Abstract
Land use changes, such as deforestation and urbanization, can influence interactions between vectors, hosts, and pathogens. The consequences may result in the appearance and rise of mosquito-borne diseases, especially in remote tropical regions. Tropical regions can be the hotspots for the emergence of diseases due to high biological diversity and complex species interactions. Furthermore, frontier areas are often haphazardly surveyed as a result of inadequate or expensive sampling techniques, which limit early detection and medical intervention. We trialed a novel sampling technique of nonpowered traps and a carbon dioxide attractant derived from yeast and sugar to explore how land use influences mosquito communities on four remote, tropical islands in the Australian Torres Strait. Using this technique, we collected > 11,000 mosquitoes from urban and sylvan habitats. We found that human land use significantly affected mosquito communities. Mosquito abundances and diversity were higher in sylvan habitats compared with urban areas, resulting in significantly different community compositions between the two habitats. An important outcome of our study was determining that there were greater numbers of disease-vectoring species associated with human habitations. On the basis of these findings, we believe that our novel sampling technique is a realistic tool for assessing mosquito communities in remote regions.
Introduction
Anthropogenic land use changes, such as deforestation, agricultural practices, road construction, hydrological transformations and urbanization, create immense impacts on biological processes and ecosystem functions. The consequences of these environmental changes are not only the loss of biodiversity and ecosystem services but also the change in vector ecology patterns. Modifications of vector–host–pathogen interactions can result in an increase of emerging infectious diseases, especially, mosquito-borne diseases.1,2 For example, the rise and emergence of malaria and yellow fever in South America and Africa and dengue in Southeast Asia can all be linked to anthropogenic land use.1,3–6
Pathogens transmitted by mosquitoes inflict significant health hazards to humans, wildlife, livestock, and pets worldwide. For example, dengue, the most important viral mosquito-borne disease, is believed to infect an estimated 400 million people yearly.7 Avian malaria is almost certainly responsible for the extinction of nearly 50% of native bird species in Hawaii.8 Rift Valley fever causes high miscarriage and death rates in cattle, sheep, and other domestic ruminants, resulting in major economic losses.9 Many of these mosquito-borne diseases are emerging in new geographical locations or are reemerging in areas where they had previously been declared absent.10
Remote tropical areas are the predicted hotspots for emerging and reemerging diseases because these are the regions, where diseases from zoonotic and vector-borne pathogens are often most concentrated, human population growth and density may be high, and surveillance often inadequate.11,12 Surveillance of mosquito communities and the arboviruses they vector yields vital data on their distribution and prevalence that is crucial for biosecurity measures and disease management. Yet, in remote regions, mosquito surveillance can be logistically demanding and expensive. Most mosquito traps require a source of electricity to operate a fan or a suction device and suitable attractants. Carbon dioxide (CO2) derived from dry ice or pressurized gas cylinders is a commonly used attractant, but in remote locations, sources of CO2 or electricity are often not available. Hence, there is a desperate need to develop and try new vector sampling methods that can be built and monitored within remote communities. Powerless traps, like our homemade passive box trap (PBT) (see full description in Ritchie and others)13 have been used successfully in several cities in Australia with CO2 obtained from pressurized gas cylinders or dry ice.13,14 As these CO2 attractants are difficult to transport to remote localities, we have also successfully trialed a novel method of producing CO2 from sugar, yeast, and water.15–17 In a recent study, we compared mosquito captures from standard traps (Center for Disease Control light trap model 512) baited with dry ice versus our sugar, yeast, and water method and found that while dry ice resulted in greater overall captures, mosquito species composition was similar between the two methods.18
In the study reported here, we used the unique combination of PBT and CO2 produced from sugar and yeast to study mosquito communities in the Torres Strait, a remote tropical island archipelago, situated between Papua New Guinea and Australia. Using this method, we investigated if the mosquito community composition, abundance, and species richness varied between urban and sylvan (forest vegetation) habitats. In the Torres Strait, mosquitoes involved in disease transmission have been studied in urban communities since the 1980s; however, no research has yet examined the mosquito community in natural habitat.
Materials and Methods
Study area.
This study was conducted during the wet season (January–April) in natural vegetation and villages on the remote islands of Saibai (9° 23′ S, 142° 37′ E), Boigu (9° 14′ S, 142° 13′ E), Badu (10° 09′ S, 142° 10′ E), and Moa (10° 09′ S, 142° 10′ E) in the Torres Strait (Figure 1 ). These islands were selected because of their location, similarity in size, replication potential, and that we were granted permission to carry out field work by indigenous elders and island authorities. Saibai and Boigu are low-lying, swampy alluvial mud islands, dominated by mangroves, saltpans, and grasslands, and are situated approximately 5 km south of the Western Province of Papua New Guinea. Badu and Moa are continental islands with eucalypt forests and grasslands and are located approximately 90 km south of Papua New Guinea.19 The climate of the islands is tropical monsoon with most of the annual rainfall (approximately 1,600 mm/year1) occurring between December and April. Temperature reaches an annual mean maximum of 30.4°C/year1 and a mean minimum of 24.6°C/year.1,20
Figure 1.
