Abstract
Anaphylactic shock represents a serious complication of echinococcosis as up to 4.6% of patients die as a result of its severity and improper handling. Once a definite diagnosis is made, effective treatments need to be immediately initiated. Here, we report the immunological characteristics and management of two patients with recurrent anaphylactic shock concurrent with the surgical removal of hydatid cysts. Both patients had systemic echinococcosis classified as cystic echinococcosis type 2 (CE2) with multiple, immature cysts (absence of calcification and necrosis). In addition, both patients had increased eosinophils and basophils before surgery, as well as elevated crude hydatid cyst fluid antigen (anti-EgCF) and hydatid cyst fluid native antigen B (anti-EgB) antibodies and high IgG levels. Although we cannot definitively predict which patients are at risk for cyst fluid leakage or anaphylactic shock at present, clinicians may consider taking precautions before surgery on encountering patients with a similar profile to prevent the occurrence of anaphylactic shock and the likelihood of a second incident. However, these observations need to be confirmed in further studies with a larger number of patients.
Introduction
Echinococcosis is a serious zoonotic disease caused by Echinococcus granulosus that is highly prevalent in the Mediterranean region, Russia, China, Africa, Australia, and South America.1–3 Although the liver is the primary organ affected then the lung, together contributing to 90% of cases, the eggs or scolices can remain latent for several years without inducing any clinical symptoms. Diagnosis is often based on identification of cysts through imaging although enzyme-linked immunosorbent assays (ELISAs) to detect cyst fluid proteins have been developed.1,3 Treatment of echinococcal cysts includes cystectomy, chemotherapy with benzimidazole compounds, as well as cyst puncture and aspiration.3
Once an E. granulosus cyst ruptures, anaphylactic shock can occur,4 resulting in death with an incidence of up to 4.6%.5 Echinococcosis-induced anaphylactic shock has been found to be an IgE-dependent allergic reaction.6,7 Most patients have specific IgE against E. granulosus antigens, and serological IgE levels have been associated with disease severity.8 Since the occurrence of echinococcosis-induced anaphylactic shock during surgery is unpredictable, few strategies for its prevention and treatment have been described.9,10 Here, we describe the immunological characteristics and management of two patients with echinococcosis with recurrent anaphylactic shock concurrent with the surgical removal of hydatid cysts, an extremely rare event. The results of this study may help inform clinicians of the likelihood of recurrent anaphylactic shock in patients with echinococcosis.
Materials and Methods
Patients and diagnosis of E. granulosus infection.
Both patients presented at the First Affiliated Hospital of Xinjiang Medical University, Urumqi, China, for elective cystectomy after a diagnosis of E. granulosus infection following noncompliance to albendazole using the following criteria: the hydatid cysts were confirmed by B ultrasound and computed tomography (CT). In addition, 90 patients with echinococcosis, who were treated surgically with or without anaphylactic shock, were also included (1:4 match). All demographic and baseline clinical parameters were obtained via their medical records. In addition, serum samples were obtained immediately when entering into the operating room, as well as when anaphylactic shock occurs to assess antibody and cytokine levels. Finally, CT scans were taken for each patient before each surgery.
Analysis of blood cell levels.
The levels of neutrophils, lymphocytes, monocytes, eosinophils, and basophils were determined before the surgery by ADVIA 2120 blood cell analyzer (Bayer HealthCare Pharmaceuticals, Montville, NJ).
Analysis of serum anti-EgCF, anti-EgP, anti-EgB, and anti-Em2 antibody levels.
Serum anti-EgCF, E. granulosus protoscolex antigen (anti-EgP), anti-EgB, and Echinococcus multilocularis metacestode antigen (anti-Em2) antibody levels were determined before the surgery with specific antibodies (all from Xinjiang Beisiming Biotechnology Development Co., Ltd., Urumqi, China).
Analysis of serum cytokine, histamine, platelet-activating factor and thromboxane B2 levels.
Interleukin (IL) 4 and 10, interferon gamma, tumor necrosis factor alpha (TNF-α) and platelet-activating factor (PAF), and thromboxane B2 (TXB2) levels were determined by ELISA kit purchased from Excell Biology (Shanghai, China).
Analysis of protein levels in the cyst fluid.
The protein levels in the cyst fluid were determined using BCA Protein Assay Kit (Bio-Rad, Richmond, CA).
Results
Case presentations and treatment.
Case 1.
