Abstract
Since the first reported epidemic of dengue in Pemba, the capital of Cabo Delgado province, in 1984–1985, no further cases have been reported in Mozambique. In March 2014, the Provincial Health Directorate of Cabo Delgado reported a suspected dengue outbreak in Pemba, associated with a recent increase in the frequency of patients with nonmalarial febrile illness. An investigation conducted between March and June detected a total of 193 clinically suspected dengue patients in Pemba and Nampula, the capital of neighboring Nampula Province. Dengue virus-type 2 (DENV-2) was detected by reverse transcriptase polymerase chain reaction in sera from three patients, and 97 others were classified as probable cases based on the presence of DENV nonstructural protein 1 antigen or anti-DENV immunoglobulin M antibody. Entomological investigations demonstrated the presence of Aedes aegypti mosquitos in both Pemba and Nampula cities.
The four dengue virus-types (DENV-1–4) are arthropod-borne RNA viruses belonging to the family Flaviviridae and transmitted by Aedes spp. mosquitos.1 Dengue is endemic in tropical and subtropical regions where an estimated 390 million infections occurred in 2010.2,3 Approximately 50% of the world's population lives in areas at risk for DENV infection.4 Although current estimates suggest that sub-Saharan Africa carries 16% of the annual worldwide burden of dengue, the epidemiology of dengue in the region is poorly understood and the disease remains neglected.4,5 Reasons for the under-recognition and underreporting of dengue in sub-Saharan Africa include a lack of clinical awareness, over diagnosis of malaria, limited laboratory diagnostic capability, and weak surveillance systems.6
In recent years, an increasing number of dengue outbreaks have been identified in Africa, including Angola, Kenya, Ethiopia, and Tanzania.7–10 In Mozambique, the last known outbreak of dengue occurred in 1984–1985 in Pemba, the capital of Cabo Delgado Province, in northern Mozambique, caused by DENV-3.11 In March 2014, the Cabo Delgado Provincial Health Authority reported a cluster of patients with nonmalarial febrile illness in Pemba, suspected as dengue. This report describes findings from the outbreak investigation.
The investigation was conducted in two phases, with the initial phase from March 15–28, 2014 focused on patients hospitalized at Pemba Provincial Hospital in Cabo Delgado Province, with unknown febrile illness suspected of dengue. A suspected dengue case was defined as a patient with acute febrile illness with a negative malaria test result, and at least two or more of the following manifestations: headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, or leucopenia. During phase one, blood specimens were collected from 20 patients with suspected dengue and tested at the national reference laboratory for DENV nonstructural protein 1 (NS1) antigen and anti-DENV immunoglobulin M (IgM) antibody by enzyme immunoassay (EIA) (Panbio Capture ELISA, Alere, Waltham, MA). Specimens from a subset of these patients were forwarded to the regional reference laboratory in South Africa for confirmatory testing by EIA and reverse transcriptase polymerase chain reaction (RT-PCR) for DENV (one step flavi-RT PCR using primer set FU1/CFD2).12 Confirmed dengue cases were defined as patients with a positive RT-PCR result for DENV.
Following confirmation of the dengue outbreak, phase two of the investigation was initiated on April 17 with prospective surveillance among outpatients presenting with febrile illness at Pemba Provincial Hospital. All patients who met the above suspected screening case definition for dengue were tested using a rapid diagnostic test (RDT) for DENV NS1 antigen and anti-DENV IgM/IgG antibody detection (SD Bioline Dengue Duo; Standard Diagnostics, Gyeonggi-do, Republic of Korea). During phase two, blood specimens were also forwarded to the national reference laboratory for DENV NS1 antigen and IgM antibody testing by EIA. However, no further specimens were forwarded to the regional reference laboratory in South Africa and no additional PCR testing was conducted during phase two. Activities in both phases of the investigation were judged by the Ministry of Health to be public health practice, nonresearch, in response to an acute disease outbreak. Therefore, the activities were exempt from further institutional review board requirements.
