Abstract
Profenofos is a direct acting phosphorothioate organophosphorus (OP) pesticide capable of inhibiting β-esterases such as acetylcholinesterase, butyrylcholinesterase, and carboxylesterase. Profenofos is known to be detoxified to the biologically inactive metabolite, 4-bromo-2-chlorophenol (BCP); however, limited data are available regarding the use of urinary BCP as an exposure biomarker in humans. A pilot study conducted in Egyptian agriculture workers, demonstrated that urinary BCP levels prior to application (3.3–30.0 μg/g creatinine) were elevated to 34.5-3566 μg/g creatinine during the time workers were applying profenofos to cotton fields. Subsequently, the in vitro enzymatic formation of BCP was examined using pooled human liver microsomes and recombinant human cytochrome P-450s (CYPs) incubated with profenofos. Of the nine human CYPs studied, only CYPs 3A4, 2B6, and 2C19 were able to metabolize profenofos to BCP. Kinetic studies indicated that CYP 2C19 has the lowest Km, 0.516 μM followed by 2B6 (Km = 1.02 μM) and 3A4 (Km = 18.9 μM). The Vmax for BCP formation was 47.9, 25.1, and 19.2 nmol/min/nmol CYP for CYP2B6, 2C19, and 3A4, respectively. Intrinsic clearance (Vmax/Km) values of 48.8, 46.9, and 1.02 ml/min/nmol CYP 2C19, 2B6, and 3A4, respectively, indicate that CYP2C19 and CYP2B6 are primarily responsible for the detoxification of profenofos. These findings support the use of urinary BCP as a biomarker of exposure to profenofos in humans and suggest polymorphisms in CYP 2C19 and CYP 2B6 as potential biomarkers of susceptibility.
Keywords: Profenofos, 4-Bromo-2-chlorophenol (BCP), Cytochrome P450 (CYP), Pooled human liver microsomes, Recombinant human CYPs
1. Introduction
Organophosphorus (OP) pesticides are the most commonly used pesticides throughout the world (Sultatos, 1994). This together with their potential to produce acute and chronic neurotoxic responses, positions these compounds as environmental chemicals of concern to human health. It is generally accepted that inhibition of acetylcholinesterase (AChE) is the primary mechanism by which OPs cause acute neurotoxicity (Jamal and Julu, 2002; Farahat et al., 2003). OP inhibition of AChE, decreases the hydrolysis of acetylcholine in both central and peripheral cholinergic synapses, resulting initially in overstimulation of nicotinic and muscarinic receptors followed by receptor down-regulation on post-synaptic membranes (Costa, 2006). Acute OP poisoning is generally thought to be mediated primarily by overstimulation of receptors secondary to AChE inhibition, whereas it has been hypothesized that chronic OP neurotoxicity is due to receptor down-regulation (Costa, 2006). Most OP pesticides require bioactivation by cytochrome P-450s (CYPs) to form the active oxon metabolite which are potent cholinesterase inhibitors (Ma and Chambers, 1994).
In contrast to most OP pesticides, the parent form of profenofos is active, and CYP-mediated metabolism results in oxidative bioactivation and detoxification reactions (Wing et al., 1984; Abass et al., 2007). Profenofos [O-(4-bromo-2-chlorophenyl) O-ethyl S-propyl phosphorothioate] is a thiophosphate OP pesticide (O=P-S-C) that was developed for pest strains resistant to chlorpyrifos and other OPs (Gotoh et al., 2001). On average, approximately 775,000 pounds of profenofos is applied to about 5% of the approximately 13,818,000 acres of cotton grown within the United States per year (U.S. EPA, 2006). It has been classified as a moderately hazardous (Toxicity Class II) pesticide by the World Health Organization (WHO) and it has a moderate level of acute toxicity following oral and dermal administration (Malghani et al., 2009; Abass et al., 2007). The use of OP pesticides, such as profenofos and chlorpyrifos, is common in the Nile River delta of Egypt, where agriculture is a vital component of its national identity and economy. Our group has previously investigated chlorpyrifos exposure and effects in Egyptian cotton field workers by determining the relationship between chlorpyrifos exposure, as determined by quantification of the chlorpyrifos specific urinary metabolite, trichloro-2-pyridinol (TCPy), and toxicity, as determined by blood cholinesterase activity and neurobehavioral performance (Farahat et al., 2003, 2010, 2011). However, biomarkers of profenofos exposure and effect have not been reported in these agriculture workers or any other occupational cohort.