A 42-year-old male Han Chinese patient, weighing 70 kg, was diagnosed with multiple systemic Echinococcus infections in the liver, lung, spleen, and pelvic area (Figure 1A and B ). The patient had no prior history of allergic disease or asthma and had never undergone surgery or had been administered anesthesia (Table 1). The first surgery was elective to remove Echinococcus in the right lung. Anesthesia was induced using the following protocol: initiation with 0.1 mg/kg midazolam, 0.2 mg/kg cisatracurium, and 6 μg/kg fentanyl followed by insertion of a left-sided double-lumen bronchial catheter in approximately 8 minutes under the guidance of a well-positioned fiber-optic bronchoscope. Anesthesia was maintained by continuous infusion of 0.25–0.5 mg/kg/minute remifentanil, 0.2 mg/kg/hour cisatracurium, and 2 mg/kg/hour propofol with constant monitoring of an electrocardiogram (ECG), aortic pressure, central venous pressure (CVP), arterial PO2, end tidal CO2 (ETCO2), and body temperature. Before the surgery, the patient received antibiotics (amoxicillin and potassium clavulanate) and methylprednisolone to prevent Echinococcus-induced allergy. All vital signs remained stable throughout the surgery. At approximately 1 hour into the surgery, a giant pulmonary hydatid cyst ruptured, which led to an increase in the patient's heart rate from 75 to 170 beats/minute, a decrease in systolic arterial pressure (SAP) from 120/80 to 55/30 mmHg, and an elevation of airway pressure from 18 to 48 cmH2O (single lung measurement). There were weak breath sounds in both lungs, particularly in the right lung, and manual respiration was subsequently adopted. The appearance of poor lung compliance and reduced pulse oxygen saturation was indicative of bronchial spasm. The saturation of oxygen (SatO2) decreased from 100% to 55%, and the patient's skin showed a large area of maculopapular rash and urticaria. No changes in CVP were found. The patient's body temperature dropped from 37°C to 36.3°C. The patient was, therefore, diagnosed with anaphylactic shock and received expectant treatment consisting of 100% oxygen to both lungs as well as 1,200 μg adrenaline and 1,500 mL crystalloid solution via intravenous (IV) infusion. Moreover, salbutamol (300 μg/time) was repeatedly administered via the airway along with IV infusion of 200 mg hydrocortisone. After a single dose of 1.0 mg norepinephrine, 0.5 mg adrenaline, 1.5 mg norepinephrine, and 80 mg dopamine were continuously infused. After 30 minutes, the patient's aortic pressure returned to 80/40 mmHg, while the restoration of hemodynamics and lung compliance occurred after 45 minutes. A small dose of adrenaline (0.5 μg/kg/hour) was constantly administered until completion of the surgery. After the cystectomy, sternal closure was performed, and the patient was subsequently transferred to the intensive care unit (ICU). The patient was back to full consciousness 4 hours after the surgery. A tracheal catheter was inserted after induction of anesthesia and was removed 0.5 hours after patient was fully awake with respiratory support.
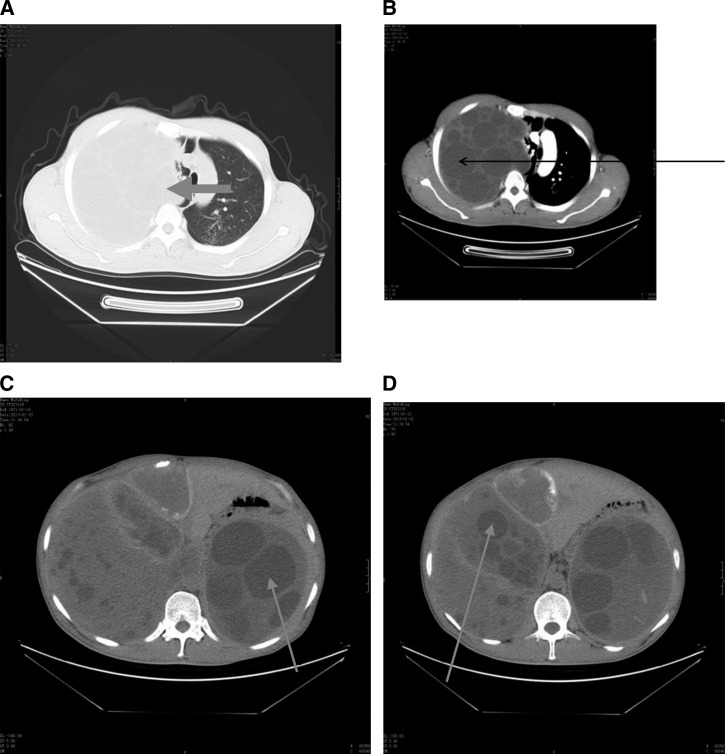
Figure 1.
Plain and enhanced computed tomography (CT) scans from patient no. 1. The CT scans were obtained before (A and B) the first surgery and (C and D) the second surgery. (A) Plain CT of the chest showing that the hydatid occupied the whole left thoracic cavity (arrow). (B) Enhanced CT of the chest showing the vesicae that were formed as a result of Echinococcus granulosus infection (arrow). (C and D) Plain CTs of the abdominal and pelvic cavities showing multiple hydatids in these cavities and vesicae that were formed as a result of E. granulosus infection (arrows).
Table 1.
