Reducing postoperative pulmonary complications by improving postoperative lung expansion and ventilation is a primary goal after major thoracic surgeries, and can directly impact morbidity and downstream health care costs. Although deep breathing exercises, with or without devices, have demonstrated efficacy, the evidence regarding the utility of incentive spirometry has been inconclusive. Accordingly, this comprehensive literature search and review examined selected randomized controlled trials investigating several aspects of incentive spirometry interventions.
Keywords: Abdomen; Heart surgery; Incentive, Respiratory therapy; Spirometry; Thoracic surgery
Abstract
BACKGROUND:
Evidence regarding the effectiveness of incentive spirometry (ISy) on postoperative pulmonary outcomes after thoracic, cardiac and abdominal surgery remains inconclusive. This is attributed to various methodological issues inherent in ISy trials. Patient compliance has also been highlighted as a possible confounding factor; however, the status of evidence regarding patient compliance in these trials is unknown.
OBJECTIVE:
To explore the status of evidence on patient compliance with ISy interventions in randomized controlled trials (RCTs) in the above contexts.
METHOD:
A systematic search using MEDLINE, EMBASE and CINAHL databases was conducted to obtain relevant RCTs from 1972 to 2015 using the inclusion criteria. These were examined for specific ISy parameters, methods used for determining compliance and reporting on compliance. Main outcome measures were comparison of ISy parameters prescribed and assessed, and reporting on compliance.
RESULTS:
Thirty-six relevant RCTs were obtained. Six ISy parameters were identified in ISy prescriptions from these trials. Almost all (97.2%) of the trials had ISy prescriptions with specific parameters. Wilcoxon signed-rank test revealed that the ISy parameters assessed were significantly lower (Z=−5.433; P<0.001) than those prescribed; 66.7% of the trials indicated use of various methods to assess these parameters. Only six (16.7%) trials included reports on compliance; however, these were also incomprehensive.
CONCLUSIONS:
There is a scarcity and inconsistency of evidence regarding ISy compliance. Compliance data should be obtained using reliable and standardized methods to facilitate comparisons between and among trials. These should be reported comprehensively to facilitate valid inferences regarding ISy intervention effectiveness.
Abstract
HISTORIQUE :
Les données relatives à l’efficacité de la spirométrie incitative (ISy) sur la capacité pulmonaire après une chirurgie cardiaque, thoracique ou abdominale ne sont pas concluantes. Ce phénomène est attribuable à divers problèmes méthodologiques inhérents aux essais d’ISy. La compliance des patients peut également constituer un facteur confusionnel. Toutefois, on ne connaît pas l’état des données sur la compliance des patients lors de ces essais.
OBJECTIF :
Explorer l’état des données sur la compliance des patients aux interventions d’ISy dans le cadre d’essais aléatoires et contrôlés (EAC) réalisés dans les contextes susmentionnés.
MÉTHODOLOGIE :
À l’aide des critères d’inclusion, les chercheurs ont fouillé systématiquement les bases de données MEDLINE, EMBASE et CINAHL pour obtenir les EAC de 1972 à 2015. Ils ont analysé certains paramètres d’ISy, les méthodes utilisées pour déterminer la compliance et les rapports de compliance. Les principales mesures de résultats étaient la comparaison des paramètres d’ISy prescrits et évalués et les rapports de compliance.
RÉSULTATS :
Les chercheurs ont extrait 36 EAC pertinents. Ils en ont tiré six paramètres dans les prescriptions d’ISy. Presque tous les essais (97,2 %) comportaient des prescriptions d’ISy aux paramètres précis. Le test de la somme des rangs de Wilcoxon a révélé que les paramètres d’ISy évalués étaient considérablement plus faibles (Z= −5,433; P<0,001) que ceux prescrits, et 66,7 % des essais indiquaient l’utilisation de diverses méthodes pour évaluer ces paramètres. Seulement six essais (16,7 %) comportaient des rapports de compliance, également incomplets.
CONCLUSIONS :
Les données sur la compliance à l’ISy sont rares et contradictoires. Il faudrait obtenir les données de compliance au moyen de méthodes fiables et standardisées afin de faciliter les comparaisons entre les essais. Les rapports devraient être détaillés pour favoriser des inférences valides sur l’efficacité des interventions d’ISy.
Postoperative pulmonary complications (PPCs) are common occurrences after major cardiac, thoracic and abdominal surgeries (1) because effects from the surgical procedures, anesthesia and pain can impede chest wall mobility and lung expansion (1,2). Triggered by shallow breaths, lung atelectasis and pulmonary dysfunction, PPCs can lead to increased rates of morbidity, mortality (1,3) and health care costs (1). A landmark study by Thoren (4) provided evidence regarding the benefits of deep breathing exercises (DBEs) in reducing PPCs in abdominal surgery patients. This led to respiratory interventions – using DBEs with or without devices – to improve postoperative lung expansion and ventilation. One device commonly used for this purpose is the incentive spirometer (IS) (1). The IS was designed by Bartlett et al (5) to facilitate a sigh manoeuvre to increase lung expansion and prevent atelectasis, especially in the presence of monotonous, constant, low-volume breathing patterns (7,8). Performance of deep breathing exercises with the IS is known as incentive spirometry (ISy).
Numerous research reviews (9–18) have been conducted to integrate information and consolidate evidence regarding the effectiveness of ISy after cardiac, thoracic and abdominal surgeries. Unfortunately, to date, the evidence remains unsupportive and inconclusive (1). Much of this has been attributed to methodological issues inherent in IS trials, which have made drawing of well-founded conclusions difficult (1,6–11,13–18). Despite this, ISy remains a routinely used postoperative respiratory therapy in many health care settings (1,8) because of its convenience and ease of use (8,10). The need for rigorous methodologies in areas, such as randomization, sample size calculations and blinding procedures, have been advocated to facilitate more valid conclusions from this often-used intervention (1,6–11,13–18).
The effect of compliance as a possible confounder of IS trial outcomes has also been highlighted, given that ISy interventions require active participation by the patients (9,10,14,15,18). Compliance, in clinical contexts, is the extent to which patients actually perform a certain required behaviour in concordance with a prescribed therapeutic regimen (19,20). The direct relationship of poor patient compliance with poor outcomes in various aspects of clinical therapy (21), including the use of medical devices by patients (22–24), has been firmly established in the literature. However, there is little information regarding patient compliance in review articles assessing randomized controlled trials (RCTs) investigating postoperative IS after cardiac, thoracic and abdominal surgeries. Hence, the objective of the present study was to explore ISy RCTs for any information or data regarding ISy interventions, and to discuss the implications of the findings. Selected trials were examined for information regarding ISy prescriptions, assessment of ISy performance or usage, and monitoring and reporting of compliance. Additionally, they were also examined for the methods used to determine compliance.
METHODS
Literature search
A systematic search was performed using MEDLINE, EMBASE and CINAHL databases to obtain relevant trials from January 1972 to January 2015. Key words used for the search included “heart surgery”, “thoracic surgery”, “abdomen”, “respiratory therapy”, “breathing exercises”, “physiotherapy”, “physical therapy”, “coronary artery bypass”, “spirometry” and “incentive”. The search timeline was set to begin from 1972 because the first documented evidence of ISy as a treatment modality appears to be a report by Van de Water (25). References from the selected trials were also searched for any additional relevant articles.
Study selection
The titles and abstracts of articles were screened and read online independently by two reviewers. If they appeared relevant to the study objectives, full-text versions were retrieved. These were assessed for suitability for inclusion using the criteria stated below. Any disagreement was resolved in a consensual manner through discussions with the third reviewer. Inclusion criteria consisted of RCTs that investigated effectiveness of ISy interventions on postoperative pulmonary outcomes after cardiac, thoracic or abdominal surgeries; clearly stated the use of IS in their interventions; involved an adult study population; and were English language. Studies were excluded if the RCTs were outside the adult population; in languages other than English; included interventions in which IS use was not clearly stated; or did not investigate postoperative pulmonary outcomes.
