Abstract
The epidemiologic status of melioidosis in Sri Lanka was unclear from the few previous case reports. We established laboratory support for a case definition and started a nationwide case-finding study. Suspected Burkholderia pseudomallei isolates were collated, identified by polymerase chain reaction assay, referred for Matrix Assisted Laser Desorption Ionization-Time of Flight analysis and multilocus sequence typing (MLST), and named according to the international MLST database. Between 2006 and early 2014, there were 32 patients with culture-confirmed melioidosis with an increasing annual total and a falling fatality rate. Patients were predominantly from rural communities, diabetic, and male. The major clinical presentations were sepsis, pneumonia, soft tissue and joint infections, and other focal infection. Burkholderia pseudomallei isolates came from all parts of Sri Lanka except the Sabaragamuwa Province, the south central hill country, and parts of northern Sri Lanka. Bacterial isolates belonged to 18 multilocus sequence types, one of which (ST 1137) was associated with septicemia and a single-organ focus (Fisher's exact, P = 0.004). Melioidosis is an established endemic infection throughout Sri Lanka, and is caused by multiple genotypes of B. pseudomallei, which form a distinct geographic group based upon related sequence types (BURST) cluster at the junction of the southeast Asian and Australasian clades.
Introduction
The geographic distribution of melioidosis is in the tropical and subtropical zone, between 30°N and 30°S, with varying prevalence due in part to the limits of public health services. The majority of cases have been reported from Thailand, northern Australia, Malaysia, and Singapore. Sri Lanka lies in the melioidosis-endemic belt between 5°N and 10°N surrounded by countries known to have endemic melioidosis and has a similar climate and environment. Despite this, very few cases of endemic infection have been reported. Melioidosis was first reported from Sri Lanka (formerly Ceylon) in 1927 when a European tea broker developed the infection.1 Sri Lanka was described as melioidosis endemic in 1971, based on this single case.2 A seroepidemiological survey shortly afterwards concluded that melioidosis was not a public health threat.3 The international Burkholderia pseudomallei collection of multilocus sequence types (STs) includes a single ST (ST 421) isolated from a Belgian international traveler who had previously visited Sri Lanka.4 By 2005, Sri Lanka was no longer considered to have endemic melioidosis.5 Subsequent reports of endemic infection from south India,6 and sporadic fatal infections in Sri Lanka raised the possibility that melioidosis was reemerging in parts of Sri Lanka.7,8 Melioidosis is commonest among rural populations at risk of exposure to the causal agent, B. pseudomallei, which is a saprophytic soil bacterium. The wide range of clinical manifestations of melioidosis has no characteristic presentation so that recognition depends heavily on laboratory confirmation by isolation of B. pseudomallei from clinical specimens. Unless diagnosed and treated early, severe melioidosis is often fatal and may go undetected. After identifying cases in the Central and Western Provinces in 2006 and 2008, we commenced prospective case finding through a World Health Organization laboratory twinning project and began to uncover additional cases.9–12 Here, we describe the results of that laboratory-based case-finding program: the geographic distribution of invasive melioidosis in Sri Lanka, the principal epidemiologic risk groups, the range of clinical presentations, their outcomes, and corresponding molecular epidemiology.
Materials and Methods
Our methods built on a previous World Health Organization laboratory twinning project, during which we established a laboratory case definition.9
Clinical case finding.
Presumptive cases were identified through a national clinical microbiology network representing the major government and teaching hospitals in Sri Lanka. Suspected B. pseudomallei isolates (oxidase-positive, gram-negative bacilli resistant to gentamicin) were prospectively collected from cases of septicemia, pneumonia, or deep abscesses and reported to the Department of Microbiology, University of Colombo between 2008 and early 2014. Occasional gentamicin-sensitive isolates resembling B. pseudomallei were identified by preliminary bacteriology procedures (see below). These isolates were maintained in glycerol brain heart infusion broth at −70°C.
Bacteriological methods.
