The non-rebreather mask is an oxygen delivery system with many applications and has been a valuable tool in specific patient care scenarios. Under certain low-flow conditions, however, a lack of washout of exhaled gases and rebreathing of carbon dioxide may occur – a situation scarcely addressed in the literature. This bench study compared two commercially available products in an attempt to shed more light on this important issue.
Keywords: Delivery, Hypercarbia, Hypoxemia, Hypoxia, Non-rebreather mask, Oxygen, OxyMask, Respiratory failure
Abstract
BACKGROUND:
The non-rebreather mask (NRBM) is used for many applications and in many patient care scenarios in which hypoxemia and resultant hypoxia are a concern. The NRBM is a low-flow oxygen delivery system that is easily deployed and capable of delivering a relatively high fraction of inspired oxygen (FiO2).The potential for ineffective carbon dioxide (CO2) removal at low flow rates is a safety concern.
OBJECTIVE:
The authors hypothesized that the use of an OxyMask (Southmedic Inc, Canada) would mitigate these safety concerns while still delivering a relatively high FiO2.
METHODS:
Bench studies were performed in a third-party laboratory by qualified engineers (Piper Medical, USA). A Harvard Respirator Pump (Harvard Apparatus, USA), oxygen source, CO2 source and a mannequin head were used to simulate varying respiratory conditions. End tidal CO2 (EtCO2), FiO2, fraction of inspired CO2 and percent drop in CO2 in the first second of exhalation were measured at different mask flow rates and respiratory rates. There were two categories of flow rates: high-flow (15 L/min) and low-flow (2 L/min). In each flow group, the above parameters were measured using a tidal volume of 400 mL, inspiratory/expiratory ratio of 1:2, EtCO2 of 5% and a breathing frequency of 15, 20 or 24 breaths/min. Mask performance measurements were obtained and compared.
CONCLUSION:
The OxyMask outperformed the traditional NRBM in each tested category. There was a higher inspired oxygen level, lower inspired CO2 level, and more efficient CO2 clearance at each mask flow level and simulated patient minute volume. This was especially true during conditions in which there were very low mask flow rates.
Abstract
HISTORIQUE :
Le masque sans réinspiration (MSRI) a de nombreuses applications et sert à de nombreux scénarios de soins aux patients chez qui l’hypoxémie et l’hypoxie qui en découle posent problème. Le MSRI est un système de distribution d’oxygène à faible débit qui est facile à installer et peut insuffler une fraction inspirée d’oxygène (FiO2) relativement élevée. Le potentiel d’élimination inefficace du dioxyde de carbone (CO2) à faible débit représente un problème d’innocuité.
OBJECTIF :
Les auteurs ont postulé que l’utilisation d’un OxyMask (SouthMedic Inc, Canada) réduirait ces problèmes d’innocuité tout en insufflant une FiO2 relativement élevée.
MÉTHODOLOGIE :
Des ingénieurs diplômés ont effectué des bancs d’essai dans le laboratoire d’un tiers (Piper Medical, États-Unis). Ils ont utilisé une pompe respiratoire Harvard (Harvard Apparatus, États-Unis), une source d’oxygène, une source de CO2 et une tête de mannequin pour simuler diverses conditions respiratoires. Ils ont mesuré le CO2 de fin d’expiration (EtCO2), la FiO2, la fraction inspirée de CO2 et la chute en pourcentage du CO2 pendant la première seconde d’exhalation à divers débits au masque et diverses fréquences respiratoires. Il y avait deux catégories de débit : élevée (15 L/min) et faible (2 L/min). Dans chacun des groupes de débit, les ingénieurs ont mesuré les paramètres précédents au moyen d’un volume courant de 400 mL, d’un ratio entre l’inspiration et l’expiration de 1:2, d’un EtCO2 de 5 % et d’une fréquence respiratoire de 15, 20 ou 24 respirations à la minute. Ils ont obtenu les mesures de rendement des masques et les ont comparées.