Demographics and baseline clinical parameters of two patients with recurrent echinococcosis-induced anaphylactic shock
| Patient no. 1 | Patient no. 2 | |||
|---|---|---|---|---|
| First incident | Second incident | First incident | Second incident | |
| Age (years) | 42 | 42 | 26 | 26 |
| Sex | Male | Female | ||
| Height (cm) | 171 | 163 | ||
| Weight (kg) | 70 | 65 | 60 | 58 |
| Blood type | O+ | A+ | ||
| Preoperative diagnosis | Mixed echinococcosis | Mixed echinococcosis | Mixed echinococcosis | Mixed echinococcosis |
| Allergic history | No | Yes | No | Yes |
| Family history of allergy | No | No | ||
| Type of echinococcosis | Multiple daughter cysts | Multiple daughter cysts | ||
| Neutrophils (109/L) | 2.77 | 6.22 | 4.73 | 3.92 |
| Lymphocytes (109/L) | 1.06 | 1.99 | 1.15 | 2.24 |
| Monocytes (109/L) | 0.22 | 0.27 | 0.3 | 0.5 |
| Eosinophils (109/L) | 0.46 | 1.44 | 0.36 | 0.48 |
| Basophils (109/L) | 0.15 | 0.18 | 0.13 | 0.16 |
| Anti-EgCF antibody | +++ | +++ | +++ | +++ |
| Anti-EgP antibody | ++ | ++ | ++ | ++ |
| Anti-EgB antibody | +++ | ++ | +++ | ++ |
| Anti-Em2 antibody | + | + | + | + |
| Cyst fluid protein levels (mg/100 mL) | 182 | 160 | 201 | 186 |
anti-EgCF = crude hydatid cyst fluid antigen; anti-EgB = hydatid cyst fluid native antigen B; anti-Em2 = Echinococcus multilocularis metacestode antigen.
Six months after the surgery, the patient was again admitted to our hospital to receive elective pulmonary cystectomy on January 12, 2013 (Figure 1C and D). The anesthesia induction and maintenance as well as routine monitoring were identical to those previously used. When the pulmonary hydatid endocyst was handled, the patient's heart rate increased from 85 to 180 beats/minute, the SAP decreased from 110/75 to 60/30 mmHg, while no apparent increase in airway pressure was observed. Both urticaria and maculopapular rash were observed all over the patient's chest, back, and four limbs, and 1,500 mL crystalloid solution was given via IV infusion. In addition, fractional doses of IV 1.0 mg adrenaline, 30 mg dopamine, 200 of hydrocortisone, and 50 mg esmolol were administered multiple times. After 45 minutes, the patient's blood pressure returned to normal while the rash remained. No catecholamines were administered, and the patient was fully awake 2 hours after surgery. A tracheal catheter was inserted as airway support after induction of anesthesia and was subsequently removed 1 hour after patient was fully awake.
Case 2.
A 26-year-old female Han Chinese patient, weighing 60 kg, was admitted and diagnosed with systemic multiple Echinococcus infections in the liver, left lung, and pelvic area (Figure 2A and B ). The patient had no prior history of allergic disease or asthma; however, she did have a prior history of receiving anesthesia during previous Caesarean section and diagnostic abdominocentesis as well as blood transfusion (Table 1). Because of abdominal swelling and distention, the patient first underwent elective surgery for abdominal Echinococcus. Anesthesia was induced with 0.1 mg/kg midazolam, 0.6 mg/kg rocuronium bromide, and 5 μg/kg sufentanil followed by the insertion of tracheal catheter. Anesthesia was maintained via administration of surfentanil at intervals and continuous administration of 36 mg/hour rocuronium bromide and 2 mg/kg/hour propofol. ECG, aortic pressure, CVP, arterial PO2, ETCO2, and body temperature were constantly monitored during the surgery. Although all vital signs were stable at the beginning of the surgery, the presence of severe abdominal adhesions induced by previous surgeries resulted in an increased heart rate from 80 to 175 beats/minute, a decreased SAP from 110/70 to 65/40 mmHg, and an elevated airway pressure from 13 to 41 cmH2O in both lungs. Upon detection of weak breath sounds in both lungs, manual respiration was initiated, and the appearance of poor lung compliance and reduction of pulse oxygen saturation to 55% indicated bronchial spasm. No changes in CVP were observed, and the patient's body temperature decreased from 37.3°C to 35.6°C. Because all signs indicated the occurrence of anaphylactic shock, 100% oxygen was administered to both lungs along with 1,000 μg adrenaline, 1,000 mL crystalloid solution, and 500 mL colloid solution administered by IV infusion. Salbutamol was also administered multiple times via the airway along with 200 mg IV hydrocortisone. After a single administration of 0.5 mg norepinephrine, adrenaline was continuously administered at a total dose of 1.0 mg. After 45 minutes, the patient's aortic pressure increased to 90/60 mmHg, which was accompanied by restoration of hemodynamics and lung compliance. Small doses of adrenaline were constantly administered until the surgery was finished. After the sternal closure, the patient was transferred to the ICU and was fully awake 3 hours after surgery. The tracheal catheter was inserted after induction of anesthesia as airway support and was removed 1 hour after patient was fully awake.
