Abstract
We report the case of an Ebola virus (EBOV) RNA–negative pregnant woman who delivered an EBOV RNA–positive stillborn infant at a community health center in rural Sierra Leone, 1 month after the mother's last possible exposure. The mother was later found to be immunoglobulins M and G positive indicating previous infection. The apparent absence of Ebola symptoms and not recognizing that the woman had previous contact with an Ebola patient led health workers performing the delivery to wear only minimal personal protection, potentially exposing them to a high risk of EBOV infection. This case emphasizes the importance of screening for epidemiological risk factors as well as classic and atypical symptoms of Ebola when caring for pregnant women, even once they have passed the typical time frame for exposure and incubation expected in nonpregnant adults. It also illustrates the need for health-care workers to use appropriate personal protection equipment when caring for pregnant women in an Ebola setting.
On January 13, 2015, the U.S. Centers for Disease Control and Prevention (CDC) field laboratory in Bo, Sierra Leone, identified Ebola virus (EBOV) RNA in an oral swab from a stillborn infant by quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) for Ebola NP and VP40 genes. The swab, taken in accordance with Sierra Leone Ministry of Health and Sanitation (MoHS) protocol to investigate all deaths regardless of symptoms or exposure during the outbreak, tested positive with cycle threshold values of 16 in both targets, indicating a high viral load. At the time of delivery, the infection status of the mother was unknown.
A team from Médecins Sans Frontières (MSF) traveled to the village where the infant was born to find the mother and investigate the chain of transmission. The 20-year-old woman was found healthy and living with her husband who had been discharged from the MSF Ebola Management Center (EMC) in Bo on December 16, 2014. Despite her insistence that she had had no symptoms of Ebola virus disease (EVD), she agreed to be tested for EBOV.
On January 15, a whole blood sample was collected from the mother in the Bo Hospital Transit Center and analyzed at the CDC field laboratory. The sample was negative for both EBOV RNA targets. CDC laboratorians re-extracted RNA and reran assays on the maternal and infant samples with the same result. Later enzyme-linked immunosorbent assays performed by CDC Atlanta on the maternal sample to detect antibodies specific to Ebola found it positive for immunoglobulin M (IgM, titer ≥ 1:1,600) and immunoglobulin G (IgG, titer ≥ 1:400), indicating the mother had had a recent EBOV infection (Table 1).1 Virus isolation attempts with the infant swab were positive. Isolation was not attempted with the maternal sample because of negative qRT-PCR and positive IgM results.
Table 1.
Test results for samples collected from persons in the chain of transmission, Bo, Sierra Leone, 2014–2015
| Patient | Specimen collection date | Specimen source | Ebola virus load result (CT value)* | Other results |
|---|---|---|---|---|
| Wife | January 15, 2015 | Blood | Negative (> 40:> 40) | IgG positive (≥ 1:400) IgM positive (≥ 1:1,600) |
| – | Repeat† | Negative (> 40:> 40) | – | |
| Stillborn Infant | January 10, 2015 | Oral swab | Positive (16:16) | – |
| – | Repeat† | Positive (20:19) | – | |
| Husband | December 7, 2014 | Blood | Positive (28:28) | – |
| Husband's Brother | December 7, 2014 | Blood | Positive (18:19) | – |
| Husband's Friend | December 7, 2014 | Blood | Positive (26:26) | – |
| Uncle | November 24, 2014 | Oral swab | Positive (26:26) | – |
CT = cycle threshold; IgG = immunoglobulin M; IgM = immunoglobulin M.
The first value corresponds with the NP gene and the second value corresponds with the VP40 gene.
The sample from the infant and wife were retested on January 17, 2015.
