Abstract
At the end of 2013, chikungunya virus (CHIKV) emerged in Saint Martin Island, Caribbean. The Asian lineage was identified. Seven months after this introduction, the seroprevalence was 16.9% in the population of Saint Martin and 39.0% of infections remained asymptomatic. This moderate attack rate and the apparent limited size of the outbreak in Saint Martin could be explained by control measures involved to lower the exposure of the inhabitants. Other drivers such as climatic factors and population genetic factors should be explored. The substantial rate of asymptomatic infections recorded points to a potential source of infection that can both spread in new geographic areas and maintain an inconspicuous endemic circulation in the Americas.
Chikungunya virus (CHIKV) is a virus of the family Togaviridae, genus Alphavirus, transmitted by Aedes mosquitoes, first isolated in Tanzania in 1953.1 The burden of the disease is related to persistent arthralgia that sometimes outlasts the initial characteristic triad: fever, arthritis, and rash.2 CHIKV strains can be divided into three genetic lineages: west African, east/central/south African (ECSA), and Asian lineage.3 Over the last decade, the ECSA lineage became prevalent worldwide causing outbreaks in Europe, Africa, Indian Ocean, and south Asia.4
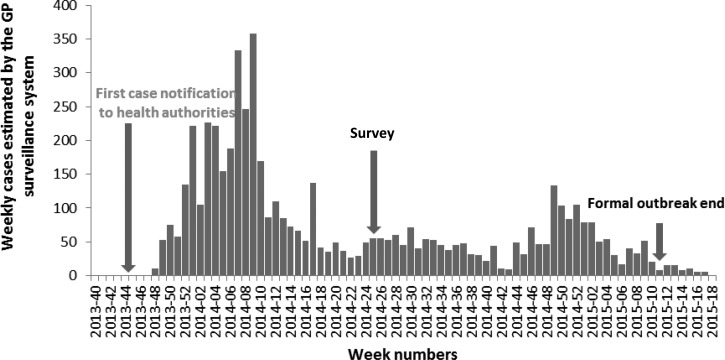
Saint Martin Island is divided into two parts: in the north, the French overseas territory of Saint Martin (∼36,000 inhabitants) and in the south, Sint Maarten (∼40,000), country of the kingdom of the Netherlands. The first cases of CHIKV infection in the Americas were identified in Saint Martin in November 2013; the Asian lineage was involved.5 The spread of CHIKV from human to human by the widely distributed vector Aedes aegypti quickly evolved into an outbreak. By February 2014, weekly clinical cases of CHIKV infections diagnosed by general practitioners peaked at 226 (Figure 1 ). Since March, CHIKV circulation decreased in Saint Martin to a weekly average of 42 clinical cases.
Figure 1.
Number of weekly incident cases of chikungunya reported by the general practitioner surveillance system, Saint Martin.
In early July, with the beginning of the wet season, we expected an increased activity of A. aegypti vector mosquitoes. To anticipate the dynamics of chikungunya in Saint Martin and inform decision making, we conducted a serosurvey to assess the level of herd immunity and the proportion of asymptomatic infections.
For this serosurvey, we constituted a convenience sample, taking advantage of the sole laboratory of Saint Martin. Between July 3 and 8, 2014 (Figure 1), all individuals attending the laboratory for any type of biological analysis were offered a serological test for CHIKV. Only people living in Saint Martin for over 6 months were included. Participants or their legal guardian signed an informed consent and completed a questionnaire collecting information on gender, age, and possibly symptoms of CHIKV infection (joint pain and fever) during the last 6 months. A 5 mL sample of venous blood was collected from adults and 2 mL for children (those less than 6 months of age were excluded). The Advisory Ethical Committee of Paris and the French Data Protection Authority approved the study. Sera were collected and kept at −20°C for 1–6 days before being sent in dry ice to the regional French National Reference Center for Arboviruses, Institut Pasteur de Guyane. Both IgM and IgG anti-CHIKV-specific antibodies were screened in sera using an “in-house” enzyme-linked immunosorbent assay (IgM antibody capture ELISA and ELISA, respectively) as described by Talarmin and others.6 If IgM or IgG were detected, the serological test was considered positive.
We performed statistical analysis using STATA version 10 (www.stata.com). After a direct standardization by gender and age using 2010 Census data of the French National Institute of Statistics and Economic Studies, we estimated the seroprevalence and the proportion of asymptomatic infections in the population.