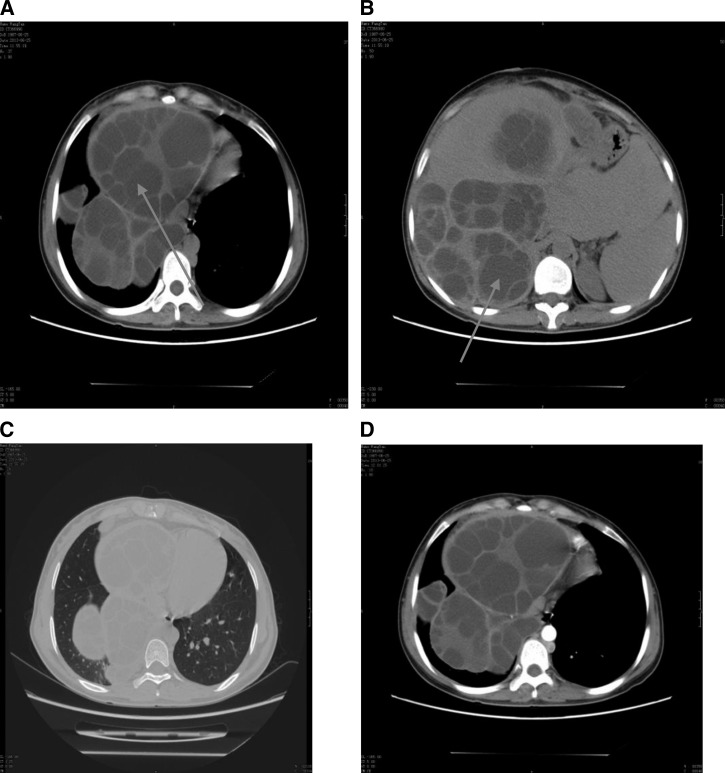
Figure 2.
Plain and enhanced computed tomography (CT) scans from patient no. 2. The CT scans were obtained before the first surgery (A and B) and the second surgery (C and D). (A and B) Plain CT showing multiple hydatids in the abdominal and pelvic cavities and vesicae that were formed as a result of Echinococcus granulosus infection (arrows). (C) Plain and (D) enhanced CTs of the chest.
Eight months after the first cystectomy, the patient self-described the expectoration of an Echinococcus cyst and the aggravating difficulty of lying in a recumbent position. Hence, the patient was again admitted to our hospital to receive elective hydatid ectocyst removal in the left lung (Figure 2C and D). Anesthesia was induced and sustained using identical protocols to those previously used. The insertion of left-sided double-lumen bronchial catheter was performed under the guidance of a well-positioned fiber-optic bronchoscope. ECG, CVP, SAP, SatO2, ETCO2, and temperature were again routinely monitored. On manipulating the pulmonary hydatid endocyst, the patient's heart rate increased from 80 to 170 beats/minute, SAP decreased from 120/70 to 60/40 mmHg, and airway pressure elevated from 18 to 45 cmH2O (single lung measurement). After detection of weak breath sounds in both lungs, particularly in the left lung, manual respiration was subsequently adopted. The concurrent appearance of poor lung compliance and reduced pulse oxygen saturation to 45% was indicative of bronchial spasm. No changes in CVP were found, and the patient's body temperature dropped from 37°C to 36.3°C. The patient was consequently diagnosed with anaphylactic shock, and 100% oxygen was administered to both lungs in addition to intermittent IV adrenaline (1,000 μg), crystalloid solution (1,000 mL), and colloid solution (1,000 mL). Salbutamol (300 μg/time) was given multiple times via the airway along with 200 mg of IV hydrocortisone. After a single dose of 0.5 mg norepinephrine, adrenaline (total dose of 1 mg) and phenylephrine (total dose of 1 mg) were then continuously administered until the surgery was finished. After 40 minutes, hemodynamics was restored and lung compliance occurred, at which time the surgery resumed. The cyst removed by the surgery was approximately 1.35 kg. Because the lower lobe of the left lung failed to re-expand, it was subsequently resected. After the surgery was done, the patient was transferred to the ICU and was completely conscious after 3 hours. A tracheal catheter was inserted after induction of anesthesia as airway support and was removed 1 hour after patient was fully awake. After 2 days, the patient was transferred from the ICU to a general hospital ward.
Analysis of blood cell and antibody levels.
As shown in Table 1, routine blood tests before both surgeries all indicated that the levels of eosinophils and basophils in both patients were higher than those in other patients with echinococcosis, but without evident allergy. In addition, both patients were positive for anti-EgCF, anti-EgP, anti-EgB, and anti-Em2 antibodies, among which anti-EgCF and anti-EgB antibodies were strongly positive (Table 1).
Analysis of protein levels in the cyst fluid.
The more clear the cyst fluid, the fewer amounts of formed elements and the lower maturity of Echinococcus, resulting in a better immune tolerance of the organism infected and a reduced chance for an allergic reaction. The protein levels detected in the cyst fluid of both patients were extremely high (Table 1), which may be one of the reasons why anaphylactic shock occurred twice in these particular individuals.
Cytokine and IgE levels.
In both patients, higher IgE and IgG and IgG1 levels were noted with each incident of anaphylactic shock (Table 2). Moreover, higher IL-4, IL-10, and TNF-α levels were consistently increased with anaphylactic shock along with the release of intermediates, such as histamine, PAF, and TXB2, to different extents (Table 2).
Table 2.