During prospective surveillance in Pemba, a patient from the neighboring provincial capital of Nampula presented with symptoms of dengue and tested positive for DENV NS1 antigen by RDT. On the basis of this finding, prospective surveillance activities were also initiated at Nampula Central Hospital on April 25 using the same methods. Prospective surveillance was conducted in both cities until June 3, 2014. During both phases of the investigation, data were collected from medical records and interviews with suspected cases. A standardized case investigation form was used to collect clinical and sociodemographic information. Clinical and laboratory data from both phases of the investigation were combined in one data set for analysis. For the final case classification, confirmed cases were defined as those with positive PCR results for DENV, and probable cases were defined as those with positive DENV NS1 antigen or anti-DENV IgM antibody results by either RDT or EIA.
Between March and June 2014, 193 patients who met the suspected screening case definition were tested for dengue infection using RDT or EIA. Among the initial 20 patients with suspected dengue, 13 were also tested by RT-PCR; three of these patients were positive for DENV-2 and classified as confirmed cases. A total of 97 other patients were classified as probable dengue cases based on the presence of DENV NS1 antigen or anti-DENV IgM antibody. The remaining 93 patients either tested negative (N = 87) or tested positive only for anti-DENV IgG antibody (N = 6) (hereafter referred to as nondengue patients or noncases).
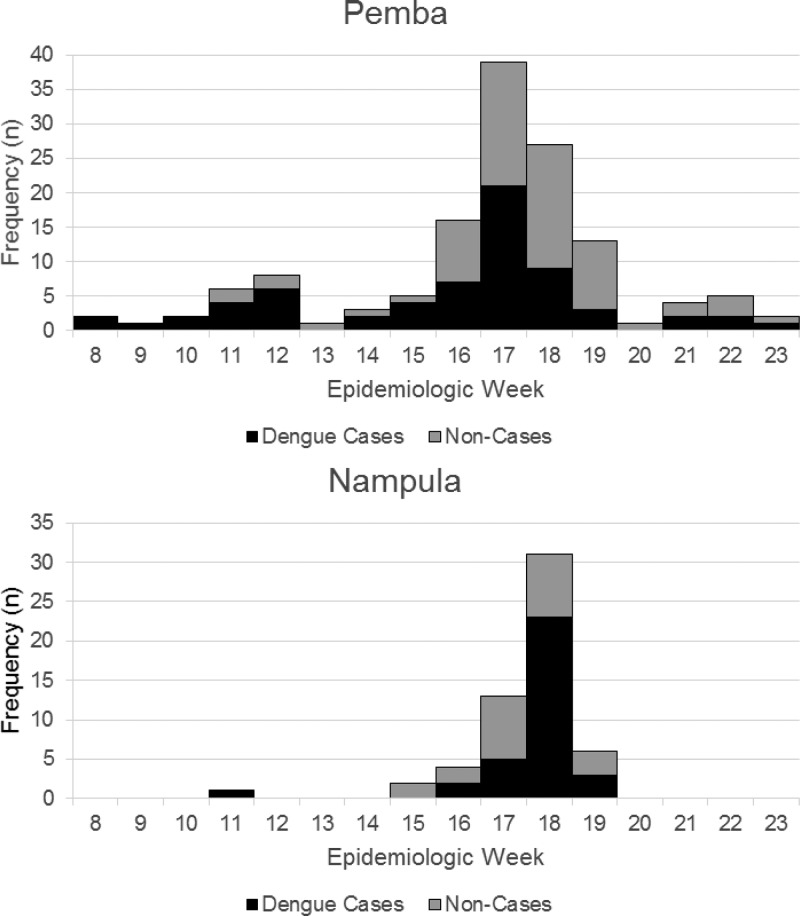
Of the 100 confirmed or probable case-patients (hereafter referred to as cases or case-patients), 66% resided in Cabo Delgado and 34% in Nampula Province (Table 1). Case-patients resided in various neighborhoods of Pemba and Nampula city, as well as two patients from two districts outside these cities. Several case-patients were identified among students and staff of a residential health-sciences training facility located in the most affected neighborhood of Pemba. Dengue case-patients in Pemba presented with onset of illness over a 15-week period, peaking during late April 2014 (Figure 1 ). The majority of case-patients in Nampula had illness onset over a 4-week period from mid-April to early-May.
Table 1.
