Abstract
Although Escherichia coli infections are common throughout the developing world, their prevalence patterns in space and over time are not well characterized. We used serial case control data collected from 16 communities in northwestern Ecuador between 2004 and 2010, to examine the prevalence of enteroinvasive E. coli (EIEC) and enterotoxigenic E. coli (ETEC). At its peak, the regional prevalence of EIEC was 8.3 infections/100 persons but this decreased to 1 infection/1,000 persons. The regional prevalence of ETEC ranged from 8 infections/1,000 persons to 3.7 infections/100 persons. The prevalence pattern of EIEC resembled that of a large epidemic whereas the prevalence of ETEC was more stable over time. Here, we provide community-based evidence for temporal shifts in the dominant E. coli pathotype from EIEC to ETEC over a multi-year time period. Furthermore, genotype analysis suggests that a given strain of EIEC and ETEC can persist in this region for long periods, up to 24 and 55 months, respectively.
Introduction
In developing regions, diarrheagenic Escherichia coli causes up to 40% of diarrhea in children under five.1 Diarrhea is the second leading cause of mortality in this age group.1 The diarrheagenic group of E. coli is made up of a number of pathotypes. Those most common, and perhaps most virulent, in developing countries include enteroinvasive E. coli (EIEC), enterotoxigenic E. coli (ETEC), Shigella, and enteropathogenic E. coli (EPEC).2,3 Escherichia coli may be transmitted directly from person-to-person via fecal–oral contact, or indirectly, through contaminated food or water.4 These transmission modes make it likely for pathogen movement between communities that are socially or geographically connected.
Transmission of ETEC is associated with the consumption of contaminated food,5–8 and water.2 Reports of person-to-person transmission of ETEC are rare.2,5,6,9 In an experiment where volunteers were infected with ETEC, investigators found no evidence of direct transmission to their close contacts.10 In contrast, there is strong evidence that EIEC is transmitted directly from person-to-person.11 Food manipulation12,13 and imported food products14,15 may also be important vehicles for EIEC. Similarly, EPEC may be spread by person-to-person contact.2 Shigella, which has a lower infectious dose than the other pathotypes (10–100 organisms), is transmitted via each of the aforementioned routes.16 Given differences in transmission pathways, we would expect these pathotypes to move between communities differently, resulting in different prevalence patterns across a geographical region and over time. For example, in a prior analysis from this region, we showed differential spatial patterns of pathogen prevalence along a remoteness gradient (based on access to roads), suggesting higher prevalence of all pathogens in villages residing along the road. The magnitude of this spatial gradient differed across pathogens; E. coli exhibited the largest gradient followed by rotavirus and Giardia.17 At that time, we did not have sufficient power to examine prevalence patterns of individual E. coli pathotypes.
The prevalence of EIEC, ETEC, EPEC, and Shigella in single locations at single time points has been well documented in the literature, but there are few studies characterizing prevalence trends in space and time (see e.g., of references 18–23). The majority of these studies focus on one pathotype. Cross-sectional studies of multiple pathotypes suggest that ETEC is the predominant circulating pathotype.6,24–27 However, previous research from northwestern Ecuador suggests that EIEC is the dominant pathogen in this region.28 Longitudinal data would be important to confirm that these patterns hold over time. In this study, we estimate the regional- and community-specific prevalence of EIEC, ETEC, Shigella, and EPEC, with a focus on the former two pathotypes. We use data from 16 communities along three different river basins in northwestern Ecuador collected during seven sampling periods between 2004 and 2010. Using prevalence trends and genotype patterns in space and time, we aim to better understand E. coli transmission between communities in the region.
Methods
Study area.
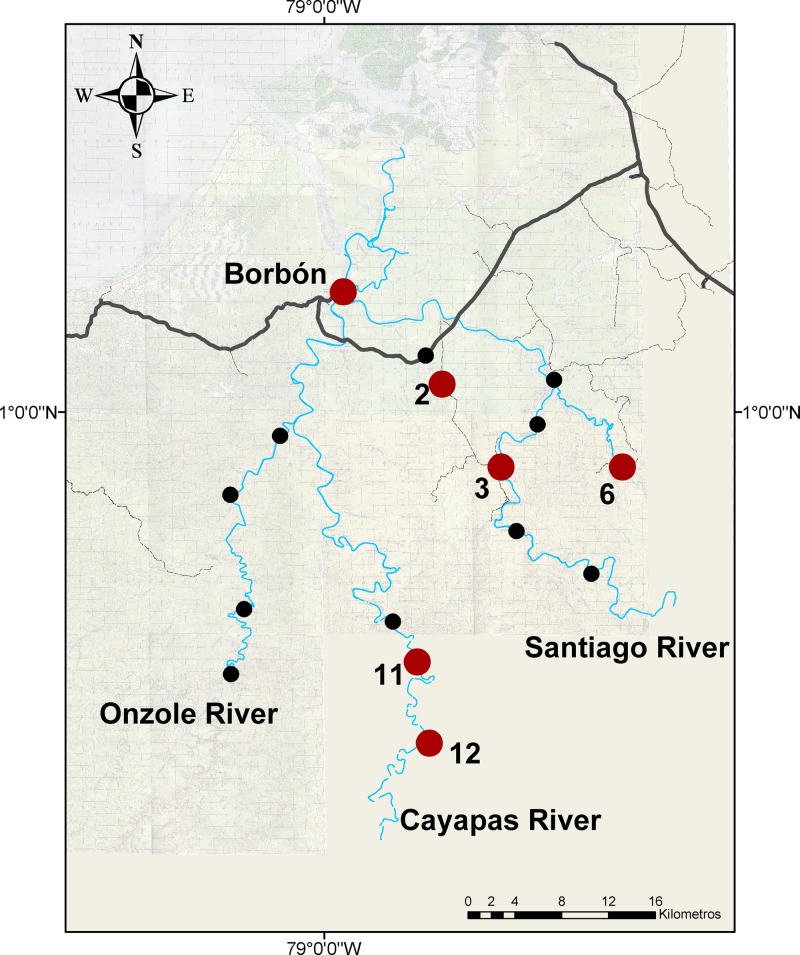
We conducted seven 15-day case control studies in 16 communities (described in Table 1) in the Canton Eloy Alfaro in the northwestern province of Esmeraldas, Ecuador between November 2004 and December 2010. Communities were selected using block randomization based on access to roads to ensure representation of the study region and to assess the role of roads as distal determinants of health within the original study.17 Two of the communities are accessed almost exclusively by road and 13 of these communities are located on one of three river systems: the Cayapas, the Onzole, and the Santiago. These river systems drain into one single river near Borbón, the 16th community in the study, which is also the main population and commercial center in the region. Borbón has had a water distribution system since 2005, supplying piped water to the majority of its households. Here, sanitation practices are mixed and range from use of flush toilets to open defecation.29 Communities upstream of Borbón tend to use the river as their primary water source and are largely reliant on unimproved sanitation facilities or practice open defecation. At the beginning of the study, all roads, rivers, and communities in the region were mapped using global positioning system. In communities other than Borbón, a census of every household was conducted before each case control study. In Borbón, the census was carried out in a random sample of 200 households (2004–2007) or 400 households (2008–2010). Oral consent was obtained from all households in our study. The University of Michigan institutional review board and Universidad San Francisco de Quito bioethics committee approved all protocols.
