Abstract
Hypertensive chronic kidney disease is one of the most prevalent medical conditions with high morbidity and mortality in the United States and worldwide. However, early events initiating the progression to hypertensive chronic kidney disease are poorly understood. We hypothesized that elevated endothelial hypoxia-inducible factor-1alpha is a common early insult triggering initial glomerular injury leading to hypertensive chronic kidney disease. To test our hypothesis we used an angiotensin II infusion model of hypertensive chronic kidney disease to determine the specific cell type and mechanisms responsible for elevation of HIF-1α and its role in the progression of hypertensive chronic kidney disease. Genetic studies coupled with RT-PCR profiling revealed that elevated endothelial HIF-1α is essential to initiate glomerular injury and progression to renal fibrosis by the transcriptional activation of genes encoding multiple vasoactive proteins. Mechanistically, we found that endothelial HIF-1α gene expression was induced by Ang II in a nuclear factor-κB-dependent manner. Finally, we discovered reciprocal positive transcriptional regulation of endothelial Hif-1α and Nf-κb genes is a key driving force for their persistent activation and disease progression. Overall, our findings revealed that the stimulation of HIF-1α gene expression in endothelial cells is detrimental to induce kidney injury, hypertension and disease progression. Our findings highlight early diagnostic opportunities and therapeutic approaches for hypertensive chronic kidney disease.
Keywords: hypertension, chronic kidney disease, hypoxia-inducible factor-1alpha, angiotensin II
Introduction
Chronic kidney disease (CKD) is one of the most prevalent medical conditions with high morbidity and mortality in the United States and worldwide1-4. Hypertension is a key pathogenic factor that contributes to the deterioration of kidney function. 10%-15% of hypertension patients develop CKD5, 6. Hypertension is also one of the most common comorbidities associated with CKD, with more than 85% of patients with stage 3-5 CKD having hypertension7. Early insults that initiate the progression to CKD are not well understood. However, it has been long speculated that glomerular injury and vasoconstriction of efferent arterioles are the two major initiating factors leading to decreased postglomerular peritubular capillary blood flow, tubulointerstitial hypoxia and eventually renal fibrosis8-10. The hypothesis that hypoxia is a common mediator of kidney disease was first published over fifteen years ago11. Since that time the hypothesis has been supported by the analysis of kidney biopsies from patients with CKD at the end of stage of the disease and from the study of experimental animal models of CKD12. For example, animal studies have demonstrated that over-activation of HIF-1α in the epithelial cells of the renal tubule interstitium contributes to CKD by stimulating epithelial to mesenchymal transition and fibrosis13. Thus, chronic hypoxia in the tubulointerstitium, leading to fibrosis and functional impairment, is widely considered to be the final common pathway to end stage renal disease. Recently, multiple studies demonstrate that growth factors, cytokines and hormones directly induce HIF-1α levels independent of hypoxia14-17. However, specific cell types and signaling pathways responsible for hypoxia-independent elevated HIF-1α in the hypertensive CKD are poorly understood.
Angiotensin II, an endogenous vasoconstrictor, is known to be elevated in hypertension and CKD patients18-22. Numerous animal studies demonstrated that infusion of Ang II into mice or rats leads to hypertension and progression to renal fibrosis23-30, indicating that Ang II infusion is a well-accepted animal model of hypertensive CKD. Notably, recent studies showed that HIF-1α levels are significantly elevated in kidneys of patients with hypertensive CKD and mice with infusion of Ang II27, 31. Thus, we hypothesized that the HIF-1α induced by Ang II is an early insult triggering hypertension and initial glomerular injury leading to CKD. To test our hypothesis we used an Ang II infusion model of CKD to determine the specific cell type and mechanisms responsible for elevation of HIF-1α and its role in the progression of CKD. These findings will provide a better understanding of the role of elevated HIF-1α expression in kidney disease progression and will influence the choice of therapeutic options for CKD treatment.
Materials and Methods
Other methods are in the supplemental data.
Animals
We obtained VE-cadherin cre+ mice and Hif-1αf/fVE-cadherin cre+ mice from Dr. Holger Eltzschig's laboratory in University of Colorado at Denver. Six to twelve mice for each group were infused with Ang II (Sigma) or Phosphate Buffered Saline (PBS). by minipump at a rate of 425ng/kg/min for 14-days27. All protocols involving animal studies were reviewed and approved by the Institutional Animal Welfare Committee of the University of Texas Houston Health Science Center. All studies in animals were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Mouse blood pressure measurements
Systolic blood pressure was non-invasively measured by a carotid catheter-calibrated tail-cuff system27, 32-36 (CODA, Kent Scientific, Torrington, CT). Mice were put in proper size holders on a warm and comfortable pad during the measurement. Blood pressure was monitored on day 0 which was considered as baseline and continuously measured on the day 3rd, 7th, 10th, and 14th. After an initial acclimatization of the mice for five cycles, blood pressure was monitored over a period of 40 cycles for about 30 min and averaged for the final blood pressure measurement. Blood pressure measurement were conducted at the same time each day. On the final day of Ang II infusion the intracarotid mean arterial blood pressure was measured in the mice after anesthesia with isoflurane (2%). The carotid artery was isolated and cannulated with a PE-50 microtip catheter. The intracarotid mean artery blood pressure (MAP) was measured with a pressure transducer connected to a Grass Model 7B chart recorder (AD Instrument Co, USA).
