Abstract
Purpose
Brain metastasis (BM) is associated with impaired quality of life and increased mortality. The study aimed to compare BM risk after erlotinib administration and chemotherapy in stage IIIB/IV pulmonary adenocarcinoma patients harboring epidermal growth factor receptor (EGFR) mutation.
Patients and methods
Eligible patients underwent match pair process with matching factors, including age, sex, performance status score, first-line or second-line treatment, first-line chemotherapy regimen (for the second-line treatment subgroup), stage IIIB or IV, and genotypes of EGFR mutation. BM and mortality risk of both groups were recorded and compared.
Results
In total 129 matched pairs were included for analysis. During a median follow-up of 21.5 months, time to BM risk was longer and incidences of BM within 2 years were lower in patients who received erlotinib than chemotherapy in total population, as well as subgroups of first-line treatment, second-line treatment, stage IIIB, stage IV, exon 19 deletion mutation, and exon 21 L858R mutation. Similar overall survival time and 2-year survival rates were seen in two groups totally or in any subgroup. Multivariate analysis showed that BM was retarded in patients who received erlotinib administration (hazard ratio, 1.695; P=0.001) and in patients who were in stage IIIB (hazard ratio, 1.751; P=0.001).
Conclusion
Erlotinib administration decreases BM risk in advanced pulmonary adenocarcinoma patients harboring sensitive EGFR mutations.
Keywords: pulmonary adenocarcinoma, brain metastasis, epidermal growth factor receptor, erlotinib, match pair analysis
Introduction
Approximately half of the patients with non-small-cell lung cancer (NSCLC) suffer from brain metastasis (BM) during their clinical disease progression, which is associated with a significantly impaired quality of life and a fourfold increase in mortality.1–3 Advances in modern neuroimaging techniques and prolonged life span under improved systemic treatments also contribute to high incidences of BM, which makes prevention and control of BM emerge as a vital therapeutic strategy for tumor control and life quality improvement.
In the era of molecular targeted therapy, epidermal growth factor receptor (EGFR)-mutant lung cancer has been labeled as a specific subset of disease, demonstrating distinct clinical and biological features. Recent studies4,5 showed that patients with EGFR-mutant NSCLC were prone to develop BM than patients with wild EGFR. EGFR tyrosine kinase inhibitors (TKIs), such as erlotinib, are associated with a high response rate of up to 80% to EGFR-mutant lung cancer.6 In addition, it may penetrate through the blood–brain barrier (BBB) more efficiently7–10 than large chemotherapeutic molecules.11,12 However, few studies have been conducted to examine the impact of upfront EGFR-targeted therapy versus conventional chemotherapy on BM risk in NSCLC patients with sensitive EGFR mutation. In prospective, randomized, and controlled trails,13,14 comparing erlotinib or gefitinib with chemotherapy for advanced EGFR-mutant NSCLC, the differences in BM risk between two groups were not reported. A retrospective study15 exploring the effect of first-line EGFR-TKI versus conventional chemotherapy on risk of metastasis to the central nervous system (CNS) in stage IV EGFR-mutant NSCLC patients showed a lower cumulative risk of CNS metastasis after first-line EGFR-TKI treatment (77 patients) compared with the chemotherapy treatment (42 patients) (P=0.032). Nevertheless, the aforementioned data should be interpreted rigorously due to the small sample size, lack of survival information, and retrospective analysis in nature. Therefore, we retrieved clinical materials and survival data of stage IIIB/IV EGFR-mutant pulmonary adenocarcinoma, comparing BM risk and survival in matched pair patients who had been administered with erlotinib or chemotherapy.
Patients and methods
Patient selection
The study was approved by the Shandong Cancer Hospital and Institute ethics committee. All patients signed an informed consent form for voluntary participation prior to their inclusion in the study. From January 2008 to August 2014, pathologically confirmed pulmonary adenocarcinoma patients with treat-naive advanced (stage IIIB/IV) disease were reviewed for eligibility. Inclusion criteria were the patients 1) who were completely evaluated for medical history and physical examination and underwent an enhanced computed tomography (CT) scan of chest and abdomen, a magnetic resonance imaging (MRI) of brain, and a single-photon emission computerized tomography scan of bone at diagnosis; 2) who had histological diagnoses from biopsies with bronchoscope, mediastinoscope, or CT-guided needle biopsy; 3) with EGFR genotype determined as sensitive mutation by direct sequencing or amplification refractory mutation system technique; 4) who were BM-free at diagnosis for stage IV patients; 5) in whom efficacy evaluation after treatment was routinely performed; and 6) in whom follow-up examinations using enhanced CT and/or MRI scans of the brain were performed every 3 months for the initial 2 years and then every 6 months afterward at least.
