Abstract
Background
The degree of pain relief required to diagnose sacroiliac joint (SIJ) dysfunction following a diagnostic SIJ block (SIJB) is not known. No gold standard exists. Response to definitive (i.e., accepted as effective) treatment might be a reference standard.
Methods
Subgroup analysis of 320 subjects enrolled in two prospective multicenter trials evaluating SIJ fusion (SIJF) in patients with SIJ dysfunction diagnosed by history, physical exam and standardized diagnostic SIJB. A 50% reduction in pain at 30 or 60 minutes following SIJB was considered confirmatory. The absolute and percentage improvements in Visual Analog Scale (VAS) SIJ pain and Oswestry Disability Index (ODI) scores at 6 and 12 months after SIJF were correlated with the average acute improvement in SIJ pain with SIJB.
Results
The average pain reduction during the first hour after SIJB was 79.3%. Six months after SIJF, the overall mean VAS SIJ pain reduction was 50.9 points (0-100 scale) and the mean ODI reduction was 24.6 points. Reductions at 12 months after SIJF were similar. Examined in multiple ways, improvements in SIJ pain and ODI at 6 and 12 months did not correlate with SIJB findings.
Conclusions
The degree of pain improvement during SIJB did not predict improvements in pain or ODI scores after SIJF. A 50% SIJB threshold resulted in excellent post-SIJF responses. Using overly stringent selection criteria (i.e. 75%) to qualify patients for SIJF has no basis in evidence and would withhold a beneficial procedure from a substantial number of patients with SIJ dysfunction.
Level of Evidence
Level 1.
Clinical Relevance
The degree of pain improvement during an SIJ block does not predict the degree of pain improvement after SIJ fusion.
Keywords: sacroiliac joint dysfunction, sacroiliac joint fusion, diagnostic sacroiliac joint block, risk factors
Introduction
Sacroiliac joint (SIJ) dysfunction is a term used to describe pain and disability resulting from abnormal function of the SIJ. Causes of SIJ dysfunction are myriad and can include osteoarthritic degeneration, traumatic SIJ disruption, SIJ laxity due to changes during pregnancy, autoimmune disease, and other etiologies. For patients with SIJ pain sufficient to seek surgical treatment, SIJ dysfunction was associated with prominent decreases in quality of life scores similar to those of other spinal conditions that are commonly treated surgically,1 suggesting a high burden of disease.2
Non-surgical treatments for SIJ dysfunction include pain medications, physical therapy, SIJ steroid injections, chiropractic treatments or prolotherapy, and radiofrequency (RF) ablation. High quality scientific evidence supporting intraarticular steroid administration, a procedure which is commonly performed in the US, is still lacking. While two small blinded randomized trials demonstrated the short-term effectiveness of RF ablation of the lateral branches of the sacral nerve roots,3, 4 12-month follow-up from one of these trials suggested that this intervention may only give rise to modest long-term pain relief.5
For patients not responding to non-surgical treatment, open and minimally invasive SIJ fusion (SIJF) surgeries are options that may provide symptomatic relief. The popularity of minimally invasive SIJ fusion continues to increase6 and several devices are now commercially available. The majority of published literature reporting the clinical outcomes of these patients employed porous coated triangular titanium implants placed across the SIJ. The 12-month results from a prospective, multicenter randomized trial showed that patients with carefully diagnosed SIJ dysfunction exhibited far larger improvements in SIJ pain, disability and quality of life after treatment with minimally invasive SIJF compared to nonsurgical management.7 A companion prospective multicenter single-arm study also showed that SIJF with this same device resulted in marked, immediate and sustained improvements in pain, disability and quality of life.8
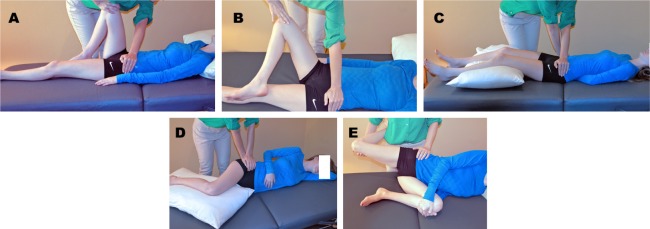
Current best practices for diagnosing the SIJ as the source of low back pain include history, physical examination using provocative testing maneuvers that stress the SIJ, and confirmatory diagnostic SIJ blocks (SIJB). Physical examination maneuvers that stress the SIJ and are considered positive when the maneuver reproduces the patient's typical pain are shown in Figure 1. Multiple studies, summarized in a recent systematic review,9 have indicated that the occurrence of at least 3 positive physical examination signs in patients suspected of having SIJ dysfunction was highly predictive of their response to SIJB. In contrast to lumbar spine conditions in which imaging is considered to be critical for diagnosis, the painful SIJ is not associated with characteristic findings on plain radiographic or cross-sectional imaging with the exception being inflammatory findings in patients with seropositive10 or seronegative11 autoimmune spondyloarthropathies. At this time, plain radiographic and cross-sectional imaging are largely used to rule out other competing diagnoses, leaving SIJB as the de facto current standard for the confirmation of SIJ dysfunction preliminarily made based upon the history and physical exam.
Fig. 1.
Physical examination tests for sacroiliac joint dysfunction: A: thigh thrust; B: flexion, abduction, and external rotation (FABER); C: pelvic gapping (distraction); D: compression; E: Gaenslen test.
As part of a diagnostic SIJB, the joint is accessed under fluoroscopic guidance (or CT) and a small amount of contrast mixed with a short-acting local anesthetic is injected with the intent of achieving intra-articular delivery. The patient's response during the minutes to hours following the injection is monitored; as with blocks of other body structures, a marked transient decrease in typical pain at rest, or pain elicited by typical triggering activities, is accepted as a positive test and suggests that the blocked joint is the source of pain. This approach is supported by multiple pain and anesthesia physician societies.12–16 Potential issues associated with SIJB include insufficient anesthesia of the entire joint or extravasation of the injectate outside of the joint which may serve to anesthetize other structures in close proximity to the SIJ. A more basic question is the degree to which the response to such a block predicts clinical outcomes. Proceeding under the assumption that the diagnostic injection of local anesthetics and treatment procedures (e.g., RF ablation of the sacral nerve roots or SIJF) work by the same mechanism of action, the responses to SIJB and these therapeutic interventions should be correlated. Following this logic, patients with little or no response to SIJB should, if they were treated, derive little or no benefit from such treatment. However, this theory has borne out for other spinal conditions. For instance, In patients with lumbar facet joint pain diagnosed by facet block, there was little correlation between pain relief during a medial branch block and subsequent response RF-based denervation of the facet.17 In this report, we used a similar analytic approach to examine the correlation between the immediate response to SIJB and the 6- and 12-month pain and disability scores of patients undergoing SIJF in two prospective multicenter clinical trials.