Primary isolation relied on conventional culture techniques for blood and other sterile fluids, tissue, and occasional soft-tissue specimens from patients with focal pyogenic infection. Bacterial isolates that were oxidase positive, gentamicin-resistant (and occasionally gentamicin-sensitive), and gram-negative bacilli were forwarded to a laboratory in Colombo, where they were subcultured to establish pure growth and maintained at −70°C for subsequent definitive tests. We deployed a portable molecular laboratory to Colombo in 2012 to screen presumptive isolates with real-time polymerase chain reaction (PCR) assays for B. pseudomallei-specific sequences. Confirmed B. pseudomallei were then transferred to Perth, Western Australia for detailed characterization. Isolates were sent as suspensions of bacteria in sterile water and duplicated in nutrient agar-embedded bacteriology swabs. Shipment was made in accordance with United Nations (UN) hazardous goods regulations and importation completed via an accredited Australian Quarantine Inspection Service laboratory. The Western Australian Public Health Laboratory then confirmed the identity of all isolates by Matrix Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) mass spectrometry and real-time PCR assays for LpxO gene and type three secretion system (TTSS) targets.13–15 Confirmed B. pseudomallei were also subjected to biogeographic attribution PCR assays for Yersinia-like fimbrial (YLF) and Burkholderia thailandensis-like flagellum and chemotaxis (BTFC) gene clusters.16
Molecular epidemiology.
MLST was performed as previously described.17 For each locus, sequencing was performed in both directions on a 3130xl sequencer using BigDye v3.1 chemistry (Applied Biosystems, Foster City, CA).
Ethics approval.
Ethics approval for the study was obtained from the Ethics Review Committee, Faculty of Medicine, at the University of Peradeniya.
Geographic analysis, biostatistics, and bioinformatics.
Physical and human geographic data were obtained from the Survey Department, Government of Sri Lanka. Statistical analysis was performed with Prism 6.0 (GraphPad, San Diego, CA). MALDI-TOF mass spectra were analyzed using proprietary software (Biotyper; Bruker Daltonic GmbH, Bremen, Germany), the manufacturer's bacterial database and an extended in-house Burkholderia species reference library.13 MLST ST profiles were analyzed and displayed using goeBURST with single-locus variants (SLVs) selected.18 Available STs and country of origin were obtained from the MLST website and details filled in from original descriptions. Isolates of unknown origin were removed from the data set except for American Type Culture Collection (ATCC) ATCC 23343 (ST 516) and 145/02 (ST 749). Recurring isolate names were either deleted for duplicate entries or differentiated by an additional character. Isolates were sorted using the UN geoscheme with the exception of Sri Lanka isolates, which were retained as a separate group. STs, which appeared in more than one sub-region, were assigned to a multi-region group. Nucleotide diversity, divergence and differentiation analysis, was performed with DNAsp V5.1 on concatenated sequences.19 Population structure was investigated using the Structure 2.3.4 with settings as previously described.20,21
Results
We identified 32 cases of culture-confirmed severe melioidosis in Sri Lanka between 2006 and March 2014. The range of clinical presentations included localized unifocal, multifocal, or septicemia and combinations of these (Table 1). The culture-positive case series included 18 cases of septicemia, 20 cases with one or more abscesses, 11 with pneumonia, and six with septic arthritis. The lower limbs were affected in 11 patients.
Table 1.
Clinical presentation, epidemiology, bacteriology, and outcomes of invasive melioidosis in Sri Lanka. The main clinical presentation categories, their corresponding clinical features, Burkholderia pseudomallei genotypes and survival outcomes of culture-positive invasive melioidosis in Sri Lanka