Patients who received erlotinib were defined as erlotinib group, while those who received chemotherapy were defined as chemotherapy group. Additional inclusion criteria for the erlotinib group were the patients 1) in whom erlotinib was administrated in first-line or second-line treatment (with cisplatin/pemetrexed or carboplatin/paclitaxel applied in first-line chemotherapy for at least two cycles); 2) in whom erlotinib was orally administrated at a prescribed daily dose of 100–150 mg until any disease progression or intolerable toxicity was evaluated by qualified oncologists; 3) without any anti-EGFR therapy, other than erlotinib, before the occurrence of BM; 4) in whom erlotinib administration lasted for >2 months, followed by efficacy evaluation based on the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines16 obtaining complete response (CR)/partial response (PR) or stable disease (SD). Additional inclusion criteria for the chemotherapy group were the patients 1) in whom chemotherapy was administrated in first-line and/or second-line treatment; 2) in whom at least two cycles of cisplatin/pemetrexed or carboplatin/paclitaxel combined regimens in first-line treatment; 3) in whom at least two cycles of single or combined regimens including docetaxel, pemetrexed, gemcitabine, and platinum in second-line treatment; and 4) without any anti-EGFR therapy before the occurrence of BM. Additionally, doses and duration of chemotherapy regimens were applied according to the clinical guidelines. Patients who received concurrent radiotherapy during first-line and/or second-line treatments were excluded.
Study design
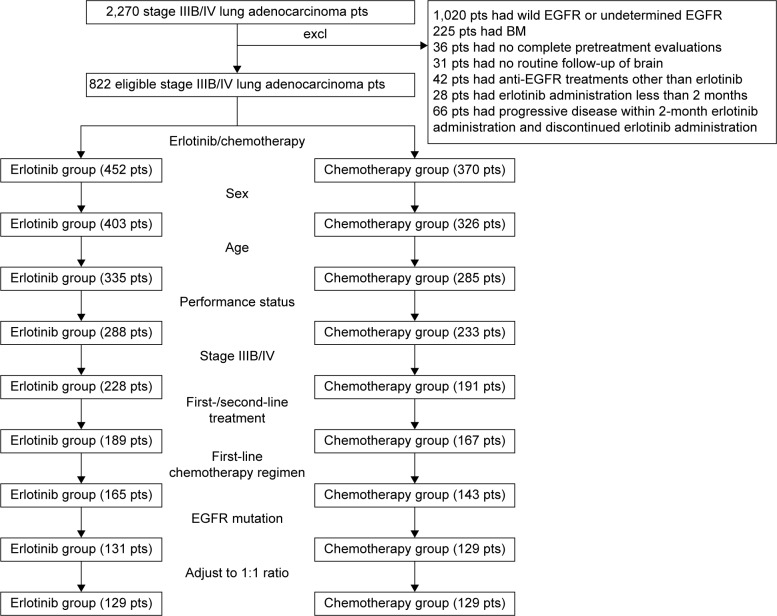
The information of all the eligible patients were retrieved from the patient database, including age, sex, smoking history, performance status (PS) score (Eastern Cooperative Oncology Group), and treatment schemes (regimens and cycles of chemotherapy; targets and doses/fractionation of radiotherapy; classifications, doses, and duration of targeted molecular treatments; response evaluation of the treatment; etc). The matching factors included age (within ±5 years), sex, PS, first-/second-line treatment, first-line chemotherapy regimen (for second-line treatment subgroup), stage IIIB/IV, and EGFR genotype. If more than one patient in a group was eligible, the best match was chosen by random selection (Figure 1). The matching procedure was conducted blindly without any information about patient outcomes.
Figure 1.
Flowchart of match pair process.
Abbreviations: excl, excluded; EGFR, epidermal growth factor receptor; BM, brain metastasis; pts, patients.
The first day of erlotinib administration or chemotherapy was set as the starting point for analysis. The end point event was the occurrence of BM, which was diagnosed by enhanced brain MRI or CT. The definition of time to BM was the time interval from first day of first-/second-line treatment to BM or the last follow-up. Overall survival (OS) was defined as the time interval from the starting point of the research study to death by any reason or the last follow-up. The PS score and adverse reactions of each patient during erlotinib administration or chemotherapy were also recorded and analyzed.