Despite the unique ability of profenofos to inhibit cholinesterase without biotransformation, few studies have investigated profenofos in animal or human models. Animal studies with profenofos are limited to the WHO Evaluation of Profenofos (WHO, 2007) and a study in rats by McDaniel and Moser (2004). Human studies are limited to cases of accidental poisoning (Eddleston et al., 2009; Gotoh et al., 2001), an agricultural worker study assessing cholinesterase inhibition with chlorpyrifos and profenofos (Lakew and Mekonnen, 1998), and an in vitro study of profenofos metabolism which measured the formation of the toxicologically active metabolite, desthiopropylprofenofos and hydroxyprofenofos (Abass et al., 2007).
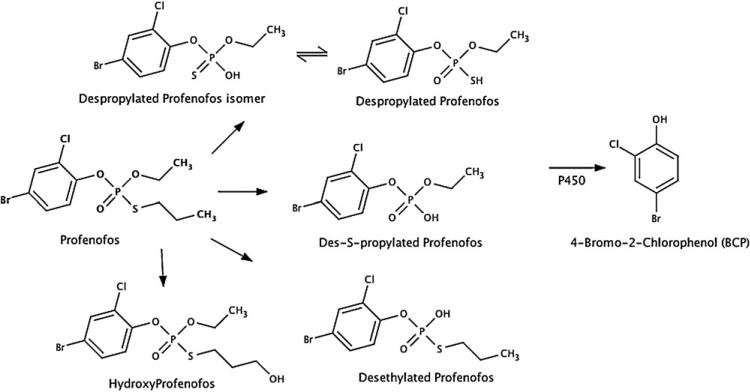
The current study will focus on the metabolism of profenofos to its detoxified metabolite 4-bromo-2-chlorophenol (BCP) (Fig. 1). BCP is a metabolite unique to profenofos and therefore may represent a sensitive and specific biomarker of exposure for individuals exposed to profenofos.
Fig. 1.
Metabolism of profenofos to its metabolites hydroxyprofenofos, desethylated profenofos, des-S-propylated profenofos, despropylated profenofos, despropylated profenofos isomer, and BCP. Figure is an adaptation from figures presented in Gotoh et al. (2001) and Abass et al. (2007).
2. Materials and methods
2.1. Chemicals
Profenofos (CAS 41198-08-7) and 4-bromo-2-chlorophenol (CAS 3964-56-5) were purchased from ChemService Inc (West Chester, PA). Tetraisopropyl pyrophosphoramide (iso-OMPA; CAS 513-00-8) was purchased from Sigma–Aldrich (St. Louis, MO) and Bis(trimethylsilyl)trifluoroacetamide (BSTFA) was obtained from SUPELCO (Bellefonte, PA). Pooled human liver microsomes (HLM, which contains liver microsomes pooled from 50 donors of mixed sex, 20 mg/ml stock) were obtained from XenoTech (Lenexa, KS). Recombinant human cytochrome P450s were purchased from BD Gentest (Woburn, MA).
2.2. Human exposure to profenofos
The human exposure study took place in the Menoufia governorate of Egypt, which is situated in the Nile River delta north of Cairo. Egypt's Ministry of Agriculture directs the use and application of pesticides in the cotton fields and employs workers in one of the following job categories (Farahat et al., 2003, 2010, 2011): (1) applicator, who applies pesticides with backpack sprayers; (2) technician, who walks each row with the applicator to direct the path of the applicator; and (3) engineer, who periodically walks the field but more often directs the application process from the edge of the field and oversees the mixing and loading of pesticides into backpack sprayers. Workers applied profenofos to cotton from July 30 to August 8, 2008. During the summer of 2008, spot urine samples were collected from workers prior to (July 24) and during the period of application (August 4). Samples were placed on wet ice in a cooler and transported to Menoufia University (Shebin El-Kom, Egypt) where they were stored at −20 °C until shipped on dry ice to the University at Buffalo (Buffalo, NY) for analyses.