Table 1.
Characteristics of 16 communities in northwestern Ecuador across seven sampling periods between 2004 and 2010
| Community | Average sample population size | Average percent of the sample population younger than 5 years of age | Road/river basin |
|---|---|---|---|
| 1 | 283 | 16.3 | Road |
| 2 | 816 | 15.2 | Road |
| 3 | 517 | 14.0 | Road/Santiago |
| 4 | 131 | 14.3 | Santiago |
| 5 | 242 | 16.1 | Santiago |
| 6 | 306 | 13.1 | Santiago |
| 7 | 75 | 9.8 | Santiago |
| 8 | 145 | 10.5 | Santiago |
| 9 | 90 | 20.4 | Cayapas |
| 10 | 96 | 13.0 | Cayapas |
| 11 | 336 | 16.4 | Cayapas |
| 12 | 138 | 14.3 | Cayapas |
| 13 | 76 | 18.3 | Onzole |
| 14 | 99 | 13.3 | Onzole |
| 15 | 427 | 11.8 | Onzole |
| Borbón | 800–2,000* | 13.6 | Road/Santiago |
Between sampling periods 1 and 5, 800 persons were followed in Borbón. During sampling period 6, this population was increased to approximately 2,000.
Sample collection.
During each 15-day case–control study, we visited every house daily to identify all cases of diarrhea in the community or in the random sample from Borbón. Fecal samples were collected from both cases and controls in the community. Cases were defined as having three or more loose stools in a 24-hour period and were not subject to exclusion criteria. Controls, selected from the same household and from the community, were defined as those without diarrhea in the past 6 days. No other inclusion or exclusion criteria were applied to controls. Between 2005 and 2008, one household and two community controls were randomly sampled per case. From 2009 to 2010, 10% of all noncases in the community were randomly sampled as controls. Each community was visited approximately every 9 months between November 2004 and December 2010. We refer to this period as a sampling period in the subsequent text. Sampling periods were as follows: 1 (November 2004 to July 2005); 2 (August 2005 to March 2006); 3 (May 2006 to December 2006); 4 (January 2007 to July 2007); 5 (September 2007 to March 2008); 6 (December 2008 to November 2009); and 7 (January 2010 to December 2010).
Microbiological analysis and genotyping.
Samples were cultured on the following media: xilose lisine desoxicholate agar, Salmonella and Shigella agar, and MacConkey agar. Escherichia coli was identified by selecting lactose-fermenting colonies and testing β-glucoronidase activity using Cromocult® Coliforms Agar (Merck, Darmstadt, Germany). Lactose-negative colonies were analyzed using API® 20 E (BioMérieux, Marcy l'Etoile, France). A sample of five lactose-positive E. coli and any lactose-negative E. coli or Shigella isolates were analyzed with polymerase chain reaction for the presence of pathotype-specific virulence genes (bfp for EPEC, estA and/or eltB for ETEC, and ipaH for EIEC and Shigella).30 Isolates identified as Shigella by the API 20 E with no corresponding ipaH gene were also included in the study. Individuals with at least one positive pathogenic E.coli isolate were considered infected. Isolates were genotyped using our validated probe hybridization array typing method with 28 gene probes27,31 on the Library on a Slide platform.32 Each typing probe generated a binary outcome of presence or absence of the probed gene. Isolates that matched on all 28 probing outcomes were considered to have the same genotype as described elsewhere27,31; genotypic results using this methodology were found to be comparable with those in this study (data not shown).
Statistical analysis.
To estimate the prevalence of each pathotype, we assigned inverse probability sampling weights to all diarrhea cases and controls. We assumed that all cases were identified during the 15-day visit to a community and thus, cases were assigned a weight of one. Control weights reflected a random sampling of individuals within houses (for household controls) and individuals from communities (for community controls) during the case–control visit. Using these weights and the standard Horvitz–Thompson theory,33 we estimated a 15-day period prevalence of infection with each pathotype. In a previous study from the region diarrhea prevalence varied significantly across age group, however, significant differences were not found in the prevalence of EIEC, ETEC, or Shigella infection by age group.34 Thus, age-specific prevalence estimates of infection are not reported here.
To obtain 95% confidence intervals (CIs) for prevalence estimates, we bootstrapped our sample with replacement 1,000 times and took the 2.5th and 97.5th percentiles of the weighted prevalence distribution as our lower and upper limits, respectively. On the basis of preliminary data collected from the region in 2003, we expected infection with each E. coli pathotype to be rare in most communities, such that our confidence interval would contain the value zero. Thus, prevalence was classified as elevated when the lower prevalence limit was greater than zero. Elevated prevalence was thought to reflect widespread infection in the population. Analyses were carried out using R. v 2.11.1.
Results
Evidence for widespread EIEC infection in the region.