Data extraction
The selected studies were examined by two of the authors (ALTN and SRSH). ISy prescriptions were identified and details regarding ISy performance and usage based on parameters stated in the ISy clinical practice guidelines 2011 (6) were extracted and tabulated. Other information extracted included details regarding assessment of compliance with these prescriptions, methods used for determining compliance and reporting on compliance. The studies were then re-examined by another author (ES) to verify the extracted data.
Data analysis
Data were analyzed using SPSS version 16.0 (IBM Corporation, USA) (26). Descriptive data were expressed as frequencies and percentages. Because data were not normally distributed, ISy parameters prescribed and assessed were compared using the nonparametric Wilcoxon signed-rank test. Every ISy parameter identified in each study was accounted for and included in the analysis. The number of ISy parameters assessed was expressed as a percentage of the number of parameters prescribed for each included study before conducting the Wilcoxon signed-rank test. Level of significance was set at α=0.05 to test the null hypothesis that there was no difference between prescribed and assessed parameters; the null hypothesis would be rejected if P<0.05.
RESULTS
The search strategy yielded 527 records. Fifty-four relevant RCTs were identified and retrieved for review. Eighteen did not meet the inclusion criteria and were excluded. Figure 1 depicts the selection process of studies included for analysis and reasons for exclusion of the 18 studies.
Figure 1).
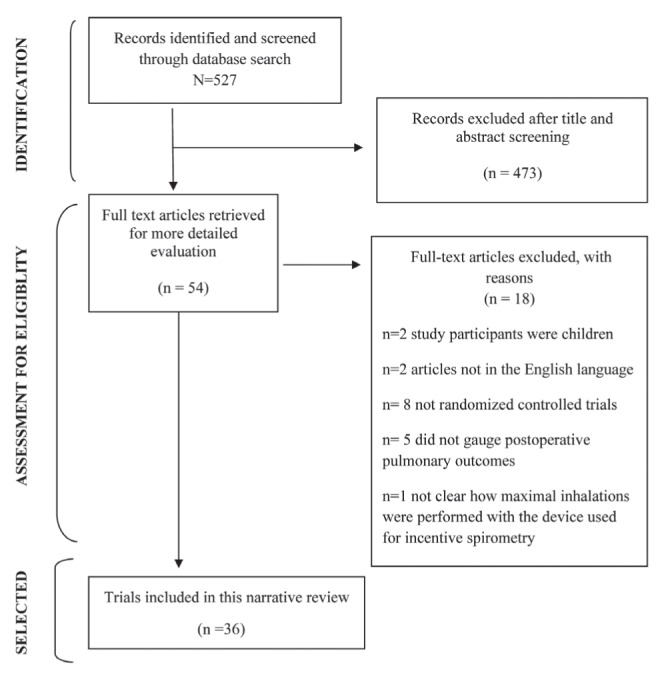
Flowchart depicting the trials retrieval process
Study characteristics
Thirty-six RCTs (25,27–61) that fulfilled the inclusion criteria were obtained. These were published over a span of 42 years (1972 to 2014). Of a total 3753 patients involved in these studies, 1957 (52.1%) had received ISy interventions. Table 1 presents details of the extracted data from each selected trial.
TABLE 1.
Brief summary of reviewed articles with details regarding incentive spirometry (ISy)
| Study, year; Total patients/patients using ISy, n/n | ISy | Method used to determine compliance | Reporting on compliance | ||
|---|---|---|---|---|---|
|
| |||||
| Type and method of IS | Parameters prescribed | Parameters assessed | |||
| Van De Water et al (25), 1972; 30/15 | Bartlett-Edwards: 4× daily; As many inspirations as possible; Hold breath as long as possible with each maximal inspiration; Inhale at rate >100 mL/s leak rate in IS |
Session frequency Inspiration frequency (frequency not specified) Breath hold (duration not specified) Flow rate |
Record of cumulative time of breath hold Flow rate |
Timing device attached to Bartlett-Edwards IS that records breath hold time | Data regarding total breath hold time (in seconds) for each postoperative day for 2 patients (one with and one without PPCs) |
| Craven et al (27), 1974; 70/35 | Bartlett-Edwards: 10 maximal inspirations/h; Breathe to volume target based on patient’s inspiratory effort each POD; Hold breath as long as possible with each maximal inspiration; Inhale at rate >100 mL/s leak rate to keep IS light on |
Session frequency Inspiration frequency Volume target Breath hold (duration not specified) Flow rate |
Inspiration frequency Volume achievements Flow rate |
Barlett-Edwards IS | General observation on possible association between inspiration frequency and volume achievements with development of PPCs |
| Dohi and Gold (28), 1978; 64/34 | Triflo: 5 maximal inspirations every waking hour, 8× daily |
Session frequency Inspiration frequency |
Not stated | Not stated | None |
| Iverson et al (29), 1978; 145/58 | Not specifed: 3 to 5 maximal inspirations every 3 h; Hold breath as long as possible with each maximal inspiration |
Session frequency Inspiration frequency Breath hold (duration not specified) |
Not stated | Not stated | None |
| Lyager et al (30), 1979; 94/43 | Bartlett-Edwards: 4 maximal inspirations every waking hour; Hold breath as long as possible with each maximal inspiration; Inhale at rate faster than 100 mL/s leak rate to keep IS light on |
Session frequency Inspiration frequency Breath hold (no specific duration) Flow rate |
Inspiration frequency Flow rate |
Barlett-Edwards IS | Average inspiration frequency for whole postoperative period |
| Gale and Sanders (31), 1980; 109/51 | Bartlett-Edwards: 20 min sessions, 4× daily; Minimum 10 maximal inspirations per session; Hold breath as long as possible with each maximal inspiration; Inhale at rate faster than 100 mL/s leak rate to keep IS light on |
Session duration Session frequency Inspiration frequency Breath hold (duration not specified) Flow rate |
Not stated | Supervision by respiratory therapist | None |
| Jung et al (32), 1980; 126/45 | Spirocare: 15 to 20 min sessions, 4× daily; As many inspirations as possible; Breathe to preset volume target set arbitrarily between 1400 to 1750 mL; Hold breath 3 s with each maximal inspiration |
Session frequency Session duration Inspiration frequency (frequency not specified) Volume target Breath hold |
Not stated | Supervision by staff | None |
| Lederer et al (33), 1980; 79/79 | Triflo; Spirocare; Bartlett-Edwards: 10 maximal inspirations every waking hour; Progressively attempt to breathe to preset volume target set at preoperative maximal inspiratory volume; Hold breath 2 to 3 s with each maximal inspiration |
Session frequency Inspiration frequency Volume target Breath hold |
Session frequency | Daily feedback from patients on frequency of use | No specific data on any ISy parameters Data available – percent-age of patients using the 3 different types of IS from POD 1 to POD 5 |
| Minschaert et al (34), 1982; 20/11 | Respirex: 6 maximal inspirations every waking hour; Hold breath 3 s with each |
Session frequency Inspiration frequency Breath hold |
Not stated | Not stated | None |
| Stock et al (35), 1982; 65/22 | Bartlett-Edwards: 15 min sessions every 2 h when awake |
Session duration Session frequency |
Not stated | Supervision by physicians and therapists | None |
| Dull and Dull (36), 1983; 49/16 | Spirocare: 10 maximal inspirations 4 × a day |
Session frequency Inspiration frequency |
Not stated | Supervision by physiotherapists | None |
| Celli et al (37), 1984; 172/45 | Not specified: Minimum of 10 maximal inspirations, 4× daily; Breathe to preset volume targets ranging from 100–800 mL starting from at least 50% of preoperative vital capacity until 70% vital capacity; Hold breath 3 s with each maximal inspiration |
Session frequency Inspiration frequency Volume target Breath hold |
Not stated | Supervision by respiratory personnel | None |
| Stock et al (38), 1984; 38/12 | Bartlett-Edwards: 15 min every 2 h when awake; Volume achievement each session target for subsequent sessions; Hold breath 3 s with each maximal inspiration; Inhale at rate faster than 100 mL/s leak rate to keep IS light on |
Session duration Session frequency Volume target Breath hold Flow rate |
Inspiration frequency Volume achievements Breath hold Flow rate |
