Abstract
Rabies is a lethal infectious disease that causes 55,000 human deaths per year and is transmitted by various mammalian species, such as dogs and bats. The host immune response is essential for avoiding viral progression and promoting viral clearance. Cytokines and chemokines are crucial in the development of an immediate antiviral response; the rabies virus (RABV) attempts to evade this immune response. The virus's capacity for evasion is correlated with its pathogenicity and the host's inflammatory response, with highly pathogenic strains being the most efficient at hijacking the host's defense mechanisms and thereby decreasing inflammation. The purpose of this study was to evaluate the expression of a set of cytokine and chemokine genes that are related to the immune response in the brains of mice inoculated intramuscularly or intracerebrally with two wild-type strains of RABV, one from dog and the other from vampire bat. The results demonstrated that the gene expression profile is intrinsic to the specific rabies variant. The prompt production of cytokines and chemokines seems to be more important than their levels of expression for surviving a rabies infection.
Introduction
Rabies is a zoonotic, highly lethal, and neglected disease that has been affecting humanity for more than 4,000 years.1 Domesticated dogs are mainly responsible for rabies transmission, especially in Asian and African countries, but other wild mammals such as bats, nonhuman primates, foxes, and wild dogs can be responsible for human cases.2–5 Pathological studies have shown that animal and human brains infected with rabies virus (RABV) develop encephalitis but exhibit only minimal neuronal loss and varying degrees of inflammation,6–8 thus demonstrating that the virus has mechanisms to evade the host immune response.9 An uninfected animal's central nervous system (CNS) has intact endothelial tight junctions, low expression of adhesion molecules, and lack of immunological activity.10,11 However, when a pathogen gains access to the CNS, an immediate immune response occurs. After RABV is recognized by the Toll-like receptors and retinoic acid-inducible gene 1-like receptors that are present in neurons,12 interferon beta (IFN-β) is produced at high levels, subsequently acting as an antiviral protein and initiating a cascade of transcription that links the innate and acquired immune responses.13–20 In addition to IFN-β, stressed or damaged neurons synthesize interferon gamma (IFN-γ), interleukin 6 (IL-6), chemokine (C-C motif) ligand 21 (CCL21), and chemokine (C-X3-C motif) ligand 1 (CX3CL1), the last of which has receptors on macrophages and glial cells that are then activated and produce several other cytokines and chemokines, such as IL-1, IL-6, IL-12, TNF-α, CCL4, CCL5, CCL7, and chemokine (C-X-C motif) ligand 10 (CXCL10). These factors lead to the upregulated expression of major histocompatibility complex by microglia and to the increased expression of adhesion molecules (especially CXCL10) that promote interactions with circulating leukocytes and promote the opening of the blood–brain barrier (BBB),10,21 thus allowing a robust immune response that can be harmful, depending on the amount of reactive oxygen species released.22–25
In most cases, CNS infections are controlled by infiltrating T cells.26 However, in RABV infections, especially those due to highly pathogenic strains, T cells in the periphery do not determine the outcome of infection, though they are activated normally,27 as shown in a study conducted in mice lacking T cells.28 This inefficacy occurs because the virus can induce a significant production of calcitonin, somatostatin, and vasoactive intestinal peptides in infected cells, thus limiting the activity of T cells29; further, the virus can even induce T cell apoptosis30,31 Immunohistochemical studies of RABV-infected brains and spinal cords demonstrated that neurons showed a large amount of RABV antigen with intact morphology and that migrating T cells showed evidence of undergoing the apoptotic process.30,32,33 In contrast to infections caused by highly pathogenic strains, T cells are important in controlling those caused by attenuated viruses, in which infected neurons are successfully eliminated by apoptosis and T cells remain intact.9,34,35 Differences in the pathogenicity of RABV strains are associated not only with viral replication and the cellular infection rate but also with the level and duration of cytokine expression, in addition to the induction of apoptosis in immune cells.25 Several studies have shown that attenuated RABV samples lead to high expression of cytokines, an increase in BBB permeability related to the presence of IFN-γ, and the infiltration of inflammatory cells into the CNS, which enables viral clearance.27,36–40
In light of the crucial role of cytokines and chemokines in immune response regulation,41,42 the aim of this study was to evaluate the expression of selected cytokines and chemokines in the CNS of mice that were experimentally infected with two different wild-type strains of RABV, one from dog (variant 2 [V2]) and the other from vampire bat (variant 3 [V3]).