CONCLUSION :
L’OxyMask était supérieur au MSRI habituel dans chaque catégorie évaluée. Le taux d’oxygène inspiré était plus élevé, le taux de CO2 inspiré, plus faible, et la clairance de CO2, plus efficace à chaque niveau de débit au masque et chaque ventilation minute simulée des patients, particulièrement lorsque le débit du masque était très faible.
The patient safety profile of a non-rebreather mask (NRBM) has been a matter of concern for some time; however, there is very little reference to these performance characteristics in the literature (1–3). Low-flow characteristics and a potential lack of effective washout of exhaled gases can lead to rebreathing of carbon dioxide (CO2) in certain conditions (1–3). This concern has previously led to aftermarket modifications to the NRBM by way of removing one of the one-way valves that are located on either side of the mask. This modification is intended to reduce or attenuate the rebreathing of exhaled gasses and potential for hypercarbic respiratory failure and lower fraction of inspired oxygen (FiO2) leading to hypoxemia. These conditions may exist when the mask flow is set inadvertently low, is accidently disconnected from its fresh gas source or the very small exhalation port is obstructed (2,4). We hypothesized that the open design of the OxyMaskTM (Southmedic Inc, Canada) would mitigate these concerns by allowing for less CO2 rebreathing while delivering inspired oxygen levels that compare favourably with the Hudson RCI® NRBMTM (Teleflex Inc, USA) (5–7).
METHODS
The CO2 source was attached to the inhalation limb of the Harvard Pump (Harvard Apparatus, USA) on the piston side of the inhalation check valve. A 0.125 inch OD sensing oxygen line was attached to the head and the sensing end was positioned 1 inch into the 0.875 inch ID simulated oral cavity of the mannequin. Gas sampling was achieved through the line to the oxygen and CO2 sensor (10 mL/min each) using a vacuum source. End tidal CO2 (EtCO2) values were set without the mask in place so as to simulate normal expected breathing. The CO2 flow was set to the desired settings (Table 1). Once CO2 flow had been set to the desired EtCO2 value, the mask was adjusted to the desired oxygen flow rate (2 L/min and 15 L/min, respectively) and placed on the mannequin head as designed. The system was allowed to equilibrate for at least 3 min before obtaining each reading. Each sample was tested three times. The mask was removed from the mannequin head completely and repositioned between each test. Each mask was tested at three different respiratory rates and minute volumes: 15 breaths/min, 20 breaths/min and 24 breaths/min. Each of these conditions were simulated at both 2 L/min of oxygen flow or 15 L/min of oxygen flow. There were a total of 36 tests (2 samples × 3 tests per sample × 2 oxygen flow settings × 3 respiratory settings = 36 tests total). After allowing each setting 3 min to equilibrate a full inhalation and exhalation CO2 waveform, EtCO2 and FiO2 measurements were captured. Performance of each device was evaluated and qualified in terms of each mask’s ability to clear CO2 from the mask during exhalation. All equipment and laboratory processes met their specifications and requirements before and after testing. CO2 measurements were calibrated before testing at 0% and 5%. Oxygen measurements were calibrated at 21% and 100% before testing. After testing, calibration curves were verified. The equipment list and patient simulation set-up are listed and described in Appendix 1, and Figures 1 and 2, respectively.
TABLE 1.
Three respiratory settings used for testing
| Parameter | Setting | ||
|---|---|---|---|
|
| |||
| 1 | 2 | 3 | |
| Respiratory rate, breaths/min | 15 | 20 | 24 |
| Tidal volume, mL | 400 | 400 | 400 |
| Inspiratory:expiratory ratio | 1:2 | 1:2 | 1:2 |
| End tidal carbon dioxide, % | 5 | 5 | 5 |
Figure 1).