Barlett-Edwards IS | Data on mean number of times 3 s breath hold achieved and mean maximal volume achieved for the 15 min sessions from POD 1 to POD 3 |
| Stock et al (39), 1985; 65/22 | Bartlett-Edwards: 15 min every 2 h when awake; Volume achievement each session target for subsequent sessions; Hold breath 3 s with each maximal inspiration; Inhale at rate faster than 100 mL/s leak rate to keep IS light on |
Session duration Session frequency Volume target Breath hold Flow rate |
Inspiration frequency Volume achievements Breath hold Flow rate |
Barlett-Edwards IS | Data on mean number of times 3 s breath hold achieved and mean maximal volume achieved for the 15 min sessions from POD 1 to POD 3 |
| Ricksten et al (40), 1986; 43/15 | Triflo: 30 maximal inspirations every waking hour |
Session frequency Inspiration frequency |
Session frequency | Each hourly session noted on record sheet by nurse or patient | None |
| Schwieger et al (41), 1986; 40/20 | Inspiron: 5 min/h at least 12 × daily |
Session duration Session frequency |
Not stated | Supervision by respiratory personnel | None |
| O’Connor et al (42), 1988; 40/20 | Inspiron: 3 maximal inspirations every waking hour; Progressively increase inspiration volume to achieve preoperative volume; Hold breath 3 s with each maximal inspiration; Inhale at rate sufficient to keep ball at tip of IS chamber for 3 s |
Session frequency Inspiration frequency Volume target Breath hold Flow rate |
Not stated | Not stated | None |
| Rau et al (43), 1988; 60/60 | Spirocare; Voldyne: 4 sessions daily; then continue as many inspirations as possible every waking hour |
Session frequency Inspiration frequency (frequency not specified) |
Not stated | Group 1 and 2 – 4 daily sessions supervised by therapist | None |
| Jenkins et al (44), 1989; 110/38 | Triflo: 10 maximal inspirations every waking hour |
Session frequency Inspiration frequency | Not stated | Patients’ self-reports (instructed to report frequency of sessions – 10 inspirations equals one session) | None |
| Hall et al (45), 1991; 876/431 | Airlife: At least 5 min every waking hour; Hold breath as long as possible with each maximal inspiration; Slow sustained maximal inhalations |
Session duration Session frequency Breath hold (duration not specified) Flow rate |
General compliance levels – specific ISy parameters not stated | Assessments on compliance made by research nurse on 0–100 mm visual linear analogue scale | No specific data on any ISy parameters – only brief mention on outcomes of ‘poor compliers’ |
| Oikkonen et al (46), 1991; 52/26 | Coach: 5 maximal inspirations/session every alternate waking hour; Hold breath 3–5 s with each maximal inspiration; Slow inspirations – based on IS flow rate guide |
|
Not stated | Not stated | None |
| Hall et al (47), 1996; 456/380 | Airlife: 10× each hour; Hold breath as long as possible with each maximal inspiration; Slow sustained maximal inhalations |
Session frequency Inspiration frequency Breath hold (duration not specified) Flow rate |
General compliance levels – specific ISy parameters not stated | Assessments on compliance made by research nurse on 0–100 mm visual linear analogue scale | No specific data on any ISy parameters Data available - mean compliance level for the entire postoperative period |
| Crowe et al (48), 1997; 185/90 | Voldyne: Use hourly |
Session frequency | Not stated | Patient and staff required to record use of IS on forms provided | None |
| Weiner et al (49), 1997; 32/17 | Coach: 30 min − ≥ 30 maximal inspirations; Hold breath as long as possible with each maximal inspiration; Slow sustained maximal inhalations |
Session duration Inspiration frequency Breath hold (duration not specified) Flow rate |
Not stated | Supervision by staff | None |
| Gosselink et al (50), 2000; 67/32 | Voldyne: 2 sets of 5–10 maximal inspirations every hour; Breathe to volume target increased daily by physiotherapist; Hold breath as long as possible with each maximal inspiration; Slow sustained maximal inhalations |
Session frequency Inspiration frequency Volume target Breath hold (duration not specified) Flow rate |
Not stated | Not stated | None |
| Matte et al (51), 2000; 96/32 | Coach: 20× every 2 h |
Session frequency Inspiration frequency |
Not stated | Not stated | None |
| Ebeo et al (52), 2002; 21/12 | Not specified: Not stated |
Not stated | Not stated | Not stated | None |
| Savci et al (53), 2006; 60/30 | Not specified: 15 min sessions twice daily on POD 1 and 2 followed by one session daily from day 3 onwards; Hold breath 3 s with each maximal inspiration |
Session duration Session frequency Breath hold |
Not stated | Not stated | None |
| Romanini et al (54), 2007; 40/20 | Voldyne: 10 min sessions with 5 min intervals |
Session duration | Not stated | Not stated | None |
| Haeffener et al (55), 2008; 34/17 | Voldyne: 15 to 20 min per session – 2 × daily |
Session duration Session frequency |
Not stated | Supervision by physiotherapists | None |
| Renault et al (56), 2009; 36/18 | Respiron: 10 maximal inspirations every 2 h; Hold breath as long as possible with each maximal inspiration; Slow sustained maximal inhalations |
Session frequency Inspiration frequency Breath hold (duration not specified) Flow rate |
Session frequency | Patients record on session frequency in ‘adherence’ log | Mean frequency of ISy sessions as obtained from adherence log |
| Kundra et al (57), 2010; 50/50 | Not specified: 15 maximal inspirations every 4 h |
Session frequency Inspiration frequency |
Not stated | Feedback from patients | None |
| Cattano et al (58), 2010; 37/37 | Airlife: Group 1: 10 maximal inspirations 5× daily Group 2: 3 maximal inspirations once a day; slow sustained maximal inhalations |
Session frequency Inspiration frequency Flow rate |
Volume achievements | Log sheet for patients to record IS volume achieved in each session Interview on IS usage Questionnaire on breathing improvement rated on 5-point Likert scale |
No specific data on any ISy parameters Data available – level of breathing improvement and patient satisfaction with prescribed IS usage |
| Kulkarni et al (59), 2010; 80/20 | Spiroball: 15 min/session, 2×daily |
Session duration Session frequency |
Not stated | Not stated | None |
| Dias et al (60), 2011; 35/12 | Voldyne: 3–5 maximal inspirations, 2× daily; Slow sustained maximal inhalations |
Session frequency Inspiration frequency Flow rate |
Not stated | Supervision by physiotherapists | None |
| Agostini et al (61), 2013; 180/92 | Coach 2: POD 1: 10 maximal inspirations, 2× daily; POD 2 onwards: 10 maximal inspirations – 1 supervised session; 10 maximal inspirations, every waking hour |
Session frequency Inspiration frequency |
Not stated | POD 1: Supervision by physiotherapists POD 2 onward: 1 session – supervision by physiotherapists; subsequent sessions – no supervision |
None |
IS Incentive spirometer; POD Postoperative day; PPCs Postoperative pulmonary complications
ISy prescriptions
IS prescriptions were not given in one trial (52), while the remainder (97.2%) had prescribed various ISy parameters. Six different ISy usage parameters were identified from these prescriptions:
Session duration – specifying the duration (in units of time) patients were to perform ISy.