Materials and Methods
Animals and viruses.
Female C57/BL6 mice, 4–6 weeks old, specific-pathogen-free, were provided by Cemib (University of Campinas animal facility) and used for experimental rabies inoculation. The animals were kept in special containers and received sterile water and irradiated food “ad libitum.”
Dog RABV and hematophagous bat RABV, both of which were wild-type strains, were isolated from human patients and characterized as V2 and V3, respectively.
Experimental design.
Experiment 1: Mice were separated into two main groups and inoculated intramuscularly (i.m.) in the right hind limb with 100 μL of 40 LD50 (lethal dose 50) of V2 inoculum or 40 LD50 of V3 inoculum. For each LD50, the animals were separated into three groups with eight animals each; one group was maintained for clinical evaluation during a 30-day period and the other two groups were euthanized at 5 and 10 days postinoculation (d.p.i.) for brain collection.
Experiment 2: To evaluate the immune response in the final stage of the disease, two groups of eight animals were inoculated via intracerebral (i.c.) route with 30 μL of V2 or V3 inoculum with the same viral titer (10−6.66 LD50/0.03 mL). The animals were observed during a maximum period of 30 days and brains were collected immediately after death.
For each group, a control was established, in which animals were inoculated only with the viral diluent by the same route as the infected animals, either i.m. or i.c.
Animals in all of the groups were weighed and evaluated daily for the onset of clinical signs of infection, such as ruffled fur, hunched back, hypo- or hyperexcitability, paralysis of one or both hind limbs, or tetraplegia.15
The animal study was approved by the São Paulo State University Ethics Committee (registration number 238/2008), which follows the guidelines established by Colégio Brasileiro de Experimentação Animal—the Brazilian Society of Laboratory Animal Science.
RNA extraction and quantitative reverse transcription polymerase chain reaction.
Brain tissue RNA was extracted with the Invitek® Kit (Berlin, Germany) and stored at −80°C. The reaction for complementary DNA (cDNA) synthesis consisted of 1 μg of extracted RNA, 1 μL of Oligo(dT) primer (Invitrogen®, Carlsbad, CA), and 1 μL of SuperScript II (Invitrogen®) and was performed according to the manufacturer's instructions. The quantitative reverse transcription polymerase chain reaction was performed with 2 μL of 1/50 diluted cDNA, 1 μL of 0.1 μg of each primer and Master Mix SYBR Green (Promega®, Madison, WI) in a final volume of 25 μL, according to the manufacturer's instructions. Primers for the 18S murine genes were supplied by IDT® and used as housekeeping genes; primers for the RABV N protein gene were manufactured as described previously.43 Mouse QuantiTect® Primer Assays from Qiagen® (Hilden, Germany) were used to evaluate the expression levels of CCL2, OAS1, IL-2, IL-6, IL-12, TNF-α, IFN-γ, IFN-β, CXCL10, CD200R, and IGF-1 genes.
All thermal cyclings and detections were performed using an Applied Biosystems StepOne Fast® (ABI7500 Fast, Foster City, CA) thermal cycler employing a thermal profile of 40 cycles of 50°C for 20 seconds, 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute.
Data analysis.
The GraphPad Prism® 5.0 (La Jolla, CA) and Instat® software (La Jolla, CA) were used as analysis tools. The Kruskal–Wallis test with P < 0.05 was selected for global evaluations of cytokine and chemokine gene expression, and the two-tailed Mann–Whitney test was used to compare the expression level of a single cytokine or chemokine at 5 and 10 d.p.i.. Values of P between 0.05 and 0.1 were considered significant.
Results and Discussion
I.M. inoculation (experiment 1).