During the survey, 203 individuals were included (participation rate = 93%) and 42 (20.69%) tested positive for anti-CHIKV antibodies (19 for IgM, 36 for IgG, and 13 for both). Table 1 presents standardized estimates of the seroprevalence in Saint Martin inhabitants. We estimated that, in July 2014, the seroprevalence of CHIKV infection in the general population of Saint Martin was 16.9% (95% confidence interval [CI] = 11.6–22.1%]. Among the 42 seropositive individuals, 17 did not report any CHIKV-related symptoms. Thus, the infection remained asymptomatic for 39.0% (95% CI = 23.9–54.1%) of infected individuals in the population.
Table 1.
Seroprevalence of IgG and/or IgM against CHIKV in Saint Martin, July 2014
| Sample size n (%) | Population of Saint Martin N (%) | Standardized seroprevalence (%)* | |
|---|---|---|---|
| Age group | |||
| 6 months to 29 years | 37 (18.2) | 18,197 (0.49) | 14.8 (3.1–26.5) |
| 30–44 years | 55 (27.1) | 9,070 (0.25) | 11.7 (3.0–20.4) |
| 45–59 years | 66 (32.5) | 6,726 (0.18) | 21.6 (11.4–31.7) |
| ≥ 60 years | 45 (22.2) | 2,983 (0.08) | 34.5 (20.5–48.7) |
| Gender | |||
| Men | 74 (36.5) | 17,519 (0.47) | 18.6 (9.5–27.8) |
| Women | 129 (63.5) | 19,461 (0.53) | 15.2 (8.9–21.5) |
| Total | 203 (100) | 36,980 | 16.9 (11.6–22.1) |
CHIKV = chikungunya virus.
Sex and age adjusted.
This is the first CHIKV serosurvey in the Western Hemisphere since the 2013 emergence in the Caribbean. Seven months after this emergence, we estimated that 16.9% of Saint Martin inhabitants were infected. This moderated herd immunity indicated an intermediate stage of the outbreak in July 2014.
Although a direct standardization by gender and age has been applied to limit the selection bias in patient recruitment, an underestimation of CHIKV seroprevalence in population could not be excluded. Access to health care could be easier for people coming to the laboratory than in the general population but should be unlikely as French regulation ensures a broad access to health care. Even if the geographic distribution of the individuals coming to the laboratory was not considered, CHIKV was widespread in all districts of Saint Martin.
CHIKV circulated in the whole island; the seroprevalence should be comparable for Sint Maarten and Saint Martin because of the small size of the Island and the lack of physical or geographical separation between both countries facilitating population mixing. However, no surveillance data are available for Sint Maarten, hence the attack rate cannot be directly extrapolated for Sint Maarten.
Seroprevalence reported in other surveys varied between 10.2% and 75% (Table 2). Except for the 2007 outbreak in Italy,8 the seroprevalence observed in Saint Martin in July 2014 was the lowest recorded. This moderate attack rate was pointing the persistence of the viral circulation in the following months (confirmed by the surveillance data; Figure 1). However, comparison with other surveys should be cautious because data were collected in different settings and at different times throughout the course of the outbreaks (elapsed time between emergence and serosurvey often missing).
Table 2.
CHIKV seroprevalence and asymptomatic rates reported in other serosurveys
| Author | Date of completion | Location | Virus lineage | Primary vector | Attack rate (N) | Proportion of asymptomatic cases | Population sampling |
|---|---|---|---|---|---|---|---|
| Kumar and others7 | 2007 (during the outbreak) | Kerala, India | IOL | Aedes albopictus | 55.8% (259/381) | 3.8% (10/260) | Systematic clustered |
| Moro and others8 | 2007 (3–5 months post-outbreak) | Emilia-Romagna, Italy | IOL | Ae. albopictus | 10.2% (33/325) | 18.2% (6/33) | Systematic random |
| Ayu and others9 | 2007 (1 year post-outbreak) | Bagan Panchor, Malaysia | Asian | Aedes spp. | 55.6% (40/72) | 17.5% (7/40) | Systematic clustered |
| Sissoko and others10 | 2007 (post-outbreak) | Mayotte | IOL | Ae. albopictus | 38.1% (440/1,154) | 27.7% (122/440) | Multistage cluster |
| Aedes aegypti | |||||||
| Gérardin and others11 | 2006 (during outbreak) | La Réunion | IOL | Ae. albopictus | 18.2% (162/888) | Not estimated | Stored sera of pregnant women |
| Gérardin and others11 | 2006 (post-outbreak) | La Réunion | IOL | Ae. albopictus | 38.2% (weighted estimate: 967/2,442) | 16.7% (162/967) | Systematic random |
| Sergon and others12 | 2004 (9 weeks after the peak of the outbreak) | Lamu Island, Kenya | ECSA | Aedes spp. | 75% (215/288) | 45.1% (118/215) | Systematic proportional to size of census unit |
| Sergon and others13 | 2005 (at the peak of the outbreak) | Grande Comore Island, Comoros | IOL | Ae. aegypti | 63.1% (209/331) | 14.3% (30/209) | Systematic multistage |
| Nakkhara and others14 | 2011 (2 years after the beginning of the outbreak | Phatthalung, Thailand | IOL | Ae. albopictus | 61.9% (314/507) | 47.1% (148/314) | Systematic (whole village) |
| Ae. aegypti |
CHIKV = chikungunya virus; ECSA = east/central/south African; IOL = Indian ocean lineage.