The patterns of disease progression during follow-up were assessed based on pathology and/or serial images, including CT, MRI, and positron emission tomography. Local–regional progression, BM development, and other distant metastases were recorded separately, regardless of the timing of disease progression. For patients suffering from BM, subsequent treatments were recorded and compared. For those who received radiotherapy for BM, including whole brain radiation therapy (WBRT) or stereotactic radiosurgery (SRS), response rates of disease were evaluated according to the RECIST.16
EGFR mutation test
Direct sequencing or amplification refractory mutation system using ADx EGFR mutations detection kit (Amoy Diagnostics, Xiamen, People’s Republic of China) was applied to determine EGFR genotype. All procedures were performed following the user manual. Sensitive genotypes, including exon 19 deletion, exon 21 L858R and L861, exon 18 G719X and G719, and exon 20 S768I mutations, can be detected. Patients who underwent EGFR determination by paraffin-embedded tissue after initial diagnosis or even after the occurrence of BM were also eligible.
Statistical methods
Statistical analysis was performed using SPSS (Version 19.0). The Fisher’s exact test was applied for every matching criterion and nonmatching parameters. The survival curves (time to BM and OS) were compared by Kaplan–Meier method and log rank test. The incidences of BM within 2 years and the 2-year survival rates were calculated from the survival curves. Factors that are associated with the survival outcomes were explored by univariate analysis. The subsequent multivariate survival analysis was also conducted by Cox regression model with the backward stepwise manner. Two-sided P-values <0.05 were considered as statistically significant.
Results
Patient characteristics
A total of 2,270 advanced pulmonary adenocarcinoma patients were screened in our institution. Based on the matching criteria, 129 matched pairs were included for final analysis (Figure 1). Table 1 summarized the matched clinical and tumor factors and the comparisons between the erlotinib and the chemotherapy groups. The median age was 58 years old (range: 22–89) totally. A balance existed between the erlotinib group and the chemotherapy group in all clinical characteristics. Sixty-six pairs and 63 pairs of patients had sensitive EGFR mutations of exon 19 deletion and exon 21 L858R mutations, respectively. Mutations in exon 21 L861 (one patient) and exon 18 G719 (one patient) were also found in the erlotinib group but failed in match pair process. No other EGFR mutation was detected.
Table 1.
Basic clinical characteristics of 129 pairs of pulmonary adenocarcinoma patients
| Characteristics | Erlotinib group | Chemotherapy group |
|---|---|---|
| Age (years) | ||
| Median (range) | 59 (22–89) | 58 (28–82) |
| <60 | 70 (54.3%) | 71 (55.0%) |
| ≥60 | 59 (45.7%) | 58 (45.0%) |
| Sex | ||
| Male | 56 (43.4%) | 56 (43.4%) |
| Female | 73 (56.6%) | 73 (56.6%) |
| Stage | ||
| IIIB | 54 (41.9%) | 54 (41.9%) |
| IV | 75 (58.1%) | 75 (58.1%) |
| EGFR genotype | ||
| Exon 19 deletion | 66 (51.2%) | 66 (51.2%) |
| Exon 21 L858R mutation | 63 (48.8%) | 63 (48.8%) |
| Performance status | ||
| 0–1 | 108 (83.7%) | 108 (83.7%) |
| 2–3 | 21 (16.3%) | 21 (16.3%) |
| Treatment | ||
| First-line | 66 (51.2%) | 66 (51.2%) |
| Second-line | 63 (48.8%) | 63 (48.8%) |
| First-line regimens in second-line subgroup | ||
| Cisplatin/pemetrexed | 43 (68.3%) | 43 (68.3%) |
| Carboplatin/paclitaxel | 20 (31.7%) | 20 (31.7%) |
Note: All P-values >0.05.
Abbreviation: EGFR, epidermal growth factor receptor.
Brain metastases rate, time, and correlation factors
At last follow-up, 73 patients in the erlotinib group and 88 patients in the chemotherapy group experienced BM. Twenty versus 33 patients in the erlotinib and the chemotherapy groups experienced BM as the first site of disease progression. In addition to BM, 49 versus 58 patients in the erlotinib and the chemotherapy groups also suffered local–regional progression and/or other distant metastases. Among patients not suffering from BM by last follow-up, 31 patients in the erlotinib group and 26 patients in the chemotherapy group experienced extracranial disease progression.
