Abstract
Anemia is often a comorbidity of human immunodeficiency virus (HIV) infection. Many cross-sectional studies have been conducted on anemia and HIV, but few, if any, have addressed incidence of anemia prospectively. A longitudinal analysis was conducted in 48,068 nonpregnant HIV-infected adults in Dar es Salaam, Tanzania, seen at Management and Development for Health–U.S. President's Emergency Plan for AIDS Relief HIV care and treatment programs between 2004 and 2011. Almost 56% (N = 27,184) of study participants had anemia (hemoglobin < 11 g/dL) at the time of enrollment at the clinic. Female gender, low body mass index (BMI), low CD4 T-cell count, high levels of liver enzyme alanine aminotransferase, antiretroviral treatment (ART) regimens, and concurrent tuberculosis treatment were all independently significantly associated with an increased risk of anemia. Low BMI and low CD4 T-cell count were independently significantly associated with an increased risk for iron deficiency anemia (IDA). Higher BMI status and ART use were associated with recovery from anemia. Anemia, including IDA, is a comorbidity that is associated with other adverse consequences (e.g., low BMI and CD4 T-cell count) among individuals with HIV infection, including those on ART. Interventions to prevent anemia and its complications need to be examined in the context of future studies.
Introduction
Anemia is often a comorbidity of human immunodeficiency virus (HIV) infection. Many cross-sectional studies have been conducted on anemia and HIV, including studies on prevalence and the relationship of anemia with disease progression and mortality, but few have addressed the incidence and determinants of anemia in Africa. The goal of this study is to assess the factors associated with anemia in HIV-infected adults (particularly in the highly active antiretroviral therapy [HAART] era in Tanzania) after they have enrolled into a care and treatment program.
Tanzania, like much of sub-Saharan Africa, has a relatively high rate of HIV.1,2 Many studies have found a higher prevalence of anemia among HIV-infected individuals than their uninfected counterparts.3,4 A review conducted in 2008 found the average proportion of HIV-infected individuals living with anemia to be 60%.5 Individuals with anemia suffer from fatigue, lethargy, depression, and impaired cognitive function.6 In a study from Tanzania, the risk of acquired immunodeficiency syndrome (AIDS)-related death was more than double for patients with anemia compared with those without anemia, and anemia was associated with shorter time to immunologic disease progression.7,8 Another study found that the risk of death to be up to 70% greater in HIV-infected anemic patients compared with non-anemic patients, and that with anemia, the need for transfusions is greater and quality of life is poorer, largely due to fatigue.6 Johannessen and others found that mortality increased with decreasing hemoglobin (Hgb) among HIV-infected Tanzanian patients. The estimated 1-year mortality was 3.7% in patients without anemia, 20.0% in those with mild anemia, 37.6% in those with moderate anemia, and 55.2% in those with severe anemia.8
The pathophysiology of HIV-associated anemia may involve four basic mechanisms: blood loss, decreased red blood cell (RBC) production, increased RBC destruction, and ineffective RBC production.9 The most obvious cause of anemia is blood loss, which may be associated with opportunistic complications such as neoplastic disease or gastrointestinal lesions that accompany Cytomegalovirus infection.9,10 Other mechanisms including nutritional deficiencies, genetic disorders, and anemia due to chronic diseases are also important in this setting.
The incidence of anemia has been reported to decrease after initiation of HAART.11 Recovery from anemia is associated with a reduced risk of HIV disease progression to approximately the same level as seen among patients who have never had anemia.11 Multiple studies found an increased prevalence of anemia in female HIV-infected persons compared with male HIV-infected persons, which is often attributed to blood loss during menstruation, pregnancy, and delivery.12,13 A study in Tanzania also found low CD4 T-cell count, high erythrocyte sedimentation rate, and vitamin D insufficiency as main predictors of anemia in HIV-positive pregnant and postpartum women.14
Many cross-sectional studies have been conducted on anemia and HIV, but few have addressed the issue of incidence of anemia prospectively. In this study, we examine factors associated with anemia and iron deficiency in HIV-infected adults after entry into an HIV care and treatment program, among individuals that have been initiated on antiretroviral treatment (ART), as well as those that are healthier and have yet to be initiated on ART.
Methods
Population.
This prospective observational study was conducted at 12 Management and Development for Health (MDH) HIV care and treatment clinics (CTCs) supported by the U.S. President's Emergency Plan for AIDS Relief in Dar es Salaam, Tanzania, between November 2004 and September 2011. The MDH program was established in 2004 and provides infrastructure, laboratory, and technical support to HIV CTCs; integrated prevention of mother to child transmission; and tuberculosis (TB) facilities in the Dar es Salaam region. Details have been previously published.15,16 For inclusion in these analyses, participants had to be 15 years old and above, not pregnant at the time of enrollment, and have at least two measurements of Hgb concentration.
Clinical procedures.
Clinical care of all HIV-infected patients at MDH-supported CTCs follows national and World Health Organization (WHO) guidelines.17,18 After enrollment, patients are evaluated monthly if they are on ART (treatment) or 4–6 months if not on ART (care and monitoring). At each visit, patients on ART are examined by a physician, undergo adherence and nutrition counseling, and receive ART refills. Patients in monitoring (not on ART) are assessed by a nurse and/or physician at their follow-up visits and examined for disease progression and other opportunistic infections (OIs) and given nutrition counseling. If they are found to be eligible for ART initiation, they will be given the appropriate laboratory tests and return after 2 weeks for follow-up and ART initiation. Monitoring laboratory tests including Hgb and alanine aminotransferase (ALT) are performed 2 weeks after ART initiation and once in 4 months thereafter. Immunologic monitoring with CD4 T-cell counts is also performed once in 4 months. Viral load testing is not performed routinely. TB screening with a chest radiograph and sputum analysis screening is performed at the time of enrollment to a care and treatment center on all patients and repeated in patients who have symptoms suggestive of TB at follow-up visits. A comprehensive patient tracking system is in place to ensure all patients who are eligible for tracking (i.e., missed visit, loss to follow-up, sick, and abnormal laboratory result) are contacted by phone or in person to encourage them to return to the clinic.
Patients were initiated on ART according to National AIDS Control Program ART initiation criteria.18 At the time of this study, standard first-line ART regimens included stavudine (D4T) or zidovudine (AZT), along with lamivudine (3TC) and efavirenz (EFV) or nevirapine (NVP). Antiretroviral drugs were provided free of charge by the Tanzanian government. Since the inception of the MDH program, no patients have experienced treatment interruptions owing to drug shortages.
Study design.
Data collection.
At baseline and follow-up visits, physicians and nurses complete standard forms. These forms captured demographic, clinical, laboratory, and therapeutic information. Data reviewers are stationed at each clinic to ensure accuracy and completeness of data recording by the health-care workers. The data are then entered into a computerized database that is secure, and all original forms are securely filed away. Unique patient identifiers are used to ensure anonymity. There are daily updates to the database by data entry clerks. To ensure data accuracy, weekly quality assurance checks of the database are performed by the data management team. Attending physicians diagnosed OIs and staged all patients according to WHO criteria at the first and follow-up visits. Additional information collected at the initial visit included demographic characteristics, anthropometric measurements, previous ART use, current use of any prophylaxis against infections, and history of TB and current TB treatment. The initial laboratory workup included measurement of CD4 T-cell count, complete blood count, and assessment of liver function using ALT levels. Women whose pregnancy status was unknown were asked to undergo a pregnancy test.