Results
Elevated endothelial HIF-1α is essential for hypertension and kidney injury and progression to renal fibrosis in Ang II-infused mice
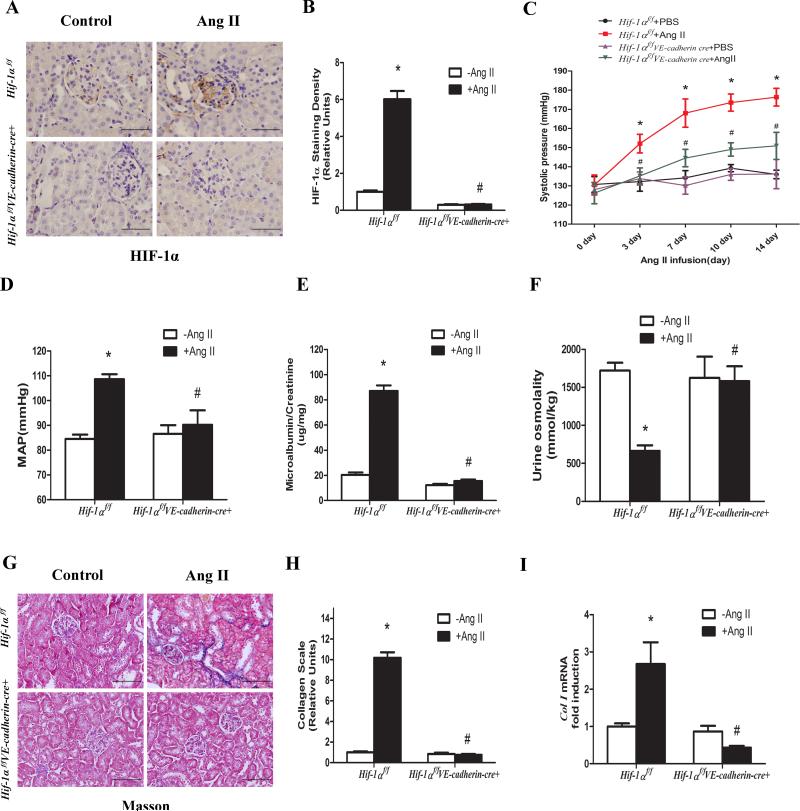
To determine the specific cell type responsible for elevated HIF-1α in the kidneys of hypertensive CKD, we infused Ang II to mice to induce hypertensive CKD. Using immunohistochemistry analysis, we found that two weeks of Ang II infusion stimulated HIF-1α protein levels in endothelial cells of kidneys compared to PBS-infused mice (Figure 1A-B). This result implicated that Ang II-induced HIF-1α in the endothelial cells may play a role in initial glomerular injury, leading to hypertension and progression to renal fibrosis.
Figure 1. Elevated endothelial HIF-1α contributes to hypertension, kidney injury and progression to renal fibrosis in Ang II-infused mice.
A, Immunohistochemical analysis showing that endothelial HIF-1α is absent in the glomeruli of Hif-1αf/fVE-cadherin cre+ mice. B, Image quantification analysis showing that HIF-1α protein levels were significantly induced in glomerular endothelial cells of Hif-1αf/f mice but not in Hif-1αf/fVE-cadherin-cre+ mice. C,Systolic blood pressure was measured at daily intervals by tail-cuff plethysmography. D, Intracarotid mean arterial blood pressure was measured on day 14 (n=8-12 per group). E & F, Ang II significantly increased microalbumin/creatinine and decreased urine osmolality in Ang II infused Hif-1αf/f mice but not Hif-1αf/fVE-cadherin cre+ mice. (n=8-12 per group). Masson's Trichrome staining (G), image quantification analysis (H) and RT-PCR analysis (I) showing significantly increased collagen deposition and mRNA levels in the kidneys of Ang II infused Hif-1αf/f mice but not Hif-1αf/fVE-cadherin-cre+ mice. Data are expressed as mean ± SEM; *P<0.05 for Ang II-infused Hif-1αf/f mice vs PBS-infused Hif-1αf/f mice; #P<0.05 for Ang II-infused Hif-1αf/fVE-cadherin cre+ mice vs Ang II-infused Hif-1αf/f mice.
Next, to precisely assess the importance of elevated HIF-1α in endothelial cells in the initiation and progression of CKD, we took a genetic approach to specifically delete HIF-1α in the endothelial cells by mating floxed-HIF-1α mice (Hif-1αf/f) with transgenic mice v-cadherin-Cre mice - (VE-cadherin-Cre+). Because basal HIF-1α levels are normally very low in kidneys compared to what we observed in kidneys of Ang II-infused mice, we conducted a coimmunofluoresence study of HIF-1α and VE-cadherin in kidneys from Ang II infused mice to determine the efficiency and specificity of the HIF-1α deletion in glomerular endothelial cells ofHif-1αf/f-VE-cadherin-Cre+ mice. The results showed that HIF-1α staining was significantly induced by Ang II in the kidneys of the Hif-1αf/f mice but not in Hif-1αf/f-VE-cadherin-Cre+ mice (Supplementary Figure S1). Moreover, HIF-1α (green fluorescence) was predominantly localized in the nuclei of the cells. Some HIF-1α positive cells were also positive for v-cadherin (red fluorescence) on the surface of glomerular endothelial cells in Ang II-infused Hif-1αf/f mice. In contrast, HIF-1α was undetectable in Ang II-infused Hif-1αf/f-VE-cadherin-Cre+ mice, while the levels of v-cadherin in Ang II-infused Hif-1αf/f-VE-cadherin-Cre+ mouse kidneys were the same as Hif-1αf/f mice (Supplementary Figure S1). These results indicate that we successfully generated mice with specific ablation of HIF-1α in the endothelial cells and Ang II-induced glomerular endothelial HIF-1α was almost completely abolished in Hif-1αf/f-VE-cadherin-Cre+ mice.