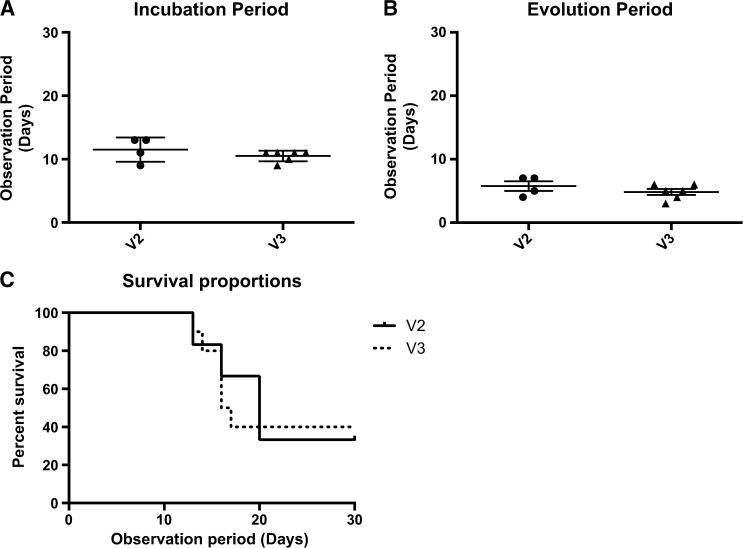
The incubation periods were 12 days and 11 days for V2 and V3, respectively, with no significant difference; thus, the length of the incubation period is variant independent. The evolution periods, which were characterized by the period between the beginning of clinical signs of infection and death, were 5 and 6 days and mortality rates were 60% and 66%, respectively, for V3 and V2, with no significant differences observed for the evolution periods. This finding demonstrates that after the entry of RABV particles into the CNS, there is no difference between dog and bat variants regarding the time required for disease progression. A study of the major characteristics of human rabies cases due to bat and dog variants also showed that after the onset of clinical signs of infection, there was no difference in the survival time between the variants.44 Our survival analysis showed a difference between V3 and V2 (P = 0.003) (Figure 1 ).
Figure 1.
(A) Incubation period of V2 and V3 groups; Mann–Whitney test was used to calculate differences between the groups. No statistical difference was found. (B) Evolution period; Mann–Whitney test showed no significant difference (P > 0.05) between the groups. (C) Survival analysis between groups during 30-day observation period; Mantel–Cox test demonstrated a statistical significance between V3 vs. V2 (P = 0.003).
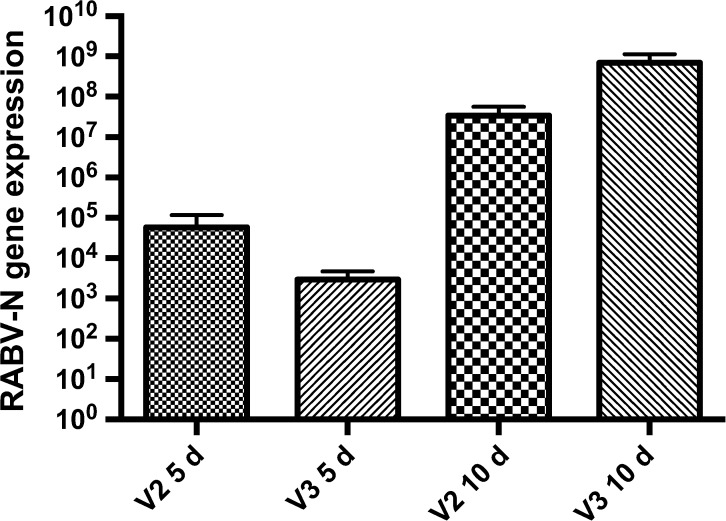
When comparing N gene expression between the groups in the same period, 5 and 10 d.p.i., no significant difference was found (Figure 2 ). A study published by Chopy and others15 examined mice that were inoculated via an i.m. route with a lethal dose of Challange Virus Standard (CVS) and were then killed in different stages of clinical disease. That study found no difference in the total amount of virus between the different phases of the disease when N gene transcripts of RABV were evaluated in those animals.
Figure 2.
Rabies virus N gene expression in the brains of mice infected with V2 and V3. Mann–Whitney test was applied to compare the results between the groups at 5 and 10 days postinoculation (d.p.i.). There was no difference between the groups at the same period, 5 d.p.i. or 10 d.p.i. or at 5 d.p.i. vs. 10 d.p.i.