Session frequency – specifying the frequency of ISy sessions per day.
Inspiration frequency – specifying the number of times inspiratory manoeuvres should be performed.
Volume targets – the inspiratory volume goal the patient should aim for.
Breath hold – the duration which the patient was to hold their breath at maximal inspiration.
Flow rate – how quickly, or the speed at which each inspiration should be performed.
Of these, only ‘session duration’ was not stipulated in the current ISy guidelines (6).
ISy parameters prescribed versus parameters assessed
Collectively, ISy parameters had been prescribed a total of 112 times. The parameter most frequently prescribed was session frequency (25,27–48,50,51,53,55–61), followed by inspiration frequency (25,27,28–34,36,37,40,42,43,44,46,47,49–51,56–58,60,61), session duration (31,32,35,38,39,41,44,45,49,53,54,55,59), breath hold (25,27,29–34,37–39,42,45–47,49,50,53,56), flow rate (25,27,30,31,38,39,42,45–47,49,50,56,58,60) and inspiratory volume target (27,32,33,37–39,50).
Only 10 (27.8%) of 36 trials that had ISy prescriptions indicated they had assessed any of these parameters. However, none had assessed all of the parameters that were prescribed in their respective prescriptions. Collectively, assessment of parameters had been performed 19 times. Parameters assessed were flow rate (25,27,29,38,39), inspiration frequency (27,30,38,39), volume achievements (27,38,39,58), session frequency (33,40,56) and breath hold (25,38,39). Some parameters were coalesced in several trials (ie, they were integrated with one another and, as such, occurred simultaneously at a certain target point). For example, breath hold and flow rate were coalesced in one trial (25) in which the duration of breath held at a specific flow rate was recorded by a special timing device. Flow rate and inspiration frequency were coalesced in two trials (27,30), in which inspirations with specific flow rates to preset volume goals were recorded; while flow rate, breath hold and inspiration frequency were coalesced in two trials (38,39) in which only inspiration volume targets achieved with a specific flow rate and three-second breath holds were recorded. Session duration was not assessed in any of the trials.
The Wilcoxon signed-rank test to compare mean percentages of prescribed parameters to assessed parameters included only 35 studies. One study (52) was excluded from analysis because it was not possible to discern individual parameters prescribed and assessed in this trial. Figure 2 shows the frequencies of the different ISy parameters that were prescribed and assessed. Results indicated that parameters prescribed were significantly higher than the parameters assessed in which P<0.05 (Z=−5.433; P<0.001) (Table 2).
Figure 2).
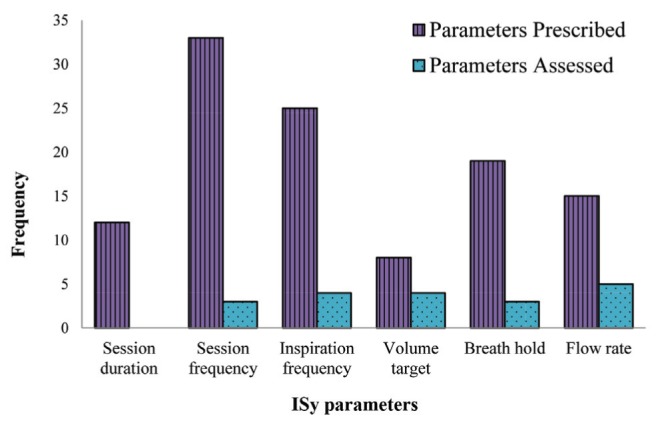
Frequency of each incentive spirometry (ISy) parameter(s) prescribed versus parameter(s) assessed in the primary trials
Table 2.
Comparison between mean percentages of incentive spirometry parameters prescribed with parameters assessed
| Mean percentage, % | P | |
|---|---|---|
|
| ||
| Parameter assessed | Parameter prescribed | |
| 100 | 12.95 | <0.0001 |
Prescriptions for ISy usage or performance parameters
Prescriptions for ISy had specified many different goals or ‘dosages’ for each parameter. Specific prescriptions or goals for session frequency, session duration and volume target were specified in all trials that had prescribed these parameters. Session duration ranged from 5 min (41,45), 10 min (55), 15 min (35,38,39,53,59), 15 min to 20 min (32,55), 20 min (31) and 30 min (50). Prescriptions for session frequencies included sessions to be conducted every hour when awake (30,33,34,40,42–45,61), every 2 h when awake (35,38,39), every alternate hour when awake (45), hourly (27,47,48,50), twice hourly (51,56), three times hourly (29), four times hourly (57), and once (53,58), twice (53,55,59,60), four times (25,31,32,36,37,43), five times (59), eight times (28) or 12 times (41) daily.
Volume targets were also prescribed in a variety of ways. Craven et al (27) progressed volume targets based on their patients’ inspiratory efforts each postoperative day, while Jung et al (32) required patients to inspire to preset volumes set arbitrarily between 1400 mL to 1750 mL. Patients in the study by Celli et al (37) had to inspire to preset volume targets ranging from 100 mL to 1800 mL, while the studies by Lederer et al (33) and O’Connor et al (42) sample had to increase inspiratory volumes progressively and aim to achieve preoperative volumes. Stock et al (38,39) used maximally achieved inspiratory volume for each session, and targets for the subsequent session and target volume goals were increased daily in the study by Gosselink et al (50); however, the basis on which this was done was not stated.
Inspiration frequency was explicitly specified in 22 trials with prescriptions for this parameter. The most prescribed inspiration frequency was 10 maximal breaths for a specified session or duration (27,31,33,36, 37,44,47,56,58,61). This was followed, in descending order by: three to five (29,60), five (28,46), 30 (40,49), three (42), four (30), six (34), 10 to 20 (50), 15 (57) and 20 breaths (51). Three trials (25,32,43), however, did not set any specific inspiration frequency because patients were instructed to perform as many inspirations as possible.
Of the 19 trials with ‘breath hold’ prescriptions, nine specified duration of breath hold. Three-second holds were specified in five (32,34,37,42,53) trials, while ranges between 3 s to 4 s (33) or 5 s (38,39,45) were specified in others. The remainder (25,27,29–31,45,47,49,50, 56) required breath holds as long as possible at the end of maximal inspirations with no specific duration indicated.
Eight of 15 trials with flow rate prescriptions specified inspiratory ‘speed’ in various ways. Six (25,27,30,31,38,39) used an IS model with a piston in its volume chamber, which activated a light bulb at a specific target volume. Because there was an air leak of 100 mL/s incorporated into the chamber, patients were required to inspire faster than the leak rate to keep the bulb lit. O’Connor et al (42) used another IS model in which the leak in the volume chamber could be preset at different flow rates depending on clinical requirements, while Oikkonen et al (46) required patients to adjust their own flow rates using a flow-rate guide at the side of the volume chamber of their IS model. The remainder of the trials (45,47,49,50,57,58,60) did not define explicit targets; patients were instructed to perform slow maximal inspirations.
Methods used to determine ISy compliance
Twenty-four (66.7%) trials indicated that ISy compliance had been monitored or measured. Six of these (25,27,30,38,39,43) had used IS devices with counter features that could record frequency of volume goal achievements, with one trial adding a special timing device to capture cumulative breath hold time to their IS (25). All of these trials were from the early 1970s to 1980s. Eleven trials (31,32,35–37,41,43,49,55,60,61) indicated direct observation methods by health care personnel to monitor ISy usage. However, patients in two trials (43,61) were subject to unsupervised sessions as well. Seven other trials (33,40,44,48,56,57,58) used various other approaches, such as the use of logs, self-reports or questionnaires, while two (45,47) used a 100 mm visual linear analogue scale to gauge level of compliance. The remaining 12 (33.3%) did not indicate if or how they had determined compliance with prescribed therapy. Figure 3 shows the methods used to determine compliance in the primary studies across the 1972 to 2014 timeline.