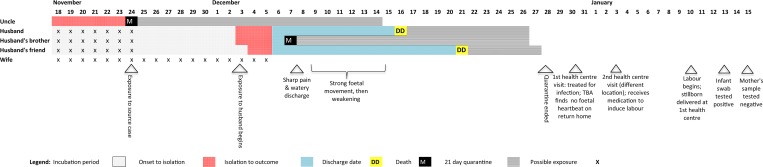
To learn more about possible transmission routes for the woman's infection, an MSF team conducted separate qualitative interviews with the woman and her husband in their village (Figure 1 ). On December 3, 2014 the husband developed symptoms (vomiting, fatigue, loss of appetite, and headache) after carrying his sick uncle to the community health center 9 days earlier. The uncle, who spent only a few hours in the pregnant woman's house, died at the health center shortly after arriving and was diagnosed EBOV positive using RT-PCR testing of a postmortem oral swab.
Figure 1.
Chains of transmission associated with an Ebola-positive stillborn infant, Bo District, Sierra Leone, November 2014 to January 2015.
The husband's brother and a friend, who helped carry the uncle to the health center but did not live with the couple or the uncle, developed symptoms on December 3 and 4, respectively. On December 6, all three men tested EBOV positive (Table 1), and their households were quarantined for 21 days. The brother died in the Transit Center; the husband and his friend were admitted to the MSF EMC and discharged cured on December 16 and 21, respectively. Both husband and wife separately reported that they did not have sexual intercourse on his return home, due to EMC health education, but also because of the woman's pregnancy.
The woman was approximately 7-month pregnant when her husband became symptomatic. Although she did not sleep in the same bed once he became ill, she did provide close contact care for him, including bathing and massage. On January 21, when CDC, MoHS, and World Health Organization officials interviewed the woman using a standardized questionnaire to collect information about potential symptoms of Ebola in the month before onset of labor, she reported experiencing intense fatigue and loss of appetite, abdominal pain, jaundice, eye pain, sensitivity to light, and confusion. During the qualitative interviews conducted by MSF on February 9, she reported experiencing severe back pain and a “gush of water” from her vagina on the day after her husband went to Bo (December 7), leakage of bloody fluid from her vagina, loss of appetite, and intense fatigue. But she did not describe the latter symptoms noted in the standardized questionnaire and in the absence of clinical opinion at the time, it is difficult to determine which report is more accurate (Table 2).
Table 2.
Symptoms reported by the subject during the case investigation compared with Sierra Leone MoHS case definition5*
| Standardized questionnaire by CDC/MoHS/WHO on January 21 | Qualitative interview by MSF investigation team on February 9 | |
|---|---|---|
| Acute fever (> 38°C) | No | No |
| Headache | No | No |
| Abdominal pain | Yes | No |
| Intense fatigue | Yes | Yes |
| Loss of appetite | Yes | Yes |
| Generalized or articular pain | No | No |
| Difficulty in swallowing | No | No |
| Difficulty in breathing | No | No |
| Nausea or vomiting | No | No |
| Hiccups | No | No |
| Miscarriage | No | No |
| Diarrhea | No | No |
| Unexplained bleeding | No | Yes |
CDC = Centers for Disease Control and Prevention; MoHS = Ministry of Health and Sanitation; MSF = Médecins Sans Frontières; WHO = World Health Organization.
A suspect case of Ebola is defined as contact with a clinical case and acute fever (> 38°C), or contact with a clinical case and three or more of the above symptoms, or acute fever and three or more of the concerning symptoms above, or any unexplained bleeding or miscarriage.5
Since the woman associated her symptoms with pregnancy and anxiety regarding her husband's illness, she did not seek medical care nor reveal her condition to anyone, including the contact tracer. When her husband returned from the EMC on December 15, the couple still did not believe any of her symptoms were related to Ebola. They were also under the impression that they could not seek medical care while in quarantine. Thus, the woman only sought medical care on December 28 after completing the 21-day quarantine. She reported being treated for an infection and vaccinated against tetanus and was told to return home until she was closer to term. After walking 3 miles home, she told the village traditional birth attendant (TBA) that the baby had stopped moving. The TBA was unable to find a fetal heartbeat and advised her to seek medical care again. She sought care at a second health center where she was given medication, which the TBA believed was to induce labor.
