cannot be easily compared with other registries due to the different methods of collection and analysis. Therefore, there is a potential for selection bias between the treatment groups.15,17,36 However, we adjusted potential confounding factors, including age, sex, comorbidities, prior medications as a proxy of RA disease activity, and concomitant medications. Furthermore, the identification of risk factors of drug discontinuation for TNF antagonists using real-world data enables the future treatment strategies of RA patients to be optimized. Second, the administrative data lacked some variables of scientific importance, including disease activity, individual smoking status, and laboratory data such as rheumatoid factor, anticitrullinated peptide antibody, human leukocyte antigen, antidrug antibody, and anti-TNF drug levels, limiting further evaluation of the role of disease activity and genetic and environmental factors on the associations, and the role of immunogenicity on the differential effect of anti-TNF category between different concomitant MTX doses. Third, we were unable to extrapolate the results to the population not included in the NHIRD. Further studies are needed to determine the extent of similarity with other ethnic populations. Fourth, because the administrative data lacked the information about exact cause of drug discontinuation, the causal inference of noninfection-related drug discontinuation risk may be biased.
In conclusion, this population-based cohort study indicates that among anti-TNF-naïve RA patients, compared with ETN users, ADA users had a significantly higher risk of drug discontinuation if the concomitant MTX dose was more than 10 mg/wk. The potential reason of this finding is that using higher dose of concomitant MTX, which is also an immunosuppressant, may increase the RR of infections other than TB in ADA users compared with ETN users, thus leading to a significantly different discontinuation risk. However, the lack of exact cause of anti-TNF discontinuation limited causal inference of such concomitant MTX dose-related modification effect. Further prospective, controlled studies are warranted to prove this hypothesis.
Supplementary materials
Table S1.
The adjusted HRs with 95% CIs of drug discontinuation for adalimumab compared with etanercept on concomitant MTX >10 mg/wk during all treatment periods, ≤1 year or >1 year, stratified by other covariates with the significance of their modification effects
| Variable | All treatment periods (n =1,731)
|
Within 1 year (n=2,056)
|
After 1 year (n=606)
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P for modification | HR (95% CI) | P for modification | HR (95% CI) | P for modification | |
| Age | 0.312 | 0.163 | 0.209 | |||
| <65 years | 1.23 (1.04–1.45) | 1.39 (1.12–1.71) | 1.19 (0.86–1.66) | |||
| ≥65 years | 1.50 (1.10–2.05) | 1.89 (1.24–2.89) | 4.14 (1.94–8.80) | |||
| Sex | 0.911 | 0.440 | 0.240 | |||
| Female | 1.27 (1.08–1.50) | 1.52 (1.23–1.89) | 1.28 (0.92–1.77) | |||
| Male | 1.36 (0.995–1.87) | 1.37 (0.89–2.08) | 1.80 (0.89–3.63) | |||