The reason explaining the moderate seroprevalence in Saint Martin may lie in the local determinants of transmission of the Asian lineage with regard to the vectors, environment, and human population.
First, transmission efficiency of the local vector, Ae. aegypti, for the Asian lineage should be considered. Remarkably, outbreaks of Asian lineage associated with Ae. aegypti were limited in New Caledonia (33 and 30 autochthonous cases, in 2011 and 2013, respectively)15,16 and in Malaysia (1998 and 2006, respectively)9 whereas the 2009 ECSA lineage had a nationwide diffusion.17 However, a recent study has demonstrated that Ae. aegypti populations from Saint Martin were well adapted to CHIKV and transmitted efficiently both Asian and ECSA lineages.18
Different climatic factors (e.g., ambient temperature, daily fluctuation of temperature, and pluviometry) could have driven outbreak courses resulting in different attack rates. Recently, a model explaining autochthonous transmission of CHIKV in the Americas with climatic drivers (mean temperature and precipitations) was created.19 For instance, the potential link between the highest rainfall level of October and November (http://www.meteofrance.com/) and the peak of cases observed in December and January (2013 and 2014; Figure 1) should be investigated.
Besides, the moderate seroprevalence observed in Saint Martin could be indicative of the efficiency of control measures applied promptly after the emergence and throughout the outbreak (i.e., insecticide treatment, breeding sites destruction, and recommendations of personal protection against mosquitoes). Sensitized by the CHIKV outbreak in La Reunion, the French public health authorities set up in 2013 a preparedness and response plan for CHIKV introduction in Saint Martin,20 which may have slow down the dissemination of the virus in the population.
Our results indicated that 40.5% of the infected people did not reported chikungunya-like symptoms within the 6 months preceding the study (39.0% of asymptomatic cases in the general population of Saint Martin). Although a recall bias cannot be excluded, severe arthralgias caused by CHIKV are usually memorable.
The proportion of asymptomatic infections reported in other surveys was overall substantially lower than the proportion obtained in Saint Martin (Table 2). However, high rate of asymptomatic infections were recorded with ECSA lineages in Thailand and Kenya (47.1% and 45.1%, respectively).12,14
Manimunda and others suggested an association between the overall seroprevalence in a population and the proportion of unapparent infection. Indeed intensity of transmission in a population, loosely approximated by the attack rate, could be inversely associated to the proportion of unapparent infection.21
The 16.9% seroprevalence estimated gives a clearer picture of susceptible people who could still be naive (83.1%) in July 2014. Because of that large part of naive population and frequent arrival of susceptible among tourists, control efforts should be pursued. The strengthening of the viral circulation observed in December 2014 has indicated the possibility of other epidemic waves. Moreover, our study highlighted a substantial rate of asymptomatic infections that may play a significant role in maintaining the transmission.22 A low endemic circulation of the Asian lineage in the Americas could not be excluded as observed in Malaysia.9
Asian CHIKV lineage is currently disseminating in the Americas23 and ECSA have emerged in Brazil.24 CHIKV outbreaks caused by ECSA and Asian lineages could become common wherever competent vectors, Ae. aegypti or Aedes albopictus, are established in the Western Hemisphere. Our study highlighted the need of a preparedness plan to mitigate the dissemination of the CHIKV. A particular attention should be paid to the substantial rate of asymptomatic infections recorded. Even if difficult to registered by the epidemiological surveillance system, asympytomatic individuals are still a potential source of infection that can spread in new geographic area.14 Finally, further studies are needed in the Americas to monitor closely for each CHIKV lineage: the spread, the related proportion of unapparent infection, and the transmission efficiency by the local vector mosquitoes.
ACKNOWLEDGMENTS
We acknowledge the technical support of the Agence Régionale de Santé.