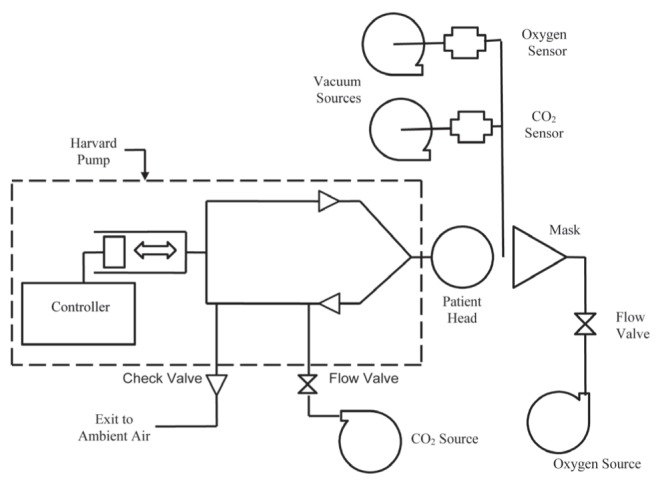
The patient simulation setup used for testing. CO2 Carbon dioxide
Figure 2).
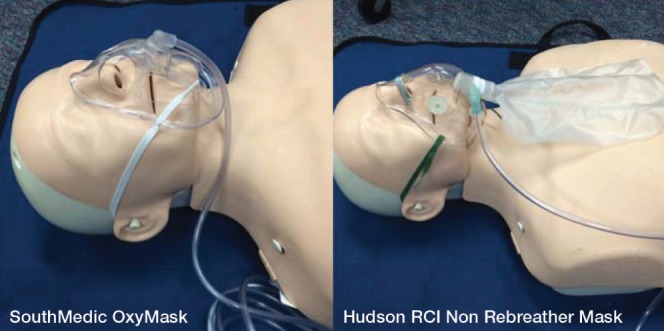
Left panel OxyMaskTM (Southmedic Inc, Canada). Right panel Hudson RCI® Non-Rebreather MaskTM (Teleflex Inc, USA)
Statistical analysis
All analyses were performed using SPSS version 20.0 (IBM Corporation, USA) for Windows (Microsoft Corporation, USA). Descriptive statistics were examined and reported for continuous data as means and SD. Differences between means were assessed using multivariate ANOVA to determine the effect of the three respiratory rates (15, 20 and 24 breaths/min) and two oxygen flows (2 L/min and 15 L/min) on four dependent variables. Significant differences among the covariates were assessed using Wilks’ λ. All statistical tests were two-tailed and based on a 0.05 significance level.
RESULTS
Table 2 summarizes combined mean data collected while testing both masks with oxygen flow rates of 2 L/min and 15 L/min and respiratory rates of 15, 20 and 24 breaths/min. Table 3 summarizes mean data for each individual test. The OxyMask delivered more or an equivalent amount of oxygen compared with the NRBM at the same conditions. The OxyMask resulted in lower or equivalent EtCO2 levels compared with the NRBM at the same conditions. CO2 levels dropped faster during exhalation with the OxyMask than with the NRBM. Performance of the two products tended to be farther apart at lower flow rates of oxygen. Significant differences among the covariates were noted (F=14.56; P<0.001; λ=0.332). When controlling for device flow and respiratory rate, there was a statistically significant effect on EtCO2 (F=29.37; P<0.001), O2 (F=24.17;P<0.001), inhaled O2 (F=54.60; P<0.001) and percent drop in CO2 (F=41.72; P<0.001).
TABLE 2.
Data collected testing both masks
| Parameter | Southmedic OxyMaskTM | Hudson RCI® NRB MaskTM | F | P |
|---|---|---|---|---|
| End tidal carbon dioxide | 5.2±0.35 | 5.8±0.61 | 29.37 | <0.001 |
| Oxygen | 61.8±16.17 | 57.0±19.03 | 24.17 | <0.001 |
| Inhaled carbon dioxide | 2.2±.52 | 3.0±1.13 | 54.60 | <0.001 |
| % drop | 83.1±6.85 | 63.8±13.52 | 41.72 | <0.001 |
Data presented as mean ± SD unless otherwise indicated
TABLE 3.