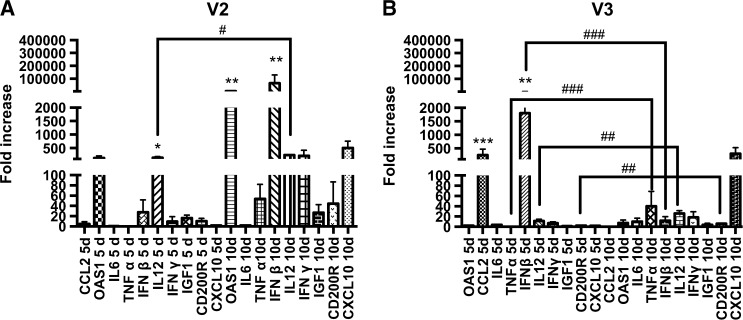
We next considered the expression of the immune markers in the V2 group. High expression levels of IL-12 (P < 0.05) at 5 d.p.i. were observed, with a further discrete enhancement at 10 d.p.i. (P = 0.09), associated with higher expression of OAS1 (P = 0.01) and IFN-β (P = 0.01), although all of the immune markers seemed to have increased expression levels at this point compared with those at 5 d.p.i. (Figure 3A ). Animals inoculated with V3 had high expression levels of IFN-β (P < 0.01) and CCL2 (P < 0.001) at 5 d.p.i. At 10 d.p.i., no difference was observed for any cytokine or chemokine between the groups. However, when expression was compared individually at 5 versus 10 d.p.i., TNF-α (P = 0.0005), IFN-β (P = 0.0002), IL-12 (P = 0.0047), and CD200R (P = 0.0047) were statistically increased at 10 d.p.i. (Figure 3B).
Figure 3.
Relative gene expression of cytokines and chemokines in both groups (V2 and V3) at 5 and 10 days postinoculation (d.p.i.). Kruskal–Wallis test was applied to analyze the global results and Mann–Whitney two-tailed test was applied to make individual comparisons at 5 vs. 10 d.p.i. (A) Expression at 5 and 10 d.p.i. in the V2 group; IL-12 (* P < 0.05) had higher expression levels at 5 d.p.i. and OAS1 (** P = 0.01) and interferon beta (IFN-β) (** P = 0.01) at 10 d.p.i.; comparing 5 d.p.i. vs. 10 d.p.i., IL-12 (# P = 0.09) showed a slight increase. (B) Expression at 5 and 10 d.p.i. in the V3 group; IFN-β (** P < 0.01) and CCL2 (*** P < 0.001) had a higher expression at 5 d.p.i. No difference was observed at 10 d.p.i., although in the comparison between 5 d.p.i. vs. 10 d.p.i. TNF-α (### P = 0.0005), IFN-β (### P = 0.0002), IL-12 (## P = 0.0047), and CD200R (## P = 0.0047) showed a higher expression at 10 d.p.i.
We then compared the results solely for those immune markers with statistically enhanced expression at 5 and 10 d.p.i. in the V2 versus V3 groups. Despite a similar mortality rate in both groups, at 5 d.p.i., V2 had a higher expression of IL-12 (P = 0.007), whereas V3 had a more prominent expression of IFN-β (P = 0.007). At 10 d.p.i., no difference was found. Considering that the animals were inoculated with the same lethal dose of both variants and had similar mortality rates, biologically, the profile of cytokines and chemokines seems to show that V2 induces the expression of a larger number of the analyzed genes and at a higher intensity, especially at 10 d.p.i., compared with V3. A study performed in mice inoculated with the same lethal dose, via i.c. or i.m. routes, using a highly pathogenic silver-haired bat RABV (SHBRV) or an attenuated CVS strain (B2C) showed that the genes upregulated by the B2C strain were higher in both number and intensity. It is also interesting to note that the OAS1 gene was upregulated 4-fold compared with IFN-β in the same group, 2-fold higher compared with OAS1 expression, and more than 6-fold higher compared with IFN-β expression in the SHBRV group.6 Although both of the RABV strains used in this present study were classified as highly pathogenic because they were isolated from human rabies patients, the gene expression of V2 and V3 was biologically distinct, even at the same viral titer and mortality rate, especially at 10 d.p.i., which was related to the induced and upregulated gene expression of V2 compared with V3. The data in this study show that the mortality rate does not appear to be associated with gene expression.
I.C. inoculation (experiment 2).