A 1 ml aliquot of each urine sample was thawed and mixed prior to the addition of 50 ng of internal standard 2,4,5 trichlorophenol. Samples were then hydrolyzed to free sulfate or glucuronide conjugated BCP (Gotoh et al., 2001) at 80 °C for 1 h with 100 μl of 12 N HCl, and extracted with 1 ml of toluene. The toluene extract was then derivatized with BSTFA at 70 °C for 1 h and analyzed by gas chromatography–micro electron capture detection (GC/μECD) as described in metabolite detection. Creatinine concentrations were measured for all workers using the Jaffe reaction (Fabiny and Ertingshausen, 1971) and urine BCP concentrations are expressed as μg/g creatinine.
2.3. In Vitro metabolism
Profenofos (5 μM) was incubated with pooled human liver microsomes (0.1 mg protein/ml) in buffer (100 mM Tris–HCl, 5 mM MgCl2, 1 mM EDTA and 50 μM iso-OMPA; pH 7.4) at 37 °C in a final volume of 0.5 ml. EDTA was included to inhibit α-esterases while iso-OMPA was used to inhibit β-esterases (Reiner et al., 1993). Profenofos was dissolved in dimethylsulfoxide (DMSO) at a final concentration in the reaction medium of 1%. Incubation conditions were optimized to assure that the reaction was linear with time and protein concentration. All incubations were initiated by adding 1 mM NADPH and quenched with 50 μl of 12 N HCl. Preliminary studies showed that the hydrolysis step was not necessary in vitro. For kinetic studies, pooled human liver microsomes (0.1 mg protein/ml) were incubated with profenofos (0.1, 0.5, 1, 2.5, 5, 10, 20, 40, and 80 μM) for 2 min. Recombinant human CYPs 3A4, 2C19, 2B6, 1A1, 1A2, 2C9, 2A6, 2D6, and 2E1 were incubated with profenofos at a low (1 μM) and high concentration (10 μM) to identify CYPs capable of metabolizing profenofos. Profenofos was then incubated at concentrations of 0.5, 1, 5, 10, 20, 40 and 80 μM with the active CYPs (1 nmol/ml) in buffer (100 mM Tris–HCl, 5 mM MgCl2, 1 mM EDTA and 50 μM iso-OMPA; pH 7.4) at 37 °C with a final volume of 0.5 ml for 2 min. 25 ng of 2,4,5 trichlorophenol was added to the mixture followed by 0.5 ml of toluene. The final mixture was centrifuged followed by extraction of the supernatant. The samples were then derivatized with BSTFA at 70 °C for 1 h and then analyzed by the GC/μECD.
2.4. Metabolite detection
BCP was analyzed using a modification of the method described by Farahat et al. (2010). The method described in Farahat et al. (2010) was transferred and modified to allow for increased sensitivity of BCP measurement on the GC/μECD. A change in the detector from GC/MS to GC/μECD required modifications in the internal standard as well as derivatizing agent. BCP was identified using a relative retention time with internal standard and measured through the use of known concentrations of BCP standards. The amount of BCP measured using the GC/μECD was comparable to the amount measured when using GC/MS. The optimized method was used for both urine and in vitro analyses and described as follows:
Samples were analyzed on an Agilent gas chromatograph model 6890 equipped with a micro-electron capture detector (μECD) and a capillary column (Rtx-5 with integra-guard, 65 m × 250 μm × 0.25 μm nominal) (Restek; Bellefonte, PA). Ultra pure helium (He) carrier gas was used at a flow rate of 1.2 ml/min. The detector temperature was set at 325 °C with ultra-pure nitrogen (N2) as its make-up gas and a flow rate of 60 ml/min. The starting oven temperature of 100 °C was held for 1 min and increased at 10 °C/min up to 300 °C and then held for 4 min. The injection port temperature was set at 250 °C with a split ratio of 1:10 and an injection volume of 1 μl. Under these conditions, the BCP limit of detection and limit of quantification were 0.16 ng/ml and 0.54 ng/ml respectively (U.S. EPA MDL).