Map of the study area in the Australian Torres Strait. Mosquitoes were collected from the islands of Saibai, Boigu, Badu and Moa (▴)
Early human land use in the Torres Strait included burning and clearing of vegetation for plant cultivation.21,22 All of the islands where our fieldwork was performed have been subjected to annual burning for thousands of years.22 In more recent times (late eighteenth and early nineteenth century), many islands were heavily deforested to provide fuel for the bêche-de-mer (sea cucumber, trepang) industry and missionary steamers, and also for the construction of boats and slipways.23 Landscape alterations to the already fragile island environments were also caused by the introduction of exotic plants (e.g., yam, taro, banana, cassava, sugarcane, sweet potato, tobacco, bamboo, and sisal) and animals (e.g., dogs, pigs, goats, Rusa deer, horses, chickens, and wallabies).21,22
Sampling methods for mosquitoes.
Field work was conducted during the wet seasons of 2013 and 2014. Adult mosquitoes were collected using nonpowered PBT (full description in Ritchie and others)13 with the following modification: the entry bowl was painted black, which is attractive to many mosquito species.24,25 The traps were baited with CO2 (derived from sugar and yeast fermentation) from 15-L water bags (containing 4 L of water, 1 kg of sugar, and 40 g of yeast), which produced ~120 mL CO2/minute. We replaced this solution after two nights as our laboratory trials at 28°C demonstrated that CO2 production decreases after 48 hours (unpublished data). Traps were placed at a height of approximately 0.5 m above ground level and spaced at least 20 m apart. On each island, we placed five traps in villages (close to residential houses and communal areas such as health centers, rangers sheds, and council offices) and five traps in natural vegetation (along the first tree line of mangrove forests and the surrounding matrix where grasslands supporting mainly native grasses and Pandanus spp.). All traps were operating for four nights on each island in two consecutive years, resulting in a total of 320 trap nights. Mosquitoes were removed from traps at the end of the four-night sampling period.
We had initially intended to include a comparison between the BG-Sentinel™ traps (BGT) and PBTs, both in natural vegetation and villages. However, due to battery failures in the field samples, we present only data for the villages (where electricity powered the traps). We placed five BGTs in each village on each island where they were in use for four consecutive nights in the 2013 wet season, only, and resulted in a total of 80 trap nights.
Mosquito identification.
Mosquitoes were identified to species level using taxonomic keys26 and with the assistance of taxonomic experts (Richard Russell, John Clancy, Paul Zborowski, and Bob Cooper). Culex annulirostris and Culex sitiens were pooled as they belong to the morphologically indistinguishable members of the Cx. sitiens subgroup.27
Statistical analyses.
We evaluated if mosquitoes were sampled adequately in both habitat types under our sampling design by constructing species accumulation curves to display the cumulative number of species collected against the measure of the sampling effort. We used linear mixed effects models (with restricted maximum likelihood) to examine relationships between the response variables: mosquito captures and diversity (Fisher's alpha) as a function of land use (village versus natural habitat). Habitat was a fixed factor in the model and island was a random factor. Variables for the capture data were log (x + 1) transformed. We examined major gradients in the composition of mosquito communities across study sites by undertaking nonmetric multidimensional ordination analysis (NMS). NMS is a robust and commonly used method for ecological community data to examine the structure and composition within and between communities. We used NMS in two ways: 1) to evaluate if the mosquito community varies in response to habitat type and island and 2) to evaluate if the vector community (species transmitting alphaviruses, flaviviruses, and protozoans) varies in response to habitat type and island. Data were log (x + 1) transformed before analysis. Monte Carlo randomization tests (250 runs) were used to determine whether the ordination axes explained significantly more variation than expected by chance. A Bonferroni correction was used to reduce the likelihood of type II errors, where P = 0.15/n (n represents the number of mosquito species multiplied by the number of axes and 0.15 is the experiment-wise error rate).28 Permutation-based nonparametric MANOVAs (PerMANOVAs)29 are a method that allows for examining within and between categories examined in the ordination, followed by pairwise comparisons to distinguish differences among mosquito communities on the four islands. We used an independent sample's t test with log-transformed data to compare mean captures of mosquitoes and NMS ordination analysis to compare the mosquito community composition between BGTs and PBTs.
All modeling was performed in R,30 using the package nlme31 and following a standard protocol for data exploration and model validation.32 For the ordination analyses and the PerMANOVAs we used PC-ORD 6.0 package33 and SPSS 22.034 statistical package was used for the t test. Where required, data were tested for normality and homogeneity of variances by using Kolmogorov–Smirnov and Levene's tests.
Ethical statement.
All mosquito collections were conducted in traditional owner's country and private residences with the owners' permission, consent, and presence. This study did not involve endangered or protected species.
Results
Mosquitoes in urban and sylvan habitats.
Our novel sampling method resulted in the capture of 11,109 mosquitoes. We found that habitat type influenced mosquito captures significantly with generally higher captures in sylvan habitat compared with village locations (linear mixed effects model; Table 1). We identified 27 species from seven genera (Table 2). The genus Aedes was the most dominant genus (N = 4,385 captures) and was represented by 13 species. Two species were most frequently captured, together contributing up to 70% of the captures, Cx. sitiens subgroup (37%), and Ae. kochi (33%). The most dominant species on the northern low-lying islands, Saibai and Boigu, was Cx. sitiens subgroup; whereas on the southern continental islands, Badu and Moa, Ae. kochi was the most dominant species. Some species, such as Anopheles spp. and Coquillettidia spp., were captured only on Saibai and Boigu and Aedes albopictus was trapped only on Badu and Moa. Culex quinquefasciatus and Mansonia uniformis were captured only on Saibai. Mosquito diversity was also higher in natural vegetation compared with villages (Fisher's alpha diversity index; Table 1).