Comparison of social and demographic characteristics between dengue case-patients and nondengue patients
| Sociodemographic characteristics | Confirmed and probable dengue case-patients (N = 100) n (%)* | Nondengue patients (N = 93) n (%)* | P value |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 28 (18–35) | 30 (13–42) | 0.24† |
| Sex | |||
| Female | 50 (51) | 48 (53) | 0.70 |
| Race | |||
| Black or mixed | 82 (83) | 79 (90) | ref |
| White | 12 (12) | 6 (7) | 0.07 |
| Asian | 5 (5) | 3 (3) | 0.39 |
| Province of residence | |||
| Cabo Delgado | 66 (66) | 70 (75) | 0.16 |
| Nampula | 34 (34) | 23 (25) | – |
| Duration of residence in current city (years) | |||
| Median (IQR) | 4 (2–11.75) | 9 (3.75–20.25) | < 0.01** |
| Mozambican nationality | |||
| Yes | 86 (87) | 80 (88) | 0.83 |
| Mozambican born outside region | |||
| Yes | 31 (36) | 13 (16) | < 0.01 |
| No | 55 (64) | 67 (84) | – |
| Education level | |||
| No schooling | 7 (7) | 18 (27) | ref |
| Primary | 17 (18) | 12 (18) | < 0.05 |
| Secondary | 36 (38) | 21 (31) | < 0.05 |
| University | 34 (36) | 17 (25) | < 0.01 |
| Health-care worker or health-care student | |||
| Yes | 20 (22) | 8 (11) | < 0.05 |
| No | 71 (78) | 67 (89) | – |
| Household member with similar illness | |||
| Yes | 6 (23) | 3 (8) | 0.09 |
| No | 20 (77) | 35 (92) | – |
IQR = interquartile range.
Numbers may not add to column total due to missing data.
Wilcoxon rank sums test. Other P values relate to likelihood-ratio χ2 test.
Figure 1.
Frequency, by week of onset, of dengue cases and noncases in Pemba and Nampula cities, 2014.
Case-patients and nondengue patients were similar in median age (28 versus 30 years, respectively; P = 0.24), sex distribution (females comprised 51% and 53%, respectively; P = 0.70), and Mozambican nationality (87% versus 88%, respectively; P = 0.83) (Table 1). Findings were similar between Pemba and Nampula patients, and therefore presented together. Compared with noncases, case-patients were significantly more likely to be Mozambicans born outside the Provinces of Cabo Delgado or Nampula (P < 0.01), to have lived for a shorter duration in their current city of residence (P < 0.01), have higher education levels (P < 0.01), and to be health-care workers or health science students (P < 0.05). Among case-patients, the median duration of residence in the current city was 4 years, while for nondengue patients, the median duration of residence was 9 years (Table 1). One possible explanation for this finding is that DENV-2 may have been circulating undetected for some time in Pemba and Nampula city and that those with longer duration of residence in these cities may be more likely to have acquired protective immunity to DENV-2. However, these differences could also reflect greater likelihood of diagnosis and case detection.
Clinical characteristics did not differ significantly for dengue case-patients and nondengue patients (Table 2). Among dengue case-patients, 30% were hospitalized and 19% had one or more hemorrhagic signs. No severe cases of dengue hemorrhagic fever were noted. One case-patient with a preexisting condition died, though without hemorrhagic or shock signs; no nondengue patients died.
Table 2.
Comparison of clinical signs and symptoms between dengue case-patients and non-dengue patients
| Clinical characteristics | Confirmed and probable dengue case-patients (N = 100) n (%) | Nondengue patients (N = 93) n (%) | P value |
|---|---|---|---|
| Headache | 92 (95) | 84 (98) | 0.49 |
| Myalgia | 75 (80) | 66 (80) | 0.85 |
| Arthralgia | 73 (76) | 73 (87) | 0.11 |
| Weakness | 66 (80) | 57 (79) | 0.96 |
| Retro-orbital pain | 60 (66) | 55 (71) | 0.58 |
| Chills | 56 (68) | 47 (69) | 0.89 |
| Photophobia | 23 (28) | 26 (38) | 0.22 |
| Abdominal pain | 22 (29) | 23 (35) | 0.41 |
| Diarrhea | 19 (25) | 20 (29) | 0.63 |
| Rash | 15 (20) | 13 (20) | 0.94 |
| Persistent vomiting | 10 (14) | 10 (15) | 0.83 |
| Hemorrhagic signs | 19 (19) | 10 (11) | 0.11 |
| Hospitalized | 27 (30) | 26 (33) | 0.68 |
P values relate to likelihood-ratio χ2 test.