2.5. Data analysis
The kinetic values Vmax and Km were determined by non-linear regression analyses (enzyme kinetics module of Sigma Plot, SyStat Software Inc., V11) of hyperbolic plots obeying Michaelis–Menten kinetics. Profenofos concentration (μM) was set as the independent variable while the rate of formation of BCP (nmol/min/mg protein or nmol/min/nmol CYP) was the dependent variable. Urinary BCP levels during the profenofos application period were compared with the levels prior to application using a Wilcoxon Signed Rank Test, with significance set at p < 0.05. Km, Vmax, and intrinsic clearance values for all three recombinant CYPs were compared using one-way ANOVA with Tukey's post hoc analysis.
3. Results
3.1. Urinary BCP analysis
The urinary BCP concentrations were quantified in Egyptian agriculture workers for each job category prior to (July 24, 2008) and during the period of profenofos application (August 4, 2008). Table 1 shows that all workers, regardless of job category, had detectable amounts of urinary BCP prior to the spray period for profenofos, indicating that the workers were exposed to low background levels of profenofos and/or BCP prior to the beginning of the spray period. The urinary concentrations of BCP increased markedly during the period of profenofos application. The applicators had the highest levels of BCP, both prior to and during profenofos application, followed by the technicians and engineers (Table 1).
Table 1.
Urinary BCP concentration (μg/g creatinine) in Egyptian agriculture workers prior to and during application of profenofos to cotton fields.
| Worker | Before spray 24-July | During spray* 4-August |
|---|---|---|
| Applicator 1 | 29.6 | 1027 |
| Applicator 2 | 30.0 | 3566 |
| Applicator 3 | 16.5 | 974 |
| Technician 1 | 13.4 | 62.1 |
| Technician 2 | 3.27 | 41.2 |
| Technician 3 | 3.79 | 172 |
| Engineer 1 | 3.56 | 80.8 |
| Engineer 2 | 4.24 | 42.8 |
| Engineer 3 | 11.2 | 34.5 |
BCP levels during spray period were significantly different from levels prior to spraying period using a Wilcoxon signed rank test (p-value = 0.008).
3.2. In Vitro metabolism
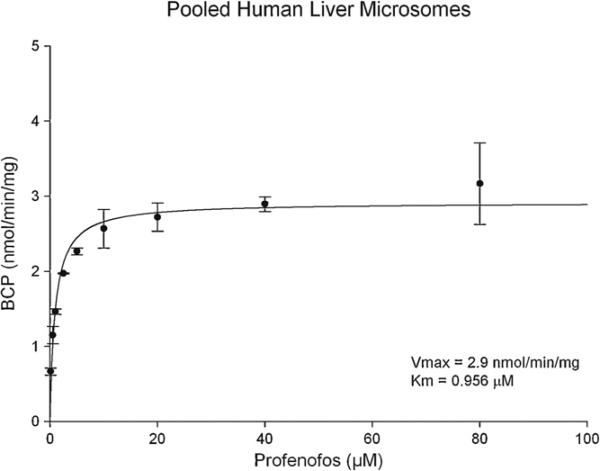
Pooled human liver microsomes and recombinant human CYPs were used to determine kinetic values (Km and Vmax) for metabolism of profenofos to BCP. A 2 min incubation time was determined to be within the linear range of metabolite formation and BCP was the major metabolite formed when profenofos was incubated with pooled HLM or recombinant human CYPs. A plot of the rate of BCP formation at various profenofos concentrations utilizing pooled HLM was used to derive Michaelis–Menten kinetic parameters (Fig. 2). The Km and Vmax values were 0.956 μM and 2.92 nmol/min/mg respectively, with an intrinsic clearance (CLint) of 3.11 ml/min/mg (Table 2).
