Abstract
A 3-y-old female Xenopus laevis was reported for a gray mass on the abdomen. The frog was used for egg collection and was otherwise experimentally naïve. On physical exam, the frog was bright and active and had a firm, gray, lobulated mass (1.5 cm × 0.5 cm × 0.5 cm) in the cutaneous tissue of the left lateral abdomen. An excisional biopsy was performed under anesthesia, and the entire mass was removed and processed for histopathology. Microscopically, the dermis was greatly expanded by connective tissue with a marked decrease in the number of glands, and occasional degenerative glands were present. When stained with Masson trichrome, the excessive connective tissue stained blue, indicating that it was composed of collagen. With Verhoeff–van Gieson staining, the connective tissue stained bright red with an absence of black-staining material, demonstrating the presence of collagen and ruling out elastic fibers. In light of the morphology of the mass and the results of the special stains, the mass was diagnosed as a collagenoma. To our knowledge, this report is the first description of a collagenoma in X. laevis.
The African clawed frog (Xenopus laevis) is a popular and widely used model in biomedical research. The primary disciplines that use this model are developmental biology, cell biology, toxicology, and neuroscience. Despite its wide use, neoplastic diseases have rarely been reported in this species. Reported neoplasms include hepatomas, teratomas, renal carcinoma, fibroma, fibrosarcoma, nephroblastoma, ovarian dysgerminoma, melanophoroma, and lymphoma.1,4,11,12,18,22 Those found in the skin include cystadenopapillomas and adenocarcinomas, which develop from the epithelium of skin mucous glands, and spontaneous pigment tumors (melanophoromas).15 However, the mass in this case did not resemble these neoplasms on histopathology. This report describes the first documented occurrence of a collagenoma in X. laevis. A collagenoma is a rare connective-tissue nevus of unknown cause that shows autosomal dominant inheritance in humans.24 Associated conditions have included cardiovascular disorders, hypogonadism, congenital exophthalmos, learning disabilities, hypertrichosis, nystagmus, café-au-lait macules, and acanthosis nigricans.3 Connective tissue nevi that are not associated with other diseases do not require treatment,14 and the prognosis for this frog is good.
Case Report
All X. laevis at our institution are housed in an AAALAC-accredited facility under controlled temperature and humidity and on a 12:12-h light:dark cycle. They are maintained at a density of no more than 1 frog per 4 L of water in an automated 400-galn continuous-flow recirculating system with 10% water changes daily (Pentair Aquatic Habitats, Apopka, FL). Input water is filtered through a Type 2 Elix water-purification system (Merck Millipore, Darmstadt, Germany), adjusted to 1600 mS with marine salt, and maintained at pH 7.4 and a temperature of 18 to 20 °C. Conductivity, pH, and temperature are monitored continuously and adjusted automatically to maintain these parameters. Water undergoes mechanical filtration, charcoal filtration, UV irradiation, and biofiltration. Frogs are fed exclusively a standard diet of frog brittle twice weekly (Nasco, Fort Atkinson, WI).
An approximately 3-y-old African clawed frog was reported for a skin mass. This frog had been imported from Nasco (Fort Atkinson, WI) and used for egg collection on an IACUC-approved animal study protocol. On this protocol, female frogs are induced to lay eggs by subcutaneous injection of chorionic gonadotropin the night before use. The next morning, eggs are manually expressed. This frog was otherwise experimentally naïve. She was separated from the remainder of the colony due to concern of an infectious etiology. The hospital tank had an increased salinity, a solution of 100 mM marine salt, because of its antiseptic properties. On physical exam, she was bright and alert with normal activity level and appetite. There was a raised, firm, round, lobulated gray mass measuring approximately 1.5 cm × 0.5 cm × 0.5 cm on her left lateral abdomen (Figure 1). At 6 d after the initial presentation, a fine-needle aspirate was performed. The frog was manually restrained, and a 25-gauge needle was inserted into the mass 3 times. A syringe was then attached to the needle, and the collected material was expelled onto a slide; a direct smear was made and examined microscopically. The mass was very firm and solid on needle penetration, and the aspirate was nondiagnostic, given that it did not yield any cells. To obtain a definitive diagnosis and prognosis, an excisional biopsy with histopathology was performed.