| Disease duration | 0.255 | 0.338 | 0.913 | |||
| <3 years | 1.12 (0.85–1.48) | 1.26 (0.88–1.79) | 1.41 (0.75–2.65) | |||
| ≥3 years | 1.38 (1.17–1.63) | 1.61 (1.28–2.02) | 1.42 (1.01–1.99) | |||
| History within 1 year before anti-TNF treatment | ||||||
| CCI | 0.114 | 0.681 | <0.001 | |||
| <2 | 1.18 (0.98–1.43) | 1.46 (1.13–1.88) | 0.90 (0.60–1.35) | |||
| ≥2 | 1.44 (1.16–1.79) | 1.58 (1.19–2.11) | 2.51 (1.58–3.99) | |||
| MTX, mg/wk | 0.013 | 0.912 | <0.001 | |||
| ≤10 | 0.94 (0.71–1.24) | 1.47 (1.02–2.11) | 0.50 (0.27–0.92) | |||
| >10 | 1.43 (1.21–1.69) | 1.51 (1.21–1.88) | 2.06 (1.47–2.90) | |||
| SSZ | 0.172 | 0.489 | 0.796 | |||
| No | 1.49 (1.09–2.03) | 1.28 (0.86–1.91) | 1.92 (0.95–3.87) | |||
| Yes | 1.23 (1.04–1.44) | 1.56 (1.25–1.93) | 1.41 (1.02–1.95) | |||
| LEF | 0.755 | 0.552 | 0.490 | |||
| No | 1.26 (1.07–1.50) | 1.53 (1.23–1.91) | 1.36 (0.94–1.98) | |||
| Yes | 1.34 (1.03–1.76) | 1.38 (0.95–2.00) | 1.62 (0.99–2.65) | |||
| HCQ | 0.046 | 0.310 | 0.093 | |||
| No | 1.88 (1.30–2.71) | 1.99 (1.19–3.34) | 2.74 (1.33–5.66) | |||
| Yes | 1.20 (1.02–1.40) | 1.42 (1.16–1.75) | 1.22 (0.88–1.69) | |||
| NSAID | 0.212 | 0.275 | 0.864 | |||
| No | a | a | a | |||
| Yes | 1.28 (1.11–1.48) | 1.49 (1.23–1.80) | 1.41 (1.05–1.89) | |||
| Pd equivalent | 0.111 | 0.426 | 0.040 | |||
| ≤5 mg/d | 1.12 (0.88–1.42) | 1.43 (1.06–1.94) | 0.80 (0.46–1.37) | |||
| >5 mg/d | 1.38 (1.45–1.65) | 1.50 (1.17–1.91) | 1.89 (1.32–2.71) | |||
| Comedication | ||||||
| SSZ | 0.261 | 0.391 | 0.472 | |||
| No | 1.42 (1.14–1.75) | 1.39 (1.05–1.83) | 1.28 (0.87–1.86) | |||
| Yes | 1.19 (0.98–1.45) | 1.61 (1.24–2.09) | 1.58 (0.99–2.53) | |||
| LEF | 0.449 | 0.492 | 0.409 | |||
| No | 1.26 (1.08–1.47) | 1.45 (1.19–1.78) | 1.49 (1.09–2.03) | |||
| Yes | 1.51 (1.02–2.21) | 1.86 (1.07–3.23) | 1.02 (0.41–2.55) | |||
| HCQ | 0.990 | 0.633 | 0.385 | |||
| No | 1.35 (1.05–1.72) | 1.41 (1.01–1.96) | 1.51 (0.99–2.29) | |||
| Yes | 1.25 (1.05–1.50) | 1.51 (1.20–1.91) | 1.25 (0.82–1.91) | |||
| NSAID | 0.101 | 0.361 | 0.683 | |||
| No | 1.17 (0.29–4.65) | 1.34 (0.42–4.24) | 7.89 (0.09–724.89) | |||
| Yes | 1.29 (1.12–1.49) | 1.51 (1.25–1.83) | 1.44 (1.06–1.94) | |||
| Pd equivalent | 0.186 | 0.806 | 0.585 | |||
| ≤5 mg/d | 1.18 (0.98–1.43) | 1.42 (1.10–1.82) | 1.41 (1.00–1.99) | |||
| >5 mg/d | 1.43 (1.15–1.78) | 1.57 (1.17–2.10) | 1.62 (0.88–3.01) | |||
Notes: Cox proportional hazard regression analyses were conducted to calculate adjusted HRs after adjusting for sex, age at anti-TNF initiation (≤65 years, >65 years), disease duration (≤3 years, >3 years), CCI (≤1, >2) within 1 year before anti-TNF use, use of LEF, SSZ, NSAID, MTX (0–10 mg/wk, >10 mg/wk), and corticosteroid (Pd equivalent ≤5 mg/d, >5 mg/d) within 1 year before and after anti-TNF use.
95% CI was very large and covered one (ie, nonsignificant).
Abbreviations: HRs, hazard ratios; CI, confidence intervals; MTX, methotrexate; TNF, tumor necrosis factor; CCl, Charlson comorbidity index; SSZ, salazopyrin; LEF, leflunomide; HCQ, hydroxychloroquine; NSAID, nonsteroid anti-inflammatory drug; Pd, prednisolone.
Table S2.