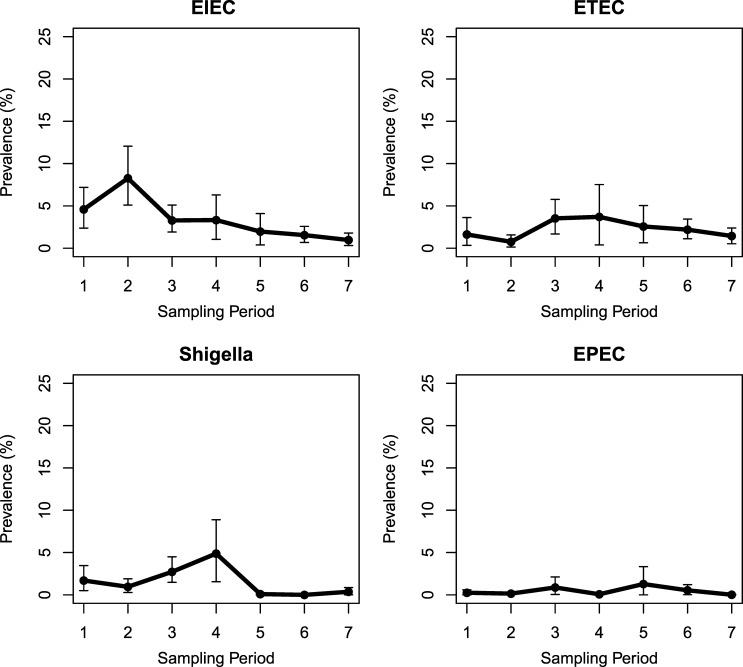
A total of 3,634 fecal samples from 769 cases and 2,865 controls were collected from 16 communities in northwestern Ecuador during seven sampling periods between November 2004 and December 2010. Of the 3,624 sampled individuals with a documented birthdate, 498 (65%) cases and 351 (12%) controls were children younger than 5 years of age. Pathogenic E. coli (including Shigella) were found in 315 (9%) samples, 136 cases (18%), and 179 controls (6%). Of these, 95 (70%) cases and 32 (18%) controls were younger than 5 years of age. The estimated regional prevalence of EIEC ranged from 0.1% to 8.3% and peaked during sampling period 2 (August 2005 to March 2006, Figure 1 ). Between sampling periods 3 and 7 (May 2006 to December 2010), ETEC was more prevalent than EIEC, though infection with either pathotype declined. The regional prevalence of ETEC was less variable over time than EIEC, ranging from 0.8% to 3.7%. Shigella prevalence varied between 0% and 4.9%, while that of EPEC was consistently low (0–1.3%). Given that the most prevalent pathogen in the region switched from EIEC to ETEC, subsequent analyses were focused on these two pathotypes.
Figure 1.
Weighted prevalence of enteroinvasive Escherichia coli (EIEC), enterotoxigenic E. coli (ETEC), Shigella, and enteropathogenic E. coli (EPEC) across seven sampling periods in 16 communities in northwestern Ecuador.
Evidence for widespread EIEC and ETEC infection in Borbón.
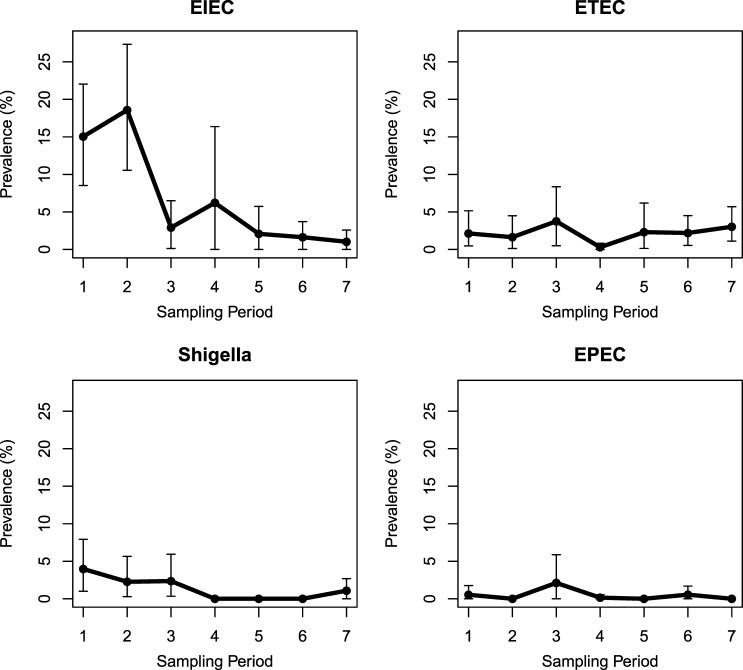
In Borbón, the temporal trend of EIEC prevalence resembled the regional pattern (Figure 2 ). Elevated prevalence of EIEC was found in Borbón during sampling periods 1 and 2 (prevalence = 15.0% and 18.6%, respectively). By our definition (a lower prevalence limit greater than 0), prevalence of EIEC was also elevated during sampling period 3, however, the lower prevalence limit just barely made our cutoff (prevalence = 2.6% (95% CI = 0.1–7.2%). ETEC infection in the population of Borbón was elevated in six of the seven sampling periods (prevalence range = 1.6–3.8%). In the communities surrounding Borbón, both EIEC and ETEC infections were less common.
Figure 2.
Weighted prevalence of enteroinvasive Escherichia coli (EIEC), enterotoxigenic E. coli (ETEC), Shigella, and enteropathogenic E. coli (EPEC) across seven sampling periods in Borbón, Ecuador.
Widespread EIEC and ETEC infection in surrounding communities.
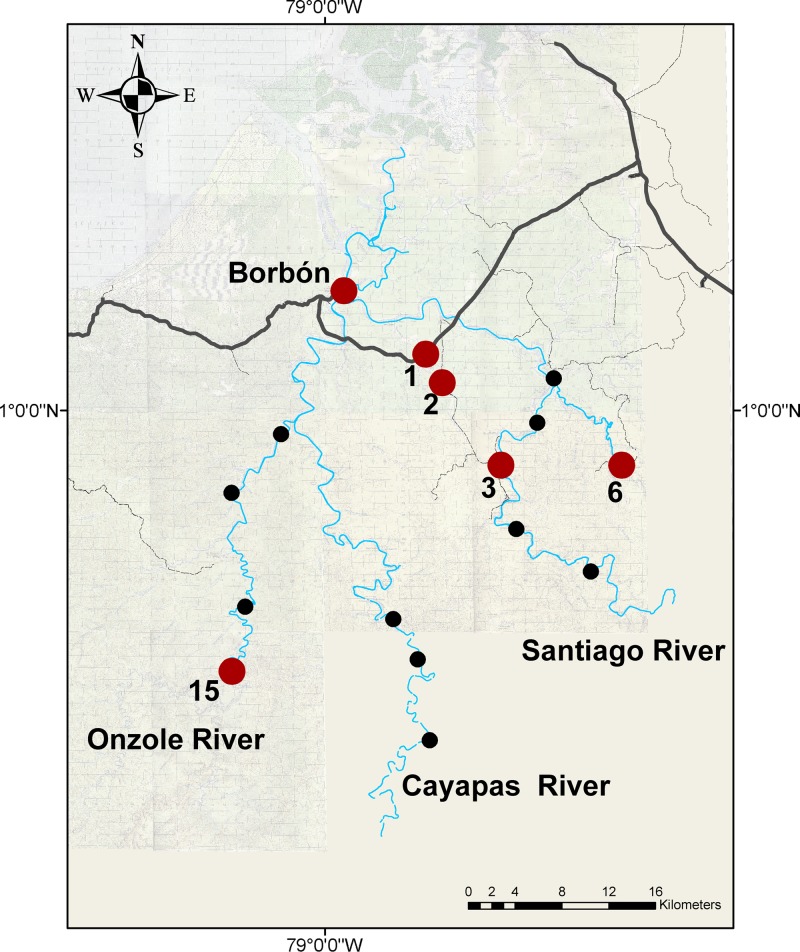
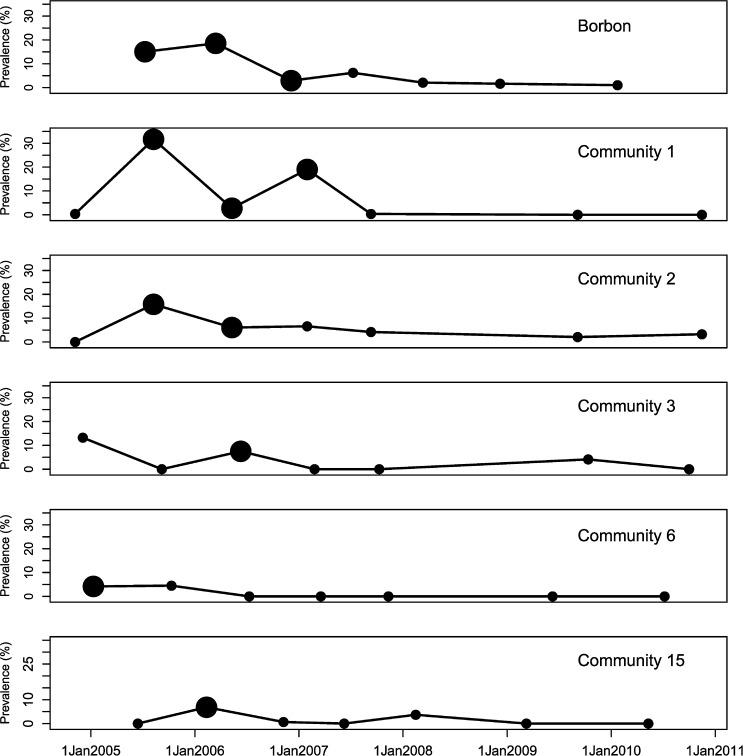
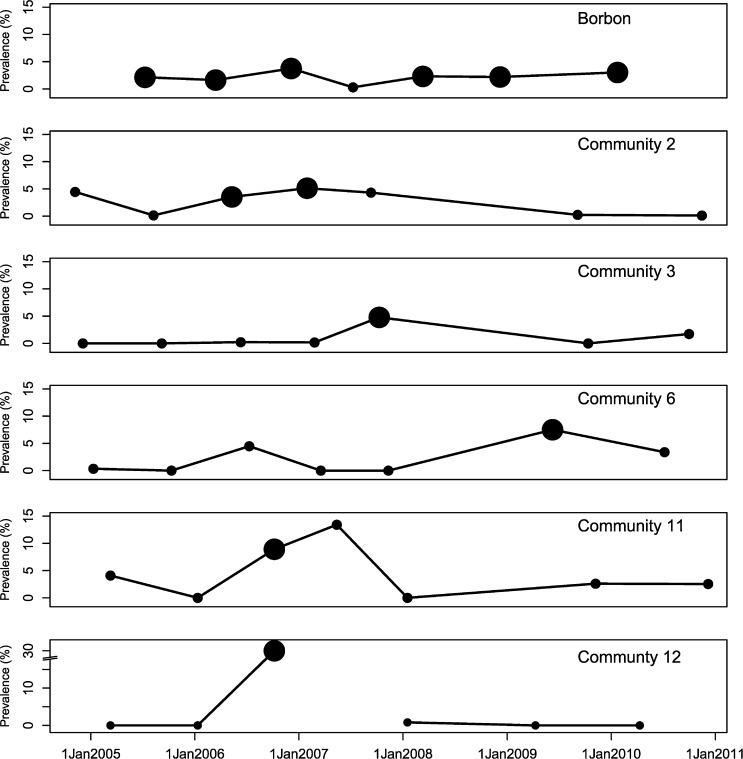
In addition to Borbón, elevated prevalence of EIEC was found in communities 1, 2, and 3 (located on the road); community 6 (Santiago river); and community 15 (Onzole river, Figure 3 ). EIEC was present in the population between sampling periods 1 and 4 (November 2004 to July 2007, Figure 4 ). Given that we observed each community for 15 days approximately every 9 months, our data are interval censored. We know the interval during which EIEC infection occurred, but not the exact date. Thus, we were not able to determine whether