Serum antibody and cytokine levels before and after anaphylactic shock
| Parameters | Patient no. 1 | Patient no. 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| First incident | Second incident | First incident | Second incident | |||||
| PRE | PERI | PRE | PERI | PRE | PERI | PRE | PERI | |
| IgE (IU/mL) | 342 | 1,780 | 286 | 2,180 | 308 | 2,364 | 352 | 2,586 |
| IgG (g/L) | 11.6 | 15.2 | 13.4 | 31.8 | 12.8 | 24.6 | 13.4 | 28.3 |
| IgG1 (g/L) | 10.2346 | 14.5711 | 11.2671 | 23.1284 | 9.0153 | 32.5234 | 11.2 | 28.1247 |
| IL-4 (ng/L) | 432.148 | 496.156 | 428.751 | 463.269 | 400.216 | 461.622 | 398.763 | 486.366 |
| IL-10 (pg/mL) | 32.1482 | 149.123 | 28.5642 | 129.37 | 48.5342 | 214.157 | 41.2346 | 198.245 |
| INF-γ (ng/L) | 135.166 | 98.3254 | 112.357 | 124.655 | 121.698 | 75.3326 | 110.325 | 158.987 |
| TNF-α (μg/mL) | 0.1328 | 24.7868 | 2.1583 | 43.2866 | 9.2143 | 23.1563 | 12.9861 | 25.1476 |
| Histamine (μg/L) | 17.83 | 34.96 | 17.88 | 37.2 | 18.09 | 34.18 | 17.24 | 26.25 |
| PAF (μg/L) | 32.2 | 353.6 | 28.7 | 296.3 | 45.3 | 305.4 | 36.7 | 312.6 |
| TXB2 (μg/L) | 123.8 | 213.6 | 151.4 | 235.1 | 140.5 | 262.4 | 138.7 | 255.8 |
IL = interleukin; INF-γ = interferon gamma; PAF = platelet-activating factor; PERI = perioperative levels; PRE = preoperative levels; TNF-α = tumor necrosis factor alpha; TXB2 = thromboxane B2.
Demographics and clinical parameters of patients with echinococcosis, who were treated surgically with or without anaphylactic shock.
We compared age, gender, and white cell count results between patients with and without anaphylactic shock during cystectomy (Table 3). Of these patients, 18 experienced anaphylactic shock once, while 72 had no anaphylactic shock. Patients with anaphylactic shock were younger (mean age = 32.6 versus 46.2 years old, P = 0.024). Similar proportions of men and women experienced anaphylactic shock. There were no differences in total white cell count, eosinophil count or proportion, or in any other white cell types. As shown in Table 4, anti-EgCF and anti-EgB levels were higher in cases without anaphylaxis. No significant differences were found in anti-EgP and anti-Em2 levels.
Table 3.
Demographics and clinical parameters of patients with echinococcosis treated surgically with or without anaphylactic shock (N = 90)
| Shock (N = 18) | Control (N = 72) | |
|---|---|---|
| Age (years) | 32.61 ± 22.41 | 46.20 ± 16.84 |
| Gender, n (%) | ||
| Male | 8 (44.44) | 36 (45.00) |
| Female | 10 (55.56) | 44 (55.00) |
| White blood cell (109/L) | 9.45 ± 4.64 | 8.95 ± 2.47 |
| Neutrophil (109/L) | 10.56 ± 19.47 | 5.42 ± 1.93 |
| Neutrophil (%) | 0.67 ± 0.17 | 0.67 ± 0.15 |
| Lymphocyte (109/L) | 2.01 ± 1.27 | 2.07 ± 1.04 |
| Lymphocyte (%) | 0.22 ± 0.11 | 0.23 ± 0.12 |
| Monocyte (109/L) | 0.57 ± 0.43 | 0.60 ± 0.46 |
| Monocyte (%) | 0.06 ± 0.02 | 0.07 ± 0.03 |
| Eosinophil, (109/L) | 0.53 ± 0.72 | 0.40 ± 0.33 |
| Eosinophil (%) | 0.05 ± 0.07 | 0.05 ± 0.06 |
| Basophil (109/L) | 0.09 ± 0.05 | 0.07 ± 0.09 |
| Basophil (%) | 0.01 ± 0.01 | 0.01 ± 0.01 |
Table 4.
Serum antibody and cytokine levels in patients with echinococcosis treated surgically with or without anaphylactic shock (N = 90)
| Shock (N = 18) | Control (N = 72) | P value† | |
|---|---|---|---|
| Anti-EgCF antibody, n (%) | |||
| + | 4 (22.22) | 11 (13.75) | 0.003* |
| ++ | 11 (61.11) | 16 (20.00) | |
| +++ | 3 (16.67) | 53 (66.25) | |
| Anti-EgP antibody, n (%) | |||
| + | 4 (22.22) | 8 (10.00) | 0.907 |
| ++ | 12 (66.67) | 72 (90.00) | |
| +++ | 2 (11.11) | 0 (0.00) | |
| Anti-EgB antibody, n (%) | |||
| − | 1 (5.56) | 1 (1.25) | 0.003* |
| + | 2 (11.11) | 9 (11.25) | |
| ++ | 12 (66.67) | 15 (18.75) | |
| +++ | 3 (16.67) | 55 (68.75) | |
| Anti-Em2 antibody, n (%) | |||
| – | 3 (16.67) | 5 (6.25) | 0.147 |
| + | 15 (83.33) | 75 (93.75) | |
anti-EgCF = crude hydatid cyst fluid antigen; anti-EgB = hydatid cyst fluid native antigen B; anti-Em2 = Echinococcus multilocularis metacestode antigen.