| Device | Flow rate, L/min | Respiratory rate, breaths/min | End tidal carbon dioxide | Oxygen | Inhaled carbon dioxide | Drop in carbon dioxide in first second of exhalation |
|---|---|---|---|---|---|---|
| OxyMaskTM* | 2 | 15 | 5.2±0.1 | 52.1±1.6 | 2.1±0.1 | 74.0±6.4 |
| Hudson RCI NRBM† | 2 | 15 | 5.8±0.1 | 44.1±0.6 | 3.3±0.1 | 41.3±5.6 |
| OxyMaskTM | 2 | 20 | 5.4±0.1 | 50.5±1.1 | 2.7±0.1 | 84.8±7.5 |
| Hudson RCI NRBM | 2 | 20 | 5.9±0.2 | 39.7±2.0 | 3.6±0.1 | 59.8±7.8 |
| OxyMaskTM | 2 | 24 | 5.8±0.1 | 39.1±1.1 | 2.9±0.1 | 92.5±3.3 |
| Hudson RCI NRBM | 2 | 24 | 6.6±0.1 | 34.0±1.8 | 4.9±0.1 | 59.0±3.8 |
| OxyMaskTM | 15 | 15 | 4.9±0.0 | 84.3±1.8 | 1.5±0.1 | 80.0±2.2 |
| Hudson RCI NRBM | 15 | 15 | 4.8±0.0 | 82.2±3.9 | 1.4±0.1 | 73.5±2.8 |
| OxyMaskTM | 15 | 20 | 4.9±0.1 | 73.2±1.6 | 1.8±0.0 | 84.4±2.3 |
| Hudson RCI NRBM | 15 | 20 | 5.1±0.2 | 73.6±0.8 | 2.2±0.2 | 80.6±3.3 |
| OxyMaskTM | 15 | 24 | 4.9±0.1 | 71.9±0.9 | 2.1±0.1 | 82.7±1.8 |
| Hudson RCI NRBM | 15 | 24 | 5.4±0.0 | 68.6±1.0 | 2.8±0.0 | 68.8±1.2 |
Data presented as mean ± SD % unless otherwise indicated.
Southmedic Inc, Canada;
Teleflex Inc, USA. NRBM Non-rebreather mask
DISCUSSION
Patient safety is paramount. It has been historically hypothesized that the use of an NRBM may be unsafe when certain elements exist that create conditions favourable for rebreathing CO2 (7–10). The literature supporting this notion is virtually nonexistent. Our bench report comparing the Southmedic OxyMaskTM and the Hudson RCI® NRBMTM has taken a step toward answering this question. First, we chose parameters that were believed to be appropriate surrogates of common patient conditions. Inhaled and exhaled oxygen levels, as well as CO2 levels, were measured. Subsequently, varying patient and equipment conditions were introduced by way of changing respiratory rates and oxygen flow rates. Higher oxygen flow rates (15 L/min) were chosen to simulate the standard practice with both masks. Lower oxygen flow rates (2 L/min) were used to simulate an inadvertent decrease from the standard. Increasing respiratory rates were tested to simulate a change in patient condition and minute volume. Our experiments demonstrated, that when the NRBM and OxyMask are used as per the standard (higher flows), they are safe oxygen delivery masks and deliver a relatively high and stable level of inspired oxygen. Additionally, CO2 appears to be adequately cleared under these conditions. Alternatively, when tested at lower flow rates, the OxyMask appears to outperform the NRBM in terms of CO2 clearance and at delivering inspired oxygen.
There were limitations to the present study. Although the measurements obtained during these experiments show a statistical significance almost across the board in favour of the OxyMask at lower flow rates, the sample numbers are low and further evaluation may be helpful to suggest a change in safe practice. We believe that our data suggests that the Southmedic OxyMask may be a safer alternative to the Hudson RCI NRBM in which conditions exist that make inadvertent low oxygen delivery flows more likely to occur.