Data collected for this analysis included baseline age; district of residence (a proxy for socioeconomic status [Temeke, Ilala, and Kinondoni]); body mass index (BMI) defined as body weight in kilograms per height in square meter; WHO clinical stage; history of prior or current TB (defined as receiving treatment of TB within 1 month after ART initiation); history of previous ART use; current co-trimoxazole prophylaxis; concurrent illness such as oral candidiasis, diarrhea, and Kaposi's sarcoma; and ART regimen at initiation and follow-up and laboratory data collected at baseline and follow-up included Hgb, mean corpuscular volume (MCV), CD4 T-cell count, ALT (> 120 U/L), creatinine (> 1.2 mmol/L), and presence of hepatitis B surface antigen.
Ascertainment of anemia status.
The primary endpoints of this study are incidence of anemia, defined as the first measure of Hgb concentration < 11 g/dL; iron deficiency anemia (IDA), defined as the first MCV less than 80 fL along with a Hgb concentration < 11 g/dL; and recovery from anemia, defined as the first measure of Hgb ≥ 11 g/dL after having previously been observed to be anemic.
Statistical analysis.
Cox proportional hazards regression methods were used to examine predictors of anemia, IDA, and recovery from anemia for both univariate and multivariate analyses and to estimate hazard ratios and their 95% confidence interval (CI).19 Time zero was based on the date of the patient's first Hgb measurement. Enrollment occurred between 2004 and 2011 in adults diagnosed with HIV/AIDS. Censoring was defined as anemia (or IDA or recovery from anemia for the respective analyses) being present or the end of the follow-up period (last patient encounter) or loss to follow-up, whichever occurred first. The proportional hazards assumptions were tested and confirmed. The Wald test statistic was used to assess the significance of associations between continuous or categorical risk factors and time to anemia/IDA/recovery from anemia, the likelihood ratio test for nominal polytomous variables, and median score trend test for ordinal categorical variables. Predictors with a univariate P value ≤ 0.20 were introduced into the multivariate model, using SAS, version 9.1, statistical software from SAS Institute Inc. (Cary, NC). In the multivariate model, we used the counting process data structure to incorporate time-varying covariates, that is, predictors that were changing or updated over time.20 Restricted cubic splines were used to assess nonlinearity for all continuous model covariates that provides flexible smooth curves.21 Significant evidence for nonlinearity at the P value ≤ 0.05 is reported. The last recorded covariate value was carried forward until a new value was available, the outcome was reached, or the individual's time was censored. Observations with covariate data that were unavailable were retained in the analyses by using missing indicators. To assess their independent effects, the final multivariate models did not simultaneously include WHO HIV disease staging, CD4 T-cell counts, or OIs; the models were run three times, once with only WHO HIV disease stage, once with CD4 T-cell count, and once with the significant OIs, adjusted for all other variables with a P value < 0.20 in the univariate model. This same technique was used for regimen and regimens containing AZT that were run in two separate models, with all other variables, including CD4 T-cell count (regimen models excluded WHO stage or OIs).
Patients were recruited for participation and enrolled in MDH-supported CTCs after providing written informed consent, which was subject to ethical reviews by the Muhimbili University of Health and Allied Sciences and the Harvard School of Public Health Institutional Review Board.
Results
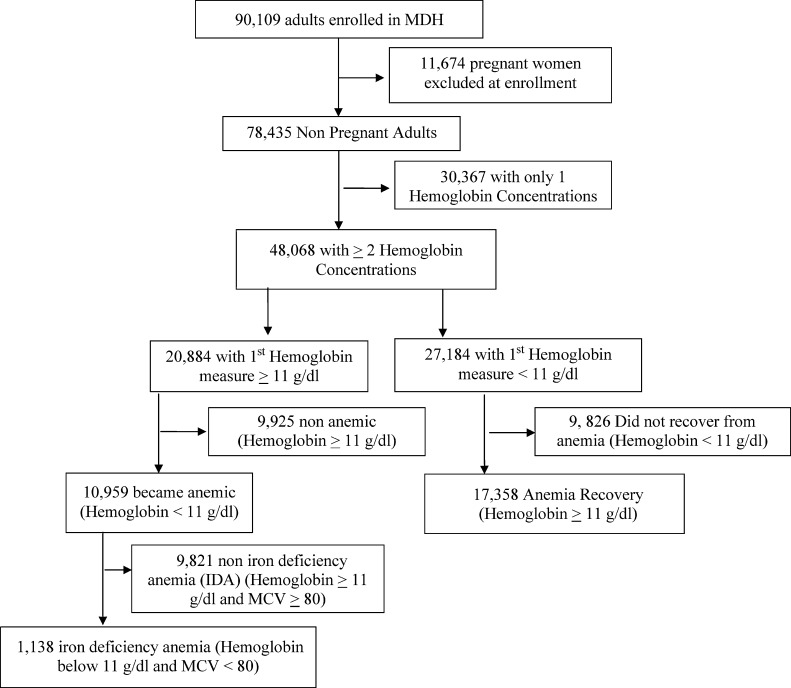
About 61% (48,068) adults out of a total of 78,435 adults enrolled in MDH with two or more Hgb concentrations available were included in the analysis. Baseline characteristics of the study population can be found in Table 1. Almost 44% (20,884) had Hgb ≥ 11 g/dL when their first measure of Hgb was taken and 56% (27,184) had Hgb of < 11 g/dL on their first measure of Hgb (Figure 1 ). Out of those that were not anemic at baseline, 53% subsequently became anemic later, and of those, 10% developed IDA during follow-up. Out of those that were anemic at baseline, almost 64% recovered from anemia during follow-up. About 66% had IDA at baseline; almost 19% recovered from IDA during follow-up.
Table 1.
Baseline characteristics of HIV-infected adults at enrollment in care and treatment program in Dar es Salaam, Tanzania
| Characteristic* | N (%) |
|---|---|
| Gender | |
| Female | 32, 885 (68.4) |
| Age group, years | |
| 30 | 9,200 (19.3) |
| 30 to < 40 | 21,073 (44.2) |
| 40 to < 50 | 12,163 (25.5) |
| 50+ | 5,258 (11) |
| Marital status | |
| Married/cohabiting | 20,448 (51) |
| Widowed/divorced/separated | 64,896 (16.2) |
| Single | 13,172 (32.8) |
| District (residence) | |
| Ilala | 14,272 (29.8) |
| Kinondoni | 21,195 (44.3) |
| Temeke | 12,429 (26) |
| BMI group, kg/m2 | |
| < 18.5 | 12,524 (26.8) |
| 18.5 to < 25 | 25,461 (54.5) |
| 25.0 to < 30 | 6,295 (13.5) |
| 30+ | 2,469 (5.2) |
| WHO HIV disease stage | |
| I | 6,479 (14.1) |
| II | 9,125 (19.9) |
| III | 21,715 (47.2) |
| IV | 8,627 (18.8) |
| CD4 count, cells/μL | |
| < 50 | 8,070 (19) |
| 50 to < 200 | 16,018 (37.6) |
| 200+ | 18,464 (43.4) |
| Medical history | |
| No previous ART use | 41,324 (88.7) |
| Previous ART use | 5,263 (11.3) |
| TB history | 10,841 (24.5) |
| Illnesses (OIs) | |
| Oral candidiasis | 2,823 (6.4) |
| Diarrhea | 2,627 (5.9) |
| Kaposi's sarcoma | 278 (0.6) |
| Biochemical tests | |
| ALT > 120 U/L | 360 (1) |
| Hepatitis B surface antigen | 650 (5.9) |
| Creatinine > 1.2 mmol/L | 3,369 (15.1) |
| Medications | |
| On septrin prophylaxis | 10,987 (22.9) |
| Taking a multivitamin | 8,289 (20.7) |
| On TB treatment | 4,662 (9.7) |
| Baseline regimen contains AZT† | 12,986 (27) |
| Baseline regimen contains EFV† | 12,210 (25.2) |
| Baseline ART regimen† | |
| D4T, 3TC, NVP | 9,164 (36.7) |
| D4T, 3TC, EFV | 2,842 (11.4) |
| AZT, 3TC, NVP | 3,046 (12.2) |
| AZT, 3TC, EFV | 9,940 (39.8) |
ALT = alanine aminotransferase; ART = antiretroviral treatment; AZT = zidovudine; BMI = body mass index; D4T = stavudine; EFV = efavirenz; HIV = human immunodeficiency virus; NVP = nevirapine; OIs = opportunistic infections; TB = tuberculosis; 3TC = lamivudine; WHO = World Health Organization.