Fig. 2.
Michaelis–Menten kinetic parameters were derived from the plot illustrating the relationship between BCP formation and profenofos (substrate) concentration using pooled human liver microsomes. Data are presented as the mean ± SEM (n = 3 different experiments).
Table 2.
Kinetics of profenofos metabolism.
| Enzyme | Km (μM) | Vmax (nmol BCP/min/mg protein) | Intrinsic clearance (ml/min/mg protein) |
|---|---|---|---|
| Pooled HLM | 0.96 ± 0.21 | 2.92 ± 0.12 | 3.11 ± 0.56 |
| Enzyme | Km (μM) | Vmax (nmol BCP/min/nmol CYP) | Intrinsic clearance (ml/min/nmol CYP) |
|---|---|---|---|
| Pooled HLM | 0.96 ± 0.21 | 6.21 ± 0.12 | 8.74 ± 0.56 |
| CYP 3A4 | 18.9 ± 3.48* | 19.2 ± 1.32 | 1.02 ± 0.11* |
| CYP 2B6 | 1.02 ± 0.13 | 47.9 ± 1.20* | 46.9 ± 1.93 |
| CYP 2C19 | 0.52 ± 0.09 | 25.1 ± 0.76 | 48.8 ± 2.99 |
Km, Vmax, and the intrinsic clearance of profenofos metabolism were determined using pooled human liver microsomes (HLM) and recombinant human CYPs 3A4, 2B6, and 2C19. Data are presented as the mean ± SEM (n = 3 separate experiments).
p < 0.0005 compared with the other two CYPs determined by one-way ANOVA with Tukey's post hoc analysis.
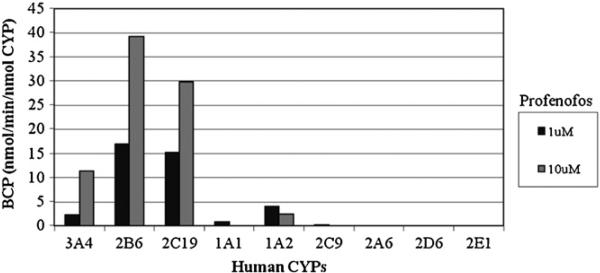
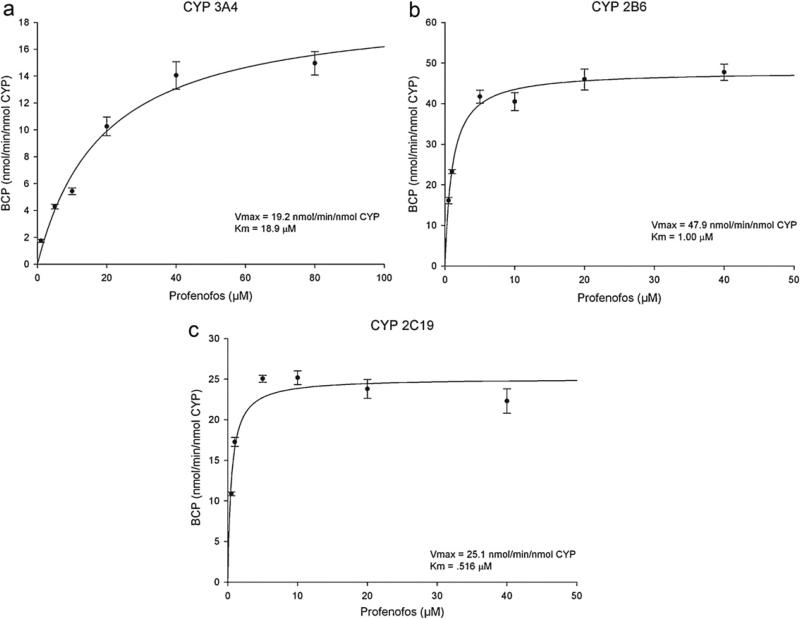
Individual human recombinant cytochrome P450 enzymes were used to screen the specific CYPs which were capable of metabolizing profenofos to BCP. CYPs 3A4, 2B6, and 2C19 were the most active in metabolizing profenofos at either 1 μM or 10 μM to BCP (Fig. 3). Kinetic parameters for profenofos metabolism were obtained from Michaelis–Menten plots illustrating the formation of BCP by CYPs 3A4, 2B6, and 2C19 (Fig. 4a–c) and are shown in Table 2. The rank order of Km was CYP 2C19 (0.52 μM) > CYP 2B6 (1.02 μM) > CYP 3A4 (18.9 μM). The rank order of Vmax was CYP 2B6 (47.9 nmol BCP/min/nmol CYP) > CYP 2C19 (25.1 nmol BCP/min/nmol CYP) > CYP 3A4 (19.2 nmol BCP/min/nmol CYP). From these two kinetic parameters, the rank order of CLint was CYP 2C19 (48.8 ml/min/nmol CYP) ≥ CYP 2B6 (46.9 ml/min/nmol CYP) > CYP 3A4 (1.02 ml/min/nmol CYP) (Table 2).