Figure 1.
The frog on physical exam. There is an oval, raised, firm, multilobular gray mass (1.5 cm × 0.5 cm × 0.5 cm) on left lateral abdomen (circle).
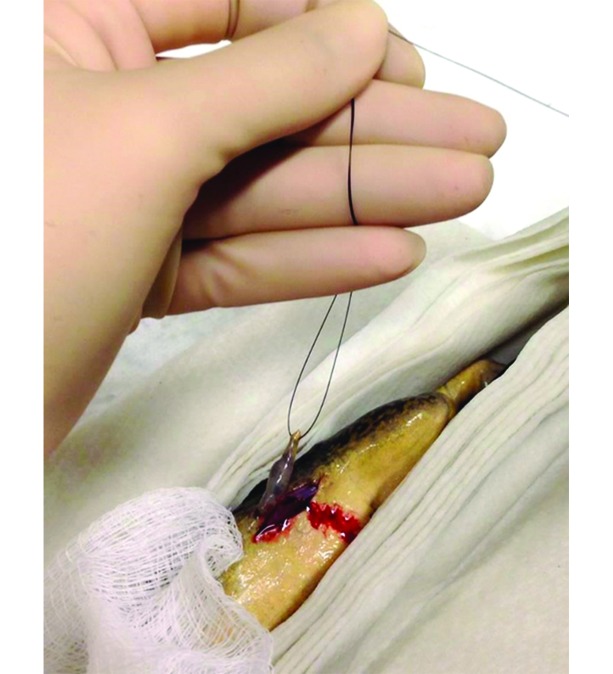
On day 8 after presentation, the frog was anesthetized in a submersion bath of MS222 buffered with sodium bicarbonate (1 g/L). The skin was prepared by using a sterile saline flush, and the frog was maintained on a bed of MS222-moistened paper towels. Bupivacaine (1 mg/kg; 0.25%, Hospira, Lake Forest, IL) was topically applied directly over the site for analgesia. The excisional biopsy was performed by placing a stay suture to elevate the mass and performing an elliptical incision around the mass (Figure 2).The skin was closed with 6 simple interrupted nylon sutures (Figure 3). Once the surgery was complete, the frog was placed in a recovery tank containing system water and monitored until she was fully recovered. The biopsy was processed for histopathology by using hematoxylin and eosin, Masson trichrome, and Verhoeff–Van Gieson stains.
Figure 2.
Excisional biopsy of MS222-anesthetized frog. A stay suture has been placed to elevate the mass for ease of removal.
Figure 3.
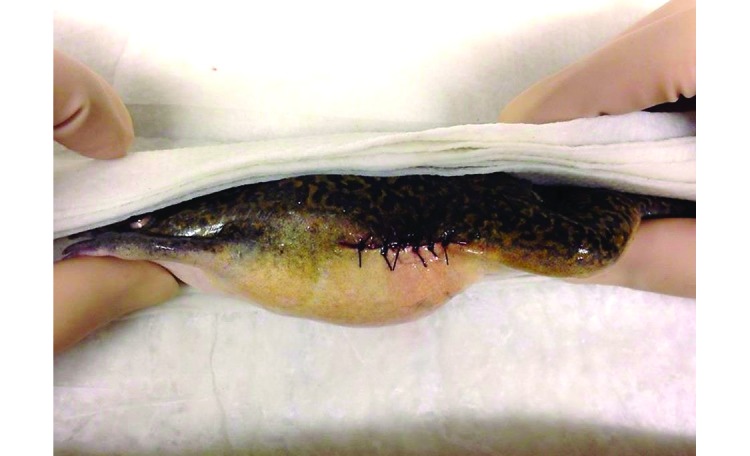
Immediately postoperatively, 6 simple interrupted nylon sutures were placed to close the skin incision.
The frog recovered from anesthesia uneventfully, and the sutures were removed 4 wk after surgery, when the skin was completely healed. She was placed back into a system tank and continues to thrive.
Pathology.