Totals do not add up to 48,068 due to missing values.
Total is sub-population of those with at least two hemoglobin measures, who initiated ART at baseline.
Figure 1.
Data on hemoglobin in adults in Management and Development for Health.
Risk factors for anemia.
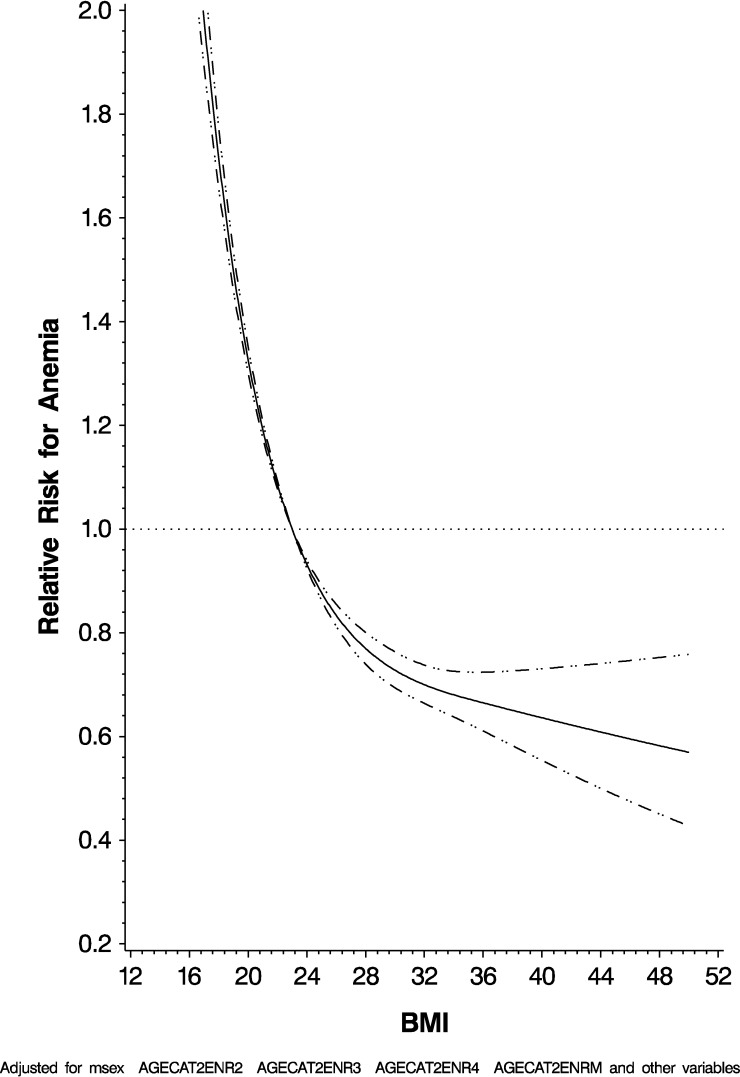
Male gender (hazard ratio [HR]: 0.55; 95% CI: 0.52, 0.57), previous ART history (HR: 0.85; 95% CI: 0.80, 0.90), and increased BMI (P < 0.0001) were independently associated with a lower risk of the occurrence of anemia. Increasing age (P < 0.0001), increasing WHO HIV disease stage (P < 0.0001), decreasing CD4 T-cell count (P < 0.0001), diarrhea (HR: 1.75; 95% CI: 1.57, 1.95), increased ALT (HR: 1.38; 95% CI: 1.17, 1.62), Kaposi's sarcoma (HR: 3.86; 95% CI: 2.43, 6.15), septrin use (HR: 1.50; 95% CI: 1.42, 1.58), ART use (HR: 1.40; 95% CI: 1.33, 1.48), and currently being treated for TB (HR: 1.19; 95% CI: 1.09, 1.29) were all independently associated with an increased risk for anemia. Figure 2 graphically represents the risk for anemia by BMI in the multivariate adjusted model, which was statistically significant for nonlinearity using the restricted cubic splines model. The complete results are given in Table 2.
Figure 2.
Risk for anemia by body mass index.
Table 2.
Risk factors for anemia (N = 10,959 became anemic)
| Characteristics | Hgb < 11/person-months | Univariate HR (95% CI) | P value | Multivariate* HR (95% CI) | P value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 7,253/84,293 | Ref | – | Ref | – |
| Male | 3,706/44,900 | 0.57 (0.55, 0.60) | < 0.0001 | 0.55 (0.52, 0.57) | < 0.0001 |
| Age group, years | |||||
| < 30 | 2,097/26,574 | Ref | < 0.0001∥ | Ref | < 0.0001¶ |
| 30 to < 40 | 4,735/56,887 | 0.86 (0.82, 0.91) | 1.06 (1.00, 1.11) | ||
| 40 to < 50 | 2,759/30,986 | 0.80 (0.75, 0.84) | 1.15 (1.08, 1.22) | ||
| 50+ | 1,281/13,627 | 0.87 (0.81, 0.93) | 1.23 (1.14, 1.32) | ||
| Marital status | |||||
| Married/cohabiting | 5,022/62,504 | Ref | < 0.0001¶ | Ref | 0.007¶ |
| Widowed/divorced/separated | 800/8,522 | 1.27 (1.18, 1.37) | 0.99 (0.92, 1.07) | ||
| Single | 2,924/32,074 | 1.23 (1.17, 1.28) | 1.04 (1.00, 1.09) | ||
| District | |||||
| Ilala | 2,665/28,650 | Ref | < 0.0001¶ | Ref | < 0.0001¶ |
| Kinondoni | 4,740/59,414 | 0.98 (0.94, 1.03) | 1.15 (1.09, 1.21) | ||
| Temeke | 3,530/41,061 | 1.94 (1.85, 2.04) | 1.59 (1.51, 1.68) | ||
| BMI group, kg/m2§ | |||||
| < 18.5 | 1,860/12,981 | 1.66 (1.58, 1.75) | < 0.0001∥ | 1.52 (1.44, 1.60) | < 0.0001∥ |
| 18.5 to < 25 | 6,056/68,339 | Ref | Ref | ||
| 25.0 to < 30 | 2,090/31,300 | 0.79 (0.75, 0.83) | 0.76 (0.72, 0.80) | ||
| 30+ | 953/16,572 | 0.72 (0.67, 0.77) | 0.66 (0.61, 0.70) | ||
| WHO HIV disease stage§ | |||||
| I | 1,603/21,398 | Ref | < 0.0001¶ | Ref | < 0.0001¶ |
| II | 2,827/38,449 | 1.17 (1.10, 1.24) | 1.08 (1.02, 1.15) | ||
| III | 5,374/60,209 | 1.40 (1.32, 1.48) | 1.15 (1.08, 1.22) | ||
| IV | 1,155/9,136 | 2.61 (2.41, 2.82) | 1.93 (1.78, 2.09) | ||
| CD4 count, cells/μL§ | |||||
| < 50 | 1,488/7,530 | 3.02 (2.84, 3.21) | < 0.0001∥ | 2.47 (2.31, 2.64) | < 0.0001∥ |
| 50 to < 200 | 3,351/26,759 | 2.02 (1.93, 2.11) | 1.78 (1.69, 1.87) | ||
| 200+ | 6,120/95,427 | Ref | Ref | ||
| Previous ART use | 1,302/18,732 | 0.61 (0.57, 0.64) | < 0.0001 | 0.85 (0.80, 0.81) | < 0.0001 |
| TB history | 2,190/22,861 | 0.93 (0.88, 0.97) | 0.002 | 0.97 (0.93, 1.01) | 0.16 |
| Oral candidiasis§ | 240/2,440 | 1.96 (1.76, 2.18) | 0.0001 | 2.33 (2.04, 2.65) | < 0.0001 |
| Diarrhea§ | 355/3,475 | 2.78 (2.45, 3.17) | < 0.0001 | 1.75 (1.57, 1.95) | < 0.0001 |