Subsequently, we chose to monitor hallmark features associated with CKD following a two-week infusion with Ang II. We found that during Ang II infusion systolic blood pressure increased significantly by day 3 and remained elevated for the duration of the 14 day experiment relative to that of mice infused with PBS (Figure 1C). However, Hif-1αf/f-VE-cadherin-Cre+ mice significantly reduced Ang II-induced systolic blood pressure (Figure 1C). We also determined mean arterial blood pressure (MAP) in carotid arteries on day 14 and found that Ang II induced MAP in Hif-1αf/f mice but not in Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 1D). Ang II infusion for two weeks in the control Hif-1αf/f mice led to proteinuria and decreased urinary osmolality (Figure 1E-F). However, the consequences of Ang II infusion were significantly reduced in Hif-1αf/f-VE-cadherin-Cre+ (Figure 1E-F). Moreover, we found that Ang II induced substantial collagen deposition and collagen I mRNA in the kidneys of the Hif-1αf/f mice but no obvious renal collagen staining and significant reduction of collagen mRNA in the Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 1G-I). Taken together, these results provide genetic evidence that elevated HIF-1α in the endothelial cells is essential to initiate kidney injury, hypertension and eventual progression to renal fibrosis in Ang II-infused mice.
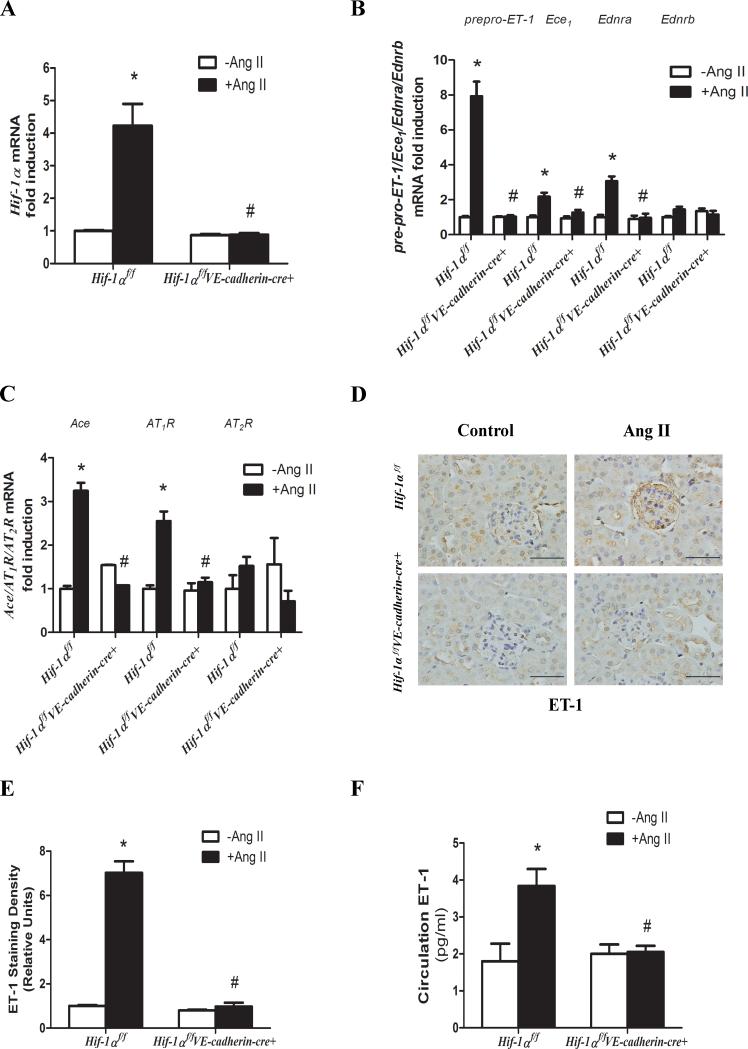
RT-PCR profiling reveals that Ang II induces renal Hif-1α gene expression and subsequently increased expression of a set of genes encoding vasoactive components
To determine the molecular basis underlying Ang II-mediated induction of HIF-1α and subsequent hypertensive CKD, we conducted high-throughput reverse-transcription polymerase chain reaction (RT-PCR) profiling. Hif-1α mRNA was among the most elevated in the kidneys of Ang II-infused Hif-1αf/f mice compared to the PBS-infused Hif-1αf/f mice (Supplementary Table S1 and Supplementary Figure S2). Furthermore, using quantitative real time RT-PCR, we confirmed that Hif-1α mRNA was significantly induced in the kidneys of Ang II-infused Hif-1αf/f mice compared to PBS-infused mice (Figure 2A). However, Ang II-induced Hif-1α mRNA was significantly reduced in Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 2A). In addition to Hif-1α, we found that the mRNAs encoding a number of vasoactive molecules were also significantly increased in the kidneys of Ang II-infused Hif-1αf/f mice. These mRNAs encode prepro-endothelin-1 (prepro-ET-1), endothelin receptor A (EDNRA), endothelin converting enzyme1 (ECE1), angiotensin type 1 receptor (AT1R) and angiotensin converting enzyme (ACE) (Figure 2B-C). Ang II-mediated induction of these mRNAs was significantly reduced in Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 2B-C). Notably, genetic deletion of Hif-1α in the endothelial cells did not affect the basal expression of these mRNAs (Figure 2B-C). These results indicate that elevated endothelial HIF-1α is essential for Ang II-induced expression of these genes encoding vasoactive molecules, but that HIF-1α is not required to maintain basal expression levels.
Figure 2. Ang II induced Hif-1α gene expression in mouse kidneys contributes to the transcriptional activation of genes encoding vasoactive factors.
A, Genetic deletion of Hif-1α in endothelial cells abolished Ang II induction of Hif-1α mRNA levels. B & C, Endothelial HIF-1α is essential for Ang II-induced expression of gene encoding preproET-1, ECE1, EDNRA, ACE, AT1R but not EDNRB or AT2R. D & E, Immunohistochemical analysis and image quantification of ET-1 in mouse kidneys. F, Circulating levels of ET-1. Data are expressed as mean ± SEM; *P<0.05 for Ang II-infused Hif-1αf/f mice vs PBS-infused Hif-1αf/f mice; #P<0.05 for Ang II-infused Hif-1αf/fVE-cadherin cre+ mice vs Ang II-infused Hif-1αf/f mice. (n=8-12 per group).