The adjusted HRs with 95% CIs of drug discontinuation followed by antibiotics prescription for adalimumab compared with etanercept on concomitant MTX >10 mg/wk during all treatment periods, ≤1 year or >1 year, stratified by other covariates with the significance of their modification effects
| Variable | All treatment period (n=1,731)
|
Within 1 year (n=2,056)
|
After 1 year (n=606)
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P for modification | HR (95% CI) | P for modification | HR (95% CI) | P for modification | |
| Age | 0.851 | 0.100 | 0.246 | |||
| <65 years | 1.48 (1.12–1.97) | 1.76 (1.20–2.59) | 1.12 (0.62–2.02) | |||
| ≥65 years | 1.72 (1.07–2.75) | 3.57 (1.80–7.06) | 3.44 (0.87–13.56) | |||
| Sex | 0.733 | 0.610 | 0.502 | |||
| Female | 1.51 (1.15–1.98) | 2.03 (1.40–2.95) | 1.20 (0.68–2.12) | |||
| Male | 1.76 (1.04–2.99) | 2.62 (1.27–5.42) | 1.90 (0.53–6.87) | |||
| Disease duration | 0.291 | 0.465 | 0.205 | |||
| <3 years | 1.91 (1.17–3.14) | 2.45 (1.23–4.87) | 3.38 (0.89–12.82) | |||
| ≥3 years | 1.47 (1.11–1.94) | 2.05 (1.40–2.99) | 1.16 (0.64–2.10) | |||
| History within 1 year before anti-TNF treatment | ||||||
| CCI | 0.562 | 0.908 | 0.074 | |||
| <2 | 1.40 (0.98–1.98) | 2.16 (1.37–3.41) | 0.83 (0.38–1.84) | |||
| ≥2 | 1.69 (1.20–2.37) | 2.29 (1.41–3.73) | 2.31 (1.11–4.82) | |||
| MTX, mg/wk | 0.841 | 0.180 | 0.304 | |||
| ≤10 | 1.42 (0.90–2.23) | 3.15 (1.63–6.11) | 0.92 (0.33–2.57) | |||
| >10 | 1.50 (1.13–2.00) | 1.86 (1.26–2.73) | 1.57 (0.84–2.91) | |||
| SSZ | 0.026 | 0.643 | 0.771 | |||
| No | 2.47 (1.49–4.08) | 2.62 (1.34–5.12) | 1.82 (0.58–5.76) | |||
| Yes | 1.31 (0.99–1.74) | 1.93 (1.31–2.84) | 1.27 (0.70–2.29) | |||
| LEF | 0.945 | 0.668 | 0.597 | |||
| No | 1.52 (1.14–2.03) | 2.17 (1.48–3.19) | 1.16 (0.60–2.24) | |||
| Yes | 1.50 (0.95–2.38) | 2.00 (1.03–3.86) | 1.97 (0.80–4.83) | |||
| HCQ | 0.106 | 0.587 | 0.745 | |||
| No | 2.83 (1.42–5.62) | 3.35 (1.24–9.10) | 1.14 (0.28–4.62) | |||
| Yes | 1.40 (1.08–1.82) | 2.02 (1.42–2.87) | 1.24 (0.70–2.18) | |||
| NSAID | 0.846 | 0.893 | 1.00 | |||
| No | a | a | b | |||
| Yes | 1.50 (1.18–1.91) | 2.10 (1.51–2.92) | 1.32 (0.80–2.23) | |||
| Pd equivalent | 0.004 | 0.244 | 0.020 | |||
| ≤5 mg/d | 1.33 (0.86–2.05) | 1.87 (1.07–3.29) | 0.31 (0.09–1.08) | |||
| >5 mg/d | 1.64 (1.22–2.20) | 2.25 (1.49–3.40) | 2.31 (1.24–4.26) | |||
| Comedication | ||||||
| SSZ | 0.182 | 0.975 | 0.108 | |||
| No | 1.82 (1.27–2.61) | 2.08 (1.30–3.32) | 0.98 (0.53–1.84) | |||
| Yes | 1.38 (0.99–1.92) | 2.21 (1.38–3.54) | 2.22 (0.75–6.62) | |||
| LEF | 0.794 | 0.251 | 0.271 | |||
| No | 1.51 (1.16–1.96) | 1.97 (1.39–2.80) | 1.36 (0.80–2.34) | |||
| Yes | 1.53 (0.81–2.86) | 3.28 (1.06–10.15) | 1 | |||
| HCQ | 0.921 | 0.434 | 0.655 | |||
| No | 1.61 (1.04–2.48) | 1.92 (1.07–3.44) | 1.49 (0.73–1.24) | |||
| Yes | 1.50 (1.12–2.00) | 2.20 (1.48–3.29) | 1.29 (0.58–2.84) | |||
| NSAID | 0.960 | 0.968 | 0.888 | |||
| No | a | a | a | |||
| Yes | 1.51 (1.19–1.93) | 2.12 (1.52–2.96) | 1.28 (0.75–2.18) | |||
| Pd equivalent | 0.879 | 0.594 | 0.868 | |||
| ≤5 mg/d | 1.59 (1.15–2.21) | 2.33 (1.48–3.66) | 1.36 (0.73–2.54) | |||
| >5 mg/d | 1.43 (1.00–2.05) | 1.86 (1.14–3.03) | 1.82 (0.62–5.32) | |||
Notes: Cox proportional hazard regression analyses were conducted to calculate adjusted HRs after adjusting for sex, age at anti-TNF initiation (≤65 years, >65 years), disease duration (≤3 years, >3 years), CCI (≤1, >2) within 1 year before anti-TNF use, use of LEF, SSZ, NSAID, MTX (0–10 mg/wk, >10 mg/wk), and corticosteroid (Pd equivalent ≤5 mg/d, >5 mg/d) within 1 year before and after anti-TNF use.