Table 1.
Results of linear mixed models fitted to mosquito abundance and diversity estimates across four tropical islands
| Covariate | df | Abundance (SE) | Fishers-alpha (SE) |
|---|---|---|---|
| Intercept | 35 | 1.418 (0.209)*** | 1.072 (0.278)** |
| Habitat | 35 | 0.506 (0.125))** | 0.448 (0.158))* |
df = degrees of freedom; SE = standard error.
Significance levels: *P < 0.01; **P < 0.001; ***P < 0.0001.
Table 2.
Mosquito species captured with the PBT in the two habitats on the four islands in the Torres Strait and the pathogens they may transmit
| Saibai | Boigu | Badu | Moa | |||||
|---|---|---|---|---|---|---|---|---|
| Species | Village | Natural vegetation | Village | Natural vegetation | Village | Natural vegetation | Village | Natural vegetation |
| Aedeomyia catasticta* | 0 | 2 | 0 | 22 | 0 | 0 | 8 | 288 |
| Aedes aegypti†‡ | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Aedes albopictus†‡ | 0 | 0 | 0 | 0 | 5 | 0 | 19 | 1 |
| Aedes alternans† | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aedes aurantius | 0 | 3 | 0 | 6 | 0 | 0 | 0 | 0 |
| Aedes culiciformis | 0 | 0 | 0 | 4 | 22 | 3 | 3 | 0 |
| Aedes kochi†§∥ | 3 | 721 | 0 | 6 | 1,416 | 1,038 | 337 | 117 |
| Aedes littlechildi | 0 | 0 | 0 | 0 | 44 | 5 | 2 | 2 |
| Aedes notoscriptus†‡§ | 0 | 1 | 0 | 45 | 15 | 13 | 25 | 3 |
| Aedes scutellaris‡ | 4 | 9 | 2 | 28 | 1 | 11 | 25 | 4 |
| Aedes tremulus†‡ | 2 | 47 | 0 | 14 | 5 | 9 | 6 | 5 |
| Aedes vigilax†‡§∥ | 4 | 0 | 0 | 20 | 0 | 5 | 0 | 2 |
| Aedes spp. | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Anopheles farauti‡∥¶ | 16 | 51 | 181 | 677 | 0 | 0 | 0 | 0 |
| Anopheles hilli†¶ | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Anopheles meraukensis† | 0 | 0 | 21 | 1 | 0 | 0 | 0 | 0 |
| Coquillettidia nr. crassipes¶** | 6 | 786 | 0 | 88 | 0 | 0 | 0 | 0 |
| Coquillettidia xanthogaster†‡ | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Culex sitiens†‡§∥†† | 291 | 1,935 | 53 | 1,746 | 10 | 5 | 4 | 18 |
| Culex hilli†† | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Culex quinquefasciatusठe] | 65 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mansonia uniformis†‡∥ f] | 3 | 109 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tripteroides magnesianus‡‡ | 0 | 3 | 0 | 12 | 0 | 7 | 0 | 55 |
| Tripteroides spp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Verrallina carmenti† | 0 | 40 | 0 | 6 | 20 | 8 | 0 | 12 |
| Verrallina funerea*†‡ | 36 | 111 | 6 | 192 | 3 | 1 | 0 | 29 |
| Verrallina lineata† | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Damaged | 3 | 48 | 5 | 45 | 0 | 3 | 0 | 0 |
| Total | 433 | 3,876 | 269 | 2,914 | 1,542 | 1,108 | 430 | 537 |
PBT = passive box trap.
Orbivirus spp.
Alphaviruses.
Flaviviruses.
Dirofilaria immitus.
Wuchereria bancrofti.
Plasmodium spp.
Brugia malayi.
Consists of the morphologically similar species Cx. annulirostris and Cx. sitiens.26
Unknown/not suspected to transmit diseases.
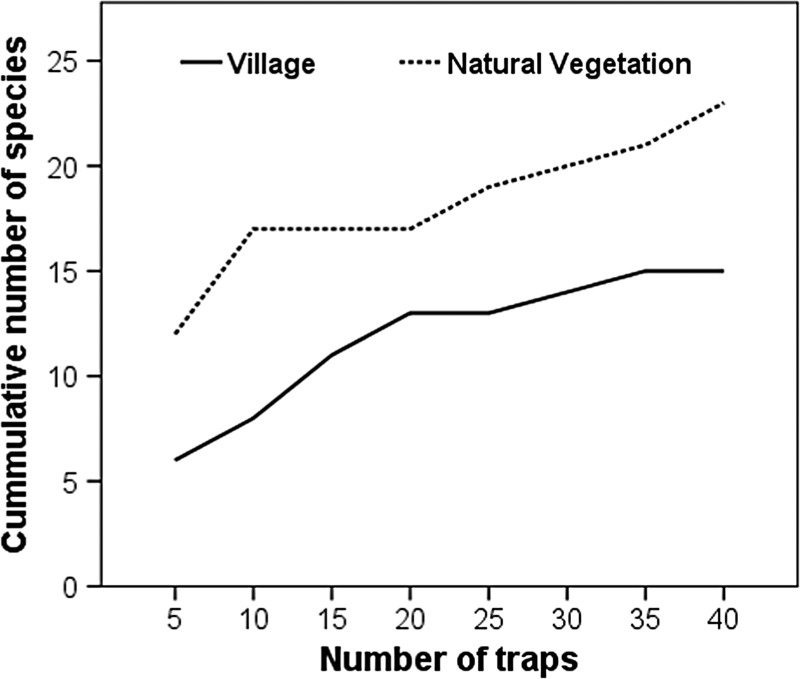
Species accumulation curves from the PBT design show two patterns. First, the difference in species capture rate between the two habitats and second, the gradual asymptoting of the curves, which suggests the traps are catching all the attracted species present in the habitats (Figure 2 ). Hence, it is possible that we have missed either rare species or those not attracted to this trap design.