Additionally, renal endothelin receptor B (EDNRB) and angiotensin type 2 receptor (AT2R), known to function downstream of ET-1 and Ang II, respectively, to mediate vasodilatory effects, were not altered in the Ang II-infused control mice. Furthermore, deletion of endothelial cell Hif-1α did not affect basal expression of the genes encoding EDNRB or AT2R (Figure 2B-C). We also found that basal levels of mRNA levels encoding HIF-1α, vasoactive molecules and collagen in the hearts of Hif-1αf/f and Hif-1αf/f-VE-cadherin-Cre+ mice were similar (Supplementary Figure S3A-D). Ang II infusion did not induce these mRNA in the hearts of Hif-1αf/f mice or Hif-1αf/f-VE-cadherin-Cre+ mice (Supplementary Figure S3). Because of its critical role in renal physiology and blood pressure regulation we chose to measure circulating and local renal levels of ET-1. Immunostaining and image quantification showed that ET-1 levels were significantly elevated in the endothelial cells of kidneys in Ang II-infused Hif-1αf/f mice but not in Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 2D-E). Moreover, circulating ET-1 was also significantly increased in Ang II-infused Hif-1αf/f mice but not in Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 2F). Overall, these results indicate that Ang II-induced accumulation of HIF-1α in the endothelial cells contributes to hypertensive CKD by stimulating the transcription of a series of genes encoding vasoactive components, including ET-1.
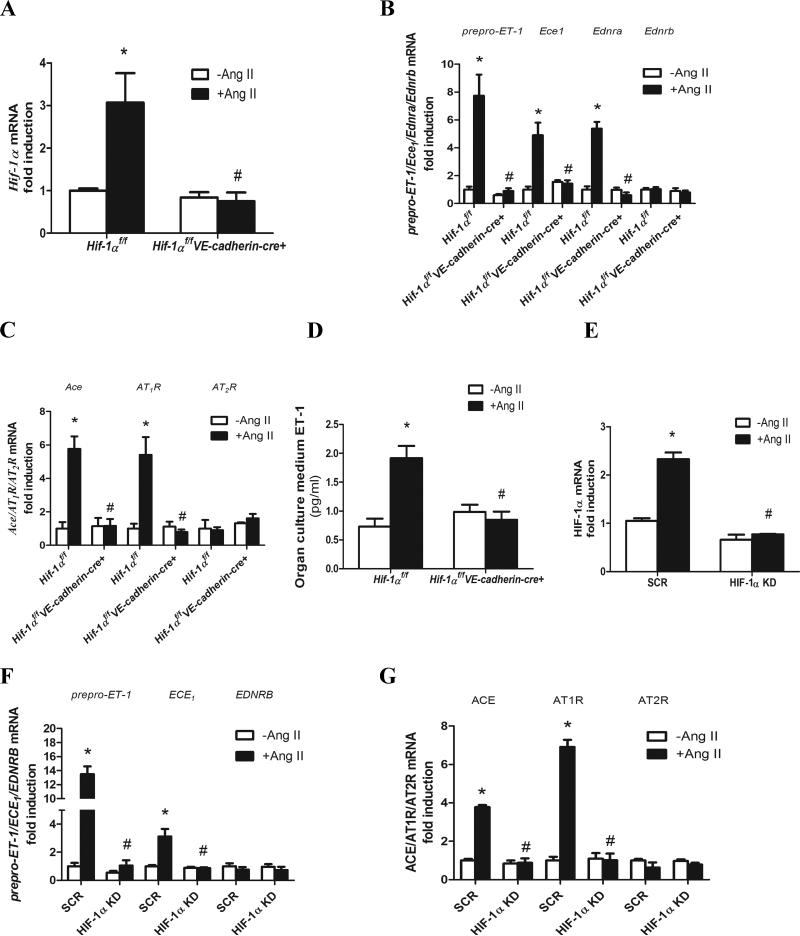
Ang II-mediated induction of Hif-1α gene expression contributes to increased expression genes encoding vasoactive molecules in isolated mouse kidney explant cultures and cultured human microvascular endothelial cells
It is difficult to decipher molecular basis underlying induction of renal HIF-1α and the subsequent increase in expression of genes encoding vasoactive molecules in intact animals. Therefore, we extended our in vivo mouse studies to the analysis of mouse kidney explant cultures. To determine the direct role of Ang II in inducing renal Hif-1α we treated kidney explants from Hif-1αf/f and Hif-1αf/f-VE-cadherin-Cre+ mice in the presence or absence of Ang II. Quantitative RT-PCR and western blot analysis revealed that Ang II induced Hif-1α mRNA and protein levels in cultured kidney explants isolated from Hif-1αf/f mice but not from Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 3A). The basal levels of Hif-1α gene expression in the cultured mouse kidney explants were similar in Hif-1αf/f mice and Hif-1αf/f-VE-cadherin-Cre+ mice. We next explored whether Ang II induced the expression of genes encoding vasoactive factors in a HIF-1α dependent manner in mouse kidney explant cultures. We found that Ang II induced the expression of prepro-ET-1, Ednra, ET-1, ECE1, AT1R and Ace genes but had no effect on the expression of Ednrb or AT2R in cultured kidney explants isolated from Hif-1αf/f mice (Figure 3B-C). Conversely, Ang II-mediated induction of mRNAs encoding these potent vasoactive molecules was significantly reduced in the cultured kidney explants isolated from Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 3B-C). ET-1 protein levels in the supernatant of cultured kidney explants isolated from HIF-1αf/f mice were significantly induced by Ang II treatment compared to the untreated mice. However, genetic ablation of endothelial Hif-1α completely abolished Ang II-induced production of ET-1 from cultured kidney explants isolated from Hif-1αf/f-VE-cadherin-Cre+ mice (Figure 3D).
Figure 3. Ang II stimulation of Hif-1α gene expression is essential for inducing a series genes encoding vasoactive factors in cultured mouse kidney explants and human microvascular endothelial cells.