Materials and Methods
Patient population
This analysis is based on a combined dataset of patients participating in two prospective multicenter clinical trials evaluating SIJF (INSITE and SIFI) conducted in the United States. INSITE (NCT01681004) is a randomized trial comparing SIJF to non-surgical management (NSM) for patients with SIJ dysfunction due to degenerative sacroiliitis or sacroiliac joint disruption. Patients were diagnosed with SIJ dysfunction by history (including a positive Fortin finger test), at least 3 positive physical examination provocative maneuvers that stress the SIJ and are predictive of SIJB response,9 and confirmatory SIJB (described further below). Other tests were performed, as required, to rule out competing diseases, and patients with known severe low back or hip pathology were excluded from the study. (For details on eligibility criteria, see the published reports.7, 8) Subjects were assigned in a 2:1 ratio to either SIJF (SIJF) using the iFuse Implant System® (SI-BONE, Inc., San Jose, California, USA) or non-surgical management (NSM). NSM consisted of pain medications, physical therapy, SIJ steroid injections and RF ablation of the lateral branches of the sacral nerve roots, delivered in serial fashion according to patient needs.
INSITE included pre-randomization and scheduled post-randomization assessments of SIJ pain measured using a 0-100 visual analog scale (VAS), Oswestry Disability Index (ODI),18 quality of life measures (SF-36 and EuroQOL-5D, whose results are discussed elsewhere7, 19) and satisfaction with treatment. The study's primary endpoint was the proportion of patients with clinical improvement, defined as a composite of an improvement in VAS SIJ pain of at least 20 points and the absence of device-related neurologic events or reoperation. The 6-month results confirmed the superiority of SIJF relative to NSM in terms of pain, disability and quality of life outcomes, with high satisfaction rates in the surgery group.20 At 12 months after surgery, the subjects who crossed over from NSM to SIJF (which was allowed by the study protocol after the 6-month visit) experienced improvements in pain, disability and quality of life which were of approximately the same magnitude as those originally observed with SIJF.7
SIFI (NCT01640353) is a companion single-arm clinical trial with enrollment criteria, study assessments and follow-up that were identical to INSITE in most respects but lacked a corresponding nonsurgical control arm. 12-month results from SIFI showed that SIJF brought about similar, clinically important and statistically significant improvements in pain, disability and quality of life with high patient satisfaction rates.8
SIJ block
In both studies, SIJB was used during the screening phase to confirm the presence of SIJ dysfunction. During the block, which was performed according to a minimum standard, patients provided a pre-block numeric rating scale (NRS) score (0-10 scale) as well as NRS scores at 30 and 60 minutes after the block. All blocks were performed under fluoroscopic guidance and the majority of blocks were performed using local anesthetic only; some blocks included steroids which are not known to give rise to any acute reductions in pain and therefore would not be expected to affect the analyses reported herein. To qualify for the study, the pre-block NRS rating had to be at least 5 and at least one post-block score had to show a ≥50% reduction compared to pre-block. The average response to SIJB was calculated as (NRSpre-block – average(NRS30/60))/NRSpreblock × 100%, as previously described by Cohen et al.17 A small number of patients who did not provide both 30 and 60 minute scores were eliminated from this analysis.
The responses to SIJF were assessed using both 6- and 12-month follow-up VAS SIJ pain and ODI scores. Using the 6-month scores is advantageous for two reasons. First of all, INSITE allowed crossover from NSM to SIJF at 6 months and the crossover rate was very high (>80%). Thus, valid comparisons of surgical and non-surgical responses were therefore only possible at 6 months. Second, we believed that recording the response to surgery after a relatively short period of time after SIJF and would serve to mitigate any confounding due to post-treatment factors such as the development of lumbar pain or other surgical issues that could diminish longer-term results (e.g., implant loosening, which was rare7) and therefore interfere with patients’ assessments of their SIJ pain. In contrast, 12-month scores may be advantageous to analyze so as to have pre-treatment predictors of longer-term responses to surgical treatment. Because INSITE and SIFI showed SIJF to be a definitive treatment for SIJ pain, this manuscript focuses primarily on the relationship between SIJB responses and responses to SIJF.
Statistical Methods
Baseline demographic and clinical characteristics were compared across studies using a Student's t test (for normally distributed continuous variables), Wilcoxon's test (for non-normal continuous variables) and Fisher's exact test (for nominal variables). Proportional odds logistic regression was used to determine the relationship between baseline demographic parameters and both pre-SIJB scores as well as SIJB response scores categorized by decile.
The relationship between the average response to SIJB and the change from baseline in VAS SIJ pain or ODI scores at 6 and 12 months was explored graphically and with Pearson's product-moment correlation coefficient. Change scores were calculated as both absolute changes as well as percent improvements. Logistic regression was used to determine the relationship between the likelihood of threshold improvements in VAS SIJ pain/ODI scores and SIJB responses (as a continuous variable and by decile of SIJB responses). Logistic regression was also used to compare the likelihood of achieving 6- and 12-month VAS SIJ pain scores ≤30 (0-100 scale) or ODI scores ≤20. All calculations were performed in R.21
Results
Combining both prospective trials, 320 subjects were enrolled at 38 centers (Table 1) of which 274 underwent immediate SIJF and 46 underwent initial non-surgical management. The mean (SD) age was 51.1 (11.2) years and 60.9% were women. The pain level documented prior to block was either <4 or not recorded in 4 cases, leaving 316 subjects available for this analysis (145 in INSITE and 171 in SIFI). The mean (SD) NRS pain levels immediately prior to block were 7.4 (1.4, range 4-10), with no variation across studies (Wilcoxon p=0.0899).
Table 1.