Indicates a significant difference between those with and without anaphylactic shock.
The data were analyzed using the Fisher's exact test or the χ2 test.
Discussion
We analyzed the immunological characteristics of recurrent anaphylactic shock in two echinococcosis-infected patients, who were successfully treated each time. With the exception of one other case of recurrent anaphylactic shock as a result of echinococcosis in Greece reported in 1986,11 ours is the only other study to date. Although both patients in our study were admitted to our hospital because of systemic echinococcosis, the locations from which the cysts were removed in the first surgery were distinct (right lung versus abdomen); however, both had pulmonary and liver echinococcosis classified as type CE2 cysts.12 The immunological profiles of the patients suggest that the shock experienced by these patients was different from type I immediate hypersensitivity shock, which is similar to that reported by Li and others13 who analyzed the records of 446 patients that received cystectomy for echinococcosis. The authors concluded that this shock may result from a combination of immediate hypersensitivity with endotoxic shock.13
Although the underlying reason for the development of anaphylactic shock in some patients with echinococcosis during cystectomy are not known at this time, studies suggest that the extent and velocity of allergy are associated with the amount of corresponding specific antibodies produced. Zhu and others14 showed that the specific antibodies produced and their titer varied with the differences in the size, number, and location of Echinococcus. However, Todorov and others15 indicated that immunological reactions have no correlation with the size of cyst but are subject to changes in the cyst itself along with surrounding tissues. Specifically, patients with clear cyst fluids possess the highest ratio of negative results or lower antibody titers, indicating the smaller the cyst, the lower the antibody levels. When a hydatid cyst has thicker fabric texture, it elicits weaker or deficient immune responses.15 Regarding the two cases described in this report, the occurrence of anaphylactic shock may have been correlated with the locality, type of Echinococcus present, and the degree of clearness in the cyst fluids.
Li and others13 reported that relatively younger age, pulmonary echinococcosis, and surgery to remove hydatid endocyst are the risk factors associated with the development of anaphylactic shock in echinococcosis patients. Because the two patients reported here were infected with systemic multiple echinococcosis, we opted to perform an endocyst excision along with partial ectocyst resection. Thus, the anaphylactic shock was likely due to leakage of the cyst fluid during the excision of endocyst. In echinococcal cyst fluid, cytotoxic formed elements, including TXB2 and 6-keto-prostaglandin F1 alpha, as well as various allergy-induced substances, such as toxalbumin, can be found.16,17 From the aspect of clinical and demographic characteristics of the two patients who developed repeated anaphylactic shock, although the two patients and their families do not have definite history of allergic diseases, their common grounds are that both patients were infected systemically with multiple echinococcosis classified as CE2, representing multiple cyst formation and lower maturity of Echinococcus. Moreover, no calcification and necrosis were found on imaging exams. In addition, the levels of eosinophils in both patients after the second occurrence of shock were higher than those detected in other patients with echinococcosis without evident allergy (0.48–1.44 versus a mean of 0.40, respectively); basophil levels in both patients at each occurrence of anaphylactic shock were higher than those detected in patients with one occurrence of anaphylactic shock or without evident allergy (0.13–0.18 versus a mean of 0.09 and 0.07, respectively). Furthermore, both patients were strongly positive for both anti-EgCF and anti-EgB antibodies.
Clinical and experimental studies have shown that the allergen causing anaphylactic shock in patients with echinococcosis is a component of EgCF, to which the patient is exposed on spontaneous rupture or surgery-induced rupture of the cyst and exposure to EgCF in the cystic fluid.18 In an animal model of echinococcosis, IV or intraperitoneal injection of raw antigens derived from cystic fluid induced anaphylactic shock.19 In addition, early studies also revealed that high molecular weight immunogenic proteins (?43 kDa) found in the raw antigens of EgCF could bind with the specific anti-Echinococcus IgE (sIgE).18,20 In addition, EgCF is usually regarded as a major antigen in the diagnosis of human cystic echinococcosis. EgCF is composed of a mixture of different components, including Echinococcus-derived protein, carbohydrates, and metabolites as well as components from the host. Although it is difficult to isolate and purify EgCF components, Echinococcus-derived lipoprotein B and antigen 5 are the main antigens after semi-purification of EgCF used in the diagnosis of echinococcosis. In addition, AgB, a heat-resistant lipoprotein of 120–160 kDa that can be degraded into several subunits of 8, 16, 24, and 32 kDa, has been regarded as an antigen with high sensitivity and specificity in the immunodiagnosis of echinococcosis.21 However, it cannot be recognized by echinococcosis-specific IgE.22 Therefore, we speculate that AgB may not be the antigen causing anaphylactic shock. Although both patients with recurrent echinococcosis-induced anaphylactic shock had high levels of both anti-EgCF and anti-EgB antibodies, we cannot conclude that the recurrent anaphylactic shock in patients with echinococcosis was caused by EgCF and/or EgB, especially given that a higher proportion of patients in the control group had high levels of these antibodies relative to those experiencing anaphylactic shock. Thus, further studies identifying the allergens causing anaphylactic shock in patients with echinococcosis are required.