A, Genetic deletion of Hif-1α in the endothelial cells abolished Ang II induction of Hif-1α mRNA levels. B & C, Endothelial HIF-1α in mouse kidney organ cultures is essential for Ang II-induced expression of genes encoding preproET-1, ECE1, EDNRA, ACE, AT1R but not EDNRB or AT2R. D, ET-1 level in the supernatants of kidney organ cultures. E, Hif-1α mRNA levels in human microvascular endothelial cells (HMECs) stably transfected with minigenes encoding scrambled control siRNA (SCR cells) or specific siRNA to knockdown HIF-1α (HIF-1a KD cells). F & G, Knockdown of HIF-1α in HIF-1α KD cells attenuated Ang II-induced expression of genes encoding prepro-ET-1, ECE1, ACE and AT1R but not EDNRB or AT2R. Data are expressed as mean±SEM.*P<0.05 for Ang II-treated mouse kidney organs or SCR cells vs untreated controls; #P<0.05 for Ang II-treated Hif-1αf/fVE-cadherin cre+ mouse kidney organs vs Ang II-infused Hif-1αf/f mouse kidney organs or SCR cells.
Next, to directly test the effect of Ang II on endothelial cells, we cultured human microvascular endothelial cells (HMECs) stably transfected with either scrambled siRNA (SCR cells) or specific siRNA to knockdown HIF-1α (HIF-1α KD cells) in the presence of absence of Ang II. Although these cells are not derived from kidneys, they represent a clonally derived source of human microvascular endothelial cells useful to examine Ang II stimulation of HIF-1α gene expression and subsequent HIF-1α-dependent stimulation of genes encoding vasoactive molecules. The results show that Ang II induced HIF-1α gene expression and the expression of a series of genes encoding vasoactive molecules (Figure 3E-G). siRNA knockdown of HIF-1α in the HMECs significantly reduced Ang II-induced expression of genes encoding vasoactive molecules (Figure 3E-G). Taken together, these results show that Ang II-induced HIF-1α gene expression is required for the transcriptional activation of genes encoding potent vasoactive factors in both cultured mouse kidney explants and human microvascular endothelial cells .
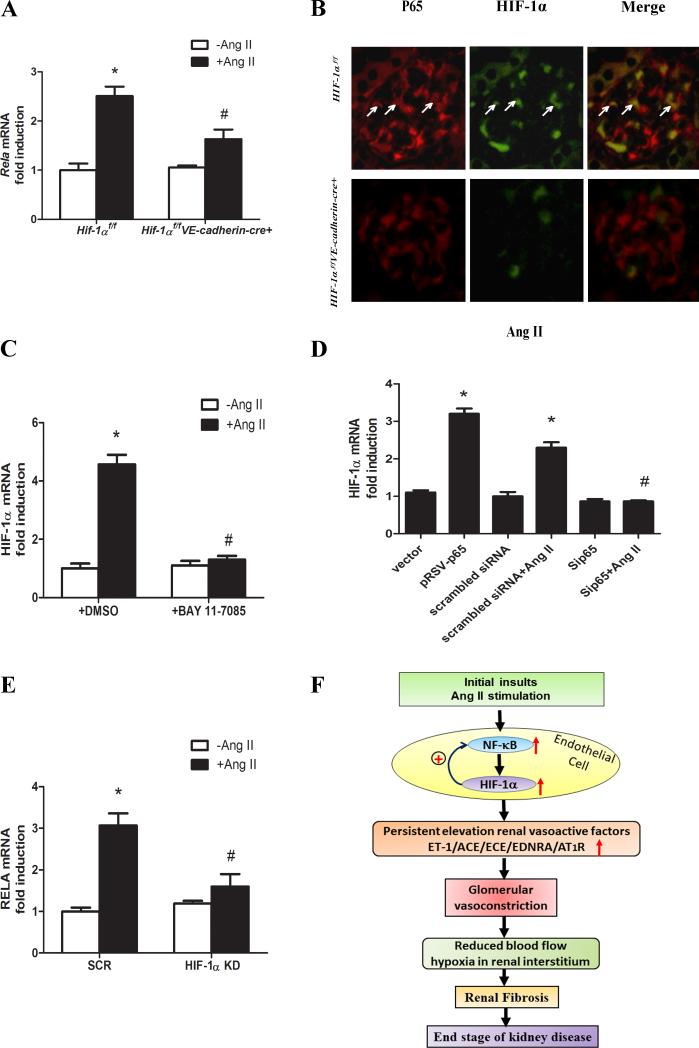
Ang II induced endothelial Hif-1α gene expression requires NF-κB
To identify the specific intracellular factors responsible for elevated Hif-1α gene expression in hypertensive CKD, we re-examined the kidney gene expression profiles in Ang II-infused mice. We found that mRNA for RELA (P65), a key subunit of the nuclear factor-κB (NF-κB) complex, was among the transcripts highly elevated in the kidneys of Ang II-infused mice (Supplementary Table S1 and Supplementary Figure S2). The elevation of renal Rela mRNA in Ang II-infused mice was confirmed by quantitative RT-PCR analysis (Figure 4A). Coimmunofluorensce analysis revealed that Ang II infusion induced glomerular NF-κB levels and that NF-κB colocalized with HIF-1α in the nuclei (Figure 4B).
Figure 4. Ang II stimulation of endothelial Hif-1α gene expression requires NF-κB and HIF-1α reciprocally upregulates NF-κB production at the transcriptional levels in a positive feedback loop.