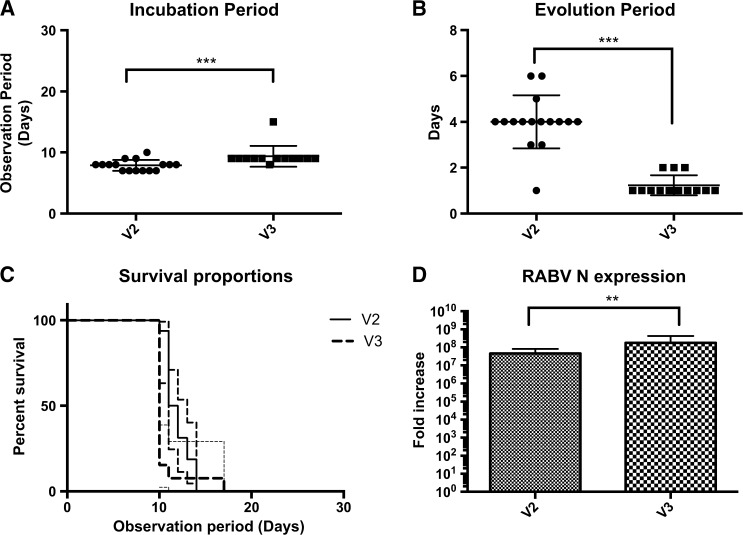
The mortality rate of animals inoculated by the i.c. route was 100% for V2 and V3, but an analysis of the survival curves showed differences in disease progression between the variants (P = 0.01). The median incubation period was 8 days for V2 and 9 days for V3 (P = 0.0002), whereas the median evolution period for V2 was 4 days and only 1 day for V3 (P < 0.0001). A study in India, analyzing data from human rabies patients showed that the incubation period is not correlated with the rapidity of the clinical course of the disease.45 Evaluation of RABV N gene expression also showed differences between the variants, with the highest expression being observed with V3 (P = 0.01) (Figure 4 ). The short evolution period is positively associated with the high N gene expression in the V3 group.
Figure 4.
(A) Incubation period of V2 and V3 groups (intracerebral route inoculation); Mann–Whitney two-tailed test showed a significant difference between the groups (*** P = 0.0002). (B) Evolution period of V2 and V3 groups; Mann–Whitney two-tailed test also showed a significant difference (*** P < 0.0001) between the groups. (C) Survival analysis curve between V2 and V3 within a 30-day observation period; Mantel–Cox test demonstrated a statistical significance between the two groups (** P < 0.01). (D) Relative rabies virus N gene expression between V2 and V3; Kruskal–Wallis test showed a higher expression in the V3 group (** P = 0.01).
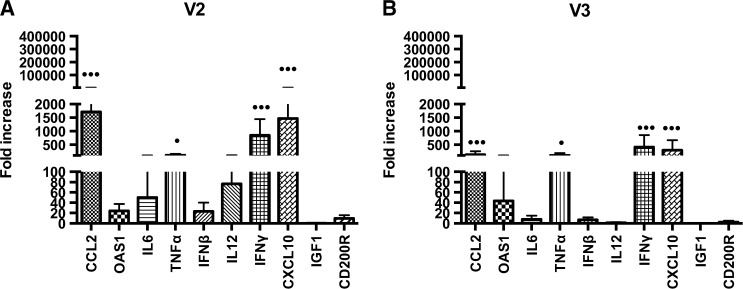
The immune markers analyzed in both variants showed increased expression of the same cytokines and chemokines, with equal P values. These were IFN-γ (P < 0.001), CXCL10 (P < 0.001), TNF-α (P < 0.05), and CCL2 (P < 0.001) (Figure 5 ).
Figure 5.
Relative gene expression of cytokines and chemokines in the brains of animals inoculated via intracerebral route with (A) V2 or (B) V3 strains. Both variants showed an increase of the following immune markers: IFN-γ (*** P < 0.001), CXCL10 (*** P < 0.001), TNF-α (* P < 0.05), and CCL2 (*** P < 0.001).