| Clinical presentation | Age | Gender | Occupation | District, province | Exposure | Travel | Comorbidities | Diagnosed | Specimen | YLF/BTFC | MLST | Outcome* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic infection with multiple foci (9) | ||||||||||||
| Septic arthritis/encephalitis/sepsis | 58 | M | Unknown | Puttalum, North Western | Rural, unknown | Unknown | Diabetes/renal transplant | February 2008 | Blood | YLF | 1134 | Died |
| Multiple skin abscesses/multifocal septic arthritis/sepsis | 58 | F | Housewife | Kandy, Central | Rural construction | Unknown | None | March 2006 | Blood/joint fluid | YLF | 1132 | Recovered |
| Pneumonia/splenic abscess/sepsis | 62 | M | Retired school principal | Kurunegala, North Western | Rural, rice farming, gardening | None | Diabetes | February 2013 | Blood | YLF | 1136 | Recovered/relapsed |
| Psoas abscess/abdominal muscle abscess/pneumonia/sepsis | 44 | M | Policeman | Galle, Southern | Rural, active service | None | Diabetes | September 2013 | Blood | YLF | 1136 | Recovered |
| Psoas abscess/chest wall abscess/sepsis | 29 | M | Builder | Gampaha, Western | Rural, construction | None | Diabetes | December 2011 | Pus/blood | YLF | 1137 | Recovered |
| Skin and soft tissue abscess/sepsis | 59 | M | Garage mechanic | Kurunegala, North Western | Urban, construction | None | Diabetes | July/August 2012 | Blood | BTFC | 1139 | Recovered |
| Splenic abscess/septicarthritis/sepsis | 54 | M | Trishaw driver | Kalutara, Western | Rural, rice farming/gardening | Kuwait | Diabetes/liver disease | March 2013 | Blood/joint fluid | YLF | 1140 | Recovered/relapsed |
| Liver abscess/septic arthritis/sepsis | 31 | M | Builder | Hambantota, Southern | Rural, construction, river bathing | None | Diabetes/alcoholism | December 2013 | Blood/joint fluid | BTFC | 1143 | Recovered/relapsed |
| Chronic liver disease/splenic abscess/sepsis | 48 | M | Physician | Kandy, Central | Suburban, flood exposure | None | None | March 2014 | Blood | YLF | 1147 | Recovered |
| Systemic infection with single-organ focus (7) | ||||||||||||
| Pneumonia/sepsis | 26 | F | Housewife | Badulla, Uva | Rural, unknown | Unknown | Diabetes | November 2007 | Blood | YLF | 1133 | Died |
| Pneumonia/sepsis | 80 | M | Farmer | Matara, Southern | Rural, rice farming | None | Alcoholism | October 2012 | Blood | YLF | 1137 | Died |
| Pneumonia/sepsis | 79 | M | School principal | Hambantota, Southern | Rural, gardening | Unknown | None | December 2012 | Blood | YLF | 1137 | Died |
| Psoas abscess/sepsis | 37 | F | Housewife | Anuradhapura, North Central | Rural, unknown | Unknown | Diabetes | May 2010 | Psoas abscess/blood | YLF | 1137 | Died |
| Pneumonia/sepsis | 62 | M | Farmer | Kandy, Central | Rural, rice farming | None | COPD* | December 2012 | Blood | YLF | 1135 | Recovered |
| Pneumonia/lung abscess/sepsis | 53 | F | Housewife | Gampaha, Western | Urban, unknown | Unknown | Diabetes | June 2010 | Blood | YLF | 1137 | Recovered |
| Liver abscess/sepsis | 40 | M | Policeman | Galle, Southern | Rural, active service, rice farming | None | Diabetes | July 2013 | Blood | YLF | 1140 | Recovered |
| Systemic infection with no localized focus (2) | ||||||||||||
| Sepsis | 45 | M | Unknown | Colombo, Western | Suburban | Unknown | Alcoholism | November 2008 | Postmortem blood | YLF | 1135 | Died |
| Sepsis | 63 | M | Civil defense | Ampara, Eastern | Rural, active service, cultivation | None | Lepromatous Leprosy | January 2014 | Blood | YLF | 1144 | Recovered |
| Multifocal tissue infections without systemic infection (5) | ||||||||||||
| Liver abscess/splenic abscess | 32 | M | Farmer | Badulla, Uva | Rural, rice farming | Unknown | Diabetes | September 2011 | Liver abscess | BTFC | 1138 | Died |
| Pneumonia/pericardial effusion | 40 | F | Unknown | Gampaha, Western | Unknown | Unknown | Diabetes | September 2013 | Pericardial fluid | YLF | 1142 | Died |
| Pneumonia/liver abscess/pleural effusion | 15 | F | Schoolgirl | Moneragala, Uva | Rural, unknown | None | Thalassaemia | December 2012 | Pleural aspirate | YLF | 1148 | Recovered |