95% CI was very large and covered one (ie, nonsignificant).
All patients used NSAID before anti-TNF initiation.
Abbreviations: HRs, hazard ratios; CI, confidence intervals; MTX, methotrexate; TNF, tumor necrosis factor; CCl, Charlson comorbidity index; SSZ, salazopyrin; LEF, leflunomide; HCQ, hydroxychloroquine; NSAID, nonsteroid anti-inflammatory drug; Pd, prednisolone.
Acknowledgments
The authors would like to thank the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan, Republic of China, for assistance with statistical analysis. The authors thank the members of the Bureau of National Health Insurance, Department of Health, and the National Health Research Institutes for providing and managing, respectively, the National Health Insurance Research Database.
Footnotes
Disclosure
Hsin-Hua Chen and Chao-Hsiun Tang received funding from Pfizer Limited, Taiwan, Republic of China. The authors report no other conflicts of interest in this work.
References
- 1.Yu KH, See LC, Kuo CF, Chou IJ, Chou MJ. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res (Hoboken) 2013;65:244–250. doi: 10.1002/acr.21820. [DOI] [PubMed] [Google Scholar]
- 2.Lai CH, Lai MS, Lai KL, Chen HH, Chiu YM. Nationwide population-based epidemiologic study of rheumatoid arthritis in Taiwan. Clin Exp Rheumatol. 2012;30:358–363. [PubMed] [Google Scholar]
- 3.Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 4.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 5.Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 6.Furst DE, Breedveld FC, Kalden JR, et al. Updated consensus statement on biological agents for the treatment of rheumatoid arthritis and other immune mediated inflammatory diseases (May 2003) Ann Rheum Dis. 2003;62(Suppl 2):ii2–ii9. doi: 10.1136/ard.62.suppl_2.ii2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinblatt ME. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc. 2013;124:16–25. [PMC free article] [PubMed] [Google Scholar]
- 8.Orme ME, Macgilchrist KS, Mitchell S, Spurden D, Bird A. Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70. Biologics. 2012;6:429–464. doi: 10.2147/BTT.S36707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kameda H, Kanbe K, Sato E, et al. Continuation of methotrexate resulted in better clinical and radiographic outcomes than discontinuation upon starting etanercept in patients with rheumatoid arthritis: 52-week results from the JESMR study. J Rheumatol. 2011;38:1585–1592. doi: 10.3899/jrheum.110014. [DOI] [PubMed] [Google Scholar]
- 10.Soliman MM, Ashcroft DM, Watson KD, Lunt M, Symmons DP, Hyrich KL. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:583–589. doi: 10.1136/ard.2010.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledingham J, Deighton C. Update on the British Society for Rheumatology guidelines for prescribing TNFα blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001) Rheumatology (Oxford) 2005;44:157–163. doi: 10.1093/rheumatology/keh464. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F. The epidemiology of drug treatment failure in rheumatoid arthritis. Bailliere’s Clin Rheumatol. 1995;9:619–632. doi: 10.1016/s0950-3579(05)80305-x. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Santana V, Gonzalez-Sarmiento E, Calleja-Hernandez M, Sanchez-Sanchez T. Comparison of drug survival rates for tumor necrosis factor antagonists in rheumatoid arthritis. Patient Prefer Adherence. 2013;7:719–727. doi: 10.2147/PPA.S47453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchesoni A, Zaccara E, Gorla R, et al. TNF-α antagonist survival rate in a cohort of rheumatoid arthritis patients observed under conditions of standard clinical practice. Ann N Y Acad Sci. 2009;1173:837–846. doi: 10.1111/j.1749-6632.2009.04621.x. [DOI] [PubMed] [Google Scholar]
- 15.Iannone F, Gremese E, Atzeni F, et al. Longterm retention of tumor necrosis factor-α inhibitor therapy in a large italian cohort of patients with rheumatoid arthritis from the GISEA registry: an appraisal of predictors. J Rheumatol. 2012;39:1179–1184. doi: 10.3899/jrheum.111125. [DOI] [PubMed] [Google Scholar]
- 16.Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856–1862. doi: 10.1136/ard.2008.098467. [DOI] [PubMed] [Google Scholar]
- 17.Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22–32. doi: 10.1002/art.27227. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29. doi: 10.1186/ar1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borah BJ, Huang X, Zarotsky V, Globe D. Trends in RA patients’ adherence to subcutaneous anti-TNF therapies and costs. Curr Med Res Opin. 2009;25:1365–1377. doi: 10.1185/03007990902896386. [DOI] [PubMed] [Google Scholar]
- 20.Tubach F, Salmon D, Ravaud P, Globe D. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009;60:1884–1894. doi: 10.1002/art.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann Rheum Dis. 2010;69:522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarra SV, Tang B, Lu L, et al. Risk of tuberculosis with anti-tumor necrosis factor-α therapy: substantially higher number of patients at risk in Asia. Int J Rheum Dis. 2014;17:291–298. doi: 10.1111/1756-185X.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreland LW. Inhibitors of tumor necrosis factor for rheumatoid arthritis. J Rheumatol Suppl. 1999;57:7–15. [PubMed] [Google Scholar]
- 24.Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–426. doi: 10.1124/jpet.301.2.418. [DOI] [PubMed] [Google Scholar]
- 25.Mitoma H, Horiuchi T, Tsukamoto H, et al. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor α-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum. 2008;58:1248–1257. doi: 10.1002/art.23447. [DOI] [PubMed] [Google Scholar]
- 26.Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin Ther. 2002;24:1720–1740. doi: 10.1016/s0149-2918(02)80075-3. discussion 1719. [DOI] [PubMed] [Google Scholar]
- 27.Wolbink GJ, Aarden LA, Dijkmans BA. Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol. 2009;21:211–215. doi: 10.1097/bor.0b013e328329ed8b. [DOI] [PubMed] [Google Scholar]
- 28.Fleischmann R, Shealy D. Developing a new generation of TNFα antagonists for the treatment of rheumatoid arthritis. Mol Interv. 2003;3:310–318. doi: 10.1124/mi.3.6.310. [DOI] [PubMed] [Google Scholar]