Acknowledgments
The authors thank Sarah Spilman MA, Data Analyst, Trauma Services, UnityPoint Health Des Moines, for her expertise in assisting with the statistical analysis.
APPENDIX 1: EQUIPMENT LIST
A) SouthMedic Adult OxyMask (SouthMedic, Barrie, Ontario)
B) Hudson RCI Adult Non-rebreathing Mask with Safety Vent (Morrisville, NC, USA)
C) 0–100 psig Pressure Gauge
D) Gilmont glass float type Rotameter (Barrington, IL, USA)
E) Low Flow Rotameter
F) AccuLAB Standard Electronic Balance TS series (Goettingen, Germany)
G) Vacuum source
H) Compressed gas source
I) Oxygen source
J) CO2 source
K) Velleman Digital Oscilloscope (Fort Worth, Texas, USA)
L) Ohmeda 5200 CO2 Monitor (Madison, WI, USA)
M) Data Acquisition System
N) Humidity/Temperature Meter
O) Oxygen Sensor
P) Harvard Respiratory Pump (Harvard Apparatus, Holliston, Massachusetts, USA),
Q) Wright Respirometer
R) Adult Mannequin Head (0.875 inch ID oral cavity, head width = 6.
Footnotes
DISCLOSURES: Keith Lamb has no financial disclosures or conflicts of interest to declare. David Piper is President of Piper Medical, Inc, Carmichael, California (USA) and performed the bench investigation(s). Mr Piper was compensated for conducting these experiments.
REFERENCES
- 1.Abe Y, Kondo T, Yamane Y, et al. The efficacy of an oxygen mask with reservoir bag in patients with respiratory failure. Tokai J Exp Clin Med. 2010;35:144–7. [PubMed] [Google Scholar]
- 2.Boumphrey SM, Morris EA, Kinsella SM. 100% inspired oxygen from a Hudson mask-a realistic goal? Resuscitation. 2003;57:69–72. doi: 10.1016/s0300-9572(02)00436-7. [DOI] [PubMed] [Google Scholar]
- 3.Wagstaff TA, Soni N. Performance of six types of oxygen delivery masks at varying respiratory rates. Anaesthesia. 2007;62:492–503. doi: 10.1111/j.1365-2044.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- 4.Wagstaff TAJ. A critical appraisal of ward-based interventions in the care of the acutely unwell patient. Diss Imperial College London. 2011 [Google Scholar]
- 5.Beecroft JM, Hanly PJ. Comparison of the OxyMask and Venturi mask in the delivery of supplemental oxygen: Pilot study in oxygen-dependent patients. Can Respir J. 2006;13:247–52. doi: 10.1155/2006/720320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul JE, Hangan H, Hajgato J. The OxyMask(™) development and performance in healthy volunteers. Med Masks (Auckl) 2009;2:9–17. [PMC free article] [PubMed] [Google Scholar]
- 7.Sonetti DA, Vines DL, Peters JI. Comparison of inspired oxygen between an OxyMask and simple mask and nasal cannula. Chest. 2009;136:126S. (Abst) [Google Scholar]
- 8.Robinson A, Ercole A. Evaluation of the self-inflating bag-valve-mask and non-rebreather mask as pre-oxygenation masks in volunteers. BMJ Open 2.5. 2012:e001785. doi: 10.1136/bmjopen-2012-001785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brotfain E, Zlotnik A, Schwartz A, et al. Comparison of the effectiveness of high flow nasal oxygen cannula vs. standard non-rebreather oxygen face mask in post-extubation intensive care unit patients. Isr Med Assoc J. 2014:718–22. [PubMed] [Google Scholar]
- 10.Susanto C, Thomas P. Assessing The use of initial oxygen therapy in COPD patients: A retrospective audit of pre-hospital and hospital emergency management. Intern Med J. 2015;45:510–6. doi: 10.1111/imj.12727. [DOI] [PubMed] [Google Scholar]