| Pneumonia/septic arthritis | 50 | M | Farmer | Mullaitivu, Northern | Rural, rice farming | None | Diabetes/alcoholism | January 2013 | Joint fluid | YLF | 202 | Recovered |
| Muscular abscess/osteomyelitis/septic arthritis/liver abscess | 61 | M | Farmer | Puttalum, North Western | Rural, rice farming, river bathing | None | Diabetes | January 2014 | Muscular abscess | YLF | 1145 | Recovered/relapsed |
| Single-organ focus without systemic infection (9) | ||||||||||||
| Liver abscess | 27 | M | Soldier | Ampara, Eastern | Rural, active service | Unknown | Diabetes | July/August 2011 | Postmortem liver abscess | YLF | 1135 | Died |
| Psoas abscess | 50 | M | Farmer | Gampaha, Western | Rural, rice farming | None | Diabetes | August 2011 | Psoas abscess | YLF | 1132 | Recovered/relapsed |
| Liver abscess | 34 | M | Business man | Kurunegala, North Western | Urban, mower repair | None | Diabetes | December 2011 | Liver abscess | YLF | 1135 | Recovered |
| Psoas abscess/transverse myelitis | 21 | M | Driver | Polonnaruwa, North Central | Rural, unknown | Unknown | None | December 2011 | Psoas abscess | YLF | 1136 | Paraplegia |
| Cervical lymphadenitis/pharyngitis | 11 | M | Schoolboy | Badulla, Uva | Rural, unknown | None | None | July 2009 | Cervical node aspirate | YLF | 1136 | Recovered |
| Inguinal lymphadenitis | 53 | F | Business woman | Puttalum, North Western | Rural, unknown | None | Diabetes | March 2013 | Inguinal lymph node pus | BTFC | 1139 | Recovered |
| Cavitating pneumonia | 25 | M | Insurance salesman | Gampaha, Western | Urban, unknown | None | Diabetes | June 2013 | Sputum | YLF | 1141 | Recovered |
| Cerebral abscess | 16 | F | Schoolgirl | Gampaha, Western | Rural, rice farming | None | Severe dengue | August 2013 | Cerebral abscess | YLF | 1142 | Recovered |
| Liver abscess | 9 | F | Schoolgirl | Puttalum, North Western | Rural, river bathing | None | Thalassaemia | February 2014 | Liver abscess | YLF | 1146 | Recovered |
BTFC = Burkholderia thailandensis-like flagellum and chemotaxis; COPD = chronic obstructive pulmonary disease; F = female; M = male; MLST = multilocus sequence typing; YLF = Yersinia-like fimbrial.
MLST genotype of B. pseudomallei.
At-risk groups.
The majority of cases (72%) were male and ranged from 9 to 80 years (median, 41–50 years). The predominant comorbidity was diabetes (63%). Other recognized comorbidities were alcoholism (5%), liver disease (1%), chronic obstructive pulmonary disease (1%), kidney disease (1%), and thalassemia (2%). The majority of cases (25/32: 78%) lived in rural areas, six were rice farmers and a further six engaged in gardening or subsistence cultivation. Service personnel belonging to the army, police, or civil defense (4%) and construction workers (2%) were represented among the affected patients. However, housewives (4%), school children (4%), business people (2%), and the professions (3%) were also represented (Table 1). Travel histories were available for 21/32 patients, only one of which had ever traveled overseas before illness; a male who had worked in the Middle East (in Kuwait) 25 years previously.
Geographic distribution.
Cases were reported from 8/9 provinces and 15/25 districts (Figure 1A , Table 1). The only areas not reporting cases were the hill country (> 500 m above sea level located in the southern wet/intermediate zones) (Figure 1B) the Sabaragamuwa Province, and most of the arid north of the country. We noted an association between culture-positive cases and lowland rice-growing areas (Figure 1C). Cases were notably absent from highland rubber and tea plantation areas.
Figure 1.
Geographic distribution of invasive melioidosis in Sri Lanka. (A) Location of culture-positive melioidosis cases by year of diagnosis notably absent from the south central hill country including Sabaragamuwa Province and most of the north, (B) distribution of cases by rainfall zones, and (C) by major crops: golden brown = rice, olive = rubber, and pink = tea.
Temporal variation.