| ALT > 120 U/L§ | 151/1,752 | 1.47 (1.25, 1.73) | < 0.0001∥ | 1.38 (1.17, 1.62) | < 0.0001¶ |
| Hepatitis B surface antigen§ | 854/9,819 | 1.09 (1.02, 1.17) | 0.22 | 1.08 (1.00, 1.16) | 0.04 |
| Creatinine > 1.2 mmol/L§ | 1,481/16,757 | 1.02 (0.96, 1.07) | < 0.0001∥ | 1.03 (0.98, 1.09) | 0.03∥ |
| Kaposi's sarcoma§ | 18/37 | 4.46 (2.81, 7.09) | < 0.0001 | 3.86 (2.43, 6.15) | 0.0001 |
| On septrin prophylaxis§ | 1,962/13,793 | 1.78 (1.69, 1.87) | < 0.0001 | 1.50 (1.42, 1.58) | < 0.0001 |
| Currently being treated for TB§ | 709/5,830 | 1.35 (1.25, 1.46) | < 0.0001 | 1.19 (1.09, 1.29) | < 0.0001 |
| Taking a multivitamin§ | 4,802/57,542 | 0.99 (0.95, 1.03) | 0.66 | ||
| On ART†‡§ | 6,389/69,974 | 1.76 (1.69, 1.84) | < 0.0001 | 1.40 (1.33, 1.48) | < 0.0001¶ |
| Regimen contains AZT†‡§ | 3,569/37,047 | 1.49 (1.42, 1.57) | < 0.0001¶ | 1.43 (1.36, 1.51) | < 0.0001¶ |
| Not on ART | 4,570/59,219 | 0.70 (0.66, 0.74) | 0.70 (0.67, 0.74) | ||
| Other regimens (non-AZT) | 2,820/32,926 | Ref | Ref | ||
| Regimen contains EFV†‡§ | 2,898/25,211 | 1.30 (1.24, 1.37) | < 0.0001¶ | 1.17 (1.12, 1.24) | < 0.0001¶ |
| Not on ART | 4,570/59,219 | 0.64 (0.61, 0.67) | 0.71 (0.68, 0.75) | ||
| Other regimens (non-EFV) | 3,491/44,763 | Ref | Ref | ||
| ART regimen†‡§ | |||||
| D4T, 3TC, NVP | 2,372/27,645 | Ref | < 0.0001¶ | Ref | < 0.0001¶ |
| D4T, 3TC, EFV | 273/1,682 | 1.10 (0.97, 1.25) | 0.95 (0.83, 1.07) | ||
| AZT, 3TC, NVP | 1,050/15,461 | 1.53 (1.42, 1.65) | 1.45 (1.35, 1.56) | ||
| AZT, 3TC, EFV | 2,519/21,586 | 1.52 (1.44, 1.61) | 1.35 (1.28, 1.43) | ||
| TDF, FTC, EFV | 106/1,943 | 1.22 (1.01, 1.49) | 1.19 (0.98, 1.44) | ||
| Other | 69/1,656 | 1.26 (0.99, 1.60) | 1.30 (1.02, 1.65) | ||
| Not on ART | 4,570/59,219 | 0.71 (0.67, 0.75) | 0.79 (0.75, 0.84) | ||
ALT = alanine aminotransferase; ART = antiretroviral treatment; AZT = zidovudine; BMI = body mass index; CI = confidence interval; D4T = stavudine; EFV = efavirenz; TDF = tenofovir; FTC = emtricitabine; Hgb = hemoglobin; HIV = human immunodeficiency virus; NVP = nevirapine; TB = tuberculosis; 3TC = lamivudine; WHO = World Health Organization.
Multivariate-adjusted hazard ratio from a proportional hazards model was adjusted for all variables in the univariate analysis with a P < 0.20.
ARTs were entered into the final model separately with all other significant variables, AZT regimen, EFV regimen, ART, and ART regimens.
Referent group for “ART regimen” is comparing each regimen to “D4T, 3TC, NVP.”
Time-varying covariates. Analysis performed using Andersen–Gill data structure with 340,137 observations during follow-up period.
Association was significantly nonlinear. P value corresponds for the test of overall significance.
P value corresponds to test for trend.
Risk factors for IDA.
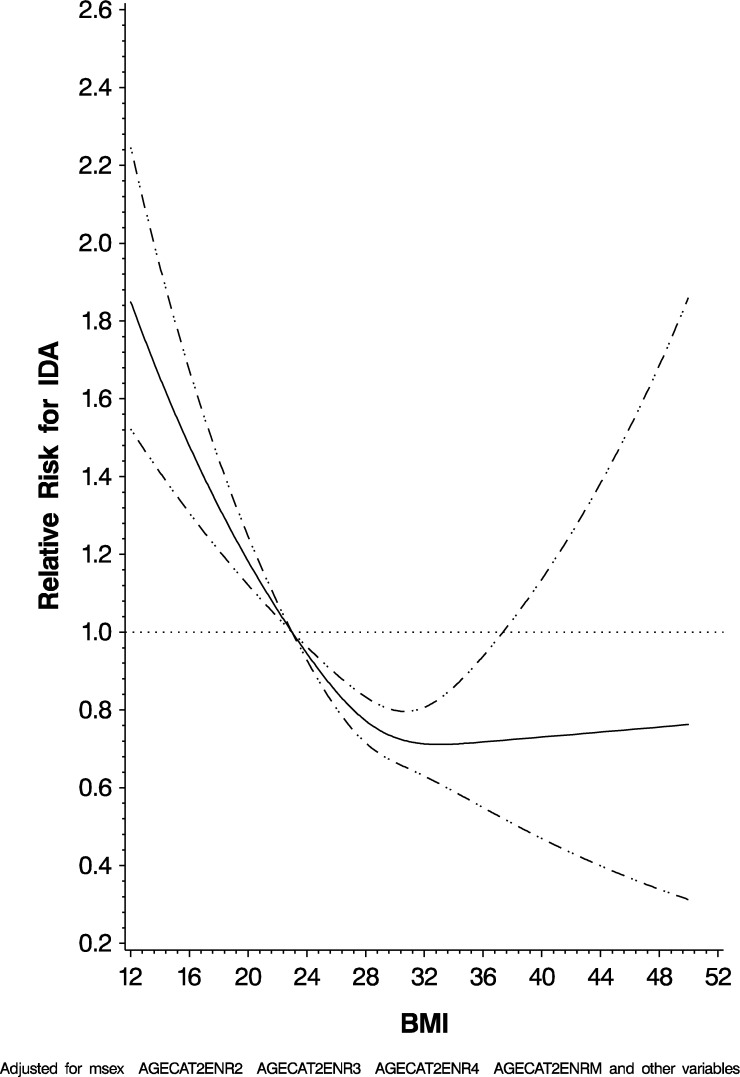
We next examined the relationships of various risk factors on IDA. Male gender (HR: 0.44; 95% CI: 0.38, 0.51), higher BMI (P < 0.0001), having been initiated on ART (HR: 0.76; 95% CI: 0.65, 0.88), and ART regimen containing EFV (HR: 1.46; 95% CI: 1.21, 1.76) were all significant predictors of IDA. Residing in Temeke District (HR: 2.19; 95% CI: 1.85, 2.58), decreasing CD4 T-cell count (P < 0.0001), and increasing ALT (P 0.03) were all risk factors for IDA. Figure 3 graphically represents the risk for IDA by BMI in the multivariate adjusted model, which was statistically significant for nonlinearity using the restricted cubic splines model. The full results are given in Table 3.