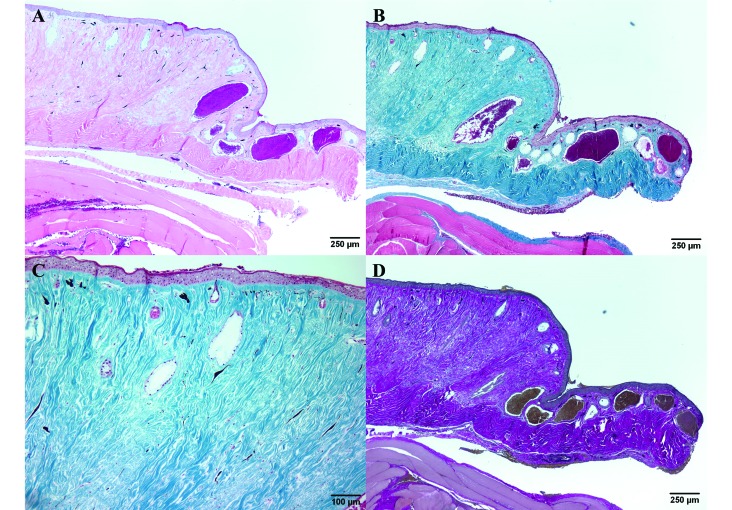
Microscopically, the loose connective-tissue layer of the dermis was markedly expanded, elevating the epidermis, and the deeper dense connective-tissue layer was mildly expanded due to abundant bundles of connective tissue. Within the expanded dermis, the number of glands was markedly decreased and occasional degenerate glands were present (Figure 4 A). The excessive connective tissue stained blue with Masson trichrome, indicating that it was composed of collagen fibers (Figure 4 B), and evaluation at increased magnification revealed a paucity of normal dermal glands (Figure 4 C). To rule out the presence of elastic fibers, a Verhoeff–van Gieson stain was used. The bright-red staining of the connective tissue with an absence of black-staining material confirmed the presence of collagen but not elastic fibers (Figure 4 D). The unaffected skin was of normal thickness and contained serous and mucus glands that were normal in number and morphology.
Figure 4.
(A) The dermis is greatly expanded by connective tissue and shows a marked decrease in the number of glands. Hematoxylin and eosin stain. (B) The excessive connective tissue stains blue, indicating that it is composed of collagen. Masson trichrome stain. (C) Higher magnification of panel B. The connective tissue of the dermis expanded by abundant blue-staining collagen; few normal dermal glands remain. Masson trichrome stain. (D) The bright-red staining of the connective tissue with an absence of black-staining material demonstrates the presence of collagen and an absence of elastic fibers. Verhoeff–van Gieson stain.
Discussion
There are several causes of skin lesions in frogs, including fungal, viral, parasitic, and bacterial infections and neoplasia. Fungal infections, particularly of Batrachochytrium dendrobatidis, can cause hyperkeratosis, sloughing, erosions, and occasional ulcerations in the epidermis of the digits and ventrum6 and affect important physiologic functions. Other clinical signs include lethargy, dehydration, dysecdysis, and occasional neurologic signs. In addition, this pathogen is associated with high mortality rates.7 Other than a skin lesion, this frog did not present with any other signs suggestive of an infection with Batrachochytrium dendrobatidis. Saprolegnia spp. and other water molds are primary skin pathogens, but a sustained low-level chronic mortality is the most common presentation.7 The characteristic lesions are grayish-white tufts of cottony mold on the skin. Water molds are often associated with previously debilitated, injured, or diseased animals and poor water quality.10 These features were not associated with the frog in this case.
Parasitic infections were also ruled out. Pseudocapillaroides xenopi is the most common parasite to cause skin lesions,9 including a mottled and roughened appearance with mild multifocal erosion and ulceration,2 but infections are typically related to stress, water temperature, and water-quality problems and usually cause significant morbidity and mortality.13
Although viral causes of skin disease in frogs are rare, mortality due to infection with Ranaviruses is the most common.13 Ranaviruses in frogs cause severe erythematous and vesicular or erosive skin lesions and have been implicated in amphibian deaths. In addition, this pathogen typically causes disease in the early life stages of amphibians, and clinical signs include hemorrhages and general signs of sepsis.10 The lesions and clinical signs characteristic of Ranavirus infection were not observed in the frog in this case.