Figure 3).
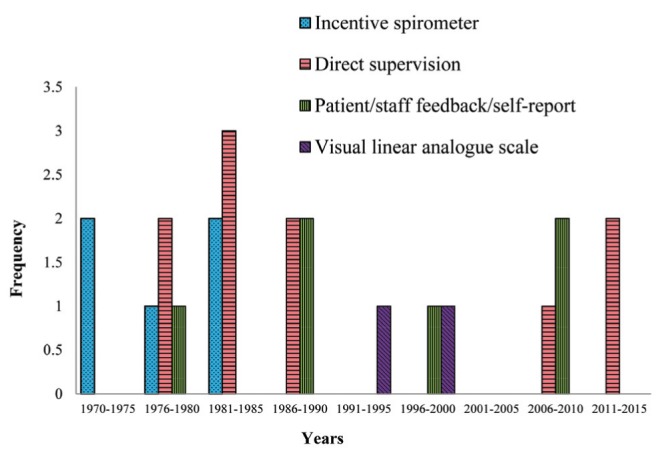
Frequency of methods used for monitoring compliance/collecting incentive spirometry usage data in primary trials between 1972 and 2015
Types of IS devices used
Eleven trials (25,27,30–33,35,36,38,39,43) conducted from early 1970s to 1980s used IS devices with counter features. Twenty-one trials (28,33,34,40–48,49–51,54–56,58–61) used various other IS models without this feature. These trials were conducted from the 1980s onward. Three different models were used in one trial (33); two of which had counter features, while one (43) had used two models (of which one was equipped with counter features). The remainder did not specify the type of IS used. Frequency of IS devices with or without counter features that were used in primary trials across the 1972 to 2014 timeline is shown in Figure 4.
Figure 4).
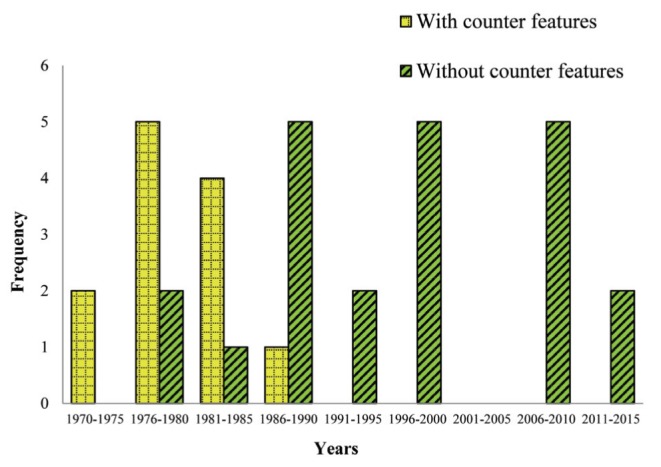
Frequency of use of incentive spirometers with and without counter features in primary trials between 1972 and 2015
Reporting of ISy compliance
Six of the 36 (16.6%) trials (25,27,30,38,39,56) presented some compliance data regarding specific ISy parameters. Van de Water et al (25) reported on total breath hold achieved for each postoperative day for only two patients: one who developed PPCs and the other who did not, for comparison purposes. They also indicated that patients with more cumulative usage time of ISy experienced fewer incidences of PPCs, but no specific dataset was available. Craven et al (27) reported that one-third of their ISy cohort were compliant with therapy and recounted general observations on possible associations between inspiration frequency and volume achievements with development of PPCs; however, no explicit data were reported. Meanwhile, Stock et al (38,39) presented datasets regarding inspiration frequency and inspiratory volume achieved by their ISy patients for each postoperative day for the duration of their trial. Lyager et al (30) also reported inspiration frequency, but a general mean value was given for the whole intervention period. They also found their ISy intervention group had a wide variation of usage frequency, which necessitated the reclassification of 15 ‘compliant’ patients into the ‘good Bartlett’ group for statistical analysis. Renault et al (56) presented mean frequency of ISy sessions achieved by their patients for the duration of study. They reported there was no significant difference in number of sessions achieved between groups and indicated that only 69.4% of their patients had completed their adherence logs. Two trials, both by Hall et al (45,47), reported mean compliance levels as assessed on a linear visual analogue scale, but had no detail on specific ISy parameters.
DISCUSSION
Findings indicate inconsistency and scarcity of evidence regarding ISy compliance in RCTs gauging the effectiveness of ISy on postoperative outcomes after cardiac, thoracic and abdominal surgeries. Only six trials had some reporting on compliance; however, these, too, were inconsistent and incomprehensive. Nevertheless, several did elicit insights into some compliance-related issues. Two trials (27,30) drew attention to the degree of noncompliance that may be existent in trials, while one (56) highlighted the possible drawbacks of certain data collection methods. Additionally, two trials (38,39) provided some insights into the possible role of ISy parameters on the effectiveness of therapy. One, which found no significant differences between continuous positive airway pressure therapy and ISy on pulmonary function after cardiac surgery, indicated that frequency of IS use and target volume achievements remained low and did not increase significantly for the duration of their trial (38). The other, in which increases in frequencies and significant increases in volume achievements were evident in the ISy group, found ISy more effective than continuous positive airway pressure therapy on functional residual capacity and atelectasis after upper abdominal surgery (39). It is difficult to draw any valid inferences as to the effects of ISy performance on outcomes because these trials had inherent methodological issues (10,13,15,17); nonetheless, this highlights the need for compliance data to facilitate critical evaluation of research findings.
Despite a majority of trials having prescribed specific ISy parameters, only a small percentage had been assessed. This suggests either a lack of emphasis on compliance data collection, or difficulties in successfully tracking and collecting such data. Of five trials that assessed ISy parameters, four (25,33,38,39) were from the 1970s and 1980s and had used the Bartlett-Edwards (BE) IS, which had incidence-counting features (31). However, the BE IS and Spirocare models, which also have counter features (62), have since given way to single-use, disposable, less-expensive models without counter features. This change is reflected by the IS models used in trials conducted from the 1980s onward. The techniques used to determine compliance also appear to coincide somewhat with the evolution of IS devices, with trials from the 1980s onward using various other strategies. However, direct monitoring or supervision appears to be a fairly consistent choice throughout the four-decade span.
The variety of compliance monitoring and data collection methods in the included IS trials also reflect the lack of standardization and consensus for such efforts. The degree of compliance cannot be ascertained unless effective monitoring is in place (63). Although there are no ‘gold standards’ for determining compliance, direct observation or measurement and electronic monitoring have been suggested as more accurate and reliable techniques (63). However, for ISy interventions, direct methods, such as supervision by staff, may not be viable options in terms of cost, time and manpower resource availability. The proposed advantage of ISy is the reduction of burden on health care resources by facilitating patients’ independent efforts in treatment regimens, and this is bound to be nullified if such strategies were to be used (10). Furthermore, administration of interventions can be influenced by those providing these interventions and pose a threat to the validity of the data collected (64). This should be considered when deciding on techniques for compliance data collection.
Electronic technologies have been used in some areas of health care involving the use of medical devices (65–67), and four earlier trials (25,30,38,39) have used IS counters to collect compliance data. Although the BE IS is deemed less suitable for current respiratory therapy practice (15), some of its features, such as the incidence counter, may be an indispensable component. In fact, the IS was conceived not only to facilitate active maximal lung expansion, but also to keep record of the manoeuvres by its inventors (68). As such, innovative methods for reinstating suitable versions of counter devices capable of monitoring and collecting compliance data could be contemplated. However, the implication of cost should also be duly considered, given that current IS models are mainly disposable, single-use units.