infection prevalence rose in Borbón before appearing in every community. The impact of interval censoring is most apparent in communities 1 and 2 where the time between finding a prevalence of zero in these communities and elevated prevalence in Borbón spans 8 months (November 2004 to July 2005). The impact is less apparent in community 15, where this period is only 3 months (April 2005 to July 2005). Interval censoring did not however, preclude us from inferring that the prevalence of EIEC infection was elevated in Borbón before community 3. Comparing community 6 with Borbón, we note that the data in Borbón are left censored such that there is uncertainty about which community experienced an elevated infection prevalence first. Elevated ETEC infection prevalence was observed in 6 communities including Borbón, located on the road, the Santiago, and Cayapas river systems (Figure 5 ) between sampling periods 1 and 7 (November 2004 to December 2010, Figure 6 ). Here, interval censoring was not an issue. We observed widespread ETEC infection in Borbón before each interval in which the same was found in communities 2, 3, 6, 11, and 12.
Figure 3.
Map of the study region in northwestern Ecuador; enteroinvasive Escherichia coli; study communities with an elevated infection prevalence are highlighted in red, all others are depicted in black.
Figure 4.
Weighted prevalence of enteroinvasive Escherichia coli in communities of northwestern Ecuador across seven sampling periods (2004–2010).
Figure 5.
Map of the study region in northwestern Ecuador; enterotoxigenic Escherichia coli; study communities with an elevated infection prevalence are highlighted in red all others are depicted in black.
Figure 6.
Weighted prevalence of enterotoxigenic Escherichia coli in communities of northwestern Ecuador across seven sampling periods (2004–2010).
EIEC and ETEC genotypes.
To further characterize community infection patterns, we identified all EIEC isolates from communities with elevated prevalence estimates (1, 2, 3, 6, and 15) between sampling periods 1 and 4. Of these 100 isolates, 57 were genotyped according to the presence or absence of 28 genes (Table 2). From 57 isolates, we identified 31 unique genotypes. Seven (23%) of these unique genotypes (G7, G11, G26, G43, G45, G49, and G50) were found in Borbón and in at least one other community with elevated infection prevalence at time points 1–18 months apart. Genotypes G7 and G54 were detected in Borbón 16 and 24 months apart, respectively, suggesting that EIEC strains can persist for as long as 2 years. Using the same approach, we characterized ETEC infection in communities with an elevated prevalence. Of the 90 isolates identified across the seven sampling periods, 48 (53%) were genotyped (Table 3). Twenty-six unique genotype patterns were found, of which, 6 (23%) were seen in both Borbón and another community. Isolates with the same genotype patterns, G11 and G24, were found in Borbón during the first and last sampling periods (approximately 55 months apart), suggesting that ETEC can also persist for years.