Characteristics of enrolled subjects in INSITE and SIFI.
| Characteristic | Non-Surgical Management (n=46) | SI Joint Fusion (n=274)* | P-value** |
|---|---|---|---|
| Age, mean (SD, range) =65 years old, n (%) |
53.8 (10.6, 29.5-71.1) 8 (17.4%) |
50.6 (11.3, 23.5-71.7) 32 (11.7%) |
0.7245 0.3325 |
| Women, n (% female) | 28 (60.9%) | 195 (71.2%) | 0.1684 |
| Race, n (%) White Black American Indian Hawaiian, Pacific Islander Other |
43 (93.5%) 2 (4.3%) 0 (0.0%) 0 (0.0%) 1 (2.2%) |
263 (96.0%) 5 (1.8%) 1 (0.4%) 1 (0.4%) 4 (1.5%) |
0.4786 |
| Ethnicity Hispanic or Latino, n (%) | 4 (8.7%) | 11 (4.0%) | 0.2459 |
| Body mass index, mean (SD, range) | 30.6 (6.6, 19.4-48.9) | 29.7 (6.5, 14.1-51.0) | 0.1549 |
| Smoking status, n (%) Current smoker Former smoker Never smoker | 3 (6.5%) 13 (28.3%) 30 (65.2%) | 70 (25.5%) 79 (28.8%) 125 (45.6%) | 0.0050 |
| Condition DS SD |
18 (39.1%) 28 (60.9%) |
79 (28.8%) 195 (71.2%) |
0.4132 |
| Prior lumbar fusion (n, %) | 17 (37.0%) | 116 (42.3%) | 0.5222 |
| Years of pain, mean (range) | 5.0 (0.5-38.9) | 5.8 (0.4-41.1) | 0.1059 |
| VAS SI joint pain score, mean (±SD) | 82.3 (10.0) | 80.7 (12.6) | 0.0589 |
| ODI score, mean (±SD) | 56.2 (14.3) | 56.0 (11.9) | 0.2422 |
Combines INSITE (n=102) and SIFI (n=172)
Fisher p-value for ordinal variables; t test for continuous variables
The average response over the hour following SIJB was 79.3% (SD 19.2%, range 28.6-100.0%) with no variation by study (p=0.8984). One hundred ninetyfive patients (61.7%) had an average SIJB reduction ≥75% and 118 (37.3%) had an average reduction <75%. 83 (26.3%) subjects exhibited a reduction in SIJ pain following SIJB that was between 50 and 75% at both 30 and 60 minutes. Univariate regression showed no correlations between the average pain reduction following a SIJB and age, gender, body mass index, pain level prior to the SIJB, duration of SIJ pain, history of lumbar fusion, underlying condition, smoking status, or opioid usage at baseline.
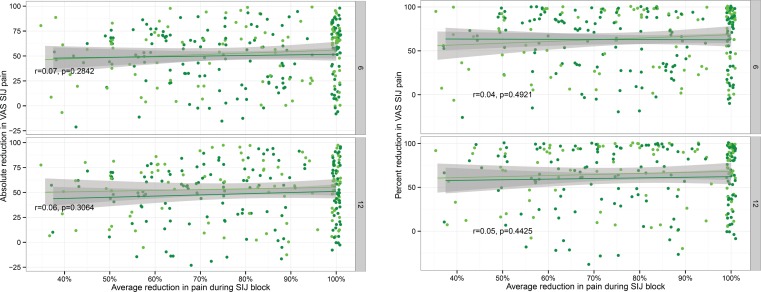
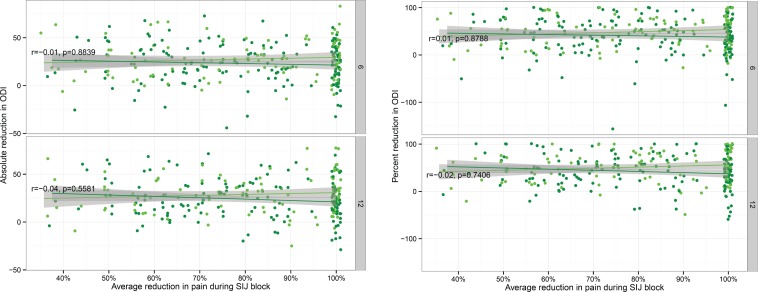
At 6 and 12 months, the mean (SD) reductions in VAS SIJ pain were 50.9 (28.6) and 50.8 (29.2) points (both p<.0001 compared to baseline). Relative to baseline, the percent reductions in VAS SIJ pain at 6 and 12 months were 62.8% and 62.9%, respectively. The 6-month improvements in SIJ pain were larger in the SIJF group than those recorded for the NSM group by approximately 38.5 points (p<.0001; see Polly et al.7 for the details of the comparisons between surgical and non-surgical treatment; the current investigation focuses primarily on the response to SIJF). Similarly, the 6- and 12-month reductions in ODI scores were 24.6 (20.5) and 25.8 (20.5) points (p<.0001 for both comparisons vs. baseline); the corresponding percentage reductions were 43.3 and 45.6%, respectively. Figure 2 shows the 6- and 12-month improvements in VAS SIJ pain from baseline values plotted as absolute change (top) or relative change (bottom) as a function of average pain response following the SIJB and stratified by treatment assignment. Figure 3 shows the corresponding changes in ODI plotted similarly. Both of these figures show no relationship between the degree of improvements in VAS SIJ pain or ODI scores and the average response to SIJB.
Fig. 2.
Six- and 12-month absolute (top) and relative (i.e., percent, bottom) improvement in VAS SIJ pain by average pain reduction during SIJB. Each dot represents an individual SIJF in INSITE (light green) or SIFI (dark green). Values are jittered slightly for visualization. Shaded areas represent 95% smoothed confidence intervals from linear regression. Plots show Pearson correlation coefficient combined across studies and associated p-values.
Fig. 3.
Six- and 12-month absolute (top) and relative (i.e., percent, bottom) improvement in Oswestry Disability Index (ODI) by average pain reduction during SIJB. Each dot represents an individual SIJF in INSITE (light green) or SIFI (dark green). Values are jittered slightly for visualization. Shaded areas represent 95% smoothed confidence intervals from linear regression. Plots show Pearson correlation coefficient combined across studies and associated p-values.