The major activity of chemokines (such as CXCL10 and CCL2) is to modulate the trafficking of T cells to the CNS, though chemokines are also important in promoting viral clearance. Studies conducted with different viral agents such as herpes simplex and West Nile showed that deletion of the gene responsible for CXCL10 expression led to the poor infiltration of T cells and to inefficient viral control, which was associated with more severe disease. However, an excess of T cells can also be deleterious because it causes greater cytotoxicity and inflammation.24,25 Several in vivo or in vitro studies have demonstrated that CXCL10 is present and upregulated after RABV infection.6,22,46 This chemokine has been shown to play an important role in BBB permeability and seems to be essential in the clearance of RABV from the CNS because it allows the entry of immune effector cells.40 Although studies6,40 have described enhanced expression of CXCL10 in models infected with attenuated RABV, but not in those infected with wild-type RABV, the results of this study revealed a statistically significant upregulation of CXCL10 and TNF-α in animals infected with wild-type strains. Immunohistochemistry assays of the brains of human patients correlated the presence of TNF-α and IL-1 (proinflammatory cytokines) with the presence of RABV and showed that the presence of these cytokines was also positively correlated with brain inflammation, but no association was established between viral load and higher levels of TNF-α.45
When these four increased cytokines and chemokines (IFN-γ, CXCL10, TNF-α and CCL2) were compared between the V2 and V3 groups, the expression levels of CCL2 (P = 0.01) and CXCL10 (P = 0.01) were higher in V2. The statistically high expression levels of these chemokines can possibly be related to lower levels of the N gene present in the V2 group, but they are not associated with a decreased mortality rate, which was 100% in both groups. Mansfield and others22 showed that high expression levels of CXCL10, IFN-γ, and IL-6 in mice in a clinical phase of encephalitis caused by European bat lyssavirus type 2 were not related to survival. Altogether, it can be concluded that even with high expression levels of cytokines and chemokines that are involved in the inflammatory process and that mediate the specific immune response, a substantial innate response was still insufficient to control infection and prevent death.
Conclusions
Mice inoculated with V2 and V3 at the same LD50 via the i.m. route presented the same mortality and clinical features but different gene expression profiles, which indicates that the immune response is intrinsic to the RABV variant.
The prompt production of cytokines and chemokines seems to be more important than their levels of expression for rabies survival, as observed in moribund animals that still succumbed to the disease, despite expressing high levels of these cytokines and chemokines related to a host-specific immune response.
ACKNOWLEDGMENTS
We acknowledge Maria Luiza Carrieri and Ivanete Kotait for kindly providing the rabies virus strains. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Process 482726/2012-1) and Fundação de Apoio à Pesquisa do Estado de São Paulo (Fapesp-Process 08/11446-1).
Authors' addresses: Camila Michele Appolinário, Susan Dora Allendorf, Marina Gea Peres, Bruna Devidé Ribeiro, Clóvis R. Fonseca, Acácia Ferreira Vicente, João Marcelo A. de Paula Antunes, and Jane Megid, Departamento de Higiene Veterinaria e Saude Publica, Faculdade de Medicina Veterinaria e Zootecnica, Universidade Estadual Paulista “Julio de Mesquita Filho”–UNESP, Botucatu, São Paulo, Brazil, E-mails: camilaapp.vet@gmail.com, susanallendorf@yahoo.com.br, marinageavet@yahoo.com.br, brunadevide@yahoo.com.br, crsfonseca2@yahoo.com.br, acaciavicente@hotmail.com, joaomarceloantunes@live.com, and jane@fmvz.unesp.br.
References
- 1.Knobell DL, Cleaveland S, Coleman PG, Fevre MI, Meltzer MI, Miranda MEG, Shaw A, Zinsstag J, Meslin F-X. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 2.Ito M, Arai YT, Itou T, Sakei T, Ito FH, Takasaki T, Kurane I. Genetic characterization and the geographic distribution of rabies virus isolates in Brazil: identification of two reservoirs, dogs and vampire bats. Virology. 2001;284:214–222. doi: 10.1006/viro.2000.0916. [DOI] [PubMed] [Google Scholar]
- 3.Schneider MC, Romijn PC, Uieda W, Tamayo H, da Silva DF, Belotto A, da Silva JB, Leanes LF. Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Rev Panam Salud Publica. 2009;25:260–268. doi: 10.1590/s1020-49892009000300010. [DOI] [PubMed] [Google Scholar]