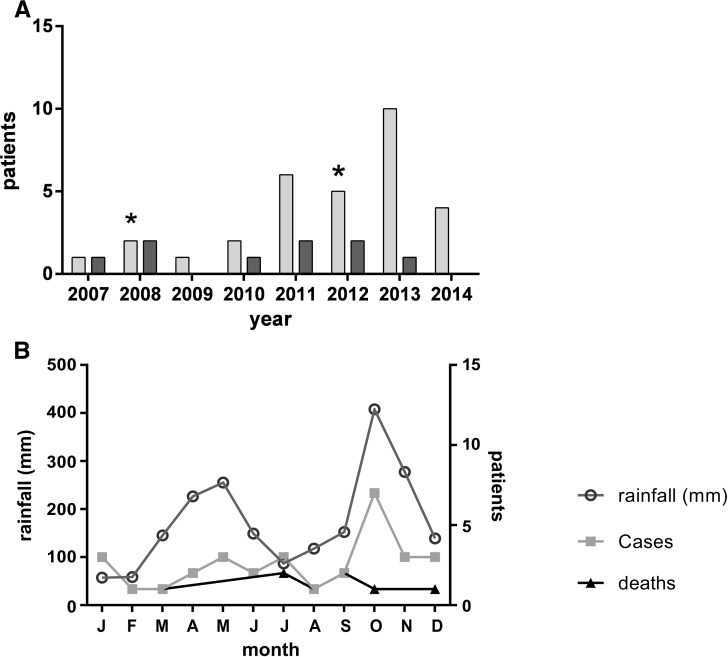
Annual culture-confirmed cases increased from one in 2006 to 10 per annum in 2013 (Figure 1A). At least one fatal infection occurred every year from 2007 to 2013, except in 2009 (Figure 2A ). The overall mortality rate was 28% and was not significantly higher in those with positive blood cultures (6/18; 33%). Mortality fell over the course of the study (χ2 test for trend: χ2 = 5.297, P = 0.02). There were 2 monthly peaks in cases corresponding to the highest seasonal rainfall as recorded closest to the single largest geographic case cluster (Figure 2B; Pearson correlation coefficient r = 0.724, P = 0.008). Cases and deaths occurred throughout the year, with an October annual peak in culture-positive cases during the study period, coinciding with the highest monthly rainfall in Western Province (Figure 2: seasonal variation).
Figure 2.
Temporal distribution of invasive melioidosis in Sri Lanka. (A) Annual total of culture-positive melioidosis cases (light gray) showing decline in mortality (dark gray) during study (χ2 trend, 2 df, P = 0.02); * = World Health Organization training workshop. (B) Culture-positive melioidosis cases from 2006 to 2014 by month, superimposed on average monthly rainfall recorded near International Airport, showing correlation between peak monthly rainfall and case total (Pearson; r = 0.724, P = 0.008).
Bacteriology.
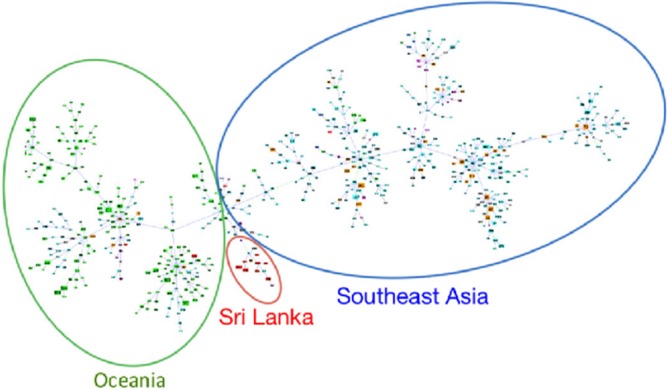
All B. pseudomallei isolates were polymyxin resistant and co-amoxyclav sensitive. One isolate was gentamicin sensitive (minimum inhibitory concentration; 6 μg/mL) and one was oxidase negative. The appearance of bacterial colonies varied from rough (25) to smooth (4) and overtly mucoid (3). MALDI-TOF and real-time PCR assays for lpxO and TTSS confirmed the identity of B. pseudomallei, including the rare gentamicin-sensitive and oxidase-negative isolates. The majority of isolates (28) belonged to the southeast (SE) Asian biogeographic YLF variant and the remainder (4) belonged to the Australian BTFC variant. MLST genotyping demonstrated a range of 18 STs with no evidence of clustering by year. Six different STs were associated with fatal infections, 15 with nonfatal infection, 13 with multisystem infection, and 10 with a single-organ focus. There was no genotypic association between ST and tissue or organ system tropism (Table 1). The commonest genotype was ST 1137, which was isolated from five patients, all of whom had bloodstream infections. Three of these were fatal. ST 1137 B. pseudomallei more commonly caused septicemia associated with a single-organ focus than non-septicemic, single-organ infection (Fisher's exact test, P = 0.02), and was also isolated more frequently from bacteremic infections associated with a single-organ focus than from all other types of presentation (Fisher's exact, P = 0.004). The goeBURST analysis with groups defined at SLV level resulted in STs 202, 1141, and 1135 appearing in a section of the tree dominated by Oceania STs, whereas ST 1133 appeared in a predominantly southeastern Asia ST section. However, STs 1132, 1134, 1136, 1137, 1140, 1145, 1146, and 1147 formed a tighter Sri Lanka predominating cluster at the junction of the Oceania/SE Asia sections (Figure 3 ). ST 1143 was closely associated with, but separate from the Sri Lankan ST cluster. STs 1148, 1144, 1142, 1139, 1138, and the previously documented ST 421 were not linked with any other STs. The only Sri Lankan ST reported from another region was ST 202 with two entries describing isolation from invasive melioidosis cases in Thailand (IDs 354 and 518). Global population structure was as described by Pearson and others,21 however, for K = 2, 37% of Sri Lankan STs were not strongly associated with either inferred population (< 90% assignment). Nucleotide diversity (π) for these Sri Lankan isolates was 0.00124 compared with 0.00253 