Table 2 shows the proportion of subjects achieving a threshold 6-month improvement in VAS SIJ pain (20 points) or ODI scores (15 points) as a function of average response to SIJB. For both outcomes, logistic regression confirmed the lack of a relationship between achieving the threshold response and the average response following SIJB (VAS SIJ pain, p=0.8407; ODI, p=0.6368). Equivalent analyses using 12 month scores (not shown) also showed no associations between the longer-term response to SIJF and the average response following SIJB.
Table 2.
Proportion of SIJF and NSM subjects in INSITE and SIFI achieving a threshold reduction in pain or ODI score at month 6 by average response during screening SIJB.
| At least 20 point reduction in VAS SIJ pain | At least 15 point reduction in ODI Score | |||
|---|---|---|---|---|
| SIJ Fusion | NSM | SIJ Fusion | NSM | |
| Average response during SIJ block | ||||
| 40-50% | 25/32 (78.1%) | 0/5 (0.0%) | 19/32 (59.4%) | 0/5 (0.0%) |
| 50-60% | 22/27 (81.5%) | 1/4 (25.0%) | 17/27 (63.0%) | 0/4 (0.0%) |
| 60-70% | 28/33 (84.8%) | 1/3 (33.3%) | 20/33 (60.6%) | 1/3 (33.3%) |
| 70-80% | 27/35 (77.1%) | 2/9 (22.2%) | 26/35 (74.3%) | 0/9 (0.0%) |
| 80-90% | 36/44 (81.8%) | 2/8 (25.0%) | 33/44 (75.0%) | 1/8 (12.5%) |
| 90-100% | 80/97 (82.5%) | 6/16 (37.5%) | 67/97 (69.1%) | 4/16 (25.0%) |
| All | 219/271 (80.8%) | 12/45 (26.7%) | 183/271 (67.5%) | 6/45 (13.3%) |
A similar analysis was performed to examine the proportion of patients who achieved VAS SIJ pain or ODI scores below selected thresholds (≤30 for VAS SIJ pain and ≤20 for ODI) at 6 (Table 3) and 12 months (not shown). 57.9% and 56.5% of subjects undergoing SIJF had a pain score ≤30 at 6 and 12 months, respectively; the proportions achieving this pain score were not predicted by average response to SIJB (logistic regression p=0.7605 and 0.7943). Likewise, 36.9% and 32.5% of subjects undergoing SIJF achieved an ODI score ≤20 at 6 and 12 months, respectively, which were also not predicted by the response to SIJB (p=0.6340 and 0.5014).
Table 3.
Proportion of SIJF and NSM subjects achieving 6 month pain or ODI scores less than indicated threshold by average response during screening SIJ block.
| VAS SIJ Pain at 6 Mo =30 | ODI at 6 Mo =20 | |||
|---|---|---|---|---|
| SIJ Fusion | NSM | SIJ Fusion | NSM | |
| Average response during SIJ block | ||||
| 40-50% | 18/32 (56.2%) | 0/5 (0.0%) | 12/32 (37.5%) | 0/5 (0.0%) |
| 50-60% | 16/27 (59.3%) | 0/4 (0.0%) | 10/27 (37.0%) | 0/4 (0.0%) |
| 60-70% | 20/33 (60.6%) | 1/3 (33.3%) | 9/33 (27.3%) | 1/3 (33.3%) |
| 70-80% | 20/35 (57.1%) | 0/9 (0.0%) | 18/35 (51.4%) | 0/9 (0.0%) |
| 80-90% | 23/44 (52.3%) | 0/8 (0.0%) | 19/44 (43.2%) | 0/8 (0.0%) |
| 90-100% | 60/97 (61.9%) | 3/16 (18.8%) | 32/97 (33.0%) | 2/16 (12.5%) |
| All | 157/271 (57.9%) | 4/45 (8.9%) | 100/271 (36.9%) | 3/45 (6.7%) |
Discussion
An increasing body of evidence derived from prospective clinical trials7, 8 (and summarized in a systematic review22) supports the effectiveness of minimally invasive SIJF as treatment option that improves pain, disability and quality of life in patients with SIJ dysfunction who have not responded to non-surgical treatment. Given the positive results from these studies, it becomes critical to carefully define the target patient population for this treatment. The best available evidence suggests that a combination of history, physical examination and confirmatory diagnostic SIJB may be utilized to identify individuals with SIJ dysfunction. However, across the entire spectrum of non-traumatic painful conditions, there is still no gold standard to confirm that a patient's pain arises from a particular part of the body. A possible reference standard could be the response to definitive treatment; nevertheless, this approach is challenging when the definitive treatment is surgical in nature and may be associated with significant adverse events.
An analysis of SIJB data collected during the screening phase of two prospective multicenter clinical trials demonstrated no relationship between the degree of response to SIJB and the subsequent response to minimally invasive SIJF. Overall, the response rate to SIJF – measured in several different ways – was high across the spectrum of SIJB responses, provided that the measured response exceeded the 50% threshold for enrollment at least one of the two assessed post-SIJB time points. The studies’ pooled sample size was large (>300 subjects, of whom 274 underwent SIJF), suggesting that if an effect were present, the combined data had sufficient power (sample size) to detect it. While it seems axiomatic that the degree of improvement in pain following an anesthetic block of a painful joint should predict the response to treatment of that joint, we were unable to demonstrate this in our analysis. We offer the following potential explanations.
The most likely explanation is that SIJB as implemented in our study served as a “binary” confirmatory test, i.e., SIJB serves to confirm the SIJ as the cause of pain in patients whose pain source was diagnosed based primarily on history and physical examination but otherwise provides little additional information. In both studies, study qualification criteria required patients to have a history of pain consistent with SIJ dysfunction, a positive Fortin finger test (in which a patient points to the posterior superior iliac spine with a single finger when indicating the location of pain),23 and typical pain occurring with at least 3 physical examination maneuvers thought to stress the SIJ. Multiple studies have compared findings on physical exam in populations similar to those we studied to SIJB results. In a systematic review/meta-analysis,9 the sensitivity and specificity of at least 3 positive physical examination maneuvers for a positive diagnostic SIJB were 85% and 76%, respectively, and the diagnostic odds ratio (i.e., the relative increase in odds of having a positive SIJB for patients with positive vs. negative physical exam tests) was 17.2. The studies summarized this meta-analysis generally used a 75% or 80% threshold as a positive SIJB. These findings, along with the high response rates to definitive treatment, suggest that SIJB as implemented in our study correctly identified the majority of patients with SIJ dysfunction. In other words, our study confirmed that patient selection using history and physical exam, confirmed via a positive diagnostic SIJB, successfully identified a patient population with a high likelihood of responding favorably to SIJF, with high 1-year success rates independent of the degree of responsiveness to SIJB (provided that there is at least a 50% reduction in acute pain at one early time point after the block).