Although strategies, such as electronic technology, questionnaires and self-reports, can be used for determining compliance, each method has its own strengths and weaknesses (69). Nonetheless, ideal methods for collecting ISy compliance data can only be ascertained if methods used are evaluated rigorously for reliability and feasibility through appropriately designed trials. This can further facilitate standardization of compliance data collection and comparisons between trials.
Compliance involves human behaviour and can be rather unpredictable, with marked intra- and intersubject variability (20). Furthermore, it is not a dichotomous entity because it can fluctuate and change (70). Complications, such as pain (71) and cognitive dysfunction (72), are common after major surgeries. These experiences are unique to individual patients (71,72) and may affect activities, such as ISy performance, to varying degrees in the postoperative period. Personal beliefs and perceptions also appear to have some effect on patients’ resolute to adhere to ISy prescriptions (73). As such, it is imperative that methods used to ascertain compliance are not only reliable, but also possess the ability to track degrees of compliance accurately throughout the course of interventions.
Methods used for determining compliance should also be stated clearly in published trials so that valid inferences can be made on the quality of data collected (69). Unfortunately, nearly one-half of the trials had no reporting on methods. This, coupled with the lack of uniformity of methods used in the remaining trials, calls for more attention to this aspect in future trials. More than one-half of the trials’ total participants underwent ISy interventions, but there was little information included regarding their compliance levels. Even low rates of poor compliance can subtly underpower trials and affect outcomes (74,75). As such, it must be measured and reported accurately and reliably; not only to facilitate more valid interpretations of intervention effects, but also to inform statistical analysis (64,74,75).
The lack of standardization and inconsistency in prescription of ISy parameters suggests uncertainties on optimal dosages for this intervention. Although inspiration frequency and volume have been cited as important for therapy efficacy (68), supportive evidence regarding optimal dosages is lacking (6). The role and effects of the other parameters are also unclear. This uncertainty can only be addressed if compliance data encompassing the various ISy parameters are systematically collected and compared so that the role of each parameter on therapy efficacy can be ascertained.
The ongoing interest and debate regarding the efficacy of postoperative ISy in addressing PPCs after cardiac, thoracic and abdominal surgery reflects the quest for more conclusive evidence on this routinely used intervention. In fact, the need for more attention to compliance perspectives in IS trials has been highlighted many times in the literature (9,10,14,15,25,27,31–33,46,48,50,56,60,61). Although ISy has been compared with several device-based respiratory therapies (25,28,29,31,32,35,37–40,46,51,52,54), these operate in different ways to achieve lung expansion (76). ISy most closely relates to spontaneous DBEs in physiological principles of lung expansion (37,77), and its carryover effects on respiration may be better than other respiratory interventions (54). Although DBEs should suffice for most postoperative patients in preventing PPCs (78), the challenge would be to determine how well a given ISy prescription is adhered to, even in the absence of the health care professional. The cost of PPCs can be substantial (79); therefore, every effort must be made to optimize the process of care (78). If found to be effective, following evaluations that include compliance perspectives, ISy may be a valuable option for addressing PPC concerns.
A strength of the present study is that it is believed to be the first to have examined ISy trials exclusively from compliance perspectives. Details pertaining to ISy interventions are presented in a concise manner to allow for objective evaluation and comparisons between trials. Limitations are the focus on ISy interventions in the context of cardiac, thoracic and abdominal surgeries, because this was our area of interest and these patient groups have been extensively studied due to their high susceptibility to PPCs. Future studies may target different therapeutic areas in which ISy is routinely used to provide more evidence. Additionally, only 10 studies had indicated assessment of ISy parameters. As such, the comparison analysis for ISy parameters prescribed versus parameters assessed could only be performed for studies in which this information was available.
CONCLUSION
The present study reveals a scarcity and inconsistency of evidence regarding ISy compliance. Compliance perspectives need to be incorporated into future trials to build a stronger evidence base for ISy interventions after cardiac, thoracic and abdominal surgery. Compliance should be determined using reliable methods capable of collecting data on various ISy parameters throughout the intervention period so that valid inferences can be made. Data collection methods should be standardized to enable comparisons between trials and reporting should be comprehensive to facilitate valid trial interpretation.
Footnotes
CONTRIBUTORS: ALTN conceived and designed the study. ALTN and SRGSH executed the search strategy, screened titles and abstracts, and collected data. ALTN, SRGSH and ES analyzed the data. The manuscript was written by ALTN. All authors made revisions and approved of the final version of the manuscript.
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Branson RD. The scientific basis for postoperative respiratory care. Respir Care. 2013;58:1974–84. doi: 10.4187/respcare.02832. [DOI] [PubMed] [Google Scholar]
- 2.Warner DO. Preventing postoperative pulmonary complications: The role of the anaesthesiologist. Anesthesiology. 2000;92:1467–72. doi: 10.1097/00000542-200005000-00037. [DOI] [PubMed] [Google Scholar]
- 3.Wynne R, Botti M. Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: Clinical significance and implications for practice. Am J Crit Care. 2004;13:384–93. [PubMed] [Google Scholar]
- 4.Thoren L. Postoperative pulmonary complications: Observations on their prevention by means of physiotherapy. Acta Chir Scand. 1954;107:193–205. [PubMed] [Google Scholar]
- 5.Bartlett RH, Krop P, Hanson EL, et al. Physiology of yawning and its application in postoperative care. Surg Forum. 1970;21:222–3. [PubMed] [Google Scholar]
- 6.Restrepo RD, Wettstein R, Wittnebel L, Tracy M. AARC (American Association for Respiratory Care) clinical practice guideline. Incentive spirometry: 2011. Respir Care. 2011;56:1600–4. doi: 10.4187/respcare.01471. [DOI] [PubMed] [Google Scholar]
- 7.Freitas ER, Soares BG, Cardoso JR, Atallah AN. Incentive spirometry for preventing pulmonary complications after coronary artery bypass graft. Cochrane Database Syst Rev. 2009;3:CD004466. doi: 10.1002/14651858.CD004466.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Freitas ERFS, Soares BGO, Cardoso JR, Atallah AN. Incentive spirometry for preventing pulmonary complications after coronary artery bypass graft. Cochrane Database Syst Rev. 2012;9:CD004466. doi: 10.1002/14651858.CD004466.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas JA, McIntosh JM. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysis. Phys Ther. 1994;74:3–10. doi: 10.1093/ptj/74.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Overend TJ, Anderson CM, Lucy SD, Bhatia C, Jonsson BI, Timmermans C. The effect of incentive spirometry on postoperative complications; a systematic review. Chest. 2001;120:971–8. doi: 10.1378/chest.120.3.971. [DOI] [PubMed] [Google Scholar]