There are several bacterial infections that cause dermal lesions in frogs. ‘Red leg syndrome’ is the name attributed to the characteristic dermal lesions most often caused by bacteria. These include swelling, petechia, and ecchymoses affecting a limb or digit. The bacteria most often associated with red leg syndrome is Aeromonas hydrophila,8 but other organisms that have been implicated include Proteus spp., E. coli, Aerobacter spp., Pseudomonas spp., Citrobacter spp., Mimi spp., Staphylococcus spp., Streptococcus spp., Enterobacter spp., Klebsiella spp., and Chryseobacterium spp.5,16,23 However, red leg syndrome often occurs secondary to physical injury, environmental stress, or husbandry-related issues, and clinical signs often progress beyond the local site of infection to systemic disease.13 This frog's lesion did not have evidence of hemorrhage, and the clinical signs were confined to the skin with no evidence of systemic illness. This presentation allowed red leg to be ruled out in this case. Mycobacterium spp. is another common bacterial etiology of skin disease in frogs. Although the most common presentation of this infection is ulcerative, nonhealing skin lesions, there have been 2 reports of nodular skin lesions in Xenopus frogs caused by Mycobacterium liflandii10 and Mycobacterium gordonae.21 In those cases, the nodular growths were characterized by granulomatous inflammation with intralesional bacterial colonies. The lack of inflammation and bacteria allowed Mycobacterium spp. to be ruled out in the current case.
Neoplasia can occur in frogs in any organ system but is rare in the absence of pollutants or an infectious agent,17 given that amphibians are resistant to the development of cancer.20 Based on its clinical presentation and microscopic features, this mass was diagnosed as a collagenoma, a specific type of connective tissue nevus due to an excess of collagen. Connective tissue nevi are rare hamartomas that consist of one or more components of the dermal connective tissue. This can include nevi consisting of excessive deposition of collagen, elastic fibers (elastoma), or glycoaminoglycans (nevus mucinosis). In humans, these lesions are classified based on clinical, genetic, and histopathologic considerations. Hamartomas of collagen, or collagenomas, can be classified as inherited or acquired. The inherited collagenomas are familial cutaneous collagenoma or shagreen patches; acquired collagenomas are classified as eruptive or isolated.25 The familial collagenomas tend to be multiple, whereas the acquired form can either be multiple lesions or a single lesion. Clinically, collagenomas present as single or multiple asymptomatic flesh-colored to white or pink papules, nodules, or plaques.19,25 Familial cutaneous collagenoma has been associated with various cardiovascular disorders including cardiomyopathy, atrioseptal defect, aortic insufficiency, and conduction defects, hypogonadism, congenital exophthalmos, learning disabilities, hypertrichosis, nystagmus, café-au-lait macules, and acanthosis nigricans. Isolated collagenomas have been associated with Down syndrome and type III Ehlers–Danlos syndrome.3 Shagreen patches are associated with tuberous sclerosis.25
To our knowledge, this report is the first description of a collagenoma in an African clawed frog. It is important to continue to investigate and report diseases of this valuable model, because an expanded knowledge base about their health will allow the research community to better care for and understand these animals.