for the rest of world population. Measures of divergence and differentiation between the Sri Lankan and the rest of world populations were DXY = 0.00252 and FST = 0.246. Comparison with isolates from individual geoscheme subregions identified minimum DXY and FST with south central Asian isolates of 0.00209 and 0.273, respectively. Closer focus on the Sri Lankan cluster produced an even lower π and FST of 0.00069 and 0.362, respectively, when compared with the rest of world population. Genetic differentiation estimates between this subpopulation and the rest of world produced test statistics with P values all less than 0.001. Binomial analysis with P = 0.017 (Sri Lankan STs/total STs), n = 18 (Sri Lankan STs), and K = 8 (Sri Lankan STs in cluster) produced a P (X = 8) of 2.62e-10.
Figure 3.
goeBurst multilocus sequence typing clustering of all logged/available/curated/entered Burkholderia pseudomallei sequence types (STs). Grouping was performed at single-locus variant level. Green oval, blue oval, and red oval delineate Oceania, southeast Asia, and Sri Lanka ST dominant regions of the tree, respectively.
Discussion
This nationwide investigation of melioidosis in Sri Lanka uncovered a wide geographic distribution of culture-confirmed cases, particularly in rice-growing rural areas surrounding the hill country in the southwest of the island. MLST genotyping revealed 18 different STs with few new alleles, consistent with an established endemic infection. The predominance of the SE Asian YLF biogeographic marker and lower phylogenetic divergence and differentiation measurements with south central Asian isolates supports an Asian origin of the infection. A cluster of Sri Lankan genotypes appears in a new, strongly region-specific branch of the BURST tree close to the intersection between Oceania (including Australian) and SE Asian type-dominated regions of the tree. The reduced diversity of Sri Lankan isolates is consistent with the region-specific BURST cluster and is further supported by significant genetic differentiation and a nonrandom result from binomial analysis. International introduction and recently recognized homoplasy in the MLST scheme may have contributed to the STs in the Oceania and SE Asia dominated branches of the BURST tree, as well as the multiregional distribution of ST 202.22 However, since most Sri Lankans travel overseas only on pilgrimage to the north of India or for work in the Middle East, introduction from other regions is unlikely. The high fatality rate during the early part of this study supports the “tip of the iceberg” concept of melioidosis first used in connection with SE Asia, a possible result of under-diagnosis.23 We used technical workshops, seminars, and a continuing educational program to increase case detection through physician, clinical scientist, and pathologist awareness including recognition of rare phenotypes such as the mucoid, gentamicin-sensitive, and oxidase-positive variants. Though the mortality rate appears to have fallen, this may reflect increased detection of localized soft tissue infection before the onset of septicemia, and early application of effective antimicrobial therapy. The association of ST 1137 with septicemia and a single-organ focus, rather than a more quiescent multifocal pyogenic infection is intriguing, and warrants further investigation in view of the relatively small numbers in ST-specific subgroups in this study. Though there were remarkably few new MLST genotype alleles from this collection of 32 distinct B. pseudomallei isolates, the allele combinations that comprise the MLST STs are distinct from other clinical isolates in the international B. pseudomallei database. Furthermore, the commoner STs such as ST 1137 and ST 1135 are dispersed over the island with no evident geographic or temporal clustering. These genotypic features argue against a single-point source