A, Genetic deletion of Hif-1α in endothelial cells abolished Ang II-induced Rela (encodes P65 subunit of NF-κB) gene expression in kidneys of Hif-1αf/fVE-cadherin-cre+ mice. B, Double staining of P65 and HIF-1α in Ang II -infused Hif-1αf/f mice and Hif-1αf/fVE-cadherin cre+ mice. C, The effect of the NF-κB inhibitor, BAY 11-7085 (5μM) on Ang II-induced HIF-1α mRNA levels in human microvascular cells (HMECs). Data are expressed as mean±SEM. *P<0.05 for Ang II treated HMEC cell vs the control cell; #P<0.05 for BAY 11-7085(5μM) treated HMEC cell vs Ang II treated HMEC cell. D, HIF-1α mRNA levels were significantly increased in the HMECs cells transiently transfected with plasmid encoding P65 (pRSB-P65) compared to the cells transiently transfected with the control vector. However, Ang II-induced elevation of HIF-1α mRNA levels were significantly attenuated in in HMECs transiently transfected with specific siRNA to knockdown P65 (Sip65) compared to the HMECs transiently transfected with scrambled siRNA as controls (scrambled siRNA). Data are expressed as mean±SEM. *P<0.05 for Ang II treated HMEC cell or HMECs with transient transfection of scrambled siRNA vs the control cells; #P<0.05 for Ang II treated HMEC cells transiently transfected with siRNA for P65 vs Ang II treated HMEC transiently transfected with scrambled siRNA. (E) Rela gene expression in the control human microvascular endothelial cells (HMECs) stably transfected with scrambled control siRNA (SCR cell) or specific siRNA to knockdown HIF-1 α (HIF-1a KD cell) treated with or without Ang II. Data are expressed as mean±SEM.*P<0.05 for Ang II-treated SCR cells vs untreated controls; #P<0.05 for Ang II-treated HIF-1a KD cell vs Ang II treated SCR cells. F, Working model: Our studies reveal that elevated endothelial HIF-1α promotes kidney injury, hypertension and progression to renal fibrosis in a mouse model of CKD based on infusion of Ang II. Moreover, we demonstrated that Ang II directly induces endothelial Hif-1α gene expression via NF-κB activation and that elevated HIF-1α reciprocally upregulates Rela gene expression in a positive feedback loop resulting in the induction of genes encoding vasoactive factors that are likely to induce glomerular vasoconstriction. We hypothesize that without interference this malicious cycle results in chronic hypertension and decreased blood flow to renal tubular interstititium and subsequently promotes renal fibrosis and end stage renal disease. Thus, circulating vasoactive factors and cytokines are likely pathogenic biomarkers for early stages of the disease prior to renal fibrosis and they are likely effective and safe targets for early intervention.
To determine whether NF-κB is required for Ang II-induced endothelial HIF-1α gene expression we used cultured human microvascular endothelial cells (HMECs). We found that inhibition of NF-κB by a specific antagonist (Bay 11-785) or by specific siRNA knockdown of P65 in HMECs significantly attenuated Ang II-induced HIF-1α mRNA expression (Figure 4C&D). In contrast, overexpression of P65 in HMECs directly induced HIF-1α mRNA levels (Figure 4D). Moreover, immunohistochemical analysis for P65 validated that Ang II increased NF-κB protein and subsequent nuclear trans-localization in HMECs (Supplementary Figure S4). Inhibition by the NF-κB specific antagonist or siRNA knockdown of P65 attenuated Ang II-induced elevation of NF-κB levels and subsequent nuclear trans-localization in HMECs (Supplementary Figure S4). Conversely, overexpression of P65 led to increased nuclear trans-localization of P65 (Supplementary Figure S4) in HMECs. Taken together, these results indicate that NF-κB is induced in the glomeruli of CKD mice and is required for HIF-1α transcriptional upregulation and persistently elevated HIF-1α proteins in the glomerular endothelial cells of mice with CKD.
Elevated HIF-1α functions in a positive feed-back loop to stimulate increased endothelial cell NF-κB gene expression in hypertensive CKD
Unexpectedly, we found that genetic ablation of endothelial specific Hif-1α led to the reduction of NF-κB gene expression, protein levels and nuclear trans-location in Ang II infused Hif-1αf/f-VE-cadherin-Cre+ mice compared to Hif-1αf/f mice (Figure 4A-B). These results suggest a reciprocal positive transcriptional regulation of NF-κB and HIF-1α gene expression in endothelial cells in hypertensive CKD. To test this possibility, we used human microvascular endothelial cells stably transfected with minigenes encoding either scrambled siRNA (SCR cells) or specific siRNA to knockdown HIF-1α (HIF-1α KD cells) treated with or without Ang II as described above. The results (Figure 4E) show that Ang II induced expression of the RELA gene, encoding the p65 subunit of NF-κB. SiRNA knockdown of HIF-1α in HMECs significantly reduced Ang II-induced NF-κB gene expression (Figure 4E). Taken together, these results indicate that a reciprocal positive regulation of HIF-1α and RELA gene expression provides a feed forward signaling loop that maintains high levels of HIF-1α and NF-κB in endothelial cells and contributes to the progression of hypertensive CKD in Ang II infused mice.
Discussion
Using targeted mouse genetic studies coupled with in vitro cellular analysis, we demonstrated for the first time that persistently elevated endothelial HIF-1α contributes to Ang II-induced glomerular injury, hypertension and promote CKD by inducing a series of vasoactive factors. Prior to our findings multiple studies focused on the role of HIF-1α in renal tubulointerstitial fibrosis and end stage kidney disease. For example, previous studies have shown that HIF-1α contributes to the profibrotic action of Ang II in the renal medullary interstitial cells. Identified target genes encode collagen I/III, tissue inhibitor of metalloproteinase-1, proliferating cell nuclear antigen and a trans-differentiation marker, vimentin37. Similarly, silencing Hif-1α gene expression using shRNA approaches attenuates Ang II-induced profibrotic effects and transforming growth factor (TGF) β1-induced epithelial to mesenchyme transition (EMT) in renal cells in vitro and in vivo31, 38. Moreover, genetic ablation of renal epithelial Hif-1α inhibits the development of renal tubulointerstitial fibrosis in unilateral ureteral obstruction rats and that over-expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis in 5/6 nephrectomy mice39. In contrast, increasing HIF-1α levels exacerbates kidney damage in a rat model of hypertension induced by high salt diet and nitric oxide withdrawal40. Taken together, these studies suggest that overproduction of HIF-1α in the epithelial cells of the renal tubule interstitium contributes to kidney disease by stimulating EMT and fibrosis. However, nothing was known about the role of HIF-1α in the glomerular endothelium in the CKD until we conducted the targeted genetic studies presented here. Thus, our findings regarding the detrimental role of endothelial cell HIF1α in hypertensive CKD is novel and immediately highlight diagnostic and interventional possibilities for disease management (Figure 4F).