In general, a diagnostic approach based on history, physical examination and confirmatory block may be superior to imaging-based approaches. Although diagnostic blocks have been employed to diagnose lumbar disc disease as a pain generator, provocative discography is not recommended by any surgical societies and this procedure has largely fallen out of favor.24 While cross-sectional imaging is universally used for diagnosing lumbar spine pathology, its utility remains controversial. Recent recommendations from the American Association of Neurosurgeons / Congress of Neurological Surgery25 do not provide specific details as to what MRI findings constitute lumbar disc disease for which surgery is clearly indicated whereas other guidelines propose the existence of Modic changes should represent such a requirement.26 However, no high-quality evidence has shown that MRI studies are predictive of better outcomes after lumbar fusion. Moreover, various MRI irregularities are common in people without any symptoms,27–29 suggesting that imaging for lumbar spine conditions may have a high false positive rate. Other than for inflammatory conditions, imaging for SIJ diagnosis is not thought to be helpful and, as with the lumbar spine, the prevalence of SIJ abnormalities appears to be high in individuals without suspected SIJ-mediated pain.30 Our results suggest that at least for SIJ dysfunction, the combination of history, physical examination and confirmatory diagnostic blocks may be sufficient to identify a patient population which exhibits a high response rate to definitive surgical treatment.
Several additional factors may explain why the hypothesized relationship was not observed. First, the hypothesis that “joint block response predicts treatment response” assumes that the same mechanism of action underlying the observed effect of pain relief is similar between SIJB and SIJF. The pain relief arising from a block results from a biochemical interaction between local anesthetics and the nerves innervating the articular portion of the SIJ, subchondral bone, capsule, and surrounding ligaments whereas pain relief that occurs following fusion relies on the mechanical stabilization of the SIJ (initially with hardware and later by adhesion of bone to the implants and actual fusion of the joint itself). Mechanical stabilization may alter load transfer across the SIJ, which could affect both the intra-articular surfaces as well as adjacent extra-articular structures such as muscles and ligaments. Thus, it is possible that SIJ stabilization/fusion may affect more distant structures. Moreover, the innervation of the SIJ is complex and variable31–33 so that anesthetizing only the articular portion of the SIJ may only incompletely block SIJ pain.34 SIJ dysfunction may give rise to a combination of noxious stimuli generated by the articular surfaces, extra-articular SI joint structures, and altered local, regional, and/or global biomechanics. Furthermore, maladaptation to SIJ dysfunction may result in altered function and pain in associated/related anatomic structures which are obviously not able to be addressed acutely by a SIJB. Regardless, whether the pain relief mechanisms underlying SIJB and SIJF are similar enough to yield a statistically significant correlation is unknown.
Other aspects of the SIJB which were not detected in this study may also modulate its effectiveness. The expected correlation relies on the delivery of “perfectly executed” blocks and “perfect joint fusion.” For SIJB, one would have to assume that all patients received an optimal block, consisting of access to the SIJ that did not itself provoke pain and delivery of anesthetic into the entire extent of the joint space (as opposed to just the lower pole near the needle access point) without any leakage that could anesthetize pain arising from other structures. Ventral leakage of anesthetic has been reported in 16% of cases, which could certainly diminish the anesthetic effects of SIJ anesthesia or cause false positive responses mediated by the lumbosacral plexus.35 Some physicians routinely ask patients undergoing SIJB to walk immediately after their injection in an attempt to facilitate the dispersion of anesthetic throughout the joint; similarly, patients may also be instructed to repeat painful activities before and after the block. Collectively, these factors may all contribute to variability in SIJB effectiveness. For SIJF, the correlational hypothesis also relies on adequate stabilization of the SIJ. While the vast majority of these trial patients received 3 implants, some procedures were complicated by suboptimal implant placement, and the poor pain relief seen in some of these subjects may be due to inadequate SIJ stabilization rather than misdiagnosis.
Another issue is that many trial participants had a history of prior lumbar fusion, a known risk factor for SIJ dysfunction36 and SIJ degeneration,37 or presented with concomitant spine and/or hip conditions, possibly because the same degenerative processes that predisposed them to the development of hip and/or spine arthritis also caused SIJ degeneration. It is possible that long-term responses to SIJF were affected to some degree by these competing diagnoses. The coexistence of multiple pain generators, which were variably addressed during trial followup,38 could result in a blurring of the relationship between SIJB (i.e., acute treatment of one pain generator) and overall response to SIJ treatment. Moreover, ODI, the trials’ primary measure of disability due to pain, does not distinguish between pain from the hip, spine or SIJ.
Fourth, for many conditions, possibly including the SIJ, it is well accepted that the pain experience varies daily and depends on psychological factors. In this study, the pain and disability assessments occurred at fixed time points (i.e. baseline, 6 months, 12 months postoperatively). Thus, it is conceivable that this day-to-day variation in pain confounded the relationship between the SIJB response and long-term pain response after SIJF.
Another possibility is that the proposed relationship between SIJB and response to SIJF exists but our trial excluded patients in whom the relationship was more likely to be shown. Our trials included only patients whose SIJ dysfunction was confirmed by a 50% or greater decrease in acute pain at 30 or 60 minutes after an SIJB whereas individuals with smaller responses were excluded. Had the “low response” group undergone SIJF, it is possible that their responses would have diminished as well. Given that a marked response to SIJB is accepted as a part of the diagnostic workup, an examination of responses to SIJF in such a “low SIJB responder group” would be challenging.
Another less likely possibility is that the SIJB might not allow for the identification of any actual biological response. Stated differently, the combination of history and physical examination might be sufficient to diagnose SIJ dysfunction such that SIJB provides no further information. This theory is somewhat refuted by the high frequencies with which blocks are used in modern medicine and the fact that many patients with various pathologic disorders appear to derive substantial pain relief from both blocks (temporary relief) and joint fusion (long-term relief). Moreover, it is unlikely that a majority of subjects would derive durable pain relief (up to 4-5 years in published retrospective cohorts [which used the same SIJB procedure to identify patients]39, 40) following SIJF if the block were not detecting a true phenomenon.