Figure 3.
Risk for iron deficiency anemia by body mass index.
Table 3.
Risk factors for IDA (N = 1,138; Hgb < 11 g/dL; and MCV < 80 fL)
| Characteristics | Hgb < 11 and MCV < 80/person-months | Univariate HR (95% CI) | P value | Multivariate* HR (95% CI) | P value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 865/12,575 | Ref | – | Ref | – |
| Male | 273/3,570 | 0.48 (0.42, 0.55) | < 0.0001 | 0.44 (0.38, 0.51) | < 0.0001 |
| Age group, years | |||||
| < 30 | 260/3,822 | Ref | < 0.0001∥ | Ref | 0.06¶ |
| 30 to < 40 | 504/7,260 | 0.80 (0.68, 0.92) | 0.97 (0.83, 1.13) | ||
| 40 to < 50 | 259/3,547 | 0.68 (0.57, 0.81) | 1.02 (0.85, 1.22) | ||
| 50+ | 100/1,202 | 0.58 (0.46, 0.73) | 0.92 (0.73, 1.17) | ||
| Marital status | |||||
| Married/cohabiting | 548/7,765 | Ref | 0.24¶ | – | – |
| Widowed/divorced/separated | 69/982 | 0.87 (0.67, 1.1) | – | ||
| Single | 313/4,308 | 1.10 (0.96, 1.260 | – | ||
| District | |||||
| Ilala | 189/2,702 | Ref | < 0.0001¶ | Ref | < 0.0001¶ |
| Kinondoni | 280/4,268 | 1.03 (0.85, 1.23) | 1.06 (0 88, 1.28) | ||
| Temeke | 668/9,164 | 2.05 (1.75, 2.41) | 2.19 (1.85, 2.58) | ||
| BMI group, kg/m2§ | |||||
| < 18.5 | 174/1,909 | 1.29 (1.09, 1.53) | < 0.0001∥ | 1.22 (1.02, 1.45) | < 0.0001∥ |
| 18.5 to < 25 | 674/9,155 | Ref | Ref | ||
| 25.0 to < 30 | 195/3,340 | 0.68 (0.58, 0.80) | 0.66 (0.56, 0.78) | ||
| 30+ | 95/1,741 | 0.68 (0.55, 0.85) | 0.61 (0.49, 0.76) | ||
| WHO HIV disease stage§ | |||||
| I | 203/2,971 | Ref | 0.32¶ | – | – |
| II | 322/4,677 | 1.07 (0.90, 1.28) | – | ||
| III | 513/7,358 | 0.96 (0.81, 1.13) | – | ||
| IV | 100/1,139 | 1.50 (1.17, 1.91) | – | ||
| CD4 count, cells/μL§ | |||||
| < 50 | 126/1,282 | 2.40 (1.96, 2.95) | < 0.0001¶ | 2.38 (1.93, 2.94) | < 0.0001¶ |
| 50 to < 200 | 267/2,961 | 1.41 (1.21, 1.64) | 1.52 (1.30, 1.78) | ||
| 200+ | 745/11,902 | Ref | Ref | ||
| Previous ART use | 115/2,289 | 0.50 (0.41, 0.61) | < 0.0001 | 0.78 (0.64, 0.95) | 0.01 |
| TB history | 219/2,887 | 0.81 (0.69, 0.93) | 0.004 | 0.92 (0.78, 1.07) | 0.27 |
| Oral candidiasis§ | 23/323 | 1.48 (0.97, 2.28) | 0.07 | 1.36 (0.88, 2.10) | 0.17 |
| Diarrhea§ | 41/706 | 1.68 (1.22, 2.30) | 0.001 | 1.55 (1.12, 2.13) | 0.008 |
| ALT > 120 U/L§ | 17/143 | 1.75 (1.08, 2.83) | 0.02∥ | 1.72 (1.06, 2.78) | 0.03∥ |
| Hepatitis B surface antigen§ | 108/1,527 | 1.27 (1.04, 1.55) | 0.02 | 1.29 (1.06, 1.58) | 0.01 |
| Creatinine > 1.2 mmol/L§ | 134/2,025 | 0.96 (0.80, 1.15) | 0.009¶ | 0.95 (0.79, 1.14) | 0.002∥ |
| On septrin prophylaxis§ | 123/1,159 | 1.32 (1.09, 1.60) | 0.005 | 1.23 (1.01, 1.50) | 0.04 |
| Currently being treated for TB§ | 84/963 | 1.60 (1.28, 2.01) | < 0.0001 | 1.46 (1.15, 1.86) | 0.002 |
| Taking a multivitamin§ | 455/5,643 | 1.11 (0.99, 1.26) | 0.84 | – | – |
| On ART | 483/7,276 | 0.85 (0.74, 0.98) | 0.03 | 0.76 (0.65, 0.88) | 0.0003 |
| Regimen contains AZT†‡§ | 213/2,746 | 1.42 (1.19, 1.70) | < 0.0001¶ | 1.44 (1.20, 1.72) | < 0.0001¶ |
| Not on ART | 655/8,869 | 1.36 (1.15, 1.60) | 1.53 (1.29, 1.81) | ||
| Other regimens (non-AZT) | 270/4,531 | Ref | Ref | ||
| Regimen contains EFV†‡§ | 183/2,017 | 1.58 (1.31, 1.90) | < 0.0001¶ | 1.46 (1.21, 1.76) | 0.0001¶ |
| Not on ART | 655/8,869 | 1.38 (1.17, 1.62) | 1.50 (1.27, 1.77) | ||
| Other regimens (non-EFV) | 300/5,259 | Ref | Ref | ||
| ART regimen†‡§ | |||||
| D4T, 3TC, NVP | 224/3,688 | Ref | < 0.0001¶ | Ref | < 0.0001¶ |
| D4T, 3TC, EFV | 29/398 | 0.93 (0.63, 1.38) | 0.85 (0.57, 1.25) | ||
| AZT, 3TC, NVP | 65/1,216 | 1.01 (0.76, 1.34) | 1.09 (0.83, 1.45) | ||
| AZT, 3TC, EFV | 148/1,529 | 1.81 (1.47, 2.23) | 1.72 (1.39, 2.12) | ||
| TDF, FTC, EFV | 6/91 | 3.74 (1.66, 8.44) | 4.18 (1.85, 9.44) | ||
| Other | 11/354 | 1.59 (0.86, 2.94) | 1.69 (0.91, 3.14) | ||
| Not on ART | 655/8,869 | 1.40 (1.18, 1.66) | 1.54 (1.29, 1.84) | ||
ALT = alanine aminotransferase; ART = antiretroviral treatment; AZT = zidovudine; BMI = body mass index; CI = confidence interval; D4T = stavudine; EFV = efavirenz; TDF = tenofovir; FTC = emtricitabine; Hgb = hemoglobin; HIV = human immunodeficiency virus; IDA = iron deficiency anemia; MCV = mean corpuscular volume; NVP = nevirapine; TB = tuberculosis; 3TC = lamivudine; WHO = World Health Organization.
Multivariate-adjusted hazard ratio from a proportional hazards model was adjusted for all variables in the univariate analysis with a P < 0.20.
ARTs were entered into the final model separately with all other significant variables, AZT regimen, EFV regimen, ART, and ART regimens.
Referent group for “ART regimen” is comparing each regimen to “D4T, 3TC, NVP.”
Time-varying covariates. Analysis performed using Andersen–Gill data structure with 212,204 observations during follow-up period.