Using high throughput RT-PCR screening, we demonstrated that elevated HIF-1α in glomerular endothelial cells is essential for the transcriptional activation of a family of genes encoding well-known vasoactive factors which are key contributors to hypertension and renal impairment in the kidneys from Ang II-induced CKD mouse models. These include the potent vasoactive peptide ET-1 and vasoconstrictive receptors, EDNRA and AT1R. Genes encoding additional components of the vasoactive system include endothelin converting enzyme and angiotensin converting enzyme. It is noteworthy that vasorelaxative components such as the AT2R and EDNRB receptors were not induced. Genetic deletion of Hif-1α in the mouse endothelial cells and knockdown of Hif-1α in HMECs significantly reduced Ang II-induced expression of genes encoding vasoactive molecules, respectively.. Thus, both in vitro and in vivo studies identify the glomerular endothelium as a site for pathogenic induction of HIF-1α by Ang II. As such, HIF-1α-mediated induction of the endothelial vasoactive factors likely stimulates vasoconstriction of the efferent arterioles, resulting in reduced blood flow to the post-glomerular capillary beds surrounding the renal tubules in the medullary interstitium, thereby promoting progression of renal fibrosis.
Using RT-PCR profiling, we discovered that Ang II stimulates transcription of the Hif-1α gene by inducing expression of the Rela gene that encodes the P65 subunit of the transcription factor NF-κB. This was shown in intact animals, in cultured mouse kidney explants and in cultured human microvascular endothelial cells. Although early studies have shown that Hif-1α gene expression in immune cells is induced by the activation of NF-κB37, nothing was known about the role of NF-κB in HIF-1α gene expression in the kidneys and endothelial cells. Here we provide the first evidence that NF-κB is a common molecule functioning downstream of Ang II to induce Hif-1α gene expression in both mouse glomerular endothelium and HMECs. Subsequently, we unexpectedly found that genetic deletion of endothelial specific Hif-1α in mice reduced Ang II-induced Rela gene expression and decreased nuclear translocation of NF-κB in mouse glomerular endothelial cells. Finally, we found that siRNA specific knockdown of HIF-1α attenuated Ang II induced Rela gene expression in HMECs. Thus, we revealed HIF-1α and NF-κB can positively regulate the production of each other in endothelial cells. Taken together, our studies reveal that NF-κB contributes to Ang II induced activation of the Hif-1α gene and that HIF-1α and NF-κB function in a positive transcriptional cycle providing a feed forward signaling loop to maintain persistent elevation of HIF-1α and NF-κB levels in endothelial cells and contribute to the progression of hypertensive CKD by inducing a series of endothelial vasoactive factors (Fig.4F).
Perspectives
We report here that increased endothelial HIF-1α production is essential for renal injury and progression of kidney fibrosis in and Ang II-induced mouse model of CKD. Mechanistically, we provide evidence for a self-perpetuating transcriptional cycle involving NF-κB that serves to maintain elevated levels of HIF-1α. Taken together, studies with mice, mouse kidney cultures and human endothelial cells have determined that the reciprocal up-regulation of HIF-1α and NF-κB in endothelial cells drives the expression of a collection of genes that encode vasoactive factors initiating glomerular injury and hypertension and promoting progression to renal fibrosis (Figure.4F).
Supplementary Material
Novelty and Significance.
What Is New?
The identification of elevated endothelial HIF-1α in hypertensive CKD and its role in the initiation and progression of hypertensive CKD in Ang II-induced hypertensive CKD mouse model.
Novel mechanisms underlying persistently elevated HIF-1α in CKD by reciprocal upregualtion of NF-κB.
Up-regulation of HIF-1α and NF-κB in endothelial cells drives the expression of a collection of genes that encode vasoactive factors initiating glomerular injury and hypertension and promoting progression to renal fibrosis.
What Is Relevant?
Our current studies have added new insight to the role of elevated endothelial HIF-1α in hypertensive CKD. Additionally, our discoveries indicate therapeutic possibilities targeting HIF-1α.
Summary
We have provided mouse genetic and human cellular evidence showing that increased HIF-1α expression in the endothelial cell plays a crucial role in hypertensive CKD. Circulating vasoactive factors are likely early pathogenic biomarkers for early stages of the disease prior to renal fibrosis and they are likely effective and safe targets for early intervention.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health Grants HL119549 (to Y.X.), RC4HD067977 and HD34130 (to Y.X and R.E.K.), and by China National Science Foundation 81228004 (to Y.X.).