The lack of a relationship between the degree of response to an anesthetic block and the degree of response to definitive treatment is not new. Cohen et al examined the relationship between the response to lumbar facet blocks and the subsequent response to RF ablation of the nerves innervating lumbar facet joints.17 In this prospective study, no cutoff reliably identified a patient population that responded better to lumbar facet RF ablation. In addition, there was very little correlation between the response to lumbar facet blocks and the eventual response to treatment, a finding that mirrors those of our study. Other investigations also demonstrate no correlation between the responses to diagnostic tests and the subsequent responses to definitive treatment in a wide variety of settings (see Table 4).
Table 4.
Response to treatment in those with high or low responses to diagnosis of the underlying condition. Chart courtesy of Steven Cohen.*
| Author | N | Procedure | Comparison | Results |
|---|---|---|---|---|
| Cohen et al. 20071 | 92 | Cervical facet RF | >50% vs. >80% | 56% success rate in > 50% group vs. 58% in > 80% group |
| Erdek et al. 20102 | 50 | Celiac plexus neurolysis | >50% vs. >80% | 56% success rate in > 50% group vs. 54% in > 80% group |
| Cohen et al. 20073 | 262 | Lumbar facet RF | >50% vs. >80% | 52% success rate in > 50% group vs. 56% in > 80% group |
| Stojanovic et al. 20104 | 77 | Lumbar facet RF | >50% vs. >80% | 47% success rates in both groups |
| Williams et al. 20115 | 244 | Spinal cord Stimulation | <50% vs. >50% vs. >75% | 18% in < 50% vs. 90% in > 50% vs. 71% in > 75% groups |
| Cohen et al. 20096 | 77 | SI joint RF | >50% vs. >80% | 51% success rate in > 50% group vs. 49% in > 80% group |
| Huang et al. 20127 | 101 | Pulsed RF of occipital nerves | <50% vs. >50% vs. >80% | 50% in < 50% vs. 48% in > 50% vs. 58% in > 75% groups |
| McGreevy 20138 | 32 | Superior hypogastric neurolysis | % pain relief | Mean pain relief of 75% for positive outcomes vs. 82% for negative outcomes |
Personal communication, Steven Cohen, MD (Johns Hopkins University), September 2, 2015.
Cohen SP, Bajwa ZH, Kraemer JJ, et al. Factors predicting success and failure for cervical facet radiofrequency denervation: a multi-center analysis. Reg Anesth Pain Med. 2007;32(6):495-503. doi:10.1016/j.rapm.2007.05.009.
Erdek MA, Halpert DE, González Fernández M, Cohen SP. Assessment of celiac plexus block and neurolysis outcomes and technique in the management of refractory visceral cancer pain. Pain Med Malden Mass. 2010;11(1):92-100. doi:10.1111/j.1526-4637.2009.00756.x.
Cohen SP, Hurley RW, Christo PJ, Winkley J, Mohiuddin MM, Stojanovic MP. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain. 2007;23(1):45-52. doi:10.1097/01.ajp.0000210941.04182.ea.
Stojanovic MP, Sethee J, Mohiuddin M, et al. MRI analysis of the lumbar spine: can it predict response to diagnostic and therapeutic facet procedures? Clin J Pain. 2010;26(2):110-115. doi:10.1097/AJP.0b013e3181b8cd4d.
Williams KA, Gonzalez-Fernandez M, Hamzehzadeh S, et al. A multi-center analysis evaluating factors associated with spinal cord stimulation outcome in chronic pain patients. Pain Med Malden Mass. 2011;12(8):1142-1153. doi:10.1111/j.1526-4637.2011.01184.x.
Cohen SP, Strassels SA, Kurihara C, et al. Outcome Predictors for Sacroiliac Joint (Lateral Branch) Radiofrequency Denervation. Reg Anesth Pain Med. 2009;34(3):206-214. doi:10.1097/AAP.0b013e3181958f4b.
Huang JHY, Galvagno SM, Hameed M, et al. Occipital nerve pulsed radiofrequency treatment: a multi-center study evaluating predictors of outcome. Pain Med Malden Mass. 2012;13(4):489-497. doi:10.1111/j.1526-4637.2012.01348.x.
McGreevy K, Hurley RW, Erdek MA, Aner MM, Li S, Cohen SP. The effectiveness of repeat celiac plexus neurolysis for pancreatic cancer: a pilot study. Pain Pract Off J World Inst Pain. 2013;13(2):89-95. doi:10.1111/j.1533-2500.2012.00557.x.
So how does this affect the manner in which the diagnosis SIJ dysfunction may be established? Our data, collected from two prospective clinical trials, provide level 1 evidence supporting two conclusions: first, the method of selecting patients for SIJF, i.e., a combination of history, physical examination and positive (≥50%) response to SIJB, is validated by the high response rate in patients thusly diagnosed. A 75% reduction in SIJ pain following a SIJB has been advocated as an appropriate threshold but this proposal has no basis in evidence, contrasts with findings reported herein, and is in conflict with studies of other painful conditions that have shown no significant relationships between diagnostic block responses and eventual pain relief from definitive treatment. In our studies, 37.3% of subjects had an average SIJB reduction of <75% and 26.3% had reductions of <75% at both 30 and 60 minutes. Application of a higher threshold (e.g., 75%) would have rendered these patients ineligible for SIJF whereas the study showed that they ultimately had responses as high as the remaining subjects.
Our analysis raises several potential avenues of further research. It is clear that standardization of SIJB is important and the addition of physical examination diagnostic maneuvers or physical performance tests (e.g., the up-and-go41 test, the sit-stand test42 or gait assessments43) before and after an SIJB may represent useful adjunctive assessments. Examining the relationship between the number of positive physical exam findings suggestive of SIJ dysfunction and response to SIJF is of interest. Finally, it would be instructive to evaluate SIJF outcomes of patients with SIJ dysfunction but who report sub-50% responses to SIJB.