From the perspective of changes in the levels of antibodies and cytokines, the occurrence of anaphylactic shock is primarily represented by elevated IgE levels accompanied by increased IgG1 levels and subsequently elevated IL-4 and IL-10,23 which was detected in both patients described here. In addition, we detected an increase in histamine, PAF, and TXB2 in both patients. In an in vivo model of echinococcosis-induced anaphylactic shock, a type I hypersensitivity reaction accompanied by endotoxic shock, which may be cytotoxic, was reported.24 It is possible that the extent of E. granulosus cyst fluid leakage leads to maturation of B cells into plasma cell, followed by IgE and accompanying specific IgG1 production that participate in antibody-dependent cell-mediated cytotoxicity, thereby resulting in the release of intermediates that further aggravates the shock. Such aggravation eventually develops into collapse of the circulation and microvascular leakage, resulting in low blood pressure represented by lowered systolic pressure and high bronchial response featured by pulmonary interstitial edema and pulmonary arterial hypertension.
Because of the massive release of endotoxins, shock symptoms cannot be relieved simply by using adrenaline. Once Echinococcosis-induced anaphylactic shock is diagnosed, supportive treatments for the airway and circulation (i.e., IV infusion of adrenaline, corticosteroids, vasoactive drugs, fluid therapy, body heat preservation, and pure oxygen delivery) need to be immediately initiated. In addition, in the event of circulation collapse and airway pressure elevation during cystectomy, which is a characteristic of cyst fluid leakage, the incidence of anaphylactic shock needs to be considered the first priority. Under such circumstances, antiallergic treatment should be immediately administered at a dose of 50 μg adrenaline IV bolus at first time (and increase amount if necessary) for adults. Furthermore, as the doses of adrenaline escalate, adrenergic-stimulating agents should be administered simultaneously to assist the circulation until the steady state is achieved.25
This study is limited in the very small number of cases presented, given that recurrent anaphylactic shock in patients with echinococcosis is rare. Therefore, further studies with a larger number of patients are needed to determine if the observations presented here are applicable in general.
In conclusion, analysis of the two cases of echinococcosis with recurrent anaphylactic shock identified certain similarities. Both patients had systemic echinococcosis classified as CE2, representing multiple cysts with less mature Echinococcus (no calcification and necrosis). In addition, both patients had increased eosinophils and basophils indicated by routine blood exams, as well as elevated anti-EgCF and anti-EgB antibodies and higher serological IgG levels, before surgery. Although we cannot definitively predict cyst fluid leakage or anaphylactic shock at present, clinicians may consider taking precautions before surgery on encountering patients with such a profile to prevent the occurrence of anaphylactic shock and the likelihood of a second incident.
Footnotes
Financial support: This study is funded by National Natural Science Foundation, project number: 81460309, “Identification, purification and immunogenicity study of specific antigen of anaphylactic shock induced by echinococcosis.”
Authors' addresses: Jianrong Ye, Qin Zhang, Long Ma, and Hong Zheng, Department of Anesthesiology, the First Affiliated Hospital of Xinjiang Medical University, Urumqi, China, E-mails: yejianrong0@sina.com, zhangqin99999999@sina.com, malong951@sina.com, and zhenghong99999@sina.com.