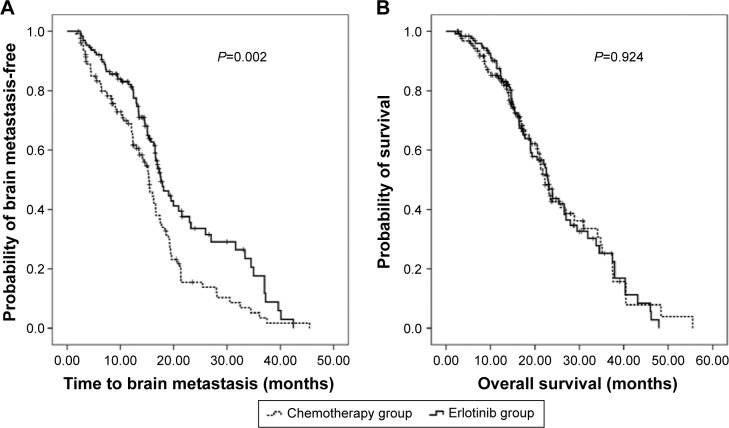
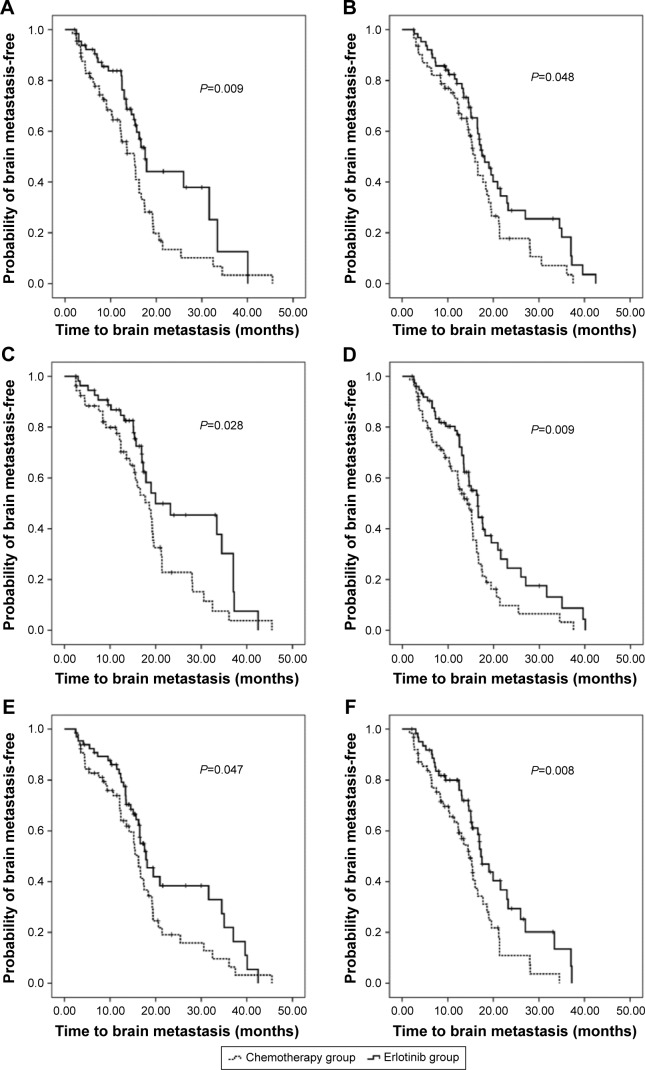
Table 2 and Figures 2 and 3 presented the incidences of BM and the time to BM in total patients and subgroups. By the end of a median follow-up interval of 21.50 months (range: 2.63–55.53 months), the incidences of BM within 2 years were 66.40% and 84.50% for the erlotinib and the chemotherapy groups (P=0.001), respectively. The median time to BM was 17.60 months for the erlotinib group, whereas 15.37 months for the chemotherapy group (P=0.002). Furthermore, subgroup analysis also showed significantly that incidences of BM within 2 years are lower and time to BM is longer in patients who received erlotinib treatment than chemotherapy in all subgroups, including first-line treatment, second-line treatment, stage IIIB, stage IV, exon 19 deletion mutation, and L858R mutation in exon 21.
Table 2.