In summary, our results suggest that a threshold of 50% is clinically relevant and employing higher thresholds runs the risk of unnecessarily rejecting patients who would otherwise benefit from undergoing a potentially helpful procedure.
Conclusions
In this study of >300 patients who were diagnosed with SIJ dysfunction by history, physical examination, and confirmatory SIJB, the 6- and 12-month improvements in SIJ pain and disability scores after SIJF were independent of the degree of improvement in acute pain during an SIJB. Based on these findings, a threshold of 50% reduction in pain following SIJB was associated with excellent post-surgical outcomes. The use of overly stringent selection criteria for determining when SIJF should be performed may serve to withhold a beneficial procedure from a substantial number of patients with SIJ dysfunction.
Disclosures
All authors conduct clinical research for SI-BONE. The studies analyzed herein are sponsored by SIBONE (San Jose, CA). SI-BONE manufactures the device used in the clinical trials. David Polly has no financial conflict. Peter Whang is a paid SI-BONE consultant participating primarily in educational events. Clay Frank is an SI-BONE consultant participating primarily in educational events, but receives only reasonable expense reimbursement as compensation. Daniel Cher is an SI-BONE employee.
References
- 1.Cher DJ, Reckling WC. Quality of life in preoperative patients with sacroiliac joint dysfunction is at least as depressed as in other lumbar spinal conditions. Med Devices Evid Res. 2015 doi: 10.2147/MDER.S92070. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cher D, Polly D, Berven S. Sacroiliac Joint pain: burden of disease. Med Devices Evid Res. 2014;7:73–81. doi: 10.2147/MDER.S55197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel N, Gross A, Brown L, Gekht G. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med Malden Mass. 2012;13(3):383–398. doi: 10.1111/j.1526-4637.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SP, Hurley RW, Buckenmaier CC, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279–288. doi: 10.1097/ALN.0b013e31817f4c7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel N. Twelve-Month Follow-Up of a Randomized Trial Assessing Cooled Radiofrequency Denervation as a Treatment for Sacroiliac Region Pain. Pain Pract Off J World Inst Pain. 2015 Jan; doi: 10.1111/papr.12269. [DOI] [PubMed] [Google Scholar]
- 6.Lorio MP, Polly DW, Jr, Ninkovic I, Ledonio CGT, Hallas K, Andersson G. Utilization of Minimally Invasive Surgical Approach for Sacroiliac Joint Fusion in Surgeon Population of ISASS and SMISS Membership. Open Orthop J. 2014;8:1–6. doi: 10.2174/1874325001408010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polly DW, Cher DJ, Wine KD, Whang PG, Frank CJ, Harvey CF, Lockstadt H, Glaser JA, Limoni RP, Sembrano JN, INSITE Study Group Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion Using Triangular Titanium Implants Vs Nonsurgical Management for Sacroiliac Joint Dysfunction: 12-Month Outcomes. Neurosurgery. 2015 Aug; doi: 10.1227/NEU.0000000000000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duhon B, Cher D, Wine K, Kovalsky D, Lock-stadt H, on behalf of the SIFI Study Group Triangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: A Prospective Study. Glob Spine J. 2015 doi: 10.1055/s-0035-1562912. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RSGM. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354–368. doi: 10.1016/j.jpain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Hermann K-GA, Bollow M. Magnetic resonance imaging of sacroiliitis in patients with spondyloarthritis: correlation with anatomy and histology. RöFo Fortschritte Auf Dem Geb Röntgenstrahlen Nukl. 2014;186(3):230–237. doi: 10.1055/s-0033-1350411. [DOI] [PubMed] [Google Scholar]
- 11.Puhakka KB, Jurik AG, Schiottz-Christensen B, Hansen GVO, Egund N, Christiansen JV, Stengaard-Pedersen K. Magnetic resonance imaging of sacroiliitis in early seronegative spondylarthropathy. Abnormalities correlated to clinical and laboratory findings. Rheumatology. 2004;43(2):234–237. doi: 10.1093/rheumatology/keh008. [DOI] [PubMed] [Google Scholar]
- 12.Pauza KJ, Aprill C, Bogduk N, Catlin R, Derby R, Dreyfuss P, Endres S, Furman M, Geraci MJ, Karasek M, Kine G, Lerman R, Lutz G, Malanga GA, McCann M, Prunskis J, Rosenfield L, Sabers S, Sawchuk T, Smith B, Young J, Weinstein SM, Bagnall D, Becker BE, Chou LH, Puttlitz KM, Thomas SA, Zuhosky JP, Casazza BA, Davis SA, deDianous DK, Lento PH, Roberts ST, Saffir M, Zuhosky JP. Educational Guidelines for Interventional Spinal Procedures. American Academy of Physical Medicine and Rehabilitation; 2008. pp. 1–48. http://www.aapmr.org/practice/guidelines/documents/edguidelines.pdf. [Google Scholar]
- 13.Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, Bryce DA, Burks PA, Caraway DL, Calodney AK, Cash KA, Christo PJ, Cohen SP, Colson J, Conn A, Cordner H, Coubarous S, Datta S, Deer TR, Diwan S, Falco FJE, Fellows B, Geffert S, Grider JS, Gupta S, Hameed H, Hameed M, Hansen H, Helm Ii S, Janata JW, Justiz R, Kaye AD, Lee M, Manchikanti KN, McManus CD, Onyewu O, Parr AT, Patel VB, Racz GB, Sehgal N, Sharma ML, Simopoulos TT, Singh V, Smith HS, Snook LT, Swicegood JR, Vallejo R, Ward SP, Wargo BW, Zhu J, Hirsch JA. An Update of Comprehensive Evidence-Based Guidelines for Interventional Techniques in Chronic Spinal Pain. Part II: Guidance and Recommendations. Pain Physician. 2013;16(2 Suppl):49–S283. [PubMed] [Google Scholar]
- 14.Bogduk N. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. 2nd ed. San Francisco: International Spine Intervention Society; 2013. Sacroiliac Joint Access; pp. 523–555. [Google Scholar]
- 15.American Society of Anesthesiologists Task Force on Chronic Pain Management, American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810–833. doi: 10.1097/ALN.0b013e3181c43103. [DOI] [PubMed] [Google Scholar]
- 16.Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Reprinted 2002. 1994. http://www.iasp-pain.org/Free-Books?navItemNumber=677.