Figure 2.
Species accumulation curves suggest that most common mosquito species attracted to our trap design in villages were sampled, whereas further sampling in natural vegetation could have resulted in capturing more species, especially rare ones.
Community composition.
Using ordination analysis (NMS), we examined the mosquito community composition in two habitats on the four islands. Both sampling years were used for the analysis, as years had no effect on the community composition (PerMANOVA: pseudo F = 1.03, df = 1, 15, P = 0.375). We found that the mosquito community strongly varied in response to island (PerMANOVA: pseudo F = 6.86, df = 3, 15, P = 0.0002) and pairwise comparisons showed that Saibai and Boigu supported a similar community composition, but different to Badu and Moa (Table 3, Figure 3 ). Natural vegetation and villages (PerMANOVA: pseudo F = 6.32, df = 1, 15, P = 0.0008) also supported different mosquito communities as seen between the continental islands on Axis 1 and the low-lying islands on Axis 2. The interaction effect of distinctly different island and habitat effects was also significant (PerMANOVA; pseudo F = 2.99, df = 3, 15, P = 0.005), with a greater relative similarity seen between villages and natural vegetation in the continental islands of Badu and Moa from the Axis 2 perspective. The two NMS ordination axes together explained 91% of the total variation (Axis 1: 48%, Axis 2: 43%). Of the 27 species examined, six were significantly correlated with these axes (Table 4): Anopheles farauti, Cx. sitiens subgroup, and Verrallina funerea were more frequently associated with Saibai and Boigu than with Badu and Moa and Ae. kochi, Aedes tremulus, and Verrallina carmenti were more frequently associated with natural vegetation.
Table 3.
Pairwise comparisons testing whether mosquito communities differ between the four islands
| Islands | t | P |
|---|---|---|
| Saibai vs. Boigu | 1.0104 | 0.3594 |
| Saibai vs. Badu | 2.4332 | 0.0260 |
| Saibai vs. Moa | 2.2071 | 0.0304 |
| Boigu vs. Badu | 2.2182 | 0.0294 |
| Boigu vs. Moa | 1.8691 | 0.0304 |
| Badu vs. Moa | 1.2051 | 0.3072 |
Values in boldface were significant.
Figure 3.
Ordination analysis of mosquito communities displaying that mosquito community composition varied strongly in response to island and habitat type. Saibai and Boigu shared a similar community composition, as did Badu and Moa.
Table 4.
Mosquito community (all species were used for the analysis): Pearson correlation of six mosquito species with two ordination axes produced by nonmetric multidimensional scaling
| Mosquito community | Vector community* | ||
|---|---|---|---|
| Species | Axis 1 | Axis 2 | Axis 1 |
| Aedes albopictus | 0.908 | ||
| Aedes kochi | 0.611 | 0.697 | |
| Aedes tremulus | −0.196 | 0.790 | |
| Anopheles farauti | −0.699 | −0.066 | −0.840 |
| Culex annulirostris | −0.897 | 0.388 | −0.980 |
| Verralina carmenti | −0.151 | 0.748 | |
| Verralina funerea | −0.776 | 0.538 | −0.847 |
Correlation values in boldface were significant (P < 0.005) using a Bonferroni-corrected alpha value (P = 0.003).
Vector community (only species vectoring alphaviruses, flaviviruses, and protozoans were considered for the analysis): Pearson correlation of four mosquito species with one ordination axis produced by nonmetric multidimensional scaling. Correlation values were significant (P < 0.005) using a Bonferroni-corrected alpha value (P = 0.009).
Finally, we found that a subset of the mosquito community (16 species), which can transmit alphaviruses, flaviviruses, and protozoans, varied significantly between the islands (PerMANOVA: pseudo F = 4.87, df = 3, 4, P = 0.030). A single significant ordination axis explained 68% of the variance. Again, Saibai and Boigu supported a similar community composition, as did Badu and Moa. Of the 16 species examined, four were significantly correlated with this single axis (Table 4). Anopheles farauti, Cx. sitiens subgroup, and Ve. funerea were more frequently associated with Saibai and Boigu whereas Ae. albopictus was mostly associated with Badu and Moa.
A comparison between our novel sampling technique and the standard BGT in 2013 found no differences in mean captures (t test; t = −1.28, df = 38, P = 0.206) and that both traps designs captured a similar mosquito community composition (PerMANOVA: pseudo F = 1.14, df = 1, 6, P = 0.374) with a single significant ordination axis explaining 84% of the variance. However, there were interesting differences in the capture success of specific disease vectors. Aedes aegypti, for example, were captured more frequently in the BGT compared with the PBT (which is not that surprising as the BGT was initially designed with Ae. aegypti in mind). Alternatively, An. farauti were captured 10 times more frequently in the PBT compared with the BGT, and both trap designs were equally successful in capturing Ae. albopictus.