Footnotes
Financial support: This study was supported by the internal resources brought by the investigators (French National Institute for Public Health Surveillance, Institut Pasteur de Guyane, Cayenne, and Laboratoire Saint Martin Biologie).
Authors' addresses: Noellie Gay, Cire Antilles-Guyane, Centre d'Affaires Agora, ZAC de l'Etang Z'Abricot, Pointe des Grives - BP 658, 97261 Fort-de-France cedex, E-mail: gnoellie@hotmail.com. Dominique Rousset, Laboratoire de Virologie, Institut Pasteur de la Guyane, Cayenne, French Guiana, E-mail: drousset@pasteur-cayenne.fr. Patricia Huc, Laboratoire Saint Martin Biologie, Diagnostic, Saint Martin, French West Indies, E-mail: labmcaraibes@orange.fr. Séverine Matheus, Centre National de Référence des Arbovirus, Laboratoire de Virologie, Institut Pasteur de la Guyane, Cayenne, French Guiana, E-mail: smatheus@pasteur-cayenne.fr. Martine Ledrans, Epidemiology Unit, Regional Office of the French National Institute for Public Health Surveillance, Martinique, French West Indies, E-mail: martine.ledrans@ars.sante.fr. Jacques Rosine, Cellule Inter Régionale d'Epidémiologie, CIRE, Martinique, French West Indies, E-mail: jacques.rosine@ars.sante.fr. Sylvie Cassadou, Regional Office of the French National Institute for Public Health Surveillance, Guadeloupe, French West Indies, E-mail: sylvie.cassadou@ars.sante.fr. Harold Noël, Département des Maladies Infectieuses, Institut de Veille Sanitaire, Saint Maurice cedex, France, E-mail: h.noel@invs.sante.fr.
References
- 1.Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond) 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK. Burden of chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis. 2009;46:26–35. [PubMed] [Google Scholar]
- 3.Powers AM, Brault AC, Tesh RB, Weaver SC. Re-emergence of chikungunya and O'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 4.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet Lond Engl. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 5.Noël H, Rizzo C. Spread of chikungunya from the Caribbean to mainland Central and South America: a greater risk of spillover in Europe? Euro Surveill. 2014;19:20855. doi: 10.2807/1560-7917.es2014.19.28.20855. [DOI] [PubMed] [Google Scholar]
- 6.Talarmin A, Labeau B, Lelarge J, Sarthou JL. Immunoglobulin A-specific capture enzyme-linked immunosorbent assay for diagnosis of dengue fever. J Clin Microbiol. 1998;36:1189–1192. doi: 10.1128/jcm.36.5.1189-1192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar NP, Suresh A, Vanamail P, Sabesan S, Krishnamoorthy KG, Mathew J, Jose VT, Jambulingam P. Chikungunya virus outbreak in Kerala, India, 2007: a seroprevalence study. Mem Inst Oswaldo Cruz. 2011;106:912–916. doi: 10.1590/s0074-02762011000800003. [DOI] [PubMed] [Google Scholar]
- 8.Moro ML, Gagliotti C, Silvi G, Angelini R, Sambri V, Rezza G, Massimiliani E, Mattivi A, Grilli E, Finarelli AC, Spataro N, Pierro AM, Seyler T, Macini P, Chikungunya Study Group Chikungunya virus in north-eastern Italy: a seroprevalence survey. Am J Trop Med Hyg. 2010;82:508–511. doi: 10.4269/ajtmh.2010.09-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayu SM, Lai LR, Chan YF, Hatim A, Hairi NN, Ayob A, Sam I-C. Seroprevalence survey of chikungunya virus in Bagan Panchor, Malaysia. Am J Trop Med Hyg. 2010;83:1245–1248. doi: 10.4269/ajtmh.2010.10-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sissoko D, Moendandze A, Malvy D, Giry C, Ezzedine K, Solet JL, Pierre V. Seroprevalence and risk factors of chikungunya virus infection in Mayotte, Indian Ocean, 2005–2006: a population-based survey. PLoS One. 2008;3:e3066. doi: 10.1371/journal.pone.0003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gérardin P, Guernier V, Perrau J, Fianu A, Le Roux K, Grivard P, Michault A, de Lamballerie X, Flahault A, Favier F. Estimating chikungunya prevalence in La Réunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis. 2008;8:99. doi: 10.1186/1471-2334-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sergon K, Njuguna C, Kalani R, Ofula V, Onyango C, Konongoi LS, Bedno S, Burke H, Dumilla AM, Konde J, Njenga MK, Sang R, Breiman RF. Seroprevalence of chikungunya virus (CHIKV) infection on Lamu Island, Kenya, October 2004. Am J Trop Med Hyg. 2008;78:333–337. [PubMed] [Google Scholar]