On January 10, 2015, she went into labor, walked to the health center where she had first sought care and delivered a stillborn infant. The fetus was deformed and macerated. Since the woman had no symptoms and was no longer in quarantine, and because the fetus was already emerging when the mother arrived, the nurse assisting wore minimal personal protection equipment (PPE) consisting of gloves and an apron during the delivery. The village TBA, who had accompanied the woman, wore no PPE during the delivery. The nurse followed MoHS guidelines and requested a safe burial for the infant and an oral swab was collected. Once the stillborn infant was identified as EBOV RNA positive, the nurse and TBA were quarantined but did not develop any EVD symptoms during the 21-day period. No additional cases were reported in the couple's village or surrounding settlements served by the health center.
To our knowledge, this is the first report of a delivery of an EBOV-infected stillbirth in a rural community health center, and the case raises a number of important issues about providing care for pregnant women outside an EMC setting during an Ebola outbreak. Most notable is the increased risk of exposure that occurs for health workers when clinical symptoms are not reported, and they do not take into account epidemiological indicators, such as previous contact with a positive case, when assessing the risk of infection.
There is limited information about maternal and fetal outcomes related to EBOV infection. Previous reports have documented only pregnancies managed in an EMC setting,2–4 noting that pregnant women tend to have more severe disease and worse outcomes than nonpregnant women. Mupapa and others reported a case fatality rate of 95.5% among pregnant women compared with 70% in nonpregnant women in an Ebola outbreak in Kikwit, Democratic Republic of the Congo, in 1995 and that the majority of the 15 EBOV-infected pregnant women presented with serious symptoms more often than EBOV-infected nonpregnant women.2
Recently, Baggi and others highlighted the potential for more positive outcomes, describing two symptomatic pregnant women admitted to an EMC in Guinea who survived vaginal delivery with supportive care. However, both delivered EBOV-infected stillborn infants, and the authors warned of the possibility of pregnant EVD survivors being referred to local health-care centers for delivery, potentially exposing maternity staff to a high risk of infection.3
The case we describe did not become severely ill in the way people in West Africa have come to expect of those suffering from Ebola. The woman did not recognize her symptoms as being related to Ebola, nor did the health workers who assisted her, despite miscarriage being a defining characteristic of Ebola during pregnancy. In the absence of the more recognizable symptoms of Ebola (i.e., fever, vomiting, and diarrhea), the woman's symptoms were misinterpreted as being caused by anxiety and pregnancy complications. We must, however, also acknowledge the difficulty of knowing for sure the clinical history of this case, given that fear of isolation and stigmatization may have influenced what the woman was willing to reveal.
The relatively long time between events and the assumption that there was no risk after completing a 21-day quarantine may also have reduced the alertness of the health workers. We believe the mother was most likely infected through contact with the uncle or with her husband after he developed symptoms and not as a result of sexual contact post recovery since the woman's description of the start of her intrauterine fetal death supports an earlier infection date. The possibility of a long delay between fetal death and delivery in EBOV-affected pregnancy has been previously seen.6 An MSF technical guidance paper cites a woman admitted to the EMC in her 5th month of pregnancy, who had an EBOV-infected stillbirth 32 days after she tested RT-PCR negative.
Although there is also scant information currently in the literature about mild or asymptomatic EBOV infections, Akerlund and others recently reported a case in Monrovia, Liberia, of a pregnant woman with suspected premature rupture of membranes who did not meet EVD case definition or report an EVD contact but tested EBOV positive with a high viral load 3 days before becoming symptomatic. Blood, urine, oral, and vaginal fluids were all positive before the onset of obvious EVD symptoms, leading the authors to suggest that pregnancy might alter EVD presentation and progression and warn of the challenges this may present for health-care workers.7
All these cases point to a critical requirement for health workers, especially in the community where laboratory results will rarely be available before care, to be informed that the risk of EBOV transmission cannot be excluded even if the pregnant woman does not appear to have symptoms and her last known exposure to an EBOV-positive contact was more than 21 days prior. It is also important that all pregnant women who are EVD survivors or have been in contact with a confirmed EVD case are followed until the end of their pregnancy and encouraged to deliver in an appropriately prepared health-care facility.