Discussion
One of the greatest hindrances to understanding the impacts of anthropogenic activities on emerging diseases is the lack of reliable, cheap, and efficient equipment for the surveillance of tropical frontiers. On four remote tropical islands of northern Australia, we used a novel homemade, nonpowered trap baited with CO2 derived from yeast and sugar to capture mosquitoes. Using this simple system, we successfully collected sufficient samples of mosquitoes (N > 11,000) to describe important mosquito community differences between urban and natural habitats on these remote islands. Specifically, we discovered that urban mosquito communities supported a higher proportion of disease-competent species compared with natural vegetation, despite having lower total captures and lower species richness. In addition, we found that low-lying islands harbored populations of major disease vectors, most notably An. farauti, the primary malaria vector in Indonesia, Papua New Guinea, and Australia.35
We were able to recognize unique species associations allowing for the identification of sylvan and urban mosquito communities. Important disease vectors such as Ae. aegypti, Cx. quinquefasciatus, and the recently arrived Ae. albopictus26,36 were almost exclusively trapped in villages. Similarly, in Thailand, the same three species were also least abundant in undisturbed compared with disturbed areas.37 These three species originate from Africa or Southeast Asia and are now described as “domestic” mosquitoes (species successfully living in close association with humans in anthropogenically modified landscapes) in many parts of the world. Human settlements provide these mosquitoes with suitable larval habitats (artificial containers), blood meal resources, and resting sites (around houses and often indoors).38 Comparative adult mosquito community studies in natural vegetations in the tropics are rare (although see37,39) as most studies concentrate on single-vector species, such as dengue vectors36,40,41 or periurban areas.42–45
Mosquito community composition not only differed between habitats, but also varied across the studied Torres Strait Islands. The northern low-lying islands situated close to Papua New Guinea (Saibai and Boigu) shared a very similar mosquito community that was distinct to that from the southern, continental islands (Badu and Moa). Disease vectors for malaria (Anopheles spp.) were captured only on the northern islands, whereas a disease vector for dengue and chikungunya (Ae. albopictus) was more frequently captured in the villages on southern islands. We have no explanation for not capturing Ae. albopictus on the northern islands and we suspect that the scarcity of swamplands in the southern islands could limit Anopheles spp. distributions. For example, Cooper and others46 found that An. farauti and Anopheles hilli often use brackish and saline waters to oviposit and Anopheles meraukensis larvae were absent from drier (< 1,000 mm rainfall/year) areas in the Gulf region of northern Australia.
The higher capture rates observed in natural vegetation habitats may be partly due to the greater availability of species-appropriate larval habitat, particularly, permanent or temporary (grassland) swamps. For example, the larvae of Coquillettidia and Mansonia are unique among mosquitoes; they are immobile and attach themselves to aquatic plants to obtain oxygen.47 Another driving factor influencing capture rates may be differential attractiveness to low CO2 levels. Mosquito species occurring in natural vegetation, such as Aedeomyia catasticta, are probably more zoophilic whereas mosquitoes occurring in villages tend to be primarily anthropophilic and these feeding preferences may influence their attraction to different outputs of CO2 production. For example, mosquitoes that display a high degree of anthropophily, such as Ae. aegypti,48,49 may be less attracted to our traps as the CO2 output of ~120 mL/minute is less than half that of an average human (~275 mL/minute).50 In contrast, zoophilic species, such as Ad. Catasticta, exploit smaller hosts (e.g., birds)39 who emit less CO2. Furthermore, lower captures in villages could also be attributed to wind intensity in the coastal human settlements compared with the wind-protected natural vegetation. The average wind speed during mosquito trapping was 13 km/hour—however, maximum wind speeds were frequently over 50 km/hour,51 which would almost certainly have had an influence on trap catches.
The most dominant mosquitoes captured in our study belong to the Cx. sitiens subgroup. Among them is Cx. annulirostris, which is the most important arbovirus vector in Australia.52 It is capable of transmitting Ross River fever, Barmah Forest virus, Murray Valley virus, Kunjin virus and Japanese encephalitis virus to humans.53,54 Culex annulirostris was responsible for the outbreaks of Japanese encephalitis in the Torres Strait in 1995 and 1998,55,56 and it was also the most dominant species captured in three different tropical habitat types (rainforest, rainforest edge, and grassland) in far north Queensland57 and from urban, periurban, and melaleuca swamps45,58 in the same region. Culex annulirostris is regarded as a generalist species with opportunistic feeding patterns defined by host availability and it can use a wide variety of larval habitats including fresh water swamps, shallow grassland pools, and large artificial containers such as livestock watering tanks. This species can also persist during dry periods.42 Its ability to use a range of hosts, habitat types, and oviposition sites may account for the dominance of Cx. sitiens subgroup mosquitoes in our study sites.