- 13.Sergon K, Yahaya AA, Brown J, Bedja SA, Mlindasse M, Agata N, Allaranger Y, Ball MD, Powers AM, Ofula V, Onyango C, Konongoi LS, Sang R, Njenga MK, Breiman RF. Seroprevalence of chikungunya virus infection on Grande Comore Island, union of the Comoros, 2005. Am J Trop Med Hyg. 2007;76:1189–1193. [PubMed] [Google Scholar]
- 14.Nakkhara P, Chongsuvivatwong V, Thammapalo S. Risk factors for symptomatic and asymptomatic chikungunya infection. Trans R Soc Trop Med Hyg. 2013;107:789–796. doi: 10.1093/trstmh/trt083. [DOI] [PubMed] [Google Scholar]
- 15.Dupont-Rouzeyrol M, Caro V, Guillaumot L, Vazeille M, D'Ortenzio E, Thiberge J-M, Baroux N, Gourinat A-C, Grandadam M, Failloux A-B. Chikungunya virus and the mosquito vector Aedes aegypti in New Caledonia (South Pacific Region) Vector Borne Zoonotic Dis Larchmt N. 2012;12:1036–1041. doi: 10.1089/vbz.2011.0937. [DOI] [PubMed] [Google Scholar]
- 16.Roth A, Hoy D, Horwood PF, Ropa B, Hancock T, Guillaumot L, Rickart K, Frison P, Pavlin B, Souares Y. Preparedness for threat of chikungunya in the pacific. Emerg Infect Dis. 2014;20 doi: 10.3201/eid2008.130696. doi:10.3201/eid2008.130696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sam I-C, Chan YF, Chan SY, Loong SK, Chin HK, Hooi PS, Ganeswrie R, Abubakar S. Chikungunya virus of Asian and central/east African genotypes in Malaysia. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2009;46:180–183. doi: 10.1016/j.jcv.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Vega-Rúa A, Lourenço-de-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yébakima A, Gustave J, Girod R, Dusfour I, Leparc-Goffart I, Vanlandingham DL, Huang Y-JS, Lounibos LP, Mohamed Ali S, Nougairede A, de Lamballerie X, Failloux A-B. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 2015;9:e0003780. doi: 10.1371/journal.pntd.0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins TA, Metcalf CJE, Grenfell BT, Tatem AJ. Estimating drivers of autochthonous transmission of chikungunya virus in its invasion of the Americas. PLoS Curr. 2015;7 doi: 10.1371/currents.outbreaks.a4c7b6ac10e0420b1788c9767946d1fc. doi:10.1371/currents.outbreaks.a4c7b6ac10e0420b1788c9767946d1fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassadou S, Boucau S, Petit-Sinturel M, Huc P, Leparc-Goffart I, Ledrans M. Emergence of chikungunya fever on the French side of Saint Martin Island, October to December 2013. Euro Surveill. 2014;19(pii):20752. doi: 10.2807/1560-7917.es2014.19.13.20752. [DOI] [PubMed] [Google Scholar]
- 21.Manimunda SP, Sugunan AP, Rai SK, Vijayachari P, Shriram AN, Sharma S, Muruganandam N, Chaitanya IK, Guruprasad DR, Sudeep AB. Outbreak of chikungunya fever, Dakshina Kannada District, south India, 2008. Am J Trop Med Hyg. 2010;83:751–754. doi: 10.4269/ajtmh.2010.09-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K. Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfus (Paris) 2013;53:2567–2574. doi: 10.1111/j.1537-2995.2012.03960.x. [DOI] [PubMed] [Google Scholar]
- 23.Díaz Y, Carrera J-P, Cerezo L, Arauz D, Guerra I, Cisneros J, Armién B, Botello AM, Araúz AB, Gonzalez V, López Y, Moreno L, López-Vergès S, Moreno BA. Chikungunya virus infection: first detection of imported and autochthonous cases in Panama. Am J Trop Med Hyg. 2015;92:482–485. doi: 10.4269/ajtmh.14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teixeira MG, Andrade AMS, Costa M da CN, Castro J-NSM, Oliveira FLS, Goes CSB, Maia M, Santana EB, Nunes BTD, Vasconcelos PFC. East/central/south African genotype chikungunya virus, Brazil, 2014. Emerg Infect Dis. 2015;21:906–907. doi: 10.3201/eid2105.141727. [DOI] [PMC free article] [PubMed] [Google Scholar]