Malaria overdiagnosis is still very common in many countries of sub-Saharan Africa, including Mozambique.13,14 Data obtained from the Provincial Malaria Control Program in Pemba indicate that 6,222 patients were tested for malaria in February 2014 and 96% of them were negative by malaria RDT. This represents an approximately 12% increase in suspect malaria patients compared with the same period in 2013, and a 7% increase in the proportion of patients tested for malaria who were negative. Our investigation found that, before confirmation of the dengue outbreak, many patients with suspected malaria and negative test results were nevertheless treated with antimalarial drugs, with no relief of clinical symptoms. These findings suggest that the dengue outbreak in Pemba likely started several weeks before it was recognized in March 2014, and may have resulted in many dengue cases among patients who were misdiagnosed with malaria. Improvements in the clinical management of febrile illness are needed to better distinguish between malaria and less common causes of febrile illness.
DENV-2 is the most widespread dengue virus-type.15 Most documented DENV-2 outbreaks have been reported in the Americas and Asia, and less commonly in sub-Saharan Africa.15 However, DENV-2 has also been reported in outbreaks in Kenya, Ethiopia, and Tanzania.8,10,16 Dengue outbreaks are frequently seen in areas characterized by rapid and unplanned urbanization, along with intense national and international migration. Substandard housing, heavy rainfall, relatively high air humidity, and poor sanitation also provide suitable environmental and microecological conditions for Aedes aegypti survival and proliferation in urban and semi-urban areas.15 These features are commonly seen in northern Mozambique, particularly in Pemba and Nampula cities.
During April to May 2014, a preliminary entomological investigation was carried out in 45 households in the urban and semi-urban areas of both Pemba and Nampula cities.17 Sampling for mosquitoes was conducted in neighborhoods where either confirmed or suspected dengue cases had occurred. We detected larvae and adults of Aedes (Stegomya) spp., later confirmed as Aedes (Stegomya) aegypti by morphological analysis. The main larval breeding sites identified were abandoned tires, cement tanks, plastic buckets, plant pots, and discarded plastic bags and bottles.
In conclusion, we confirmed an outbreak of dengue, due to DENV-2, in the capital cities of the northern Mozambican provinces of Cabo Delgado and Nampula, approximately 30 years after the last documented dengue outbreak due to DENV-3. Lack of routine laboratory diagnostic capacity and clinical awareness of dengue likely delayed the identification of this outbreak in a region where malaria is highly endemic. Adult Aedes mosquitos and larval breeding sites were observed in both cities during the outbreak. The shorter duration of residence in the current city we observed among case-patients is suggestive of prior or endemic circulation of DENV-2. Improved monitoring and disease surveillance is needed to further assess dengue endemicity in the region. Dengue should be incorporated into routine surveillance and considered in the differential diagnosis of acute febrile illness in northern Mozambique. Additional recommendations include ongoing monitoring of vector population dynamics, temporal and spatial variation of mosquito breeding sites, and their environmental and bioecological correlates, as well as key measures to effectively control Aedes mosquitos.
ACKNOWLEDGMENTS
We thank the staff from the Provincial Health Directorates in Cabo Delgado and Nampula for their assistance during the investigation, and all the staff and patients at the health facilities who contributed to this investigation. This work was supported by funds from European Foundation Initiative Into Neglected Tropical Diseases (EFINDT).