References
- 1.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson RC. The taxonomy, phylogeny and transmission of Echinococcus. Exp Parasitol. 2008;119:439–446. doi: 10.1016/j.exppara.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Saenz de San Pedro B, Cazana JL, Cobo J, Serrano CL, Quiralte J, Contreras J, Martinez F. Anaphylactic shock by rupture of hydatid hepatic cyst. Follow-up by specific IgE serum antibodies. Allergy. 1992;47:568–570. doi: 10.1111/j.1398-9995.1992.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Zhang Z, Wu W, Shi B, Li J, Zhou X, Wen H, McManus DP. Epidemiology and control of echinococcosis in central Asia, with particular reference to the People's Republic of China. Acta Trop. 2015;141:235–243. doi: 10.1016/j.actatropica.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Mooraki A, Rahbar MH, Bastani B. Spontaneous systemic anaphylaxis as an unusual presentation of hydatid cyst: report of two cases. Am J Trop Med Hyg. 1996;55:302–303. doi: 10.4269/ajtmh.1996.55.302. [DOI] [PubMed] [Google Scholar]
- 7.Sacco R, Papaleo V, Hager J, Rousseau F, Moessner R, Militerni R, Bravaccio C, Trillo S, Schneider C, Melmed R, Elia M, Curatolo P, Manzi B, Pascucci T, Puglisi-Allegra S, Reichelt KL, Persico AM. Case-control and family-based association studies of candidate genes in autistic disorder and its endophenotypes: TPH2 and GLO1. BMC Med Genet. 2007;8:11. doi: 10.1186/1471-2350-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortona E, Vaccari S, Margutti P, Delunardo F, Rigano R, Profumo E, Buttari B, Rasool O, Teggi A, Siracusano A. Immunological characterization of Echinococcus granulosus cyclophilin, an allergen reactive with IgE and IgG4 from patients with cystic echinococcosis. Clin Exp Immunol. 2002;128:124–130. doi: 10.1046/j.1365-2249.2002.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgio A, Calisti G, de Stefano G, Farella N, Scognamiglio U, Giorgio V. Percutaneous treatment of hydatid liver cysts: an update. Recent Pat Antiinfect Drug Discov. 2012;7:231–236. doi: 10.2174/157489112803521913. [DOI] [PubMed] [Google Scholar]
- 10.Wen H, Aji T, Shao YM. Diagnosis and management against the complications of human cystic echinococcosis. Front Med China. 2010;4:394–398. doi: 10.1007/s11684-010-0180-9. [DOI] [PubMed] [Google Scholar]
- 11.Giulekas D, Papacosta D, Papaconstantinou C, Barbarousis D, Angel J. Recurrent anaphylactic shock as a manifestation of echinococcosis. Report of a case. Scand J Thorac Cardiovasc Surg. 1986;20:175–177. doi: 10.3109/14017438609106498. [DOI] [PubMed] [Google Scholar]
- 12.Riganò R, Buttari B, De Falco E, Profumo E, Ortona E, Margutti P, Scottà C, Teggi A, Siracusano A. Echinococcus granulosus-specific T-cell lines derived from patients at various clinical stages of cystic echinococcosis. Parasite Immunol. 2004;26:45–52. doi: 10.1111/j.0141-9838.2004.00682.x. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Zheng H, Cao X, Liu Z, Chen L. Demographic and clinical characteristics of patients with anaphylactic shock after surgery for cystic echinococcosis. Am J Trop Med Hyg. 2011;85:452–455. doi: 10.4269/ajtmh.2011.10-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu B, Xu MQ. Experimental observation on relationship between antibody immunity response of hydatid disease and the size or quantity of hydatid cyst. Endemic Dis Bull. 1997;12:20–22. [Google Scholar]
- 15.Todorov T, Raicev I, Tenev S, Kosturkova M, Dakov I, Dimitrov A. Immunoreactivity in pulmonary echinococcosis. 2. Evaluation of antibody response. Bull World Health Organ. 1979;57:741–750. [PMC free article] [PubMed] [Google Scholar]
- 16.Macintyre A, Dixon J, Green J. Mitosis and differentiation in T-cells under cytotoxic action of Echinococcus granulosus hydatid fluid. Vet Parasitol. 2001;96:277–289. doi: 10.1016/s0304-4017(01)00384-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhao H, Kelibiena TEX, Wu XH. Pathological analysis of human hydatid disease: a report of 37 cases. J Xin Jiang Med Univer. 1999;26:22. [Google Scholar]
- 18.Zheng H, Xu ZX, Yang GX, Wen H. Recognition of specific antigens by specific IgG and IgE during anaphylactic shock induced by Echinococcus granulosus in sheep. Chin J Parasitol Parasitic Dis. 2002;6:358–360. [PubMed] [Google Scholar]
- 19.Gulipali MMTYM, Wulamu MMT. Advance in the serological diagnosis of echinococcosis. J Pathog Biol. 2011;6:297–299. [Google Scholar]
- 20.Vuitton DA. Echinococcosis and allergy. Clin Rev Allergy Immunol. 2004;26:93–104. doi: 10.1007/s12016-004-0004-2. [DOI] [PubMed] [Google Scholar]
- 21.Paschinger K, Gonzalez-Sapienza GG, Wilson IB. Mass spectrometric analysis of the immunodominant glycan epitope of Echinococcus granulosus antigen Ag5. Int J Parasitol. 2012;42:279–285. doi: 10.1016/j.ijpara.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YH, Meng CR, Zhang ZX. Diagnostic value of ELISA using antigen B in cystic echinococcosis: a meta-analysis. Chin J Evid Based Med. 2013;13:332–338. [Google Scholar]
- 23.Marshall JD, Secrist H, Dekruyff RH, Wolf SF, Umetsu DT. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J Immunol. 1995;155:111–117. [PubMed] [Google Scholar]
- 24.Khabiri AR, Bagheri F, Assmar M, Siavashi MR. Analysis of specific IgE and IgG subclass antibodies for diagnosis of Echinococcus granulosus. Parasite Immunol. 2006;28:357–362. doi: 10.1111/j.1365-3024.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Zheng H, Gu M, Wen H, Liu Z, Liu T. Comparisons of serum total IgE, IgG and IgG1 levels in patients with and without echinococcosis-induced anaphylactic shock. Am J Trop Med Hyg. 2012;87:104–108. doi: 10.4269/ajtmh.2012.11-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]