- 11.Pasquina P, Tramer MR, Walder B. Prophylactic respiratory physiotherapy after cardiac surgery: Systematic review. BMJ. 2003;327:1–6. doi: 10.1136/bmj.327.7428.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence VA, Cornell JE, Smetana GS. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: A systematic review for the American College of Physicians. Ann Inter Med. 2006;144:596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 13.Pasquina P, Tramer MR, Granier JM, Walder B. Respiratory physiotherapy to prevent pulmonary complications after abdominal surgery – a systematic review. Chest. 2006;130:1887–99. doi: 10.1378/chest.130.6.1887. [DOI] [PubMed] [Google Scholar]
- 14.Renault JA, Costa-Val R, Rossetti MB. Respiratory physiotherapy in the pulmonary dysfunction after cardiac surgery. Rev Bras Cardiovasc. 2008;23:562–9. doi: 10.1590/s0102-76382008000400018. [DOI] [PubMed] [Google Scholar]
- 15.Guimaraes MM, El Dib R, Smith AF, Matos D. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database Syst Rev. 2009;3:CD006058. doi: 10.1002/14651858.CD006058.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Agostini P, Singh S. Incentive spirometry following thoracic surgery: What should we be doing? Physiotherapy. 2009;95:76–82. doi: 10.1016/j.physio.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho CRF, Paisani DM, Lunardi AC. Incentive spirometry in major surgeries: A systematic review. Rev Bras Fisioter. 2011;15:343–350. doi: 10.1590/s1413-35552011005000025. [DOI] [PubMed] [Google Scholar]
- 18.do Nascimento Junior P, Módolo NSP, Andrade S, Guimaraes MM, Braz LG, El Dib R. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database of Syst Rev. 2014;2:CD006058. doi: 10.1002/14651858.CD006058.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urquhart J. Patient non-compliance with drug regimens: Measurement, clinical correlates, economic impact. Eur Heart J. 1996;17:8–15. doi: 10.1093/eurheartj/17.suppl_a.8. [DOI] [PubMed] [Google Scholar]
- 20.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: Three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331–342. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 21.van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: A review of reviews. BMC Health Serv Res. 2007;7:55. doi: 10.1186/1472-6963-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: Patient compliance, devices, and inhalation technique. Chest. 2000;117:542–50. doi: 10.1378/chest.117.2.542. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casutt C, Pancherz H, Gawora M, Ruf S. Success rate and efficiency of activator treatment. Eur J Orthod. 2007;29:614–21. doi: 10.1093/ejo/cjm066. [DOI] [PubMed] [Google Scholar]
- 25.Van De Water MJ, Watring WG, Linton LA, Murphy M, Byron RL. Prevention of postoperative pulmonary complications. Surg Gynecol Obstet. 1972;135:229–33. [PubMed] [Google Scholar]
- 26.SPSS Inc . Released 2007. SPSS for Windows, Version 16.0. Chicago: SPSS Inc; [Google Scholar]
- 27.Craven JL, Evans GA, Davenport PJ, Williams RH. The evaluation of the incentive spirometer in the management of postoperative pulmonary complications. Br J Surg. 1974;61:793–7. doi: 10.1002/bjs.1800611012. [DOI] [PubMed] [Google Scholar]
- 28.Dohi S, Gold MI. Comparison of two methods of postoperative respiratory care. Chest. 1978;73:592–5. doi: 10.1378/chest.73.5.592. [DOI] [PubMed] [Google Scholar]
- 29.Iverson LIG, Ecker RR, Fox HE, May IA. A comparative study of IPPB, the incentive spirometer and blow bottles: The prevention of atelectasis following cardiac surgery. Ann Thorac Surg. 1978;25:197–9. doi: 10.1016/s0003-4975(10)63521-7. [DOI] [PubMed] [Google Scholar]
- 30.Lyager S, Wernberg M, Rajani N, et al. Can postoperative pulmonary conditions be improved by treatment with the Bartlett-Edwards incentive spirometer after upper abdominal surgery? Acta Anaesthesiol Scand. 1979;23:312–9. doi: 10.1111/j.1399-6576.1979.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 31.Gale GD, Sanders DE. Incentive spirometer: Its value after cardiac surgery. Can J Anaesth. 1980;27:475–80. doi: 10.1007/BF03007047. [DOI] [PubMed] [Google Scholar]
- 32.Jung R, Wight J, Nusser R, Rosoff L. Comparison of three methods of respiratory care following upper abdominal surgery. Chest. 1980;78:31–5. doi: 10.1378/chest.78.1.31. [DOI] [PubMed] [Google Scholar]
- 33.Lederer DH, Van De Water JM, Indech RB. Which deep breathing device should the postoperative patient use? Chest. 1980;77:610–3. doi: 10.1378/chest.77.5.610. [DOI] [PubMed] [Google Scholar]
- 34.Minschaert M, Vincent JL, Ros AM, Kahn RJ. Influence of incentive spirometry on pulmonary volumes after laparotomy. Acta Anaesthesiol Belg. 1982;3:203–9. [PubMed] [Google Scholar]
- 35.Stock MC, Downs JB, Gauer PK, Cooper RB. Prevention of atelectasis after upper abdominal operations. Anesthesiology. 1982;57:A457. [Google Scholar]
- 36.Dull JL, Dull WL. Are maximal inspiratory breathing exercises or incentive spirometry better than early mobilization after cardiopulmonary bypass? Phys Ther. 1983;63:655–9. doi: 10.1093/ptj/63.5.655. [DOI] [PubMed] [Google Scholar]
- 37.Celli BR, Rodriguez KS, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry and deep breathing exercises in preventing pulmonary complications after abdominal surgery. Am Rev Respir Dis. 1984;130:12–5. doi: 10.1164/arrd.1984.130.1.12. [DOI] [PubMed] [Google Scholar]
- 38.Stock MC, Downs JB, Cooper RB, et al. Comparison of continuous positive airway pressure, incentive spirometry and conservative therapy after cardiac operations. Crit Care Med. 1984;12:969–72. doi: 10.1097/00003246-198411000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Stock MC, Downs JB, Gauer PK, Alster JM, Imrey PB. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry and conservative therapy. Chest. 1985;87:151–7. doi: 10.1378/chest.87.2.151. [DOI] [PubMed] [Google Scholar]
- 40.Ricksten SE, Bengtsson A, Soderberg C, Thorden M, Kvist H. Effects of periodic positive airway pressure by mask on postoperative pulmonary function. Chest. 1986;89:774–81. doi: 10.1378/chest.89.6.774. [DOI] [PubMed] [Google Scholar]
- 41.Schwieger I, Gamulin Z, Forster A, Meyer P, Gemperle M, Suter PM. Absence of benefit of incentive spirometry in low-risk patients undergoing elective cholecystectomy. Chest. 1986;89:652–6. doi: 10.1378/chest.89.5.652. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor M, Tattersall MP, Carter JA. An evaluation of the incentive spirometer to improve lung function after cholecystectomy. Anaesthesia. 1988;43:785–7. doi: 10.1111/j.1365-2044.1988.tb05759.x. [DOI] [PubMed] [Google Scholar]
- 43.Rau JL, Thomas Lynda, Haynes LH. The effect of method of administering incentive spirometry on postoperative pulmonary complications in coronary artery bypass patients. Respir Care. 1988;33:771–8. [Google Scholar]
- 44.Jenkins SC, Soutar SA, Loukota JM, Johnson LC, Moxham J. Physiotherapy after coronary artery surgery: Are breathing exercises necessary? Thorax. 1989;44:634–9. doi: 10.1136/thx.44.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall JC, Tarala R, Harris J, Tapper J, Christiansen K. Incentive spirometry versus routine chest physiotherapy for prevention of pulmonary complications after abdominal surgery. Lancet. 1991;337:953–6. doi: 10.1016/0140-6736(91)91580-n. [DOI] [PubMed] [Google Scholar]
- 46.Oikkonen M, Karjalainen K, Kähärä V, Kuosa R, Schavikin L. Comparison of incentive spirometry and intermittent positive pressure breathing after coronary artery bypass graft. Chest. 1991;99:60–5. doi: 10.1378/chest.99.1.60. [DOI] [PubMed] [Google Scholar]