- 29.Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 30.Krieckaert CL, Jamnitski A, Nurmohamed MT, Kostense PJ, Boers M, Wolbink G. Comparison of long-term clinical outcome with etanercept treatment and adalimumab treatment of rheumatoid arthritis with respect to immunogenicity. Arthritis Rheum. 2012;64:3850–3855. doi: 10.1002/art.34680. [DOI] [PubMed] [Google Scholar]
- 31.Krieckaert CL, Nurmohamed MT, Wolbink GJ. Methotrexate reduces immunogenicity in adalimumab treated rheumatoid arthritis patients in a dose dependent manner. Ann Rheum Dis. 2012;71:1914–1915. doi: 10.1136/annrheumdis-2012-201544. [DOI] [PubMed] [Google Scholar]
- 32.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 33.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 34.Chen DY, Chen YM, Tsai WC, et al. Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis. Ann Rheum Dis. 2015;74:e16. doi: 10.1136/annrheumdis-2013-203893. [DOI] [PubMed] [Google Scholar]
- 35.Burmester GR, Kivitz AJ, Kupper H, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis. 2015;74:1037–1044. doi: 10.1136/annrheumdis-2013-204769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kristensen LE, Kapetanovic MC, Gulfe A, Soderlin M, Saxne T, Geborek P. Predictors of response to anti-TNF therapy according to ACR and EULAR criteria in patients with established RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology (Oxford) 2008;47:495–499. doi: 10.1093/rheumatology/ken002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
The adjusted HRs with 95% CIs of drug discontinuation for adalimumab compared with etanercept on concomitant MTX >10 mg/wk during all treatment periods, ≤1 year or >1 year, stratified by other covariates with the significance of their modification effects
| Variable | All treatment periods (n =1,731)
|
Within 1 year (n=2,056)
|
After 1 year (n=606)
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P for modification | HR (95% CI) | P for modification | HR (95% CI) | P for modification | |
| Age | 0.312 | 0.163 | 0.209 | |||
| <65 years | 1.23 (1.04–1.45) | 1.39 (1.12–1.71) | 1.19 (0.86–1.66) | |||
| ≥65 years | 1.50 (1.10–2.05) | 1.89 (1.24–2.89) | 4.14 (1.94–8.80) | |||
| Sex | 0.911 | 0.440 | 0.240 | |||
| Female | 1.27 (1.08–1.50) | 1.52 (1.23–1.89) | 1.28 (0.92–1.77) | |||
| Male | 1.36 (0.995–1.87) | 1.37 (0.89–2.08) | 1.80 (0.89–3.63) | |||
| Disease duration | 0.255 | 0.338 | 0.913 | |||
| <3 years | 1.12 (0.85–1.48) | 1.26 (0.88–1.79) | 1.41 (0.75–2.65) | |||
| ≥3 years | 1.38 (1.17–1.63) | 1.61 (1.28–2.02) | 1.42 (1.01–1.99) | |||
| History within 1 year before anti-TNF treatment | ||||||
| CCI | 0.114 | 0.681 | <0.001 | |||
| <2 | 1.18 (0.98–1.43) | 1.46 (1.13–1.88) | 0.90 (0.60–1.35) | |||
| ≥2 | 1.44 (1.16–1.79) | 1.58 (1.19–2.11) | 2.51 (1.58–3.99) | |||
| MTX, mg/wk | 0.013 | 0.912 | <0.001 | |||
| ≤10 | 0.94 (0.71–1.24) | 1.47 (1.02–2.11) | 0.50 (0.27–0.92) | |||
| >10 | 1.43 (1.21–1.69) | 1.51 (1.21–1.88) | 2.06 (1.47–2.90) | |||
| SSZ | 0.172 | 0.489 | 0.796 | |||
| No | 1.49 (1.09–2.03) | 1.28 (0.86–1.91) | 1.92 (0.95–3.87) | |||
| Yes | 1.23 (1.04–1.44) | 1.56 (1.25–1.93) | 1.41 (1.02–1.95) | |||
| LEF | 0.755 | 0.552 | 0.490 | |||