We sampled over two consecutive wet seasons that may not reflect the community composition at other times, although the monsoonal climate in this region has distinct dry seasons with low mosquito densities. The main purpose of this study was not to capture as many mosquitoes as possible, but to evaluate if our inexpensive method could be a valuable tool for mosquito surveillance in remote areas. We believe our sampling has been adequate for this purpose, but acknowledge that more extensive sampling could detect other patterns. We recognize that CO2 produced from the yeast and sugar mixture is less concentrated than CO2 from dry ice. Our prior study18 found that while this may affect capture rates, it did not affect our ability to describe the community composition, although, very rare species may not be captured. We think the efficacy of the passive trap used in this study could be further enhanced by the addition of secondary attractants, such as octenol, ammonia, or lactic acid because they are effective for some species and are easily transported.13,59,60 These secondary attractants may yield higher collection totals and could be used to target more species-specific captures. In addition, we concede that there is always mosquito sampling bias depending on the type of traps used. Nevertheless, the simple CO2 attractant and passive trap combination described in this study is a cheap, effective, and reliable surveillance device for use in remote areas without adequate access to electricity and conventional CO2 sources (i.e., dry ice and CO2 gas cylinders).
This study demonstrated that human settlements significantly altered the mosquito community composition in tropical landscapes, increasing the presence and abundance of anthropophilic species. Surveillance in these remote landscapes has an important role in detecting and managing disease outbreaks and we believe that the development and testing of homemade trap designs and attractants that are affordable and reliable will be crucial to this task.
ACKNOWLEDGMENTS
We thank the residents and rangers of the Torres Strait Islands: Saibai, Boigu, Badu Moa, and Thursday for their permission to visit the islands. We also wish to acknowledge staff of the Torres Strait Regional Authority, Department of Agriculture for field support and permits; Bob Cooper, John Clancy, and Paul Zborowski for providing assistance in mosquito identification; and Signe Dalsgaard, Laila Herringer, FranÇoise Yoko Ishida, and Michal Segoli for field work.
Footnotes
Financial support: This research was supported by an Australian Postgraduate Award to DBM, and grants to SGWL from the Torres Strait Regional Authority, National Environmental Research Program, James Cook University, Reef and Rainforest Research Centre, and the Australian Research Council Future Fellowship.
Authors' addresses: Dagmar B. Meyer Steiger and Susan G. W. Laurance, , College of Marine and Environmental Sciences, James Cook University, Cairns, Queensland , Australia, E-mails: dagmar.meyersteiger@my.jcu.edu.au and susan.laurance@jcu.edu.au., Scott Alex Ritchie, School of Public Health, Tropical Medicine and Rehabilitation Sciences, James Cook University, Queensland, Australia, and Tropical Population Health Network, Queensland Health, Cairns, Australia, E-mail: scott.ritchie@jcu.edu.au.
References
- 1.Patz J, Graczyk T, Geller N, Vittor A. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 2.Williams CR. The Asian tiger mosquito (Aedes albopictus) invasion into Australia: a review of likely geographic range and changes to vector-borne disease risk. Trans R Soc S Aust. 2012;136:128–136. [Google Scholar]
- 3.Walsh J, Molyneux D, Birley M. Deforestation: effects on vector-borne disease. Parasitol. 1993;106:S55. doi: 10.1017/s0031182000086121. [DOI] [PubMed] [Google Scholar]
- 4.Morse S. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasconcelos PF, Travassos da Rosa A, Rodrigues SG, Travassos da Rosa ES, Dégallier N, Travassos da Rosa JF. Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cad Saude Publica. 2001;17:S155–S164. doi: 10.1590/s0102-311x2001000700025. [DOI] [PubMed] [Google Scholar]
- 6.Reisen WK. Landscape epidemiology of vector-borne diseases. Annu Rev Entomol. 2010;55:461–483. doi: 10.1146/annurev-ento-112408-085419. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Riper CI, van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr. 1986;56:327–344. [Google Scholar]
- 9.Bird BH, Ksiazek TG, Nichol ST, MacLachlan NJ. Rift Valley fever virus. J Am Vet Med Assoc. 2009;234:883–893. doi: 10.2460/javma.234.7.883. [DOI] [PubMed] [Google Scholar]
- 10.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchie SA, Cortis G, Paton C, Townsend M, Shroyer D, Zborowski P, Hall-Mendelin S, van den Hurk AF. A simple non-powered passive trap for the collection of mosquitoes for arbovirus surveillance. J Med Entomol. 2013;50:185–194. doi: 10.1603/me12112. [DOI] [PubMed] [Google Scholar]
- 14.van den Hurk AF, Hall-Mendelin S, Townsend M, Kurucz N, Edwards J, Ehlers G, Rodwell C, Moore FA, McMahon JL, Northill JA. Applications of a sugar-based surveillance system to track arboviruses in wild mosquito populations. Vector Borne Zoonotic Dis. 2014;14:66–73. doi: 10.1089/vbz.2013.1373. [DOI] [PubMed] [Google Scholar]
- 15.Saitoh Y, Hattori J, Chinone S, Nihei N, Tsuda Y, Kurahashi H, Kobayashi M. Yeast-generated CO2 as a convenient source of carbon dioxide for adult mosquito sampling. J Am Mosq Control Assoc. 2004;20:261–264. [PubMed] [Google Scholar]
- 16.Oli K, Jeffery J, Vythilingam I. Research note: a comparative study of adult mosquito trapping using dry ice and yeast generated carbon dioxide. Trop Biomed. 2005;22:249–251. [PubMed] [Google Scholar]
- 17.Smallegange RC, Schmied WH, van Roey KJ, Verhulst NO, Spitzen J, Mukabana WR, Takken W. Sugar-fermenting yeast as an organic source of carbon dioxide to attract the malaria mosquito Anopheles gambiae. Malar J. 2010;9:292. doi: 10.1186/1475-2875-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer Steiger D, Ritchie S, Laurance S. Overcoming the challenges of mosquito (Diptera: Culicidae) sampling in remote localities: a comparison of CO2 attractants on mosquito communities in three tropical forest habitats. J Med Entomol. 2014;51:39–45. doi: 10.1603/me12216. [DOI] [PubMed] [Google Scholar]
- 19.Stanton D, Fell D, Gooding D. Vegetation Communities and Regional Ecosystems of the Torres Strait Islands, Queensland, Australia. Greenslopes, Australia: 3D Environmental Consultants; 2009. [Google Scholar]
- 20.Bureau of Meteorology Climate Statistics for Australian Locations. 2014. http://www.bom.gov.au/climate/averages/tables/cw_027058.html Available at. Accessed November 18, 2014.