ACKNOWLEDGMENTS
We thank Tamba Aruna, Hannah Bockarie, and MSF Bo health promotion and mental health teams; Charles B. Beard (CDC Sierra Leone Ebola Response Associate Director for Science); and the District Health Management Team case investigators: Christian Allie, Festus Bundeh, and Abubakar Dukulay for their dedicated work on this and many other Ebola cases.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Footnotes
Authors' addresses: Hilary Bower and Emily Veltus, Médecins Sans Frontières, Operational Centre Amsterdam, MSF Ebola Management Centre Project, Bo, Sierra Leone, E-mails: hilarybower@gmail.com and emilyveltus@gmail.com. Julian E. Grass, Shelley Campbell, David Wang, Christopher D. Paddock, Bobbie R. Erickson, Johanna S. Salzer, Jessica Belser, Dean Seneca, and Ute Stroeher, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: hij3@cdc.gov, iaz6@cdc.gov, iuc9@cdc.gov, cdp9@cdc.gov, bee2@cdc.gov, hio7@cdc.gov, jax6@cdc.gov, zkg8@cdc.gov, and xy8@cdc.gov. Aaron Brault and Alison Jane Basile, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: zlu5@cdc.gov and ajj1@cdc.gov. Eunice Chege, World Health Organization, Bo, Sierra Leone, E-mail: nyambue@yahoo.com. Gbessay Saffa, Sierra Leone Ministry of Health and Sanitation, District Health Management Team Surveillance Unit, Bo, Sierra Leone, E-mail: gbessaysaffa@yahoo.com. Tom Decroo, Médecins Sans Frontières, Operational Centre Brussels, Luxembourg Operational Research Unit, Brussels, Belgium, E-mail: tom.decroo@luxembourg.msf.org. Grazia M. Caleo, Médecins Sans Frontières, Operational Centre Amsterdam, Manson's Unit, London, United Kingdom, E-mail: Grazia.Caleo@london.msf.org.
References
- 1.Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ. ELISA for the detection of antibodies to Ebola viruses. J Infect Dis. 1999;179((Suppl 1)):S192–S198. doi: 10.1086/514313. [DOI] [PubMed] [Google Scholar]
- 2.Mupapa K, Mukundu W, Bwaka MA, Kipasa M, De Roo A, Kuvula K, Kibadi K, Massamba M, Ndaberey D, Colebunders R, Muyembe-Tamfum JJ. Ebola hemorrhagic fever and pregnancy. J Infect Dis. 1999;179((Suppl 1)):S11–S12. doi: 10.1086/514289. [DOI] [PubMed] [Google Scholar]
- 3.Baggi FM, Taybi A, Kurth A, Van Herp M, Di Caro A, Wolfel R, Gunther S, Decroo T, Declerck H, Jonckheere S. Management of pregnant women infected with Ebola virus in a treatment center in Guinea, June 2014. Euro Surveill. 2014;19:20983. doi: 10.2807/1560-7917.es2014.19.49.20983. [DOI] [PubMed] [Google Scholar]
- 4.Oduyebo T, Pineda D, Lamin M, Leung A, Corbett C, Jamieson DJ. A pregnant patient with Ebola virus disease. Obstet Gynecol. 2015:1–3. doi: 10.1097/AOG.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 5.Sierra Leone Ministry of Health and Sanitation, World Health Organization . Clinical Management of Patients in the Ebola Treatment Centers and Other Care Centers in Sierra Leone: A Pocket Guide (Interim Emergency Guidelines) Freetown, Sierra Leone: Ministry of Health and Sanitation; 2014. [Google Scholar]
- 6.Caluwaerts S, Lagrou D. Guidance Paper—Pregnant Women in Ebola Treatment Center—MSF-OCB 2014—v1.4. 2014. https://www.rcog.org.uk/globalassets/documents/news/etc-preg-guidance-paper.pdf Available at. Accessed March 10, 2015.
- 7.Akerlund E, Prescott J, Tampellini L. Shedding of Ebola virus in an asymptomatic pregnant woman. N Engl J Med. 2015;372:2467–2469. doi: 10.1056/NEJMc1503275. [DOI] [PubMed] [Google Scholar]