Association was significantly nonlinear. P value corresponds for the test of overall significance.
P value corresponds to test for trend.
Factors associated with recovery from anemia.
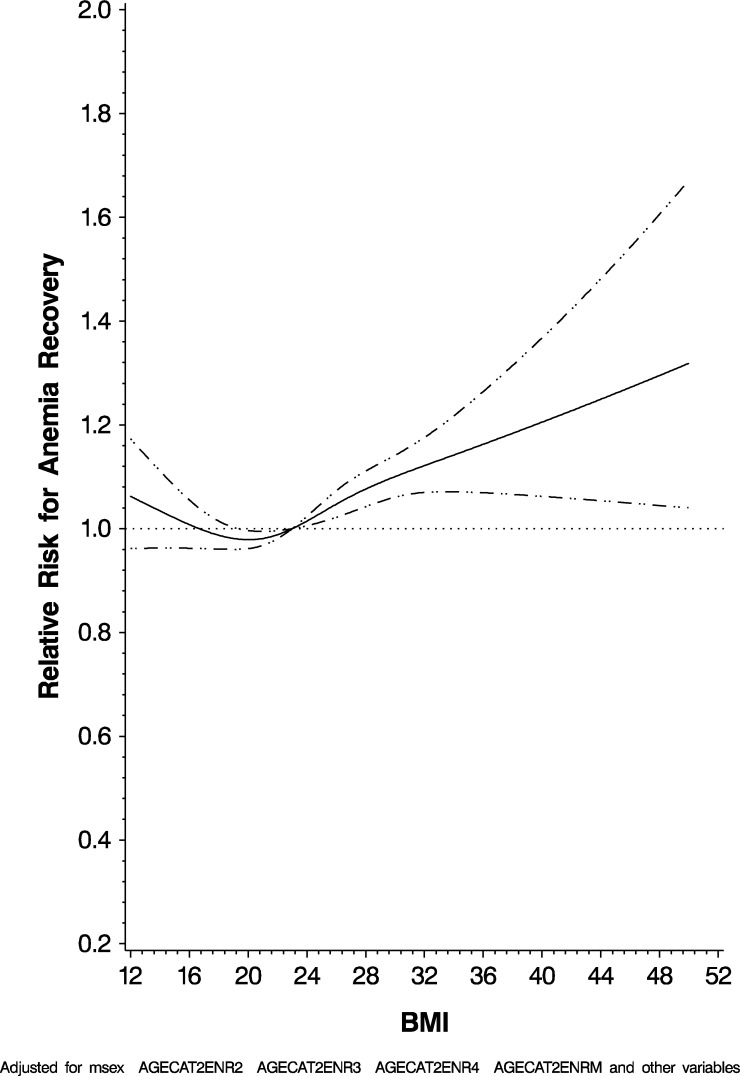
We similarly found male gender (HR: 1.72; 95% CI: 1.65, 1.78), increasing CD4 T-cell count (P < 0.0001), increasing WHO HIV disease stage (P 0.0001), history of TB (HR: 1.10; 95% CI: 1.07, 1.15), on septrin (HR: 1.13; 95% CI: 1.08, 1.18), currently being treated for TB (HR: 1.37; 95% CI: 1.30, 1.45), having been initiated on ART (HR: 1.36; 95% CI: 1.32, 1.41), and being on any ART regimen containing AZT (HR: 0.85; 95% CI: 0.82, 0.88) were significantly independently associated with recovery from anemia. In addition, we found that taking a multivitamin (HR: 0.85; 95% CI: 0.83, 0.88) was protective for this outcome. Figure 4 graphically represents recovery from anemia by BMI in the multivariate adjusted model, which was statistically significant for nonlinearity using the restricted cubic splines model. The full results are given in Table 4.
Figure 4.
Risk for anemia recovery by body mass index.
Table 4.
Risk factors for recovery from anemia (N = 17,358 recovered from anemia)
| Characteristics | Hgb > 11/person-months | Univariate HR (95% CI) | P value | Multivariate* HR (95% CI) | P value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 12,702/142,436 | Ref | – | Ref | – |
| Male | 4,656/33,826 | 2.98 (2.88, 3.09) | < 0.0001 | 1.72 (1.65, 1.78) | < 0.0001 |
| Age group, years | |||||
| < 30 | 3,263/35,375 | Ref | < 0.0001∥ | Ref | 0.55¶ |
| 30 to < 40 | 7,720/80,103 | 0.99 (0.95, 1.03) | 0.95 (0.91, 0.99) | ||
| 40 to < 50 | 4,421/42,385 | 1.12 (1.07, 1.17) | 0.96 (0.92, 1.01) | ||
| 50+ | 1,749/16,224 | 1.28 (1.21, 1.36) | 0.94 (0.89, 1.00) | ||
| Marital status | |||||
| Married/cohabiting | 7,058/70,752 | Ref | 0.93¶ | – | – |
| Widowed/divorced/separated | 1,277/13,539 | 0.84 (0.79, 0.89) | – | ||
| Single | 4,940/49,299 | 1.01 (0.97, 1.05) | – | ||
| District | |||||
| Ilala | 5,329/55,364 | Ref | < 0.0001¶ | Ref | < 0.0001¶ |
| Kinondoni | 7,574/82,900 | 0.85 (0.82, 0.88) | 0.91 (0.88, 0.95) | ||
| Temeke | 4,415/37,894 | 1.20 (1.15, 1.25) | 1.18 (1.13, 1.22) | ||
| BMI group, kg/m2§ | |||||
| < 18.5 | 3,464/25,044 | 1.05 (1.04, 1.13) | < 0.0001∥ | 0.98 (0.94, 1.02) | 0.008¶ |
| 18.5 to < 25 | 10,118/101,422 | Ref | Ref | ||
| 25.0 to < 30 | 2,785/35,286 | 0.98 (0.94, 1.02) | 1.10 (1.05, 1.14) | ||
| 30+ | 991/14,510 | 0.95 (0.89, 1.02) | 1.16 (1.08, 1.24) | ||
| WHO HIV disease stage§ | |||||
| I | 1,415/17,758 | Ref | < 0.0001¶ | Ref | 0.0001¶ |
| II | 3,010/34,317 | 1.06 (1.00, 1.13) | – | 1.00 (0.94, 1.07) | – |
| III | 10,243/100,169 | 1.37 (1.29, 1.44) | – | 1.11 (1.04, 1.17) | – |
| IV | 2,689/24,006 | 1.79 (1.67, 1.91) | – | 1.37 (1.28, 1.47) | – |
| CD4 count, cells/μL§ | |||||
| < 50 | 2,884/18,971 | 2.34 (2.23, 2.45) | < 0.0001∥ | 1.98 (1.89, 2.08) | < 0.0001∥ |
| 50 to < 200 | 6,598/52,843 | 1.77 (1.71, 1.83) | 1.57 (1.52, 1.63) | ||
| 200+ | 7,876/104,449 | Ref | Ref | ||
| Previous ART use | 1,868/20,278 | 0.93 (0.88, 0.97) | 0.003 | 1.04 (0.99, 1.10) | 0.09 |
| TB history | 4,559/40,101 | 1.31 (1.26, 1.35) | < 0.0001 | 1.10 (1.07, 1.15) | < 0.0001 |
| Oral candidiasis§ | 614/6,590 | 2.16 (1.99, 2.34) | < 0.0001 | 2.09 (1.92, 2.27) | < 0.0001 |
| Diarrhea§ | 612/6,261 | 1.57 (1.45, 1.70) | < 0.0001 | 1.40 (1.29, 1.52) | < 0.0001 |
| ALT > 120 U/L§ | 186/1,927 | 1.30 (1.12, 1.50) | 0.0004∥ | 1.22 (1.06, 1.41) | 0.007∥ |
| Hepatitis B surface antigen§ | 1,005/9,805 | 1.05 (0.99, 1.12) | 0.11 | 1.02 (0.96, 1.09) | 0.49 |
| Creatinine > 1.2 mmol/L§ | 3,012/29,559 | 1.10 (1.06, 1.14) | < 0.0001∥ | 1.08 (1.04, 1.12) | 0.0003¶ |
| Kaposi's sarcoma§ | 15/49 | 1.32 (0.80, 2.20) | 0.28 | ||
| On septrin prophylaxis§ | 2,283/16,241 | 1.31 (1.25, 1.37) | < 0.0001 | 1.13 (1.08, 1.18) | < 0.0001 |
| Currently being treated for TB§ | 2,033/14,693 | 1.41 (1.34, 1.48) | < 0.0001 | 1.37 (1.30, 1.45) | < 0.0001 |
| Taking a multivitamin§ | 5,924/62,914 | 0.86 (0.83, 0.88) | < 0.0001 | 0.85 (0.83, 0.88) | < 0.0001 |
| On ART | 12,004/123,353 | 1.76 (1.70, 1.83) | < 0.0001 | 1.36 (1.32, 1.41) | < 0.0001 |
| Regimen contains AZT†‡§ | 4,843/52,700 | 0.88 (0.85, 0.92) | < 0.0001¶ | 0.85 (0.82, 0.88) | < 0.0001¶ |
| Not on ART | 5,354/52,909 | 0.54 (0.52, 0.56) | 0.62 (0.60, 0.65) | ||
| Other regimens (non-AZT) | 7,161/70,653 | Ref | Ref | ||
| Regimen contains EFV†‡§ | 5,507/51,035 | 0.99 (0.95, 1.02) | < 0.0001¶ | 0.87 (0.84, 0.91) | < 0.0001¶ |
| Not on ART | 5,354/52,909 | 0.56 (0.54, 0.59) | 0.63 (0.60, 0.65) | ||
| Other regimens (non-EFV) | 6,497/72,318 | Ref | Ref | ||
| ART regimen†‡§ | |||||
| D4T, 3TC, NVP | 5,132/53,531 | Ref | < 0.0001¶ | Ref | < 0.0001¶ |
| D4T, 3TC, EFV | 1,624/12,051 | 1.19 (1.12, 1.26) | 1.02 (0.96, 1.08) | ||
| AZT, 3TC, NVP | 1,282/17,456 | 0.87 (0.82, 0.92) | 0.87 (0.82, 0.93) | ||
| AZT, 3TC, EFV | 3,561/35,244 | 0.91 (0.87, 0.95) | 0.81 (0.77, 0.84) | ||
| TDF, FTC, EFV | 322/3,740 | 0.70 (0.63, 0.79) | 0.63 (0.56, 0.70) | ||
| Other | 83/1,331 | 0.83 (0.67, 1.03) | 0.72 (0.58, 0.89) | ||
| Not on ART | 5,354/52,909 | 0.55 (0.52, 0.57) | 0.61 (0.58, 0.63) | ||