- 4.Brasil . 2010. Brasil: Ministério da Saúde. Secretaria da Vigilância Sanitária.http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/svs/raiva Available at. Accessed May 22, 2014. [Google Scholar]
- 5.Aguiar TDF, Costa EC, Rolim BN, Romijn PC, Morais NB, Teixeira MFS. Risco de transmissão do vírus da raiva oriundo de sagüi (Callithrix jacchus), domiciliado e semi-domiciliado, para o homem na região metropolitana de Fortaleza, estado do Ceará [in Portuguese] Rev Soc Bras Med Trop. 2011;44:356–363. doi: 10.1590/s0037-86822011005000031. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZW, Sarmento L, Wang Y, Li XQ, Dhingra V, Tseggai T, Jiang B, Fu ZF. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol. 2005;79:12554–12565. doi: 10.1128/JVI.79.19.12554-12565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson AC, Randle E, Lawrance G, Rossiter JP. Neuronal apoptosis does not play an important role in human rabies encephalitis. J Neurovirol. 2008;14:368–375. doi: 10.1080/13550280802216502. [DOI] [PubMed] [Google Scholar]
- 8.Hemachudha T, Ugolini G, Wacharapluesadee S, Sungkarat W, Shuangshoti S, Laothamatas J. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013;12:498–513. doi: 10.1016/S1474-4422(13)70038-3. [DOI] [PubMed] [Google Scholar]
- 9.Lafon M. Evasive strategies in rabies virus infection. Adv Virus Res. 2011;79:33–53. doi: 10.1016/B978-0-12-387040-7.00003-2. [DOI] [PubMed] [Google Scholar]
- 10.Griffin D. Immune response to RNA-virus infections of the CNS. Natl Rev. 2003;3:493–502. doi: 10.1038/nri1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 12.Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3- positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taniguchi T, Takaoka A. The interferon-α/β system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002;14:111–116. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- 14.Masatani T, Ito N, Shimizu K, Ito Y, Nakagawa K, Sawaki Y, Koyama H, Sugiyama M. Rabies virus nucleoprotein functions to evade activation of the RIG-I-mediated antiviral response. J Virol. 2010;84:4002–4012. doi: 10.1128/JVI.02220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopy D, Detje CN, Lafage M, Kalinke U, Lafon M. The type I interferon response bridles rabies virus infection and reduces pathogenicity. J Neurovirol. 2011;17:353–367. doi: 10.1007/s13365-011-0041-6. [DOI] [PubMed] [Google Scholar]
- 16.Nakamichi K, Inoue S, Takasaki T, Morimoto K, Kurane I. Rabies virus stimulates nitric oxide production and CXC chemokine ligand 10 expression in macrophages through activation of extracellular signal regulated kinase 1 and 2. J Virol. 2004;78:9376–9388. doi: 10.1128/JVI.78.17.9376-9388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faul EJ, Wanjalla CN, Suthar MS, Gale M, Jr, Wirblich C, Schnell J. Rabies virus infection induce type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog. 2010;6:e1001016. doi: 10.1371/journal.ppat.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biron CA. Interferons α and β as immune regulators—a new look. Immunity. 2001;14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 19.Faber M, Pulmanausahakul R, Nagao K, Prosniak M, Rice AB, Koprowski H, Schnell MJ, Dietzschold B. Identification of viral genomic elements responsible for rabies virus neuroinvasiveness. PLoS Pathog. 2004;101:16328–16332. doi: 10.1073/pnas.0407289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, McGettigan JP, Faber M, Schnell MJ, Dietzschold B. Infection of monocytes or immature dendritic cells (DCs) with an attenuated rabies virus results in dc maturation and a strong activation of the NFkappaB signaling pathway. Vaccine. 2008;26:419–426. doi: 10.1016/j.vaccine.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CX, Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol. 2002;67:161–172. doi: 10.1016/s0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 22.Mansfield KL, Johnson N, Nunez A, Hicks D, Jackson AC, Fooks AR. Up-regulation of chemokine gene transcripts and T cell infiltration into the central nervous system and dorsal root ganglia are characteristics of experimental European bat lyssavirus type 2 infection of mice. J Neurovirol. 2008;14:218–228. doi: 10.1080/13550280802008297. [DOI] [PubMed] [Google Scholar]