References
- 1.Balls M. 1962. Spontaneous neoplasms in amphibia: a review and descriptions of 6 new cases. Cancer Res 22:1142–1154. [PubMed] [Google Scholar]
- 2.Brayton C. 1992. Wasting disease associated with cutaneous and renal nematodes in commercially obtained Xenopus laevis. Ann N Y Acad Sci 653:197–201. [DOI] [PubMed] [Google Scholar]
- 3.Calonje E, Brenn T, Lazar AJ, McKee PH. 2011. Mckee's pathology of the skin. London (UK): Saunders. [Google Scholar]
- 4.Cheong SW, Fukui A, Asashima M, Pfeiffer CJ. 2000. Spontaneous thyroid-containing teratoma associated with impaired development in the African clawed frog, Xenopus laevis. J Comp Pathol 123:110–118. [DOI] [PubMed] [Google Scholar]
- 5.Crawshaw GJ. 1992. The role of disease in amphibian decline. p 60–62. In Bishop CA, Petit KE. Declines in Canadian amphibian populations: designing a national monitoring strategy. Occasional Paper no. 76. Ottawa (Canada): Canadian Wildlife Service. [Google Scholar]
- 6.Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, Speare R. 1999. Emerging infectious diseases and amphibian population declines. Emerg Infect Dis 5:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Densmore CL, Green DE. 2007. Diseases of amphibians. ILAR J 48:235–254. [DOI] [PubMed] [Google Scholar]
- 8.Emerson H, Norris C. 1905. ‘Red leg’—an infectious disease of frogs. J Exp Med 7:32–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox JG, Anderson LC, Loew FM, Quimby FW. 2002. Laboratory animal medicine. San Diego (CA): Academic Press. [Google Scholar]
- 10.Fremont-Rahl JJ, Ek C, Williamson HR, Small PL, Fox JG, Muthupalani S. 2010. Mycobacterium liflandii outbreak in a research colony of Xenopus (Silurana) tropicalis frogs. Vet Pathol 48:856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyos A, Robert J. 2009. Tumorigenesis and antitumor immune responses in Xenopus. Front Biosci (Landmark Ed) 14:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DE, Harshbarger JC. 2001. Spontaneous neoplasia in amphibia. p 335–400. In: Whitaker BR, Wright KN. Amphibian medicine and captive husbandry. Malabar (FL): Krieger Publishing. [Google Scholar]
- 13.Green SL. 2010. The laboratory Xenopus spp. Boca Raton (FL): CRC Press. [Google Scholar]
- 14.Gurel MS, Mulayim MK, Ozardali I, Bitiren M. 2007. Familial cutaneous collagenoma: new affected family with prepubertal onset. J Dermatol 34:477–481. [DOI] [PubMed] [Google Scholar]
- 15.Khudoley VV, Mizgireuv IV. 1980. On spontaneous skin tumours in amphibia. Neoplasma 27:289–293. [PubMed] [Google Scholar]
- 16.Mauel MJ, Miller DL, Frazier KS, Hines ME., 2nd 2002. Bacterial pathogens isolated from cultured bullfrogs (Rana castesbeiana). J Vet Diagn Invest 14:431–433. [DOI] [PubMed] [Google Scholar]
- 17.Merck. [Internet] 2015. Overview of amphibians. Merck veterinary manual. [Cited 02 March 2015]. Available at: http://www.merckvetmanual.com/mvm/exotic_and_laboratory_animals/amphibians/overview_of_amphibians.html?qt=Overview%20of%20amphibians.&alt=sh
- 18.Meyer-Rochow VB, Asashima M, Moro SD. 1991. Nephroblastoma in the clawed frog Xenopus laevis. J Exp Anim Sci 34:225–228. [PubMed] [Google Scholar]
- 19.Pierard GE, Lapiere CM. 1985. Nevi of connective tissue. A reappraisal of their classification. Am J Dermatopathol 7:325–333. [DOI] [PubMed] [Google Scholar]
- 20.Ruben LN, Clothier RH, Balls M. 2007. Cancer resistance in amphibians. Altern Lab Anim 35:463–470. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Morgado JM, Gallagher A, Johnson LK. 2009. Mycobacterium gordonae infection in a colony of African clawed frogs (Xenopus tropicalis). Lab Anim 43:300–303. [DOI] [PubMed] [Google Scholar]
- 22.Stacy BA, Parker JM. 2004. Amphibian oncology. Vet Clin North Am Exot Anim Pract 7:673–695 [vi-vii.]. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SK, Green DE, Wright KM, Whitaker BR. 2001. Bacterial diseases. p 159–179. In: Wright KM, Whitaker BR. Amphibian medicine and captive husbandry. Malabar (FL): Krieger Publishing. [Google Scholar]
- 24.Uitto J, Santa-Cruz DJ, Eisen AZ. 1979. Familial cutaneous collagenoma: genetic studies on a family. Br J Dermatol 101:185–195. [DOI] [PubMed] [Google Scholar]
- 25.Uitto J, Santa Cruz DJ, Eisen AZ. 1980. Connective tissue nevi of the skin. Clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol 3:441–461. [PubMed] [Google Scholar]