Table 2.
Enteroinvasive Escherichia coli genotypes found in communities of northwestern Ecuador (2004–2007)
| Sampling period | ||||
|---|---|---|---|---|
| Community | 1 November 2004 to July 2005 | 2 August 2005 to March 2006 | 3 May 2006 to December 2006 | 4 January 2007 to July 2007 |
| Borbón | G7, G11, G12, G20, G23, G24, G25, G43, G47, G49, G49, G49, G49, G49, G50, G50, G52, G54, G94, X, X, X, X, X, X, X | X, X, X, X, X, X, X, X, X, X, X, X, X, X, X, X, X, X, X | G7, G45, X, X | G26, G54 |
| 1 | G78 | G3, G19, G28, G45, G45 | G43, X, X, X | G7, X, X, X, X |
| 2 | – | G2, G15, G45, G50 | G7, G26, X, X, X | G7, G7, G30 |
| 3 | G49, X | – | G3, G10, G27, G27, G36, G39, X, X | – |
| 6 | G2, G7, G34, G79, X | X | – | – |
| 15 | – | G3, G3, G11, G58, G78 | G5 | – |
Genotypes in bold indicate identification in Borbón and in at least one other community with an elevated infection prevalence, G = genotype, X = an individual with missing genotype data.
Table 3.
Enterotoxigenic Escherichia coli genotypes found in communities of northwestern Ecuador (2004–2010)
| Sampling period | |||||||
|---|---|---|---|---|---|---|---|
| Community | 1 November 2004 to July 2005 | 2 August 2005 to March 2006 | 3 May 2006 to December 2006 | 4 January 2007 to July 2007 | 5 September 2007 to March 2008 | 6 December 2008 to November 2009 | 7 January 2010 to December 2010 |
| Borbón | G11, G20, G23, G23, G24, G25, X | X, X, X, X | X, X, X, X, X, X, X | G15, G26 | G11, G16, G24, G27 | X, X, X, X | G11, G23, G24, G24, G24, G30, G32, G34, G36, G37, X, X, X |
| 2 | G20, G24, G24 | G5 | G3, G14, X, X, X, X, X | G19, G19, G19, G21 | G18, G24, G25 | X, X | G7 |
| 3 | – | – | X | X | G2, G15, G21, G21, G22 | – | G32 |
| 6 | G4 | – | X, X | – | – | X, X, X, X | G32 |
| 11 | G22 | – | X, X, X, X | G11 | – | X | G31 |
| 12 | – | – | G17, X, X | – | X | – | – |
Genotypes in bold indicate identification in Borbón and in at least one other community with an elevated infection prevalence, G = genotype, X = an individual with missing genotype data.
Discussion
We found evidence for widespread EIEC infection across our study region in Northwestern Ecuador between November 2004 and July 2007. The source of infection may have been Borbón, the main commercial and population center of the region. Borbón geographically connects communities on three different river systems with those on the main road. Elevated prevalence of EIEC infection in Borbón preceded that in at least one of the other communities. Genotype patterns revealed indistinguishable strains circulating in Borbón and these other communities, as well as evidence of long-term persistence of EIEC strains in Borbón (on the order of years). Although limited by the number of isolates available for genotyping, our observation is likely an underestimation of the frequency of transmission in the region. During those periods in which EIEC infection was low, ETEC was the dominant pathogen in the region, and may be endemic in Borbón. Our estimates of prevalence across the region and in time may suggest that like EIEC, ETEC is transmitted from Borbón to other communities.
An earlier report from our region describes high prevalence of EIEC in 2005 and proposes that EIEC is the predominant E. coli pathotype in our region.28 The longitudinal nature of this study has allowed us to characterize this high prevalence as a regional epidemic occurring between November 2004 and July 2007. To date, there are several studies from the United States that have also described the spatial extent and duration of EIEC epidemics. Gordillo and others12 provide evidence for the movement of EIEC from Mexico into Houston 2 months before a large food-related outbreak. Harris and others11 postulate that a 12-week outbreak at a Missouri school was related to a staff member's acquisition of traveler's diarrhea in the Bahamas. Finally, Marier and others15 describe a domestic outbreak related to the consumption of imported French cheese across 14 States lasting 40 days. These studies, nevertheless, may underrepresent the magnitude and length of EIEC infection in the population, as incidence of this pathogen is difficult to measure when based only on case reports.29 Unlike these previous studies, we include individuals with symptomatic and asymptomatic carriage of EIEC, allowing us to better capture the extent of the epidemic. On the basis of this information, our data suggest that EIEC epidemics may persist for a much longer time period than has been previously observed.
The prolonged epidemic of EIEC in our study region may have been sustained by a multistrain epidemic in Borbón between November 2004 and December 2006. Previous works have shown that densely populated geographical sites can be central to transmission of other infectious diseases.35–38 Broutin and others37 demonstrate that pertussis epidemics occurring over a 15-year period began in two urban centers of Senegal before spreading to 28 surrounding villages. Wallace36 describes tuberculosis spread from Manhattan, where incidence was high in the 1980s, to districts in the Bronx and in Brooklyn. Chevallier and others38 pointed to two specific epicenters (one of which includes the current study region) of the cholera pandemic that swept through Ecuador in the 1990s.38
The introduction of EIEC infections into Borbón may be related to its connectivity to Colombia (40 km north) and to the rest of Ecuador (to the east), via a recently constructed primary road. This hypothesis is consistent with observations by Bharti and others,39 who show that regional persistence of measles and meningococcal meningitis in Niger are related to high connectivity with Nigeria and human movement via primary roads.40 The association between sexually transmitted diseases and human migration along national highways has also been shown.41–45 Persistence of EIEC infections in Borbón may be attributed to its higher population density compared with surrounding communities.
The spread of infection outward from Borbón may be the result of human movement between this urban center and surrounding communities. Borbón is the only site on the three river basins with market stalls, restaurants, hotels, and a hospital and is therefore, the most likely destination for services and provisions in the region. For anyone traveling into and out of the study region, passage through Borbón's river depot and bus station is often necessary. As expected, high prevalence of EIEC infection was observed in all road communities in our sample, these being the least remote17 from Borbón. Alternatively, one could argue that EIEC infections may arise independently in surrounding remote communities and be transmitted to Borbón via human movement or the downstream current of the river. It is theoretically possible for the pathogens to be transmitted back to the remote communities. Although less biologically plausible, future research could address this question of temporal directionality with finer temporal sampling.