Time to BM and incidences of BM within 2 years in total population and subgroups
| Subgroup | Time to BM (median, month)
|
P-value | Incidences of BM within 2 years (%)
|
P-value | ||
|---|---|---|---|---|---|---|
| Erlotinib group | Chemotherapy group | Erlotinib group | Chemotherapy group | |||
| Total patients (129 pairs) | 17.60 (15.55–19.65) | 15.37 (13.99–16.75) | 0.002 | 66.40 (55.23–77.57) | 84.50 (76.27–92.73) | 0.001 |
| First-line treatment (66 pairs) | 17.60 (15.59–19.61) | 15.20 (11.71–18.69) | 0.009 | 55.80 (38.94–72.66) | 86.50 (75.33–97.67) | <0.001 |
| Second-line treatment (63 pairs) | 18.13 (15.61–20.65) | 16.00 (14.44–17.56) | 0.048 | 61.20 (47.28–75.12) | 82.30 (70.15–94.45) | 0.016 |
| Stage IIIB (54 pairs) | 19.97 (6.54–33.40) | 18.63 (14.61–22.65) | 0.028 | 54.60 (36.37–72.83) | 77.20 (63.09–91.31) | 0.014 |
| Stage IV (75 pairs) | 16.50 (14.62–18.38) | 14.20 (11.45–16.95) | 0.009 | 75.50 (61.98–89.02) | 90.30 (80.70–99.90) | 0.027 |
| Exon 19 deletion (66 pairs) | 17.90 (15.08–20.72) | 16.23 (14.12–18.34) | 0.047 | 61.60 (46.12–77.08) | 80.90 (68.94–92.86) | 0.034 |
| Exon 21 L858R mutation (63 pairs) | 17.30 (14.70–19.90) | 14.60 (12.00–17.20) | 0.008 | 70.60 (55.12–86.08) | 89.10 (80.08–98.12) | 0.014 |
Note: Values within parentheses show 95% CI.
Abbreviations: BM, brain metastasis; CI, confidence interval.
Figure 2.
Time to BM (A) and overall survival (B) of the erlotinib group versus chemotherapy group.
Abbreviation: BM, brain metastasis.
Figure 3.
Time to BM in the erlotinib group versus the chemotherapy group in subgroups of first-line treatment (A), second-line treatment (B), stage IIIB (C), stage IV (D), exon 19 deletion (E), and exon 21 L858R mutation (F).
Abbreviation: BM, brain metastasis.
Univariate analysis demonstrated that stage IIIB (P=0.001) and erlotinib treatment (P=0.002) were significantly related to reduced BM risk. As for multivariate analysis, the time to BM was longer for patients with erlotinib administration (hazard ratio [HR], 1.695; 95% confidence interval [CI], 1.238–2.320; P=0.001) and stage IIIB (HR, 1.751; 95% CI, 1.266–2.423; P=0.001).
Treatments and response
Erlotinib/gefitinib was applied after the occurrence of BM in chemotherapy group in 79 patients. Of all patients who experienced BM, 62 versus 73 patients in the erlotinib and the chemotherapy groups received brain irradiation combined with erlotinib/gefitinib (30 vs 14 patients) or chemotherapy (15 vs 32 patients) or without any systemic treatment. For patients who had single BM, SRS (ten in the eroltinib group and 14 in the chemotherapy group), or WBRT plus SRS boost (three in the eroltinib group and two in the chemotherapy group) were delivered. Objective response rates (CR + PR) of brain irradiation were 54.8% (six CRs and 28 PRs) and 56.2% (seven CRs and 34 PRs) in patients administrating erlotinib and chemotherapy (P=0.986), respectively.
Survival analysis and adverse reactions
A median OS of 22.60 months (95% CI, 20.95–24.25 months) and a 2-year survival rate of 43.10% (95% CI, 35.06%–51.14%) were calculated for total population. The median OS was 22.93 months and 22.27 months for the erlotinib and the chemotherapy applied patients, with 2-year survival rates of 43.70% and 42.60%, respectively (P=0.924, P=1.000; Figure 2, Table 3). Subgroup analysis showed no statistical differences in OS and 2-year survival rates between the different treatments in subgroups of first-line treatment (P=0.796, P=0.596), second-line treatment (P=0.894, P=0.591), stage IIIB (P=0.507, P=1.000), stage IV (P=0.391, P=0.866), exon 19 deletion mutation (P=0.993, P=1.000), or exon 21 L858R mutation (P=0.828, P=1.000).
Table 3.