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Marilia Massangaie, Gabriela Pinto, Fernando Padama, Geraldo Chambe, Mariana da Silva, and Inocêncio Mate, National Directorate of Public Health, Ministry of Health, Maputo, Mozambique, E-mails: mariliamassangaie@yahoo.com.br, gabbydocarmo@gmail.com, ferpadama@yahoo.com.br, geraldochambe@yahoo.com.br, m.sillva@yahoo.com.br, and inomate@yahoo.com.br. Celia Chirindza and Sadia Ali, Virus Isolation Laboratory, National Institute of Health, Maputo, Mozambique, E-mails: celia.chir@gmail.com and sadia.abdul.ali@gmail.com. Sãozinha Agostinho, Ministry of Health, Cabo Delgado Provincial Health Directorate, Pemba, Mozambique, E-mail: saozinhagostinho@gmail.com. Daniel Chilaule, Nampula Provincial Health Directorate, Ministry of Health, Nampula, Mozambique, E-mail: dachilaule@hotmail.com. Jacqueline Weyer and Chantel le Roux, National Institute for Communicable Diseases, Centre for Emerging and Zoonotic Diseases, Johannesburg, South Africa, E-mails: jacquelinew@nicd.ac.za and chantell@nicd.ac.za. Ana Paula Abilio, Cynthia Baltazar, and Eduardo Samo Gudo, National Institute of Health, Maputo, Mozambique, E-mails: anabilio1408@gmail.com, cynthiasema@yahoo.com, and esamogudojr@gmail.com. Timothy J. Doyle, Centers for Disease Control and Prevention, Center for Global Health, Maputo, Mozambique, E-mail: tdoyle@cdc.gov. Julie Cliff, Faculty of Medicine, Community Health Department, Maputo, Mozambique, E-mail: julie.cliff@gmail.com. Janusz Paweska, National Institute for Communicable Diseases, Special Pathogens Unit, Johannesburg, South Africa, E-mail: januszp@nicd.ac.za.
References
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Win GR, Simmons CP, Scot TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman A, Isturiz RE. Update on the global spread of dengue. Int J Antimicrob Agents. 2010;36((Suppl 1)):S40–S42. doi: 10.1016/j.ijantimicag.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaenisch T, Junghanss T, Wills B, Brady OJ, Eckerle I, Farlow A, Hay SI, McCall PJ, Messina JP, Ofula V, Sall AA, Sakuntabhai A, Velayudhan R, Wint W, Zeller H, Margolis HS, Sankoh O, Dengue in Africa Study Group Dengue expansion in Africa-not recognized or not happening? Emerg Infect Dis. 2014;20:e140487. doi: 10.3201/eid2010.140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17:1349–1354. doi: 10.3201/eid1708.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC Ongoing dengue epidemic - Angola, June 2013. MMWR Morb Mortal Wkly Rep. 2013;62:504–507. [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson BK, Ocheng D, Gichogo A, Okiro M, Libondo D, Kinyanjui P, Tukei PM. Epidemic dengue fever caused by dengue type 2 virus in Kenya: preliminary results of human virological and serological studies. East Afr Med J. 1982;59:781–784. [PubMed] [Google Scholar]
- 9.Hertz JT, Munishi OM, Ooi EE, Howe S, Lim WY, Chow A, Morissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Gubler DJ, Crump JA. Chikungunya and dengue fever among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2012;86:171–177. doi: 10.4269/ajtmh.2012.11-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanesa-thasan N, Chang GJ, Smoak BL, Magill A, Burrous MJ, Hoke CH. Molecular and epidemiologic analysis of dengue virus isolates from Somalia. Emerg Infect Dis. 1998;4:299–303. doi: 10.3201/eid0402.980220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubler DJ, Sather GE, Kuno G, Cabral JR. Dengue 3 virus transmission in Africa. Am J Trop Med Hyg. 1986;35:1280–1284. doi: 10.4269/ajtmh.1986.35.1280. [DOI] [PubMed] [Google Scholar]
- 12.Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hume JC, Barnish G, Mangal T, Armazio L, Streat E, Bates I. Household cost of malaria overdiagnosis in rural Mozambique. Malar J. 2008;7:33. doi: 10.1186/1475-2875-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barat L, Chipipa J, Kolczak M, Sukwa T. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? Am J Trop Med Hyg. 1999;60:1024–1030. doi: 10.4269/ajtmh.1999.60.1024. [DOI] [PubMed] [Google Scholar]
- 15.Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, Bhatt S, Katzelnick L, Howes RE, Battle KE, Simmons CP, Hay SI. Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol. 2014;22:138–146. doi: 10.1016/j.tim.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chipwaza B, Mugasa JP, Selemani M, Amuri M, Mosha F, Ngatunga SD, Gwakisa PS. Dengue and Chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis. 2014;8:e3335. doi: 10.1371/journal.pntd.0003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higa Y, Abílio AP, Futami K, Lázaro MAF, Minakawa N, Gudo ES. Abundant Aedes (Stegomyia) aegypti aegypti mosquitoes in the 2014 dengue outbreak area of Mozambique. Trop Med Health. 2014;43:107–109. doi: 10.2149/tmh.2014-29. [DOI] [PMC free article] [PubMed] [Google Scholar]