- 21.Haddon A. Hunting and fishing. Reports of the Cambridge Anthropological Expedition to the Torres Straits. In: Haddon AC, editor. Volume 4: Arts and Crafts. Cambridge, UK: Cambridge University Press; 1912. pp. 152–171. [Google Scholar]
- 22.Harris DR. Subsistence strategies across Torres Strait. In: Allen J, Golsen J, Jones R, editors. Sunda and Sahul: Prehistoric Studies in Southeast Asia, Melanesia and Australia. London, UK: Academic Press; 1977. pp. 421–463. [Google Scholar]
- 23.Shnukal A. The post-contact created environment in the Torres Strait Central Islands. Memoirs of the Queensland Museum Cultural Heritage Series. 2004;3:317–346. [Google Scholar]
- 24.Browne SM, Bennett GF. Response of mosquitoes (Diptera: Culicidae) to visual stimuli. J Med Entomol. 1981;18:505–521. doi: 10.1093/jmedent/18.6.505. [DOI] [PubMed] [Google Scholar]
- 25.Hoel DF, Obenauer PJ, Clark M, Smith R, Hughes TH, Larson RT, Diclaro JW, Allan SA. Efficacy of ovitrap colors and patterns for attracting Aedes albopictus at suburban field sites in north-central Florida. J Am Mosq Control Assoc. 2011;27:245–251. doi: 10.2987/11-6121.1. [DOI] [PubMed] [Google Scholar]
- 26.Lee DJ, Hicks M, Griffiths M, Russell R, Marks E. The Culicidae of the Australasian Region. Vol. 1–12. Canberra,Australia: Australian Government Publishing Service Press; 1980-1989. [Google Scholar]
- 27.Chapman H, Kay B, Ritchie S, van den Hurk A, Hughes J. Definition of species in the Culex sitiens subgroup (Diptera: Culicidae) from Papua New Guinea and Australia. J Med Entomol. 2000;37:736–742. doi: 10.1603/0022-2585-37.5.736. [DOI] [PubMed] [Google Scholar]
- 28.Chandler RC. Practical considerations in the use of simultaneous inference for multiple tests. Anim Behav. 1995;49:524–527. [Google Scholar]
- 29.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- 30.R Development Core Team . An Introduction to R, Notes on R: A Programming Environment for Data Analysis and Graphics Version 2.10. 1. Bristol, UK: R Development Core Team; 2009. [Google Scholar]
- 31.Pinheiro J, Bates D. Linear Mixed-Effects Models: Basic Concepts and Examples. Mixed-Effects Models in S and S-PLUS. New York, NY: Springer; 2000. pp. 3–56. [Google Scholar]
- 32.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. New York, NY: Springer Science and Business Media New York; 2009. [Google Scholar]
- 33.McCune B, Mefford M. PC-ORD. Multivariate Analysis of Ecological Data. Gleneden Beach, OR: MjM Software; 2011. Version 6.0. [Google Scholar]
- 34.IBM . Armonk, NY: IBM Corporation; 2013. SPSS Statistics for Windows Version 22.0. [Google Scholar]
- 35.Beebe NW, Foley DH, Ellis JT. Populations of the southwest Pacific malaria vector Anopheles farauti ss revealed by ribosomal DNA transcribed spacer polymorphisms. Heredity. 2000;84:244–253. doi: 10.1046/j.1365-2540.2000.00665.x. [DOI] [PubMed] [Google Scholar]
- 36.Ritchie SA, Moore P, Carruthers M, Williams C, Montgomery B, Foley P, Ahboo S, van den Hurk AF, Lindsay MD, Cooper B. Discovery of a widespread infestation of Aedes albopictus in the Torres Strait, Australia. J Am Mosq Control Assoc. 2006;22:358–365. doi: 10.2987/8756-971X(2006)22[358:DOAWIO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Thongsripong P, Green A, Kittayapong P, Kapan D, Wilcox B, Bennett S. Mosquito vector diversity across habitats in central Thailand endemic for dengue and other arthropod-borne diseases. PLoS Negl Trop Dis. 2013;7:e2507. doi: 10.1371/journal.pntd.0002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next? Microbes Infect. 2010;12:272–279. doi: 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Standfast H, Barrow G. Mosquito collections in a high-rainfall area of north Queensland. J Med Entomol. 1969;6:39–43. doi: 10.1093/jmedent/6.1.37. [DOI] [PubMed] [Google Scholar]
- 40.Hanna JN, Ritchie SA, Merritt AD, van den Hurk A, Phillips DA, Serafin IL, Norton RE, McBride W, Gleeson FV, Poidinger M. Two contiguous outbreaks of dengue type 2 in north Queensland. Med J Aust. 1998;168:221–225. doi: 10.5694/j.1326-5377.1998.tb140134.x. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Rejon J, Lorono-Pino M, Farfan-Ale J, Flores-Flores L, Rosado-Paredes E, Rivero-Cardenas N, Najera-Vazquez R, Gomez-Carro S, Lira-Zumbardo V, Gonzalez-Martinez P. Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg. 2008;79:940–950. [PubMed] [Google Scholar]