OS and 2-year survival rates of total population and subgroups
| Subgroup | OS (median, month)
|
P-value | 2-year survival rates (%)
|
P-value | ||
|---|---|---|---|---|---|---|
| Erlotinib group | Chemotherapy group | Erlotinib group | Chemotherapy group | |||
| Total patients (129 pairs) | 22.93 (21.16–24.70) | 22.27 (20.04–24.50) | 0.924 | 43.70 (33.12–54.28) | 42.60 (30.45–54.75) | 1.000 |
| First-line treatment (66 pairs) | 22.57 (17.35–27.79) | 21.17 (15.10–27.24) | 0.796 | 38.20 (21.93–54.47) | 44.60 (27.35–61.85) | 0.596 |
| Second-line treatment (63 pairs) | 23.97 (18.93–29.01) | 22.27 (20.13–24.41) | 0.894 | 48.20 (34.09–62.31) | 41.30 (24.44–58.16) | 0.591 |
| Stage IIIB (54 pairs) | 26.63 (21.83–31.43) | 25.70 (11.17–40.23) | 0.507 | 50.30 (33.84–66.76) | 50.80 (32.57–69.03) | 1.000 |
| Stage IV (75 pairs) | 22.57 (18.32–26.82) | 20.6 (15.52–25.68) | 0.391 | 39.00 (25.28–52.72) | 36.20 (20.32–52.08) | 0.866 |
| Exon 19 deletion (66 pairs) | 23.07 (16.73–29.41) | 22.27 (19.80–24.74) | 0.993 | 44.10 (29.01–59.19) | 42.10 (26.22–57.98) | 1.000 |
| Exon 21 L858R mutation (63 pairs) | 22.93 (20.94–24.92) | 21.73 (16.44–27.02) | 0.828 | 43.50 (28.41–58.59) | 43.90 (25.28–62.52) | 1.000 |
Note: Values within parentheses show 95% CI.
Abbreviations: OS, overall survival; CI, confidence interval.
Univariate analysis shows that longer OS is significantly associated with stage IIIB (P=0.003) but not with age (P=0.592), sex (P=0.416), PS score (P=0.603), erlotinib/chemotherapy administration (P=0.924), EGFR mutation genotypes (P=0.937), or first-/second-line treatment (P=0.604). In multivariate analysis, OS remained longer for patients with stage IIIB disease (HR, 1.719; 95% CI, 1.192–2.479; P=0.004).
Approximately half of patients maintained stable PS score before, during, and after erlotinib administration. Forty-one patients experienced improved PS score during erlotinib treatment because of symptoms relieve. Nineteen patients suffered severe but tolerable adverse reactions with decreased PS score. As for the chemotherapy group, six patients obtained elevated PS score and 44 patients suffered deteriorated PS. The difference between the erlotinib group and the chemotherapy group was statistically significant (P<0.001). The most common adverse event with erlotinib was rash (81.4%) and diarrhea (40.3%), with most cases classified as grade 1 or 2 in severity. Chemotherapy was associated with a high incidence of gastrointestinal adverse reactions (86.8%) and hematologic toxicities (75.2%). Grades 3–4 leukopenia and thrombocytopenia were significantly more frequent with chemotherapy compared with erlotinib (1.6% vs 31.8%, P<0.001; 0.8% vs 24.8%, P<0.001).
Subgroup analysis
For the erlotinib group, according to different clinical responses to erlotinib after 2-month administration, subgroup analysis showed that the median times to BM, median OS, incidences of BM within 2 years, and 2-year survival rates were 19.03 months, 23.07 months, 59.00%, and 44.40% for the CR/PR patients, while 15.37 months, 22.57 months, 85.40%, and 40.80% for the SD patients, respectively (P=0.001, P=0.465, P=0.007, P=0.835). Further analysis showed that time to BM is longer and the incidences of BM within 2 years are lower in the CR/PR group than in the SD group, with substantial statistical significance in subgroups of exon 19 deletion mutation (P=0.009, P=0.043) and L858R mutation in exon 21 (P=0.028, 0.047). For OS and 2-year survival rates, no statistical significance existed in the subgroups of exon 19 deletion mutation (P=0.247, P=0.579) or exon 21 L858R mutation (P=0.972, P=1.000).
When analysis narrowed to the second-line treatment group, similar median times to BM, median OS, incidences of BM within 2 years, and 2-year survival rates were seen in patients receiving cisplatin/pemetrexed than those receiving carboplatin/paclitaxel (18.63 vs 15.13 months, P=0.192; 23.97 vs 20.60 months, P=0.159; 75.70% vs 76.70%, P=1.000; and 49.00% vs 38.40%, P=0.593, respectively). Subgroup analysis showed that time to BM was longer and incidences of BM within 2 years were lower in erlotinib administration than chemotherapy in patients receiving cisplatin/pemetrexed (P=0.080, P=0.293) or carboplatin/paclitaxel (P=0.275, P=1.000) without statistical significance. For OS and 2-year survival rates, no statistical significance existed in the subgroups of cisplatin/pemetrexed (P=0.897, P=1.000) and carboplatin/paclitaxel (P=0.761, P=0.353).
Discussion
The treatment for patients with EGFR-mutant pulmonary adenocarcinoma has been significantly improved. However, BM still occurs in many patients months or even years after their initial treatment. Mutant EGFR genotype and BM were correlated significantly in patients with pulmonary adenocarcinomas as exhibited in a recent study.4 More EGFR mutations were reported in patients with existed BM than extracranial metastases. In this retrospective matched pair study, we found that erlotinib treatment reduced BM risk in pulmonary adenocarcinoma patients with stage IIIB/IV EGFR-mutant NSCLC. It suggested that erlotinib administration might prevent or delay the occurrence of BM.