In contrast to the large epidemic caused by EIEC, smaller outbreaks of ETEC were observed. These small but frequent outbreaks may be due to the repeated introduction of ETEC into Borbón by infected persons and contaminated foods or persistence in the local environment. The confluence of these small outbreaks may drive ETEC to be endemic in Borbón. ETEC appears to be endemic in other urban and developing sites of Latin America.19,24,25,46,47 Differences in epidemic behavior between EIEC and ETEC may be explained by infectious dose and environmental tolerance. EIEC has a lower endpoint on the infectious dose range than ETEC (106–1010 organisms compared with 108–1010)2 and thus, amplification of an epidemic through person-to-person transmission may be more likely. ETEC, on the other hand, may be able to survive longer in the environment.48–51 Through widespread consumption of untreated potable water, uncooked seafood products, and survival in the soil, environmental sources may provide a constant and low dose of ETEC to residents of Borbón. This hypothesis is supported by our observation of the same ETEC genotype in Borbón found 55 months apart. As with EIEC, ETEC may have spread from Borbón to surrounding communities.
We observed a relatively low prevalence of Shigella (<5%) and an even lower prevalence of EPEC (<1%) in our study region. A low prevalence of Shigella and EPEC infection is generally in agreement with three studies of children living in Latin America.3,24,52 However, we report a higher prevalence of Shigella compared with EPEC whereas two of these three studies reported findings to the contrary. Our higher rates of Shigella compared with EPEC may be due to our unique inclusion of adults in the study population. Previous research from our study region suggests that adults are more often infected with Shigella compared with EPEC.34 Furthermore, when our analysis was limited to children younger than 5 years of age, prevalence of Shigella was lower than that of EPEC (data not shown). It is also possible that a small and short-lived outbreak of Shigella took place during our study period causing a peak in prevalence over time.
Weighted prevalence estimates presented here were based on a series of 15-day case–control studies in each community carried out approximately every 9 months. Although short sampling windows may inhibit disentanglement of temporal and spatial variability, previous research in the region has shown greater variability of risk factors for all-cause diarrhea across space rather than time.53 In addition, while the sampling method used may not have captured small fluctuations in prevalence estimates due to factors such as seasonality, our spatial and temporal resolution did allow us to identify and characterize a regional epidemic of EIEC, which we hypothesize began in Borbón before spreading to other communities in the region. We also found that ETEC is the predominant pathogen in the region during nonepidemic periods. Although we did not capture the proportion of isolates positive for E. coli pathotypes throughout the entire study period, a separate analysis restricted to the last year of the study showed a high proportion (66%) of infected individuals with more than one positive isolate. Given that isolates were selected at random, they likely represent the most prominent colonies within the individual and therefore, finding at least one positive isolate suggests a high pathogen load. Although our analysis did not include other E. coli pathotypes such as enterohemorrhagic E. coli (EHEC), atypical EPEC, and EAEC, unpublished genotype data from later sampling periods suggest that EHEC is not circulating in our study region, and though atypical EPEC and EAEC are circulating, they may be less pathogenic than the pathotypes considered here (L. Zhang, unpublished data). Other researchers have found year-to-year variation in the prevalence of one particular E. coli pathotype,18,20–23 or have focused on various pathotypes isolated from diarrhea cases.22 Here, we provide community-based evidence for temporal shifts in the dominant E. coli pathotype over a multi-year time period. Furthermore, we hypothesize differential movement of these pathotypes between a commercial center and its satellite villages.
ACKNOWLEDGMENTS
We would like to thank Karina Ponce and Patricio Rojas for isolating E. coli strains in this study as well as the Ecologica, Desarrollo, Salud y Sociedad (EcoDESS) project field team, administered out of the Universidad San Francisco de Quito, for their invaluable contributions to specimen and data collection. We would also like to acknowledge Sharon Boylan for her help with data management.
Footnotes
Financial support: This work was supported by the National Institutes of Health [R01-AI050038] and the University of Michigan Interdisciplinary Training Program in Infectious Diseases, funded by National Institute of Allergy and Infectious Diseases [T32AI 049816].
Authors' addresses: Darlene Bhavnani, Clinton Health Access Initiative, Panama City, Panama, E-mail: dbhavnani@clintonhealthaccess.org. Rosa de los Ángeles Bayas and Gabriel Trueba, Microbiology Institute, Universidad San Francisco de Quito, Av. Interoceanica y Pampite S/N. Circulo de Cumbaya, Ecuador, E-mails: rose_skywalker@hotmail.com and gtrueba@usfqedu.ec. Velma K. Lopez, Betsy Foxman, Carl Marrs, and Joseph N. S. Eisenberg, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, MI, E-mails: lopezvel@umich.edu, bfoxman@umich.edu, cfmarrs@umich.edu, and jnse@umich.edu. Lixin Zhang, Department of Epidemiology and Biostatistics. Michigan State University, East Lansing, MI, E-mail: lzhang@umich.edu. William Cevallos, Universidad Central del Ecuador, Sodiro N14-121 e Iquique, Quito, Ecuador, E-mail: wcevallos@uce.edu.ec.