Fig. 3.
Differential activity of specific recombinant human CYPs for biotransformation of profenofos at 1 (black) or 10 (gray) μM to BCP. CYPs 3A4, 2B6, and 2C19 produced the most BCP per minute per nmole of CYP.
Fig. 4.
Michaelis–Menten kinetic parameters were derived from the plot illustrating the relationship between BCP formation and profenofos (substrate) concentration using recombinant human CYP 3A4, 2B6, and 2C19 (panel a, b, and c respectively). Data are presented as the mean ± SEM (n = 3 three different experiments).
4. Discussion
This is among the first studies to assess the occupational exposure of agriculture workers to profenofos using urinary BCP levels as a sensitive and specific biomarker of profenofos exposure. Egyptian cotton field workers had a wide range of urinary BCP concentrations during the period of profenofos application to cotton fields, with the applicators exhibiting the highest concentrations. This is consistent with their greater potential for exposure to profenofos, and the general lack of personal protective equipment use amongst these workers, which allows for direct contact of the skin with spray residue containing profenofos (Fenske et al., 2012). Similar findings were previously reported in the same workers exposed to chlorpyrifos, with applicators exhibiting the highest level of urinary TCPy, followed by technicians and engineers (Farahat et al., 2010, 2011). Since BCP is a metabolite specific to profenofos, it was somewhat surprising to find low levels of urinary BCP prior to the period of profenofos application. The occasional, non-occupational use of pesticides at home and pesticide residues on food crops may account for the elevated levels of BCP in workers prior to the start of profenofos spray periods on cotton fields. Nonetheless, workers in all three job categories, but especially the applicators, had a marked increase in urinary BCP levels associated with the period of profenofos application. This observation supports BCP as a sensitive and specific biomarker of profenofos exposure.
Prior studies of profenofos metabolism reported the bioactivation of profenofos to the toxic metabolites desthipropylprofenofos, and hydroxyprofenofos in rat, mouse, and human liver fractions (Abass et al., 2007). Our study is the first to assess the in vitro metabolism of profenofos to the detoxified metabolite BCP by human liver microsomes and purified human recombinant CYPs.
Our screen of nine recombinant human CYPs identified CYPs 3A4, 2B6, and 2C19 as three enzymes capable of detoxifying profenofos to BCP. Michaelis–Menten kinetic parameters for each enzyme provided an estimate of Km, Vmax, and the metabolic efficiency of each enzyme expressed as the intrinsic clearance (Vmax/Km). While all three CYPs were capable of catalyzing the formation of BCP, CYPs 2C19 and 2B6 had the highest affinity with Km values of 0.52 and 1.02 μM, respectively, in contrast to CYP3A4, which exhibited a Km of 18.9 μM. The relative efficiency of each enzyme for profenofos metabolism, as measured by intrinsic clearance, indicates that CYP 2B6 and 2C19 are the primary enzymes responsible for the detoxification of profenofos.