- 23.Fu ZF, Weihe E, Zheng YM, Schafer MK, Sheng H, Corisdeo S, Rauscher FJ, Koprowski H, Dietzschold B. Differential effects of rabies and borna disease viruses on immediate- early- and late-response gene expression in brain tissues. J Virol. 1993;67:6674–6681. doi: 10.1128/jvi.67.11.6674-6681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu X, Wang H, Fu ZF. Role of chemokines in rabies pathogenesis and protection. Adv Virus Res. 2011;79:73–89. doi: 10.1016/B978-0-12-387040-7.00005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Toriumi H, Kuang Y, Chen H, Fu ZF. The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J Virol. 2009;83:11808–11818. doi: 10.1128/JVI.01346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Chan YK, Lu B, Diamond MS, Klein RS. CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile encephalitis. J Immunol. 2008;180:2641–2649. doi: 10.4049/jimmunol.180.4.2641. [DOI] [PubMed] [Google Scholar]
- 27.Roy A, Hooper DC. Lethal silver-haired bat rabies virus infection can be prevented by opening the blood-brain barrier. J Virol. 2007;81:7993–7998. doi: 10.1128/JVI.00710-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lafon M. Modulation of the immune response in the nervous system by rabies virus. Curr Top Microbiol Immunol. 2005;289:239–258. doi: 10.1007/3-540-27320-4_11. [DOI] [PubMed] [Google Scholar]
- 29.Weihe E, Bette M, Preuss MA, Faber M, Schafer MK, Rehnelt J, Schnell MJ, Dietzschold B. Role of virus-induced neuropeptides in the brain in the pathogenesis of rabies. Dev Biol (Basel) 2008;131:73–81. [PubMed] [Google Scholar]
- 30.Lafon M. Immune evasion, a critical strategy for rabies virus. Dev Biol (Basel) 2008;131:413–419. [PubMed] [Google Scholar]
- 31.Sarkar SN, Sen GC. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol Ther. 2004;103:245–259. doi: 10.1016/j.pharmthera.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Baloul L, Lafon M. Apoptosis and rabies virus neuroinvasion. Biochimie. 2003;85:777–788. doi: 10.1016/s0300-9084(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes ER, Andrade HF, Jr, Lancellotti CLP, Quaresma JAS, Demanchki S, Vasconselos PFC, Duarte MIS. In situ apoptosis of adaptive immune cells and the cellular escape of rabies virus in CNS from patients with human rabies transmitted by Desmodus rotundus. Virus Res. 2011;156:121–126. doi: 10.1016/j.virusres.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Hooper DC, Roy A, Barkhouse DA, Li J, Kean RB. Rabies virus clearance from the central nervous system. Adv Virus Res. 2011;79:56–66. doi: 10.1016/B978-0-12-387040-7.00004-4. [DOI] [PubMed] [Google Scholar]
- 35.Sugiura N, Uda A, Inoue S, Kojima D, Hamamoto N, Kaku Y, Okutani A, Park C, Yamada A. Gene expression analysis of host immune response in the central nervous system following lethal CVS-11 infection in mice. Jpn J Infect Dis. 2011;64:463–472. [PubMed] [Google Scholar]
- 36.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differenced in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 37.Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-α is neither necessary nor sufficient. J Immunol. 2007;178:7334–7343. doi: 10.4049/jimmunol.178.11.7334. [DOI] [PubMed] [Google Scholar]
- 38.Roy A, Phares TW, Koprowski H, Hooper DC. Failure to open the blood-brain barrier and deliver immune effectors to the CNS tissues leads to the lethal outcome of silver-haired bat rabies virus infection. J Virol. 2007;81:1110–1118. doi: 10.1128/JVI.01964-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spindler KR, Hsu TH. Viral disruption of blood-brain barrier. Trends Microbiol. 2012;20:282–290. doi: 10.1016/j.tim.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuang Y, Lackay SN, Zhao L, Fu ZF. Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection. Virus Res. 2009;144:18–26. doi: 10.1016/j.virusres.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 42.Borish LC, Steinke JW. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–S475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 43.Soares RM, Bernardi F, Sakamoto SM, Heinemann MB, Cortez A, Alves LM, Meyer AD, Ito FH, Richtzenhain LJ. A heminested polymerase chain reaction for the detection of Brazilian isolates from vampires bats and herbivores. Mem Inst Oswaldo Cruz. 2002;97:109–111. doi: 10.1590/s0074-02762002000100019. [DOI] [PubMed] [Google Scholar]
- 44.Udow SJ, Marrie RA, Jackson AC. Clinical features of dog- and bat-acquired rabies in humans. Clin Infect Dis. 2013;57:689–696. doi: 10.1093/cid/cit372. [DOI] [PubMed] [Google Scholar]
- 45.Solanki A, Radotra BD, Vasishta RK. Correlation of cytokine expression with rabies virus distribution in rabies encephalitis. J Neuroimmunol. 2009;217:85–89. doi: 10.1016/j.jneuroim.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 46.Zhao P, Yang Y, Feng H, Zhao L, Qin J, Zhang T, Wang H, Yang S, Xia X. Global gene expression changes in BV2 microglial cell line during rabies virus infection. Infect Genet Evol. 2013;20:257–269. doi: 10.1016/j.meegid.2013.09.016. [DOI] [PubMed] [Google Scholar]