References
- 1.O'Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005;16:125–136. doi: 10.1053/j.spid.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Hunter PR. Drinking water and diarrhoeal disease due to Escherichia coli. J Water Health. 2003;1:65–72. [PubMed] [Google Scholar]
- 3.Ochoa TJ, Ecker L, Barletta F, Mispireta ML, Gil AI, Contreras C, Molina M, Amemiya I, Verastegui H, Hall ER, Cleary TG, Lanata CF. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from Periurban areas in Lima, Peru. Nephrol Dial Transplant. 2009;49:1694–1702. doi: 10.1086/648069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis V, Cairncross S, Yonli R. Domestic hygiene and diarrhoea - pinpointing the problem. Trop Med Int Health. 2000;5:22–32. doi: 10.1046/j.1365-3156.2000.00512.x. [DOI] [PubMed] [Google Scholar]
- 5.Dalton CB, Mintz ED, Wells JG, Bopp CA, Tauxe RV. Outbreaks of enterotoxigenic Escherichia coli infection in American adults: a clinical and epidemiologic profile. Epidemiol Infect. 1999;123:9–16. doi: 10.1017/s0950268899002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain S, Chen L, Dechet A, Hertz AT, Brus DL, Hanley K, Wilson B, Frank J, Greene KD, Parsons M, Bopp CA, Todd R, Hoekstra M, Mintz ED, Ram PK. An outbreak of enterotoxigenic Escherichia coli associated with sushi restaurants in Nevada, 2004. Nephrol Dial Transplant. 2008;47:1–7. doi: 10.1086/588666. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald KL, Eidson M, Strohmeyer C, Levy ME, Wells JG, Puhr ND, Wachsmuth K, Hargrett NT, Cohen ML. A multistate outbreak of gastrointestinal illness caused by enterotoxigenic Escherichia coli in imported semisoft cheese. J Infect Dis. 1985;151:716–720. doi: 10.1093/infdis/151.4.716. [DOI] [PubMed] [Google Scholar]
- 9.Gupta SK, Keck J, Ram PK, Crump JA, Miller MA, Mintz ED. Part III. Analysis of data gaps pertaining to enterotoxigenic Escherichia coli infections in low and medium human development index countries, 1984–2005. Epidemiol Infect. 2008;136:721–738. doi: 10.1017/S095026880700934X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine MM, Rennels MB, Cisneros L, Hughes TP, Nalin DR, Young CR. Lack of person-to-person transmission of enterotoxigenic Escherichia coli despite close contact. Am J Epidemiol. 1980;111:347–355. doi: 10.1093/oxfordjournals.aje.a112906. [DOI] [PubMed] [Google Scholar]
- 11.Harris JR, Mariano J, Wells JG, Payne BJ, Donnell HD, Cohen ML. Person-to-person transmission in an outbreak of enteroinvasive Escherichia coli. Am J Epidemiol. 1985;122:245–252. doi: 10.1093/oxfordjournals.aje.a114095. [DOI] [PubMed] [Google Scholar]
- 12.Gordillo ME, Reeve GR, Pappas J, Mathewson JJ, DuPont HL, Murray BE. Molecular characterization of strains of enteroinvasive Escherichia coli O143, including isolates from a large outbreak in Houston, Texas. J Clin Microbiol. 1992;30:889–893. doi: 10.1128/jcm.30.4.889-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder JD, Wells JG, Yashuk J, Puhr N, Blake PA. Outbreak of invasive Escherichia coli gastroenteritis on a cruise ship. Am J Trop Med Hyg. 1984;33:281–284. doi: 10.4269/ajtmh.1984.33.281. [DOI] [PubMed] [Google Scholar]
- 14.Tulloch EF, Jr, Ryan KJ, Formal SB, Franklin FA. Invasive enteropathic Escherichia coli dysentery. An outbreak in 28 adults. Ann Intern Med. 1973;79:13–17. doi: 10.7326/0003-4819-79-1-13. [DOI] [PubMed] [Google Scholar]
- 15.Marier R, Wells JG, Swanson RC, Callahan W, Mehlman IJ. An outbreak of enteropathogenic Escherichia coli foodborne disease traced to imported French cheese. Lancet. 1973;302:1376–1378. [Google Scholar]
- 16.Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis: challenges and management issues. Indian J Med Res. 2004;120:454–462. [PubMed] [Google Scholar]
- 17.Eisenberg JN, Cevallos W, Ponce K, Levy K, Bates SJ, Scott JC, Hubbard A, Vieira N, Endara P, Espinel M, Trueba G, Riley LW, Trostle J. Environmental change and infectious disease: how new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proc Natl Acad Sci USA. 2006;103:19460–19465. doi: 10.1073/pnas.0609431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaheen HI, Khalil SB, Rao MR, Abu Elyazeed R, Wierzba TF, Peruski LF, Jr, Putnam S, Navarro A, Morsy BZ, Cravioto A, Clemens JD, Svennerholm AM, Savarino SJ. Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J Clin Microbiol. 2004;42:5588–5595. doi: 10.1128/JCM.42.12.5588-5595.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Duarte OG, Arzuza O, Urbina D, Bai J, Guerra J, Montes O, Puello M, Mendoza K, Castro GY. Detection of Escherichia coli enteropathogens by multiplex polymerase chain reaction from children's diarrheal stools in two Caribbean-Colombian cities. Foodborne Pathog Dis. 2010;7:199–206. doi: 10.1089/fpd.2009.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banga Singh KK, Ojha SC, Deris ZZ, Rahman RA. A 9-year study of shigellosis in northeast Malaysia: antimicrobial susceptibility and shifting species dominance. Z Gesundh Wiss. 2011;19:231–236. doi: 10.1007/s10389-010-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qadri F, Das SK, Faruque AS, Fuchs GJ, Albert MJ, Sack RB, Svennerholm AM. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol. 2000;38:27–31. doi: 10.1128/jcm.38.1.27-31.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WS, Puthucheary SD. Bacterial enteropathogens isolated in childhood diarrhoea in Kuala Lumpur–the changing trend. Med J Malaysia. 2002;57:24–30. [PubMed] [Google Scholar]
- 23.Vinh H, Nhu NT, Nga TV, Duy PT, Campbell JI, Hoang NV, Boni MF, My PV, Parry C, Nga TT, Van Minh P, Thuy CT, Diep TS, Phuong le T, Chinh MT, Loan HT, Tham NT, Lanh MN, Mong BL, Anh VT, Bay PV, Chau NV, Farrar J, Baker S. A changing picture of shigellosis in southern Vietnam: shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC Infect Dis. 2009;9:204. doi: 10.1186/1471-2334-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paniagua GL, Monroy E, Garcia-Gonzalez O, Alonso J, Negrete E, Vaca S. Two or more enteropathogens are associated with diarrhoea in Mexican children. Ann Clin Microbiol Antimicrob. 2007;6:17. doi: 10.1186/1476-0711-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilchez S, Reyes D, Paniagua M, Bucardo F, Mollby R, Weintraub A. Prevalence of diarrhoeagenic Escherichia coli in children from Leon, Nicaragua. J Med Microbiol. 2009;58:630–637. doi: 10.1099/jmm.0.007369-0. [DOI] [PubMed] [Google Scholar]
- 26.Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37:3458–3464. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan U, Zhang L, France AM, Ghosh D, Shalaby W, Xie J, Marrs CF, Foxman B. Probe hybridization array typing: a binary typing method for Escherichia coli. J Clin Microbiol. 2007;45:206–214. doi: 10.1128/JCM.01543-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vieira N, Bates SJ, Solberg OD, Ponce K, Howsmon R, Cevallos W, Trueba G, Riley L, Eisenberg JN. High prevalence of enteroinvasive Escherichia coli isolated in a remote region of northern coastal Ecuador. Am J Trop Med Hyg. 2007;76:528–533. [PMC free article] [PubMed] [Google Scholar]