ALT = alanine aminotransferase; ART = antiretroviral treatment; AZT = zidovudine; BMI = body mass index; CI = confidence interval; D4T = stavudine; EFV = efavirenz; TDF = tenofovir; FTC = emtricitabine; Hgb = hemoglobin; HIV = human immunodeficiency virus; NVP = nevirapine; TB = tuberculosis; 3TC = lamivudine; WHO = World Health Organization.
Multivariate-adjusted hazard ratio from a proportional hazards model was adjusted for all variables in the univariate analysis with P <0.20.
ARTs were entered into the final model separately with all other significant variables, AZT regimen, EFV regimen, ART, and ART regimens.
Referent group for “ART regimen” is comparing each regimen to “D4T, 3TC, NVP.”
Time-varying covariates. Analysis performed using Andersen–Gill data structure with 345,428 observations during follow-up period.
Association was significantly nonlinear. P value corresponds for the test of overall significance.
P value corresponds to test for trend.
Discussion
Previous studies have reported that between 44% and 83% of people living with HIV are anemic, with the average being 60%, which is consistent with what is reported in this study.4,8,11–13,22 We examined incidence of anemia in HIV-infected adults who were in a care and treatment program with 53% becoming anemic and 10.4% becoming iron deficient. In addition, we examined the incidence of recovery from anemia in the same population, where 64% recovered from anemia during follow-up and 19% of those that were iron deficient recovered from iron deficiency.
Male gender was associated with a lower occurrence of anemia even in the presence of HIV infection and inversely with recovery from anemia. This is consistent, with previous study findings where anemia is less prevalent in men.23–25 Multiple studies have reported an increased prevalence of anemia in female HIV-infected patients compared with male HIV-infected patients, which is most likely attributed to blood loss of iron during menstruation, pregnancy, and delivery.12,13 In a study in Tanzania, low CD4 T-cell count, high erythrocyte sedimentation rate, and vitamin D insufficiency were noted as main predictors of anemia in HIV-infected pregnant and postpartum women.14
Patients who were being treated for TB were at higher risk for anemia. Previous studies have shown that anemia, along with other factors such as HIV disease progression and CD4 T-cell count, decreased survival.5,8,9 Receiving ARTs before enrolling at an MDH site was protective for the development of anemia, which is consistent with previous studies on the relationship between anemia and survival.8 Receiving ARTs before enrolling at an MDH site was protective for recovery from anemia as well. We believe this is because patients on ART before ART programs were implemented in Tanzania had sufficient time to recover from advanced disease progression and were likely wealthier patients who could afford the expensive treatment, also means that they likely had a better diet.
The results of our study are consistent with other relevant studies that have shown the relationship between anemia and HIV.1,4,8,9,11,13,26,27 Patients on D4T, 3TC, EFV were protected from developing both anemia and IDA compared with patients on D4T, 3TC, NVP, which is the most frequently prescribed regimen in this population. In addition, a number of other regimens, including tenofovir, emtricitabine, EFV, and other regimens not part of the standard treatments, were associated with an increased risk of anemia and IDA compared with D4T, 3TC, NVP. There is concern that AZT-containing ART28,29 may increase the risk for anemia. Our study also found a statistically significant and independent association with these regimens compared with D4T, 3TC, and NVP. These results can be used to determine which regimens are safe to use when anemia is present.
Uneke and others3 stated that anemia can manifest clinically as fatigue or lethargy, depression, and impaired cognitive function. If patients are depressed and tired, their performance will be diminished and their outlook can indirectly lead to adherence problems and poor maintenance of their disease. Our study also found that HIV disease progression as defined by reduced CD4 T-cell counts is a risk factor for anemia and IDA as well as WHO stage and some OIs. Treatment of comorbidities such as TB and other OIs reduced overall health as seen through the WHO HIV disease stage and CD4 T-cell count can all assist in reducing the risk of anemia and IDA.
There are some limitations to our study. Selection bias may be a limitation in the recovery from anemia (analysis in Table 4), where the baseline population was sicker, thus more likely to die and lost to follow-up, possibly biasing the results. In addition, patients with advanced HIV disease would likely be immediately treated for TB as well as initiated on ART, which potentially could have led to recovery from anemia as well as possibly slowing disease progression (analysis in Table 4). A major strength of this study is the wealth of data available on HIV-infected adults in Tanzania. In the HAART era, this is a unique population that consists of almost 100,000 Tanzanians to date, both on ART as well as in care and monitoring.