| No | 1.26 (1.07–1.50) | 1.53 (1.23–1.91) | 1.36 (0.94–1.98) | |||
| Yes | 1.34 (1.03–1.76) | 1.38 (0.95–2.00) | 1.62 (0.99–2.65) | |||
| HCQ | 0.046 | 0.310 | 0.093 | |||
| No | 1.88 (1.30–2.71) | 1.99 (1.19–3.34) | 2.74 (1.33–5.66) | |||
| Yes | 1.20 (1.02–1.40) | 1.42 (1.16–1.75) | 1.22 (0.88–1.69) | |||
| NSAID | 0.212 | 0.275 | 0.864 | |||
| No | a | a | a | |||
| Yes | 1.28 (1.11–1.48) | 1.49 (1.23–1.80) | 1.41 (1.05–1.89) | |||
| Pd equivalent | 0.111 | 0.426 | 0.040 | |||
| ≤5 mg/d | 1.12 (0.88–1.42) | 1.43 (1.06–1.94) | 0.80 (0.46–1.37) | |||
| >5 mg/d | 1.38 (1.45–1.65) | 1.50 (1.17–1.91) | 1.89 (1.32–2.71) | |||
| Comedication | ||||||
| SSZ | 0.261 | 0.391 | 0.472 | |||
| No | 1.42 (1.14–1.75) | 1.39 (1.05–1.83) | 1.28 (0.87–1.86) | |||
| Yes | 1.19 (0.98–1.45) | 1.61 (1.24–2.09) | 1.58 (0.99–2.53) | |||
| LEF | 0.449 | 0.492 | 0.409 | |||
| No | 1.26 (1.08–1.47) | 1.45 (1.19–1.78) | 1.49 (1.09–2.03) | |||
| Yes | 1.51 (1.02–2.21) | 1.86 (1.07–3.23) | 1.02 (0.41–2.55) | |||
| HCQ | 0.990 | 0.633 | 0.385 | |||
| No | 1.35 (1.05–1.72) | 1.41 (1.01–1.96) | 1.51 (0.99–2.29) | |||
| Yes | 1.25 (1.05–1.50) | 1.51 (1.20–1.91) | 1.25 (0.82–1.91) | |||
| NSAID | 0.101 | 0.361 | 0.683 | |||
| No | 1.17 (0.29–4.65) | 1.34 (0.42–4.24) | 7.89 (0.09–724.89) | |||
| Yes | 1.29 (1.12–1.49) | 1.51 (1.25–1.83) | 1.44 (1.06–1.94) | |||
| Pd equivalent | 0.186 | 0.806 | 0.585 | |||
| ≤5 mg/d | 1.18 (0.98–1.43) | 1.42 (1.10–1.82) | 1.41 (1.00–1.99) | |||
| >5 mg/d | 1.43 (1.15–1.78) | 1.57 (1.17–2.10) | 1.62 (0.88–3.01) | |||
Notes: Cox proportional hazard regression analyses were conducted to calculate adjusted HRs after adjusting for sex, age at anti-TNF initiation (≤65 years, >65 years), disease duration (≤3 years, >3 years), CCI (≤1, >2) within 1 year before anti-TNF use, use of LEF, SSZ, NSAID, MTX (0–10 mg/wk, >10 mg/wk), and corticosteroid (Pd equivalent ≤5 mg/d, >5 mg/d) within 1 year before and after anti-TNF use.
95% CI was very large and covered one (ie, nonsignificant).
Abbreviations: HRs, hazard ratios; CI, confidence intervals; MTX, methotrexate; TNF, tumor necrosis factor; CCl, Charlson comorbidity index; SSZ, salazopyrin; LEF, leflunomide; HCQ, hydroxychloroquine; NSAID, nonsteroid anti-inflammatory drug; Pd, prednisolone.
Table S2.
The adjusted HRs with 95% CIs of drug discontinuation followed by antibiotics prescription for adalimumab compared with etanercept on concomitant MTX >10 mg/wk during all treatment periods, ≤1 year or >1 year, stratified by other covariates with the significance of their modification effects
| Variable | All treatment period (n=1,731)
|
Within 1 year (n=2,056)
|
After 1 year (n=606)
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P for modification | HR (95% CI) | P for modification | HR (95% CI) | P for modification | |
| Age | 0.851 | 0.100 | 0.246 | |||
| <65 years | 1.48 (1.12–1.97) | 1.76 (1.20–2.59) | 1.12 (0.62–2.02) | |||
| ≥65 years | 1.72 (1.07–2.75) | 3.57 (1.80–7.06) | 3.44 (0.87–13.56) | |||
| Sex | 0.733 | 0.610 | 0.502 | |||
| Female | 1.51 (1.15–1.98) | 2.03 (1.40–2.95) | 1.20 (0.68–2.12) | |||