- 29.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tornieporth NG, John J, Salgado K, de Jesus P, Latham E, Melo MC, Gunzburg ST, Riley LW. Differentiation of pathogenic Escherichia coli strains in Brazilian children by PCR. J Clin Microbiol. 1995;33:1371–1374. doi: 10.1128/jcm.33.5.1371-1374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNamara SE, Srinivasan U, Zhang L, Whittam TS, Marrs CF, Foxman B. Comparison of probe hybridization array typing to multilocus sequence typing for pathogenic Escherichia coli. J Clin Microbiol. 2009;47:596–602. doi: 10.1128/JCM.01693-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Srinivasan U, Marrs CF, Ghosh D, Gilsdorf JR, Foxman B. Library on a slide for bacterial comparative genomics. BMC Microbiol. 2004;4:12. doi: 10.1186/1471-2180-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horvitz DG, Thompson DJ. A generalization of sampling without replacement from a finite universe. J Am Stat Assoc. 1952;47:663–685. [Google Scholar]
- 34.Bhavnani D, Goldstick JE, Cevallos W, Trueba G, Eisenberg JN. Synergistic effects between rotavirus and coinfecting pathogens on diarrheal disease: evidence from a community-based study in northwestern Ecuador. Am J Epidemiol. 2012;176:387–395. doi: 10.1093/aje/kws220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klovdahl AS, Graviss EA, Yaganehdoost A, Ross MW, Wanger A, Adams GJ, Musser JM. Networks and tuberculosis: an undetected community outbreak involving public places. Soc Sci Med. 2001;52:681–694. doi: 10.1016/s0277-9536(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 36.Wallace D. The resurgence of tuberculosis in New York City: a mixed hierarchically and spatially diffused epidemic. Am J Public Health. 1994;84:1000–1002. doi: 10.2105/ajph.84.6.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broutin H, Elguero E, Simondon F, Guegan JF. Spatial dynamics of pertussis in a small region of Senegal. Proc Biol Sci. 2004;271:2091–2098. doi: 10.1098/rspb.2004.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chevallier E, Grand A, Azais JM. Spatial and temporal distribution of cholera in Ecuador between 1991 and 1996. Eur J Public Health. 2004;14:274–279. doi: 10.1093/eurpub/14.3.274. [DOI] [PubMed] [Google Scholar]
- 39.Bharti N, Djibo A, Ferrari MJ, Grais RF, Tatem AJ, McCabe CA, Bjornstad ON, Grenfell BT. Measles hotspots and epidemiological connectivity. Epidemiol Infect. 2010;138:1308–1316. doi: 10.1017/S0950268809991385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bharti N, Broutin H, Grais RF, Ferrari MJ, Djibo A, Tatem AJ, Grenfell BT. Spatial dynamics of meningococcal meningitis in Niger: observed patterns in comparison with measles. Epidemiol Infect. 2011;140:1–10. doi: 10.1017/S0950268811002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Z, Wang L, Chen RY, Li D, Qin Q, Ding Z, Ding G, Zang C, Wang N. Tracking the evolution of HIV/AIDS in China from 1989–2009 to inform future prevention and contro efforts. PLoS One. 2011;6:e25671. doi: 10.1371/journal.pone.0025671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nepal B. Population mobility and spread of HIV across the Indo-Nepal border. J Health Popul Nutr. 2007;25:267–277. [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson AG, Morris CN. Mapping transactional sex on the Northern Corridor highway in Kenya. Health Place. 2007;13:504–519. doi: 10.1016/j.healthplace.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Pandey A, Benara SK, Roy N, Sahu D, Thomas M, Joshi DK, Sengupta U, Paranjape RS, Bhalla A, Prakash A. Risk behaviour, sexually transmitted infections and HIV among long-distance truck drivers: a cross-sectional survey along national highways in India. AIDS. 2008;22(Suppl 5):S81–S90. doi: 10.1097/01.aids.0000343766.00573.15. [DOI] [PubMed] [Google Scholar]
- 45.Cook RL, Royce RA, Thomas JC, Hanusa BH. What's driving an epidemic? The spread of syphilis along an interstate highway in rural North Carolina. Am J Public Health. 1999;89:369–373. doi: 10.2105/ajph.89.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Estrada-Garcia T, Lopez-Saucedo C, Thompson-Bonilla R, Abonce M, Lopez-Hernandez D, Santos JI, Rosado JL, DuPont HL, Long KZ. Association of diarrheagenic Escherichia coli pathotypes with infection and diarrhea among Mexican children and association of atypical enteropathogenic E. coli with acute diarrhea. J Clin Microbiol. 2009;47:93–98. doi: 10.1128/JCM.01166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regua-Mangia AH, Gomes TA, Vieira MA, Andrade JR, Irino K, Teixeira LM. Frequency and characteristics of diarrhoeagenic Escherichia coli strains isolated from children with and without diarrhoea in Rio de Janeiro, Brazil. J Infect. 2004;48:161–167. doi: 10.1016/s0163-4453(03)00138-5. [DOI] [PubMed] [Google Scholar]
- 48.Ohno A, Marui A, Castro ES, Reyes AA, Elio-Calvo D, Kasitani H, Ishii Y, Yamaguchi K. Enteropathogenic bacteria in the La Paz River of Bolivia. Am J Trop Med Hyg. 1997;57:438–444. doi: 10.4269/ajtmh.1997.57.438. [DOI] [PubMed] [Google Scholar]
- 49.Begum YA, Talukder KA, Nair GB, Qadri F, Sack RB, Svennerholm AM. Enterotoxigenic Escherichia coli isolated from surface water in urban and rural areas of Bangladesh. J Clin Microbiol. 2005;43:3582–3583. doi: 10.1128/JCM.43.7.3582-3583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frank JF, Marth EH, Olson NF. Survival of enteropathogenic and non-pathogenic Escherichia coli during the manufacture of Camembert cheese. J Food Prot. 1977;40:835–842. doi: 10.4315/0362-028X-40.12.835. [DOI] [PubMed] [Google Scholar]
- 51.Kornacki JL, Marth EH. Fate of nonpathogenic and enteropathogenic Escherichia coli during the manufacture of colby-like cheese. J Food Prot. 1982;45:310–316. doi: 10.4315/0362-028X-45.4.310. [DOI] [PubMed] [Google Scholar]
- 52.Orlandi PP, Silva T, Magalhaes GF, Alves F, de Almeida Cunha RP, Durlacher R, da Silva LH. Enteropathogens associated with diarrheal disease in infants of poor urban areas of Porto Velho, Rondonia: a preliminary study. Mem Inst Oswaldo Cruz. 2001;96:621–625. doi: 10.1590/s0074-02762001000500005. [DOI] [PubMed] [Google Scholar]
- 53.Markovitz AR, Goldstick JE, Levy K, Cevallos W, Mukherjee B, Trostle JA, Eisenberg JN. Where science meets policy: comparing longitudinal and cross-sectional designs to address diarrhoeal disease burden in the developing world. Int J Epidemiol. 2012;41:504–513. doi: 10.1093/ije/dyr194. [DOI] [PMC free article] [PubMed] [Google Scholar]