Anemia in HIV-infected adults should be monitored carefully to avoid other poor outcomes of HIV disease. There have been limited prospective longitudinal studies on the relationship between HIV and anemia. Retrospective and cross-sectional studies have shown an association with anemia and HIV at baseline, decreased survival, and increased disease progression in patients with HIV infection.4,7,8,30,31 This study confirms the importance of anemia in HIV-infected patients in treatment programs, showing various risk factors for anemia but additionally showing that certain ART treatment regimens increase the risk of anemia and IDA. Patients on D4T, 3TC, and EFV were protected against both anemia and IDA, compared with those on D4T, 3TC, and NVP, which is the most frequently prescribed regimen in this population. In addition, a number of other regimens were seen to increase the risk of anemia and IDA compared with D4T, 3TC, and NVP. It is important to better understand how various treatment regimens impact anemia and IDA in this population and which regimens are best suited in the presence of anemia. To better understand the etiology and impact of various interventions of anemia in HIV-infected populations that have access to care and treatment, further studies should be undertaken.
Anemia, including IDA, is a comorbidity that is associated with increased adverse consequences among individuals with HIV infection including those on ART. Interventions to prevent anemia and its complications need to be examined in the context of future studies.
Footnotes
Financial support: This work was supported by the U.S. President's Emergency Plan for AIDS relief (PEPFAR) through the Harvard School of Public Health and by the Ministry of Health and Social Welfare, Tanzania, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K24HD058795).
Authors' addresses: Paul Petraro, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, E-mail: pvp863@mail.harvard.edu. Christopher Duggan, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, and Division of Gastroenterology, Hepatology and Nutrition, Boston Children's Hospital, Boston, MA, E-mail: christopher.duggan@childrens.harvard.edu. Donna Spiegelman, Departments of Nutrition, Biostatistics, and Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, E-mail: stdls@hsph.harvard.edu. Ellen Hertzmark, Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, E-mail: stleh@channing.harvard.edu. Abel Makubi, Guerino Chalamilla, Helen Siril, David Sando, and Said Aboud, Allied Sciences, Muhimbili University of Health and Allied Sciences, Da es Salaam, Tanzania, E-mails: makubi55@gmail.com, gchalamilla@gmail.com, neemasiril@gmail.com, stdms@channing.harvard.edu, and aboudsaid@yahoo.com. Wafaie W. Fawzi, Departments of Nutrition, Epidemiology, and Global Health and Population, Harvard TH Chan School of Public Health, Boston, MA, E-mail: mina@hsph.harvard.edu.
References
- 1.Government of Tanzania HIV/AIDS in Tanzania. 2012. http://www.tanzania.go.tz/home/pages/2245 Available at. Accessed November 12, 2015.
- 2.UNICEF United Republic of Tanzania Statistics, 2010. 2012. www.unicef.org/infobycountry/tanzania_statistics.html Available at. Accessed February 2, 2012.
- 3.Uneke CJ, Duhlinska DD, Igbinedion EB. Prevalence and public-health significance of HIV infection and anaemia among pregnant women attending antenatal clinics in southeastern Nigeria. J Health Popul Nutr. 2007;25:328–335. [PMC free article] [PubMed] [Google Scholar]
- 4.Buskin SE, Sullivan PS. Anemia and its treatment and outcomes in persons infected with human immunodeficiency virus. Transfusion. 2004;44:826–832. doi: 10.1111/j.1537-2995.2004.03359.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MG, Chambers L, Bacon J, Rueda S, Ragan M, et al. Issues of comorbidity in HIV/AIDS: an overview of systematic reviews. Toronto, ON: Ontario HIV Treatment Network; 2010. [Google Scholar]
- 6.Smith RE., Jr The clinical and economic burden of anemia. Am J Manag Care. 2010;16((Suppl)):S59–S66. [PubMed] [Google Scholar]
- 7.O'Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. 2005;40:219–225. doi: 10.1097/01.qai.0000166374.16222.a2. [DOI] [PubMed] [Google Scholar]
- 8.Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, Aglen HE, Gundersen SG, Bruun JN. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Nephrol Dial Transplant. 2004;38:1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 10.Kreuzer KA, Rockstroh JK. Pathogenesis and pathophysiology of anemia in HIV infection. Ann Hematol. 1997;75:179–187. doi: 10.1007/s002770050340. [DOI] [PubMed] [Google Scholar]
- 11.Moore RD, Forney D. Anemia in HIV-infected patients receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;29:54–57. doi: 10.1097/00042560-200201010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Levine AM, Berhane K, Masri-Lavine L, Sanchez M, Young M, Augenbraun M, Cohen M, Anastos K, Newman M, Gange SJ, Watts H. Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2001;26:28–35. doi: 10.1097/00126334-200101010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, Vlahov D. Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus-infected and -uninfected women. Nephrol Dial Transplant. 2002;34:260–266. doi: 10.1086/338151. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein JL, Mehta S, Duggan CP, Spiegelman D, Aboud S, Kupka R, Msamanga GI, Fawzi WW. Predictors of anaemia and iron deficiency in HIV-infected pregnant women in Tanzania: a potential role for vitamin D and parasitic infections. Public Health Nutr. 2011;15:928–937. doi: 10.1017/S1368980011002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalamilla G, Hawkins C, Okuma J, Spiegelman D, Aveika A, Christian B, Koda H, Kaaya S, Mtasiwa D, Fawzi W. Mortality and treatment failure among HIV-infected adults in Dar es Salaam, Tanzania. J Int Assoc Physicians AIDS Care. 2011;11:296–304. doi: 10.1177/1545109711406733. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, Aris E, Ewald T, Mugusi F, Mtasiwa D, Fawzi W. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. AIDS. 2011;25:1189–1197. doi: 10.1097/QAD.0b013e3283471deb. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2006. [PubMed] [Google Scholar]
- 18.The United Republic of Tanzania Ministry of Health and Social Welfare . National Guidelines for the Management of HIV and AIDS. 3rd edition. National AIDS Control Programme (NACP); 2008. Tanzania Ministry of Health, Dar es Salaam, TZ. [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 20.Andersen PK, Gill RD. R. D. Cox's regression model counting process: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 21.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91:301–308. [PubMed] [Google Scholar]
- 23.Lewis S, Bain B, Bates I. Dacie and Lewis: Practical Haematology. 10th edition. Philadelphia, PA: Elsevier; 2006. [Google Scholar]
- 24.Lewis SM, Dacie JV. Practical Haematology. 6th edition. London, UK: Churchill Livingstone; 1984. [Google Scholar]
- 25.Rozenburg G. Microscopic Haematology. A Practical Guide for the Laboratory. London, UK: Churchill Livingstone; 2003. [Google Scholar]
- 26.Lundgren JD, Mocroft A, Gatell JM, Ledergerber B, D'Arminio Monforte A, Hermans P, Goebel FD, Blaxhult A, Kirk O, Phillips AN. EuroSIDA Study Group A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis. 2002;185:178–187. doi: 10.1086/338267. [DOI] [PubMed] [Google Scholar]
- 27.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, Pradier C, dArminio Monforte A, Ledergerber B, Lundgren JD. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13:943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 28.Dainiak N, Worthington M, Riordan MA, Kreczko S, Goldman L. 3′-Azido-3′-deoxythymidine (AZT) inhibits proliferation in vitro of human haematopoietic progenitor cells. Br J Haematol. 1988;69:299–304. doi: 10.1111/j.1365-2141.1988.tb02366.x. [DOI] [PubMed] [Google Scholar]
- 29.Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Hirsch MS. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- 30.Johansson KA, Robberstad B, Norheim OF. Further benefits by early start of HIV treatment in low income countries: survival estimates of early versus deferred antiretroviral therapy. AIDS Res Ther. 2010;7:3. doi: 10.1186/1742-6405-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupka R, Msamanga GI, Mugusi F, Petraro P, Hunter DJ, Fawzi WW. Iron status is an important cause of anemia in HIV-infected Tanzanian women but is not related to accelerated HIV disease progression. J Nutr. 2007;137:2317–2323. doi: 10.1093/jn/137.10.2317. [DOI] [PubMed] [Google Scholar]