that might be amenable to environmental control measures.24 In the absence of bacteriological evidence for a single introduction or point source, the only guidance for disease control comes from descriptive epidemiology. In Sri Lanka, melioidosis is an infection of rural populations. Small-scale rice farming, subsistence agriculture, and gardening are common; food security is high and prosperity is increasing. Males, farmers, and patients with diabetes are well represented in our series of culture-positive melioidosis cases. The notable frequency of lower limb lesions such as psoas abscess and septic arthritis are likely consequences of a barefoot lifestyle, common throughout Sri Lanka, where traditional rice farming methods are still widely used. Small-scale rice cultivation by barefoot farmers accounts for up to one third of these cases. A notable geographic gap is in the north of the country. This may be due to the lack of clinical microbiology services in this region, though one culture-positive case was confirmed in Mullaitivu. In contrast, a lack of clinical laboratory services cannot be used to explain the apparent absence of culture-positive melioidosis in the southcentral hill area, where rubber and tea plantations predominate and no melioidosis has been detected to date. These forms of agriculture require much less contact with soil, and plantations are located on well-drained slopes. The majority of culture-positive cases reported in the present series reside in an intermediate rainfall zone where the monthly rainfall at a nearby weather monitoring station correlates with the monthly case total, echoing a similar observation from the Northern Territory of Australia.25
The epidemiology of melioidosis has been compared with an iceberg, since the majority of cases are hidden from view.23 Starting with the World Health Organization's laboratory capability-building program, clinical laboratory molecular technology has been introduced to Sri Lanka with a deployable molecular laboratory,9 and a national melioidosis surveillance program has since been established. Laboratory capacity building in other parts of Sri Lanka will strengthen melioidosis surveillance, particularly where culture-positive cases have not yet been detected. Continued MLST genotyping studies, supplemented by whole genome sequencing, are needed to investigate the association between ST 1137 and septicemic melioidosis with a single-organ focus, and to analyze the Sri Lankan branch of the B. pseudomallei BURST tree. In conclusion, melioidosis should be considered endemic throughout Sri Lanka. Male patients with diabetes from rural communities who go about barefoot are at particular risk.
ACKNOWLEDGMENTS
We are grateful to our colleagues Michael Wise and David Ravine for their advice on MLST data analysis and to the staff of the WHO Lyons office for their support and advice. The early stages of this study were supported in part by the World Health Organization laboratory capability-building twinning program, and the mobile laboratory equipment used in Sri Lanka was loaned by Lab Without Walls Inc.
Footnotes
Financial support: The initial stage of this work was supported by a project grant from the World Health Organization laboratory capability-building twinning program, and matched by PathWest Laboratory Medicine WA.
Authors' addresses: Enoka M. Corea, Department of Microbiology, Faculty of Medicine, University of Colombo, Colombo, Sri Lanka, E-mail: enokacorea@hotmail.com. Adam J. Merritt, Yi-Horng Ler and Timothy J. J. Inglis, Department of Microbiology, PathWest Laboratory Medicine WA, Nedlands, Australia, E-mails: adam.merritt@health.wa.gov.au, yihorng.ler@health.wa.gov.au and tim.inglis@health.wa.gov.au. Vasanthi Thevanesam, Department of Microbiology, Faculty of Medicine, University of Peradeniya, Peradeniya, Sri Lanka, E-mail: vasanthi.thevanesam@yahoo.com.