- 47.Hall JC, Tarala RA, Tapper J, et al. Prevention of respiratory complications after abdominal surgery: A randomized clinical trial. BMJ. 1996;312:148–53. doi: 10.1136/bmj.312.7024.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crowe JM, Bradley CA. The effectiveness of incentive spirometry with physical therapy for high-risk patients after coronary artery bypass surgery. Phys Ther. 1997;77:260–8. doi: 10.1093/ptj/77.3.260. [DOI] [PubMed] [Google Scholar]
- 49.Weiner PW, Man A, Weiner M, et al. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. J Thorac and Cardiovasc Surg. 1997;113:552–7. doi: 10.1016/S0022-5223(97)70370-2. [DOI] [PubMed] [Google Scholar]
- 50.Gosselink R, Schrever K, Cops P, et al. Incentive spirometry does not enhance recovery after thoracic surgery. Crit Care Med. 2000;28:679–83. doi: 10.1097/00003246-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Matte P, Jacquet L, Van Dyck M, Goenen M. Effects of conventional physiotherapy, continuous positive airway pressure and non-invasive ventilator support with bilevel positive airway pressure after coronary artery bypass grafting. Acta Anaesthesiol Scand. 2000;44:75–81. doi: 10.1034/j.1399-6576.2000.440114.x. [DOI] [PubMed] [Google Scholar]
- 52.Ebeo CT, Benotti PN, Byrd RP, Jr, Elmaghraby Z, Lui J. The effect of bi-level positive airway pressure on postoperative pulmonary function following gastric surgery for obesity. Respir Med. 2002;96:672–6. doi: 10.1053/rmed.2002.1357. [DOI] [PubMed] [Google Scholar]
- 53.Savci S, Sakinc S, Ince DI, Arikan H, Can Z, Buran Y, Kuralay E. Active cycle of breathing techniques and incentive spirometer in coronary artery bypass graft surgery. Fizyoterapi Rehabilitasyon. 2006;17:61–9. [Google Scholar]
- 54.Romanini W, Muller AP, Carvalho KA, et al. The effects of intermittent positive pressure and incentive spirometry in the postoperative of myocardial revascularization. Arq Bras Cardiol. 2007;89:94–9. doi: 10.1590/s0066-782x2007001400006. [DOI] [PubMed] [Google Scholar]
- 55.Haeffener MP, Ferreira GM, Barreto SSM, Arena R, Dall’Ago P. Incentive spirometry with expiratory positive pressure reduces pulmonary complications, improves pulmonary function and 6-minute walk distance in patients undergoing coronary artery bypass surgery. Am Heart J. 2008;156:900.e1–900.e8. doi: 10.1016/j.ahj.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Renault JA, Costa-Val R, Rossetti MB, Houri Neto M. Comparison between deep breathing exercises and incentive spirometry after CABG surgery. Rev Bras Cir Cardiovasc. 2009;24:165–72. doi: 10.1590/s0102-76382009000200012. [DOI] [PubMed] [Google Scholar]
- 57.Kundra P, Vitheeswaran M, Nagappa M, Sistla S. Effect of preoperative and postoperative incentive spirometry on lung functions after laparoscopic choecystectomy. Surg Laparosc Endosc Percutan Tech. 2010;20:170–2. doi: 10.1097/SLE.0b013e3181db81ce. [DOI] [PubMed] [Google Scholar]
- 58.Cattano D, Altamirano A, Vannucci A, Melnikov V, Cone C, Hagberg CA. Preoperative use of incentive spirometry does not affect postoperative lung function in bariatric surgery. Transl Res. 2010;156:265–72. doi: 10.1016/j.trsl.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Kulkarni S, Fletcher E, McConnell A, Poskitt KR, Whyman MR. Pre-operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery – a randomised pilot study. Ann R Coll Surg Eng. 2010;92:700–5. doi: 10.1308/003588410X12771863936648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dias CM, Vieira RO, Oliveira JF, Lopes AJ, Menexes SL, Guimaraes FS. Three physiotherapy protocols: Effects on pulmonary volumes after cardiac surgery. J Bras Pneumol. 2011;37:54–60. doi: 10.1590/s1806-37132011000100009. [DOI] [PubMed] [Google Scholar]
- 61.Agostini P, Naidu B, Cieslik H, et al. Effectiveness of incentive spirometry in patients following thoracotomy and lung resection including those at high risk for developing pulmonary complications. Thorax. 2014;68:580–5. doi: 10.1136/thoraxjnl-2012-202785. [DOI] [PubMed] [Google Scholar]
- 62.Glover DW. The History of Respiratory Therapy: Discovery and Evolution. Bloomington: Author House; 2010. [Google Scholar]
- 63.LaFluer J, Oderda GM. Methods to measure patient compliance with medication regimens. J Pain Pall Care Pharmacother. 2004;18:81–7. [PubMed] [Google Scholar]
- 64.Dodd S, White IR, Williamson P. Nonadherence to treatment protocol in published randomised controlled trials: A review. Trials. 2012;13:84. doi: 10.1186/1745-6215-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddel KR, Toelle BG, Marks GB, Ware SI, Jenkins CR, Woolcock AJ. Analysis of adherence to peak flow monitoring when recording is electronic. BMJ. 2002;324:146–7. doi: 10.1136/bmj.324.7330.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nordmann J, Baudouin C, Renard JP, et al. Measurement of treatment compliance using a medical device for glaucoma patients associated with intraocular pressure control: A survey. Clin Ophthalmol. 2010;4:731–9. doi: 10.2147/opth.s11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel M, Pilcher J, Travers J, et al. Use of metered-dose inhaler electronic monitoring in a real-world asthma randomised controlled trial. J Allergy Clin Immunol Pract. 2013;1:83–91. doi: 10.1016/j.jaip.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Bartlett RH, Gazzaniga AB, Geraghty TR. Respiratory manoeuvres to prevent postoperative pulmonary complications: A critical review. JAMA. 1973;224:1017–21. [PubMed] [Google Scholar]
- 69.Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poccock SJ, Abdalla M. The hope and hazards of using compliance data in randomised controlled trials. Statist Med. 1998;17:303–17. doi: 10.1002/(sici)1097-0258(19980215)17:3<303::aid-sim764>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 71.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–25. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- 72.Caza N, Taha R, Qi Y, Blaise G. The effects of surgery and anesthesia on memory and cognition. Prog Brain Res. 2008;169:409–422. doi: 10.1016/S0079-6123(07)00026-X. [DOI] [PubMed] [Google Scholar]
- 73.Tung HH, Jan MS, Huang CM, Shih CC, Chang CY, Liau CY. Using the theory of planned behaviour to predict the use of incentive spirometry among cardiac surgery patients in Taiwan. Heart Lung. 2011;40:440–7. doi: 10.1016/j.hrtlng.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Smith DL. Patient non-adherence in clinical trials: Could there be a link to postmarketing patient safety? Drug Inf J. 2012;46:27–34. [Google Scholar]
- 75.Albert JM. Accounting for noncompliance in the design of clinical trials. Drug Inf J. 1997;31:157–165. [Google Scholar]
- 76.Denehy I, Berney S. The use of positive pressure devices by physiotherapists. Eur Respir J. 2001;17:821–9. doi: 10.1183/09031936.01.17408210. [DOI] [PubMed] [Google Scholar]
- 77.Shelledy OC, Mikles SP. Patient assessment and respiratory care plan development. In: Mishoe SC, Welch MA Jr, editors. Critical Thinking in Respiratory Care – a Problem-based Learning Approach. Singapore: McGraw-Hill Education (Asia); 2003. pp. 181–234. [Google Scholar]
- 78.O’Donohue WJ., Jr Postoperative pulmonary complications. When are preventive and therapeutic measures necessary? Postgrad Med. 1992;91:167–170. doi: 10.1080/00325481.1992.11701233. [DOI] [PubMed] [Google Scholar]
- 79.Kalish RL, Daley J, Duncan CC, Davis RB, Coffman GA, Iezzoni LI. Costs of potential complications of care for major surgery patients. Am J Med Qual. 1995;10:48–54. doi: 10.1177/0885713X9501000108. [DOI] [PubMed] [Google Scholar]