- 42.Jardine A, Lindsay M, Heyworth J, Weinstein P. Dry-season mosquito breeding associated with irrigation in the northeast Kimberley region of western Australia: potential impact on mosquito-borne disease transmission. EcoHealth. 2004;1:387–398. [Google Scholar]
- 43.Keating J, Macintyre K, Mbogo C, Githure J, Beier J. Characterization of potential larval habitats for Anopheles mosquitoes in relation to urban land-use in Malindi, Kenya. Int J Health Geogr. 2004;3:1–13. doi: 10.1186/1476-072X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kay BH, Boyd AM, Ryan PA, Hall RA. Mosquito feeding patterns and natural infection of vertebrates with Ross River and Barmah Forest viruses in Brisbane, Australia. Am J Trop Med Hyg. 2007;76:417–423. [PubMed] [Google Scholar]
- 45.Jansen CC, Prow NA, Webb CE, Hall RA, Pyke AT, Harrower BJ, Pritchard IL, Zborowski P, Ritchie SA, Russell RC. Arboviruses isolated from mosquitoes collected from urban and peri-urban areas of eastern Australia. J Am Mosq Control Assoc. 2009;25:272–278. doi: 10.2987/09-5908.1. [DOI] [PubMed] [Google Scholar]
- 46.Cooper R, Frances S, Waterson D, Piper R, Sweeney A. Distribution of anopheline mosquitoes in northern Australia. J Am Mosq Control Assoc. 1996;12:656–663. [PubMed] [Google Scholar]
- 47.Russell RC. Constructed wetlands and mosquitoes: health hazards and management options—an Australian perspective. Ecol Eng. 1999;12:107–124. [Google Scholar]
- 48.Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- 49.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt-Nielsen K. Animal Physiology: Adaptation and Environment. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- 51.Willy Weather Australia http://wind.willyweather.com.au/qld/far-north.html Available at. Accessed November 18, 2014.
- 52.Kay BH, Standfast HA. Ecology of arboviruses and their vectors in Australia. In: Harris KF, editor. Current Topics in Vector Research. New York, NY: Springer Publishing Company; 1987. pp. 1–36. [Google Scholar]
- 53.Ritchie SA, Phillips D, Broom A, Mackenzie J, Poidinger M, van Den Hurk A. Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am J Trop Med Hyg. 1997;56:80–84. doi: 10.4269/ajtmh.1997.56.80. [DOI] [PubMed] [Google Scholar]
- 54.van den Hurk A, Nisbet D, Hall R, Kay B, Mackenzie J, Ritchie S. Vector competence of Australian mosquitoes (Diptera: Culicidae) for Japanese encephalitis virus. J Med Entomol. 2003;40:82–90. doi: 10.1603/0022-2585-40.1.82. [DOI] [PubMed] [Google Scholar]
- 55.Hanna JN, Ritchie SA, Phillips DA, Shield J, Bailey MC, Mackenzie JS, Poidinger M, McCall BJ, Mills PJ. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med J Aust. 1996;165:256–261. doi: 10.5694/j.1326-5377.1996.tb124960.x. [DOI] [PubMed] [Google Scholar]
- 56.Hanna J, Ritchie S, Phillips D, Lee J, Hills S, van den Hurk A, Pyke A, Johansen C, Mackenzie J. Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust. 1999;170:533. doi: 10.5694/j.1326-5377.1999.tb127878.x. [DOI] [PubMed] [Google Scholar]
- 57.Meyer Steiger DB, Johnson P, Hilbert DW, Ritchie S, Jones D, Laurance SGW. Effects of landscape disturbance on mosquito community composition in tropical Australia. J Vector Ecol. 2012;37:69–76. doi: 10.1111/j.1948-7134.2012.00201.x. [DOI] [PubMed] [Google Scholar]
- 58.Harley D, Ritchie S, Phillips D, van den Hurk A. Mosquito isolates of Ross River virus from Cairns, Queensland, Australia. Am J Trop Med Hyg. 2000;62:561. doi: 10.4269/ajtmh.2000.62.561. [DOI] [PubMed] [Google Scholar]
- 59.Braks M, Meijerink J, Takken W. The response of the malaria mosquito, Anopheles gambiae, to two components of human sweat, ammonia and L-lactic acid, in an olfactometer. Physiol Entomol. 2001;26:142–148. [Google Scholar]
- 60.Dekker T, Steib B, Carde R, Geier M. L-lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol. 2002;16:91–98. doi: 10.1046/j.0269-283x.2002.00345.x. [DOI] [PubMed] [Google Scholar]