References
- 1.Denny CR, Nicholls L. Melioidosis in a European. Ceylon J Sci. 1927;2:37–40. [Google Scholar]
- 2.Howe C, Sampath A, Spotnitz M. The pseudomallei group: a review. J Infect Dis. 1971;124:598–606. doi: 10.1093/infdis/124.6.598. [DOI] [PubMed] [Google Scholar]
- 3.Van Peenen PF, See R, Soysa PE, Irving GS. Seroepidemiological survey of hospital-associated populations in Colombo, Sri Lanka. Southeast Asian J Trop Med Public Health. 1976;1:16–20. [PubMed] [Google Scholar]
- 4.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Spratt BG. B. pseudomallei MLST, 2015. 2003. http://bpseudomallei.mlst.net/misc/info.asp Available at. Accessed March 24, 2015. [DOI] [PMC free article] [PubMed]
- 5.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopalakrishnan R, Sureshkumar D, Thirunarayan MA, Ramasubramanian V. Melioidosis: an emerging infection in India. J Assoc Physicians India. 2013;61:612–614. [PubMed] [Google Scholar]
- 7.Peetermans WE, Van Wijngaerden E, Van Eldere J, Verhaegen J. Melioidosis brain and lung abscess after travel to Sri Lanka. Clin Infect Dis. 1999;28:921–922. doi: 10.1086/517247. [DOI] [PubMed] [Google Scholar]
- 8.Jayasekara K, Perera S, Wijesundere A. Fatal Burkholderia pseudomallei septicemia. Ceylon Med J. 2006;51:69–70. [PubMed] [Google Scholar]
- 9.Inglis TJ, Merritt A, Montgomery J, Jayasinghe I, Thevanesam V, McInnes R. Deployable laboratory response to emergence of melioidosis in central Sri Lanka. J Clin Microbiol. 2008;46:3479–3481. doi: 10.1128/JCM.01254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corea E, Thevanesam V, Perera S, Jayasinghe I, Ekanayake A, Masakorala J, Inglis T. Melioidosis in Sri Lanka: an emerging infection. Sri Lankan J Infect Dis. 2012;2:2–8. [Google Scholar]
- 11.Nandasiri S, Wimalaratna H, Manjula M, Corea E. Transverse myelitis secondary to melioidosis: a case report. BMC Infect Dis. 2012;12:232. doi: 10.1186/1471-2334-12-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caldera AS, Kumanan T, Corea E. A rare cause of septic arthritis: melioidosis. Trop Doct. 2013;43:164–166. doi: 10.1177/0049475513505091. [DOI] [PubMed] [Google Scholar]
- 13.Inglis TJ, Healy PE, Fremlin LJ, Golledge CL. Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis for rapid confirmation of Burkholderia pseudomallei in septicemic melioidosis. Am J Trop Med Hyg. 2012;86:1039–1042. doi: 10.4269/ajtmh.2012.11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merritt A, Inglis TJ, Chidlow G, Harnett G. PCR-based identification of Burkholderia pseudomallei. Rev Inst Med Trop Sao Paulo. 2006;48:239–244. doi: 10.1590/s0036-46652006000500001. [DOI] [PubMed] [Google Scholar]
- 15.Novak RT, Glass MB, Gee JE, Gal D, Mayo MJ, Currie BJ, Wilkins PP. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J Clin Microbiol. 2006;44:85–90. doi: 10.1128/JCM.44.1.85-90.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuanyok A, Auerbach RK, Brettin TS, Bruce DC, Munk AC, Detter JC, Pearson T, Hornstra H, Sermswan RW, Wuthiekanun V, Peacock SJ, Currie BJ, Keim P, Wagner DM. A horizontal gene transfer event defines two distinct groups within Burkholderia pseudomallei that have dissimilar geographic distributions. J Bacteriol. 2007;1894:9044–9049. doi: 10.1128/JB.01264-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Spratt BG. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–2079. doi: 10.1128/JCM.41.5.2068-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francisco AP, Bugalho M, Ramirez M, Carrico JA. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinformatics. 2009;10:152. doi: 10.1186/1471-2105-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson T, Giffard P, Beckstrom-Sternberg S, Auerbach R, Hornstra H, Tuanyok A, Price EP, Glass MB, Leadem B, Beckstrom-Sternberg JS, Allan GJ, Foster JT, Wagner DM, Okinaka RT, Sim SH, Pearson O, Wu Z, Chang J, Kaul R, Hoffmaster AR, Brettin TS, Robison RA, Mayo M, Gee JE, Tan P, Currie BJ, Keim P. Phylogeographic reconstruction of a bacterial species with high levels of lateral gene transfer. BMC Biol. 2009;7:78. doi: 10.1186/1741-7007-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Smet B, Sarovich DS, Price EP, Mayo M, Theobald V, Kham C, Heng S, Thong P, Holden MT, Parkhill J, Peacock SJ, Spratt BG, Jacobs JA, Vandamme P, Currie BJ. Whole-genome sequencing confirms that Burkholderia pseudomallei multilocus sequence types common to both Cambodia and Australia are due to homoplasy. J Clin Microbiol. 2015;53:323–326. doi: 10.1128/JCM.02574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dance DA. Melioidosis: the tip of the iceberg? Clin Microbiol Rev. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O'Reilly L, Cameron B. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis. 2000;6:56–59. doi: 10.3201/eid0601.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis. 2003;9:1538–1542. doi: 10.3201/eid0912.020750. [DOI] [PMC free article] [PubMed] [Google Scholar]