Footnotes
Disclosures
None.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Whaley-Connell AT, Sowers JR, Stevens LA, McFarlane SI, Shlipak MG, Norris KC, Chen SC, Qiu Y, Wang C, Li S, Vassalotti JA, Collins AJ. Kidney Early Evaluation Program I. Ckd in the united states: Kidney early evaluation program (keep) and national health and nutrition examination survey (nhanes) 1999-2004. Am J Kidney Dis. 2008;51:S13–20. doi: 10.1053/j.ajkd.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Whitman IR, Feldman HI, Deo R. Ckd and sudden cardiac death: Epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol. 2012;23:1929–1939. doi: 10.1681/ASN.2012010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 6.Perry HM, Jr., Miller JP, Fornoff JR, Baty JD, Sambhi MP, Rutan G, Moskowitz DW, Carmody SE. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension. 1995;25:587–594. doi: 10.1161/01.hyp.25.4.587. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O'Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT., Jr. Chronic Renal Insufficiency Cohort Study I. Hypertension awareness, treatment, and control in adults with ckd: Results from the chronic renal insufficiency cohort (cric) study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol. 2008;110:e1–7. doi: 10.1159/000148256. [DOI] [PubMed] [Google Scholar]
- 9.Theilig F. Spread of glomerular to tubulointerstitial disease with a focus on proteinuria. Ann Anat. 2010;192:125–132. doi: 10.1016/j.aanat.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Ruggenenti P, Remuzzi G. The role of protein traffic in the progression of renal diseases. Annu Rev Med. 2000;51:315–327. doi: 10.1146/annurev.med.51.1.315. [DOI] [PubMed] [Google Scholar]
- 11.Fine LG, Orphanides C, Norman JT. Progressive renal disease: The chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74–78. [PubMed] [Google Scholar]
- 12.Shoji K, Tanaka T, Nangaku M. Role of hypoxia in progressive chronic kidney disease and implications for therapy. Curr Opin Nephrol Hypertens. 2014;23:161–168. doi: 10.1097/01.mnh.0000441049.98664.6c. [DOI] [PubMed] [Google Scholar]
- 13.Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza GL. Targeting hif-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Brune B. Cytokines and hormones in the regulation of hypoxia inducible factor-1alpha (hif-1alpha). Cardiovasc Hematol Agents Med Chem. 2006;4:189–197. doi: 10.2174/187152506777698344. [DOI] [PubMed] [Google Scholar]
- 16.Sakoda Y, Anand S, Zhao Y, Park JJ, Liu Y, Kuramasu A, van Rooijen N, Chen L, Strome SE, Hancock WW, Chen L, Tamada K. Herpesvirus entry mediator regulates hypoxia-inducible factor-1alpha and erythropoiesis in mice. J Clin Invest. 2011;121:4810–4819. doi: 10.1172/JCI57332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–19578. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- 18.Chanda R, Fenves AZ. Hypertension in patients with chronic kidney disease. Curr Hypertens Rep. 2009;11:329–336. doi: 10.1007/s11906-009-0056-z. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases: Expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 21.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Zhang R, Zhang YY, Huang XR, Wu Y, Chung AC, Wu EX, Szalai AJ, Wong BC, Lau CP, Lan HY. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin ii-induced hypertensive cardiac disease. Hypertension. 2010;55:953–960. doi: 10.1161/HYPERTENSIONAHA.109.140608. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JS, Zhang YL, Wang HX, Xia YL, Wang L, Jiang YN, Li HH, Liu Y. Identification of genes related to the early stage of angiotensin ii-induced acute renal injury by microarray and integrated gene network analysis. Cell Physiol Biochem. 2014;34:1137–1151. doi: 10.1159/000366327. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Huang XR, Lan HY. Smad3 mediates ang ii-induced hypertensive kidney disease in mice. Am J Physiol Renal Physiol. 2012;302:F986–997. doi: 10.1152/ajprenal.00595.2011. [DOI] [PubMed] [Google Scholar]
- 25.Jennings BL, Anderson LJ, Estes AM, Yaghini FA, Fang XR, Porter J, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome p450 1b1 contributes to renal dysfunction and damage caused by angiotensin ii in mice. Hypertension. 2012;59:348–354. doi: 10.1161/HYPERTENSIONAHA.111.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. At1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin ii-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Zhang W, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y. Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via a2b adenosine receptor contributes to chronic hypertension. Circ Res. 2013;112:1466–1478. doi: 10.1161/CIRCRESAHA.111.300166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin ii and egf receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 29.Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2b adenosine receptor-mediated induction of il-6 promotes ckd. J Am Soc Nephrol. 2011;22:890–901. doi: 10.1681/ASN.2010080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah BH, Catt KJ. Tace-dependent egf receptor activation in angiotensin-ii-induced kidney disease. Trends Pharmacol Sci. 2006;27:235–237. doi: 10.1016/j.tips.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Q, Wang Z, Xia M, Li PL, Van Tassell BW, Abbate A, Dhaduk R, Li N. Silencing of hypoxia-inducible factor-1alpha gene attenuated angiotensin ii-induced renal injury in sprague-dawley rats. Hypertension. 2011;58:657–664. doi: 10.1161/HYPERTENSIONAHA.111.177626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou CC, Irani RA, Zhang Y, Blackwell SC, Mi T, Wen J, Shelat H, Geng YJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody-mediated tumor necrosis factor-alpha induction contributes to increased soluble endoglin production in preeclampsia. Circulation. 2010;121:436–444. doi: 10.1161/CIRCULATIONAHA.109.902890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Parchim NF, Iriyama T, Luo R, Zhao C, Liu C, Irani RA, Zhang W, Ning C, Zhang Y, Blackwell SC, Chen L, Tao L, Hicks MJ, Kellems RE, Xia Y. Excess light contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension. 2014;63:595–606. doi: 10.1161/HYPERTENSIONAHA.113.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. American journal of hypertension. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 36.Iriyama T, Sun K, Parchim NF, Li J, Zhao C, Song A, Hart LA, Blackwell SC, Sibai BM, Chan LN, Chan TS, Hicks MJ, Blackburn MR, Kellems RE, Xia Y. Elevated placental adenosine signaling contributes to the pathogenesis of preeclampsia. Circulation. 2015;131:730–741. doi: 10.1161/CIRCULATIONAHA.114.013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li PL, Li N. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin ii in renal medullary interstitial cells. Kidney Int. 2011;79:300–310. doi: 10.1038/ki.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via hif-1 stimulation of epithelial-to-mesenchymal transition. The Journal of clinical investigation. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of hif-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295:F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dallatu MK, Choi M, Oyekan AO. Inhibition of prolyl hydroxylase domain-containing protein on hypertension/renal injury induced by high salt diet and nitric oxide withdrawal. J Hypertens. 2013;31:2043–2049. doi: 10.1097/HJH.0b013e32836356a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.