Knowledge of the in vitro metabolism of profenofos to BCP has significant potential to inform in vivo studies that use urinary BCP as a biomarker of exposure to profenofos. Individual variability in urinary BCP excretion may not only be due to differences in exposure to profenofos, but may also be influenced by genetic differences in CYP2B6 and CYP2C19, which are highly polymorphic (Hodgson and Rose, 2007; Zanger et al., 2007; Goldstein et al., 1997; Crane et al., 2012). Furthermore, serial and/or combined exposures to multiple pesticides have potential to alter enzyme activities and the resulting excretion of BCP. Cho et al., 2007, suggest that chlorpyrifos and profenofos have the potential to inhibit the catalytic activity of CYP2B6 and CYP2C19, which also metabolizes chlorpyrifos (Foxenberg et al., 2007). Essentially no CYP 2B6 activity remained after in vitro chlorpyrifos or profenofos treatment, while 49 and 43 percent of the respective CYP2C19 activity remained after chlorpyrifos and profenofos pretreatment of HLMs (Abass et al., 2009). Chlorpyrifos was also sprayed in the Egyptian cotton fields approximately 2 weeks prior to the beginning of the profenofos application period (Farahat et al., 2010, 2011). Therefore, prior exposure to chlorpyrifos could inhibit CYPs 2B6 and 2C19 mediated metabolism of profenofos. This in turn might increase inhibition of cholinesterase resulting in increased risk of neurotoxicity. Examining the interactive effects of serial exposures to chlorpyrifos and profenofos experienced by Egyptian agricultural workers will be critical to predicting the neurotoxic risks associated with these real-world occupational exposures.
Acknowledgments
We thank the Egyptian Ministry of Agriculture for their participation.
Funding information
This research was supported by R01 ES016308 (Anger and Lein, MPI) from the National Institute of Environmental Health Sciences (NIEHS) and EPA STAR grant R833454 (Olson). Corie Ellison was supported by a Research Supplement to Promote Diversity in Health-Related Research from NIEHS (ES016308-02S). The protocol and consent forms used in this research have been approved by the Oregon Health & Science (USA) and Menoufia University (Egypt) IRBs. The research was also supported by NSF Bridge to Doctorate and Ronald E. McNair Scholars Program.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
- Abass K, Reponen P, Jalonen J, Pelkonen O. In vitro metabolism and interaction of profenofos by human, mouse and rat liver preparations. Pestic. Biochem. Physiol. 2007;87:238–247. [Google Scholar]
- Abass K, Turpeinen M, Pelkonen O. An evaluation of the cytochrome P450 inhibition potential of selected pesticides in human hepatic microsomes. J. Environ. Sci. Health B. 2009;44:553–563. doi: 10.1080/03601230902997766. [DOI] [PubMed] [Google Scholar]
- Cho TM, Rose RL, Hodgson E. The effect of chlorpyrifos-oxon and other xenobiotics on the human cytochrome P450-dependent metabolism of naphthalene and deet. Drug Metabol. Drug Interact. 2007;22:235–262. doi: 10.1515/dmdi.2007.22.4.235. [DOI] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin. Chim. Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Crane AL, Klein K, Olson JR. Bioactivation of chlorpyrifos by CYP2B6 variants. Xenobiotica. 2012 doi: 10.3109/00498254.2012.702246. http://dx.doi.org/10.3109/00498254.2012.702246. [DOI] [PMC free article] [PubMed]