| Male | 1.76 (1.04–2.99) | 2.62 (1.27–5.42) | 1.90 (0.53–6.87) | |||
| Disease duration | 0.291 | 0.465 | 0.205 | |||
| <3 years | 1.91 (1.17–3.14) | 2.45 (1.23–4.87) | 3.38 (0.89–12.82) | |||
| ≥3 years | 1.47 (1.11–1.94) | 2.05 (1.40–2.99) | 1.16 (0.64–2.10) | |||
| History within 1 year before anti-TNF treatment | ||||||
| CCI | 0.562 | 0.908 | 0.074 | |||
| <2 | 1.40 (0.98–1.98) | 2.16 (1.37–3.41) | 0.83 (0.38–1.84) | |||
| ≥2 | 1.69 (1.20–2.37) | 2.29 (1.41–3.73) | 2.31 (1.11–4.82) | |||
| MTX, mg/wk | 0.841 | 0.180 | 0.304 | |||
| ≤10 | 1.42 (0.90–2.23) | 3.15 (1.63–6.11) | 0.92 (0.33–2.57) | |||
| >10 | 1.50 (1.13–2.00) | 1.86 (1.26–2.73) | 1.57 (0.84–2.91) | |||
| SSZ | 0.026 | 0.643 | 0.771 | |||
| No | 2.47 (1.49–4.08) | 2.62 (1.34–5.12) | 1.82 (0.58–5.76) | |||
| Yes | 1.31 (0.99–1.74) | 1.93 (1.31–2.84) | 1.27 (0.70–2.29) | |||
| LEF | 0.945 | 0.668 | 0.597 | |||
| No | 1.52 (1.14–2.03) | 2.17 (1.48–3.19) | 1.16 (0.60–2.24) | |||
| Yes | 1.50 (0.95–2.38) | 2.00 (1.03–3.86) | 1.97 (0.80–4.83) | |||
| HCQ | 0.106 | 0.587 | 0.745 | |||
| No | 2.83 (1.42–5.62) | 3.35 (1.24–9.10) | 1.14 (0.28–4.62) | |||
| Yes | 1.40 (1.08–1.82) | 2.02 (1.42–2.87) | 1.24 (0.70–2.18) | |||
| NSAID | 0.846 | 0.893 | 1.00 | |||
| No | a | a | b | |||
| Yes | 1.50 (1.18–1.91) | 2.10 (1.51–2.92) | 1.32 (0.80–2.23) | |||
| Pd equivalent | 0.004 | 0.244 | 0.020 | |||
| ≤5 mg/d | 1.33 (0.86–2.05) | 1.87 (1.07–3.29) | 0.31 (0.09–1.08) | |||
| >5 mg/d | 1.64 (1.22–2.20) | 2.25 (1.49–3.40) | 2.31 (1.24–4.26) | |||
| Comedication | ||||||
| SSZ | 0.182 | 0.975 | 0.108 | |||
| No | 1.82 (1.27–2.61) | 2.08 (1.30–3.32) | 0.98 (0.53–1.84) | |||
| Yes | 1.38 (0.99–1.92) | 2.21 (1.38–3.54) | 2.22 (0.75–6.62) | |||
| LEF | 0.794 | 0.251 | 0.271 | |||
| No | 1.51 (1.16–1.96) | 1.97 (1.39–2.80) | 1.36 (0.80–2.34) | |||
| Yes | 1.53 (0.81–2.86) | 3.28 (1.06–10.15) | 1 | |||
| HCQ | 0.921 | 0.434 | 0.655 | |||
| No | 1.61 (1.04–2.48) | 1.92 (1.07–3.44) | 1.49 (0.73–1.24) | |||
| Yes | 1.50 (1.12–2.00) | 2.20 (1.48–3.29) | 1.29 (0.58–2.84) | |||
| NSAID | 0.960 | 0.968 | 0.888 | |||
| No | a | a | a | |||
| Yes | 1.51 (1.19–1.93) | 2.12 (1.52–2.96) | 1.28 (0.75–2.18) | |||
| Pd equivalent | 0.879 | 0.594 | 0.868 | |||
| ≤5 mg/d | 1.59 (1.15–2.21) | 2.33 (1.48–3.66) | 1.36 (0.73–2.54) | |||
| >5 mg/d | 1.43 (1.00–2.05) | 1.86 (1.14–3.03) | 1.82 (0.62–5.32) | |||
Notes: Cox proportional hazard regression analyses were conducted to calculate adjusted HRs after adjusting for sex, age at anti-TNF initiation (≤65 years, >65 years), disease duration (≤3 years, >3 years), CCI (≤1, >2) within 1 year before anti-TNF use, use of LEF, SSZ, NSAID, MTX (0–10 mg/wk, >10 mg/wk), and corticosteroid (Pd equivalent ≤5 mg/d, >5 mg/d) within 1 year before and after anti-TNF use.
95% CI was very large and covered one (ie, nonsignificant).
All patients used NSAID before anti-TNF initiation.
Abbreviations: HRs, hazard ratios; CI, confidence intervals; MTX, methotrexate; TNF, tumor necrosis factor; CCl, Charlson comorbidity index; SSZ, salazopyrin; LEF, leflunomide; HCQ, hydroxychloroquine; NSAID, nonsteroid anti-inflammatory drug; Pd, prednisolone.