It is reasonable to suppose that EGFR-targeted therapy may be more effective for EGFR-mutant lung cancer patients in reducing BM risk because of the confirmed systemic response and molecular permeability through intact BBB. Present study showed prolonged time to BM and a lowered 2-year cumulative risk of BM development in patients receiving erlotinib than chemotherapy. The cause-specific HR for erlotinib versus chemotherapy was 1.695, suggesting an increased risk of 69.5% for chemotherapy than erlotinib. Thus, the observed lower BM incidence seems to be due, at least in part, to the effect of the EGFR TKI. The promising effect of erlotinib was also seen in subgroups of patients with first-line treatment, second-line treatment, stage IIIB disease, stage IV disease, exon 19 deletion mutation, and L858R mutation in exon 21. For patients administrated with erlotinib, CR/PR of extracranial disease predicted lower BM risk than effect evaluation of SD. Cisplatin/pemetrexed and carboplatin/paclitaxel applied in first-line treatment resulted similar BM risks in patients of the second-line treatment group. When considered for OS, no statistical significances were seen between the erlotinib and the chemotherapy groups or in any subgroup. Existed studies17,18 showed potential better prognostic values in patients harboring exon 19 deletion mutation compared with patients with L858R mutation. In our analysis, similar survival outcomes were found between both EGFR mutations. During follow-up, approximately two-thirds of patients who suffered from BM received subsequent WBRT or SRS, either alone or combined with EGFR TKI or systemic chemotherapy. Objective response rates were >50%, which was similar to previous studies.19 In addition, erlotinib provided significant improvement in quality of life compared with chemotherapy in terms of simple PS score. In consistent with existed reports, toxicities of erlotinib were exhibited as rash, diarrhea, etc, which were milder in severity and easier to be controlled when compared with chemotherapy.
Erlotinib accumulation in BM has been clearly exhibited through 11C-erlotinib imaging using clinical positron emission tomography.8 Furthermore, after erlotinib administration, high erlotinib concentrations in the cerebrospinal fluid and the subsequent BM regression were certified.7,8 For patients with asymptomatic BM, single erlotinib administration was also active and well tolerated.20 It is worth noting that EGFR TKIs may also pass through an intact BBB in patients not suffering from BM.9,10 Concentrations of erlotinib had been determined to be substantial enough to inhibit tumor cell proliferation in cerebrospinal fluid after an intravenous dose in healthy adult rhesus monkeys.10,21 However, the contributing effects of EGFR-targeted therapy on the risk of BM remain undefined in clinical practice.
The 2.23-month longer median time to BM in the patients administrating erlotinib failed to transfer into survival benefit in our study. Although BM occurrence largely shortened the survival of patients, subsequent treatments, including radiotherapy, systemic chemotherapy, and anti-EGFR treatment, succeeded in controlling BM to a certain extent. Disease progression in local disease and distant metastases other than brain also account for the balanced survival between two groups. Additionally, several randomized studies13,22–24 demonstrated that the first-line EGFR TKI resulted significant improvement in both disease response to treatment and progression-free survival than conventional platinum-based combined chemotherapy, but not in OS. The rather high proportion of patients in the chemotherapy arm crossing over to the EGFR TKI arm during disease progression provided the interpretation. Similarly in our study, 79 patients from the chemotherapy group took EGFR TKI orally after BM occurrence. The crossing over to erlotinib treatments makes the survival benefit not significant enough to be analyzed in statistics. Furthermore, for some patients in our study who died without BM occurrence, the 6.0-month time interval between median time to BM and median OS was shorter than the survival time after BM occurrence.
Although the data from this study are well compared and promising, it is important to take into consideration the limitations of the study. First, although our study used a match pairing procedure to reduce the effect of several variables on the outcomes, the retrospective single-center nonrandomized design weakened the persuasion when interpreting the results. Therefore, comparisons were carried out in subgroups of stage IIIB, stage IV, first-line and second-line treatments, and different EGFR mutations, which broadened the potential benefit population, thus helped to explore the best benefit population and best time point for erlotinib administration. Second, due to the 6-year long time span of our study, majority patients in the chemotherapy group did not undergo EGFR mutation screening at initial diagnosis because of underestimating its importance. Currently, EGFR mutation genotype is routinely determined in pulmonary adenocarcinoma. Furthermore, for lung cancer patients with BM, highly sensitive polymerase chain reaction techniques provide the feasibility of determination of EGFR mutation genotype in BM,25 which provides detail biological information of BM and thus guide treatment decisions in clinical practice.