- 17.Cohen SP, Strassels SA, Kurihara C, Griffith SR, Goff B, Guthmiller K, Hoang HT, Morlando B, Nguyen C. Establishing an optimal “cutoff ” threshold for diagnostic lumbar facet blocks: a prospective correlational study. Clin J Pain. 2013;29(5):382–391. doi: 10.1097/AJP.0b013e31825f53bf. [DOI] [PubMed] [Google Scholar]
- 18.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–2952. doi: 10.1097/00007632-200011150-00017. discussion 2952. [DOI] [PubMed] [Google Scholar]
- 19.Cher DJ, Polly DWJ. Improvement in Health State Utility after Sacroiliac Joint Fusion: Comparison to Normal Populations. Glob Spine J. 2015 Jun; doi: 10.1055/s-0035-1556581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whang PG, Cher D, Polly D, Frank C, Lockstadt H, Glaser J, Limoni R, Sembrano J. Sacroiliac Joint Fusion Using Triangular Titanium Implants vs. Non-Surgical Management: Six-Month Outcomes from a Prospective Randomized Controlled Trial. Int J Spine Surg. 2015;9 doi: 10.14444/2006. Article 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org/ [Google Scholar]
- 22.Heiney J, Capobianco R, Cher D. Systematic review of minimally invasive sacroiliac joint fusion using a lateral transarticular approach. Int J Spine Surg. 2015;9 doi: 10.14444/2040. Article 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortin JD, Falco FJ. The Fortin finger test: an indicator of sacroiliac pain. Am J Orthop Belle Mead NJ. 1997;26(7):477–480. [PubMed] [Google Scholar]
- 24.Alamin TF, Kim MJ, Agarwal V. Provocative lumbar discography versus functional anesthetic discography: a comparison of the results of two different diagnostic techniques in 52 patients with chronic low back pain. Spine J Off J North Am Spine Soc. 2011;11(8):756–765. doi: 10.1016/j.spinee.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser MG, Eck JC, Groff MW, Watters WC, Dailey AT, Resnick DK, Choudhri TF, Sharan A, Wang JC, Mummaneni PV, Dhall SS, Ghogawala Z. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 1: introduction and methodology. J Neurosurg Spine. 2014;21(1):2–6. doi: 10.3171/2014.4.SPINE14257. [DOI] [PubMed] [Google Scholar]
- 26.NASS Coverage Committee. Lumbar Fusion: Defining Appropriate Coverage Positions. Burr Ridge, IL: North American Spine Society; https://www.spine.org/Documents/PolicyPractice/CoverageRecommendations/L.… Accessed September 1, 2015. [Google Scholar]
- 27.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 28.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(3):403–408. [PubMed] [Google Scholar]
- 29.Boden SD. The use of radiographic imaging studies in the evaluation of patients who have degenerative disorders of the lumbar spine. J Bone Joint Surg Am. 1996;78(1):114–124. doi: 10.2106/00004623-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Eno J-J, Boone C, Bellino M, Bishop J. The Prevalence of Sacroiliac Joint Degeneration in Asymptomatic Adults. J Bone Joint Surg Am. 2015;97(11):932–936. doi: 10.2106/JBJS.N.01101. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto N, Yamashita T, Takebayashi T, Sekine M, Ishii S. An electrophysiologic study of mechanoreceptors in the sacroiliac joint and adjacent tissues. Spine. 2001;26(20):E468–E471. doi: 10.1097/00007632-200110150-00008. [DOI] [PubMed] [Google Scholar]
- 32.Grob KR, Neuhuber WL, Kissling RO. [Innervation of the sacroiliac joint of the human] Z Für Rheumatol. 1995;54(2):117–122. [PubMed] [Google Scholar]
- 33.Roberts SL, Burnham RS, Ravichandiran K, Agur AM, Loh EY. Cadaveric study of sacroiliac joint innervation: implications for diagnostic blocks and radiofrequency ablation. Reg Anesth Pain Med. 2014;39(6):456–464. doi: 10.1097/AAP.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 34.Dreyfuss P, Henning T, Malladi N, Goldstein B, Bogduk N. The Ability of Multi-Site, Multi-Depth Sacral Lateral Branch Blocks to Anesthetize the Sacroiliac Joint Complex. Pain Med. 2009;10(4):679–688. doi: 10.1111/j.1526-4637.2009.00631.x. [DOI] [PubMed] [Google Scholar]
- 35.Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. AJNR Am J Neuroradiol. 1999;20(8):1429–1434. [PMC free article] [PubMed] [Google Scholar]
- 36.DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med Malden Mass. 2011;12(2):224–233. doi: 10.1111/j.1526-4637.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 37.Ha K-Y, Lee J-S, Kim K-W. Degeneration of sacroiliac joint after instrumented lumbar or lumbosacral fusion: a prospective cohort study over fiveyear follow-up. Spine. 2008;33(11):1192–1198. doi: 10.1097/BRS.0b013e318170fd35. [DOI] [PubMed] [Google Scholar]
- 38.Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine. 2009;34(1):E27–E32. doi: 10.1097/BRS.0b013e31818b8882. [DOI] [PubMed] [Google Scholar]
- 39.Vanaclocha VV, Verdú-López F, Sánchez-Pardo M, Gozalbes-Esterelles L, Herrera JM, Rivera-Paz M, Martínez-Gómez D. Minimally Invasive Sacroiliac Joint Arthrodesis: Experience in a Prospective Series with 24 Patients. J Spine. 2014;3(5) doi: 10.4172/2165-7939.1000185. [DOI] [Google Scholar]
- 40.Rudolf L, Capobianco R. Five-Year Clinical and Radiographic Outcomes After Minimally Invasive Sacroiliac Joint Fusion Using Triangular Implants. Open Orthop J. 2014;8:375–383. doi: 10.2174/1874325001408010375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 42.Csuka M, McCarty DJ. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78(1):77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 43.Kibsgård TJ, Røise O, Sturesson B, Röhrl SM, Stuge B. Radiosteriometric analysis of movement in the sacroiliac joint during a single-leg stance in patients with long-lasting pelvic girdle pain. Clin Biomech Bristol Avon. 2014;29(4):406–411. doi: 10.1016/j.clinbiomech.2014.02.002. [DOI] [PubMed] [Google Scholar]