- Eddleston M, Worek F, Eyer P, Thiermann H, Von Meyer L, Jeganathan K, Sheriff MHR, Dawson AH, Buckley NA. Poisoning with the S-Alkyl organophosphorus insecticides profenofos and prothiofos. QJM: Int. J. Med. 2009;102:785–792. doi: 10.1093/qjmed/hcp119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiny DL, Ertingshausen G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 1971;17:696–700. [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, Lasarev MR, Rohlman DS, Anger WK, Lein PJ, Olson JR. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ. Health Perspect. 2011;119:801–806. doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, Fenske RA, Olson JR, Galvin K, Bonner MR, Rohlman DS, Farahat TM, Lein PJ, Anger WK. Chlorpyrifos exposures in Egyptian cotton field workers. Neurotoxicology. 2010;31:297–304. doi: 10.1016/j.neuro.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup. Environ. Med. 2003;60:279–286. doi: 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Farahat FM, Galvin K, Fenske EK, Olson JR. Contributions of inhalation and dermal exposure to chlorpyrifos dose in Egyptian cotton field workers. Int. J. Occup. Environ. Health. 2012;18:198–209. doi: 10.1179/1077352512Z.00000000030. [DOI] [PubMed] [Google Scholar]
- Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR. Human hepatic cytochrome P450-specific metabolism of parathion and chlorpyrifos. Drug Metab. Dispos. 2007;35:189–193. doi: 10.1124/dmd.106.012427. [DOI] [PubMed] [Google Scholar]
- Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, Evans DA. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics. 1997;7:59–64. doi: 10.1097/00008571-199702000-00008. [DOI] [PubMed] [Google Scholar]
- Gotoh M, Sakata M, Endo T, Hayashi H, Seno H, Suzuki O. Profenofos metabolites in human poisoning. Forensic Sci. Int. 2001;116:221–226. doi: 10.1016/s0379-0738(00)00377-7. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Rose RL. The importance of cytochrome P450 2B6 in the human metabolism of environmental chemicals. Pharmacol. Ther. 2007;113:420–428. doi: 10.1016/j.pharmthera.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Jamal GA, Julu POO. Low level exposures to organophosphorus esters may cause neurotoxicity. Toxicology. 2002;181:23–33. doi: 10.1016/s0300-483x(02)00447-x. [DOI] [PubMed] [Google Scholar]
- Lakew K, Mekonnen Y. The health status of Northern Omo State Farm workers exposed to chlorpyrifos and profenifos. Ethiop. Med. J. 1998;36:175–184. [PubMed] [Google Scholar]
- Ma T, Chambers JE. Kinetic parameters of desulfuration and dearylation of parathion and chlorpyrifos by rat liver microsomes. Food Chem. Toxicol. 1994;32:763–767. doi: 10.1016/s0278-6915(09)80009-4. [DOI] [PubMed] [Google Scholar]
- Malghani S, Chatterjee N, Yu HX, Luo ZJ. Isolation and identification of profenofos degrading bacteria. Braz. J. Microbiol. 2009;40:893–900. doi: 10.1590/S1517-838220090004000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel KL, Moser VC. Differential profiles of cholinesterase inhibition and neurobehavioral effects in rats exposed to fenamiphos or profenofos. Neurotoxicol. Teratol. 2004;26:407–415. doi: 10.1016/j.ntt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Profenofos World Health Organization – Joint Meeting on Pesticide Residue. 2007:403–443. [Google Scholar]
- Reiner E, Pavkovic E, Radic Z, Simeon V. Differentiation of esterases reacting with organophosphorus compounds. Chem. Biol. Interact. 1993;87:77–83. doi: 10.1016/0009-2797(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Sultatos LG. Mammalian toxicology of organophosphorus pesticides. J. Toxicol. Environ. Health. 1994;43:271–289. doi: 10.1080/15287399409531921. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency Method Detection Limit (MDL), Title 40, Code of Federal Regulations, Part 136, Appendix B, Revision 1.11.
- United States Environmental Protection Agency Interim Registration Eligibility Decision: Profenofos. 2006 Aug; EPA 738-R-00-006. [Google Scholar]
- Wing KD, Glickman AH, Casida JE. Phosphorothiolate pesticides and related-compounds – oxidative bioactivation and aging of the inhibited acetylcholinesterase. Pestic. Biochem. Physiol. 1984;21:22–30. [Google Scholar]
- Zanger UM, Klein K, Saussele T, Blievernicht J, Hofmann MH, Schwab M. Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics. 2007;8:743–759. doi: 10.2217/14622416.8.7.743. [DOI] [PubMed] [Google Scholar]