Conclusion
In summary, present matched pair study demonstrated the role of erlotinib administration in delaying and/or preventing BM in stage IIIB/IV EGFR-mutant pulmonary adenocarcinoma patients. It is necessary to conduct well-designed prospective clinical trials with specific BM end points, scheduled CNS imaging, and confirmed EGFR mutation status to validate our findings.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (81301868 and 81502667) and Natural Science Foundation of Shandong, People’s Republic of China (ZR2014HP041).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mamon HJ, Yeap BY, Janne PA, et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;23(7):1530–1537. doi: 10.1200/JCO.2005.04.123. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 3.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 4.Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9(2):195–199. doi: 10.1097/JTO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Park IK, Park MS, et al. Activating mutations within the EGFR kinase domain: a molecular predictor of disease-free survival in resected pulmonary adenocarcinoma. J Cancer Res Clin Oncol. 2009;135(12):1647–1654. doi: 10.1007/s00432-009-0611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller VA, Riely GJ, Zakowski MF, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008;26(9):1472–1478. doi: 10.1200/JCO.2007.13.0062. [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non- small-cell lung cancer. Mol Clin Oncol. 2014;2(1):116–120. doi: 10.3892/mco.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber B, Winterdahl M, Memon A, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol. 2011;6(7):1287–1289. doi: 10.1097/JTO.0b013e318219ab87. [DOI] [PubMed] [Google Scholar]
- 9.McKillop D, Hutchison M, Partridge EA, et al. Metabolic disposition of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, in rat, dog and man. Xenobiotica. 2004;34(10):917–934. doi: 10.1080/00498250400009171. [DOI] [PubMed] [Google Scholar]
- 10.Meany HJ, Fox E, McCully C, et al. The plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite (OSI-420) after intravenous administration of erlotinib in non-human primates. Cancer Chemother Pharmacol. 2008;62(3):387–392. doi: 10.1007/s00280-007-0616-3. [DOI] [PubMed] [Google Scholar]
- 11.Soffietti R, Ruda R, Trevisan E. Brain metastases: current management and new developments. Curr Opin Oncol. 2008;20(6):676–684. doi: 10.1097/CCO.0b013e32831186fe. [DOI] [PubMed] [Google Scholar]
- 12.Kaal EC, Niel CG, Vecht C. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4(5):289–298. doi: 10.1016/S1474-4422(05)70072-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802) a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 14.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 15.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18(16):4406–4414. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Liu WS, Zhao LJ, Pang QS, Yuan ZY, Li B, Wang P. Prognostic value of epidermal growth factor receptor mutations in resected lung adenocarcinomas. Med Oncol. 2014;31(1):771. doi: 10.1007/s12032-013-0771-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Sheng J, Kang S, et al. Patients with exon 19 deletion were associated with longer progression-free survival compared to those with L858R mutation after first-line EGFR-TKIs for advanced non-small cell lung cancer: a meta-analysis. PLoS One. 2014;9(9):e107161. doi: 10.1371/journal.pone.0107161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma S, Xu Y, Deng Q, Yu X. Treatment of brain metastasis from non-small cell lung cancer with whole brain radiotherapy and Gefitinib in a Chinese population. Lung Cancer. 2009;65(2):198–203. doi: 10.1016/j.lungcan.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803) Ann Oncol. 2013;24(4):993–999. doi: 10.1093/annonc/mds529. [DOI] [PubMed] [Google Scholar]
- 21.Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997;57(21):4838–4848. [PubMed] [Google Scholar]
- 22.Rosell R, Moran T, Queralt C, et al. Spanish Lung Cancer Group Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 23.Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line – is there a difference? J Clin Oncol. 2013;31(8):1081–1088. doi: 10.1200/JCO.2012.43.0652. [DOI] [PubMed] [Google Scholar]
- 24.Wu JY, Yu CJ, Yang CH, et al. First- or second-line therapy with gefitinib produces equal survival in non-small cell lung cancer. Am J Respir Crit Care Med. 2008;178(8):847–853. doi: 10.1164/rccm.200803-389OC. [DOI] [PubMed] [Google Scholar]
- 25.Kamila WK, Michał S, Paweł K, et al. EGFR activating mutations detected by different PCR techniques in Caucasian NSCLC patients with CNS metastases: short report. Clin Exp Metastasis. 2013;30(8):1063–1071. doi: 10.1007/s10585-013-9603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]