Abstract
The origin of the age-associated degenerative processes in meniscal tissue is poorly understood and may be related to an imbalance of anabolic and catabolic metabolism. The aim of the current study was to compare medial menisci isolated from juvenile pigs and degenerated medial menisci from adult pigs in terms of gene expression profile and ultrastructure. Medial menisci were isolated from the knee joints of juvenile and adult pigs (n = 8 for each group). Degeneration was determined histologically according to a scoring system. In addition, the gene expression profiles of 14 genes encoding extracellular matrix proteins, catabolic matrix metalloproteinases and mediators of inflammation were analyzed. Changes in the ultrastructure of the collagen network of the meniscal tissue were analyzed by using transmission electron microscopy. The histologic analysis of menisci showed significantly higher grade of degeneration in tissue isolated from adult porcine knee joints compared with menisci isolated from juvenile knee joints. In particular, destruction of the collagen network was greater in adult menisci than in juvenile menisci. Degenerated menisci showed significantly decreased gene expression of COL1A1 and increased expression of MMP2, MMP13, and IL8. The menisci from adult porcine knee joints can serve as a model for meniscal degeneration. Degenerative changes were manifested as differences in histopathology, gene expression and ultrastructure of collagen network.
Abbreviations: MMP, matrix metalloproteinase; SOX, sex-determining region box; VEGF, vascular endothelial growth factor
Much research is focused on the degeneration of connective tissue, particularly of the cartilage tissue that stabilizes the knee joint. The goal of the current study was to determine whether menisci in adult pigs show patterns of degeneration in the absence of previous major injuries, which might cause a secondary form of degeneration.
In the mammalian knee joint, the incongruence between the femoral condyle and the tibial plateau is partially balanced by 2 C-shaped fibrocartilaginous menisci. These menisci absorb impact forces, help to distribute the mechanical load on the tibial plateau, and act as stabilizers of the knee joint.35,54 Therefore, menisci are thought to play an important role in the development of osteoarthritis in knee joints,39 as indicated by the results following meniscal resection.58 Both injuries13 and degeneration4 of meniscal tissue increase the risk of osteoarthritis of the knee joint. Because the self-repair mechanisms of meniscal tissue appear to be inadequate,9 more than 1 million surgical interventions on menisci are performed every year in the United States.27 In addition, degenerative changes in the meniscal tissue are believed to contribute to meniscal lesions.
The main component of the meniscus is water (70%); and most of its dry weight is due to the protein collagen, mainly collagen I (98%).57 The ultrastructure of meniscal tissue consists of collagen fibers that are orientated circumferentially in the superficial layers. The intermediate layer consists of tangentially orientated collagen fibers.15 Other proteins involved in the extracellular matrix of meniscal tissue include collagen II and the proteoglycan aggrecan.15In addition, biglycan and fibromodulin have been found in porcine menisci.42
The primary cells in meniscal tissue are chondrocytes which, in concert with fibroblasts, produce the extracellular matrix of fibrocartilage.55 In addition, chondrocytes produce the lubricant lubricin,56 which also is expressed in meniscal tissue.24,49
Meniscal degeneration can be understood as an imbalance between anabolic and catabolic processes, as it has already been shown for articular cartilage.2 Changes in the gene expression of menisci from osteoarthritic knee joints including genes involved in immune and inflammatory responses as well as in tissue development have been previously shown.52 Furthermore, the production of the matrix proteins collagen types I, II, and III decreases during the progression of human meniscal degeneration.43 Changes in the gene expression profile of anabolic genes (for example, collagen I, collagen II, aggrecan) and catabolic genes (for example, matrix metalloproteinases) are early indicators of osteoarthritis.2,14,22,26,34,51 Therefore, additional studies analyzing the gene expression profiles of healthy and degenerated menisci are warranted to evaluate whether such marker genes also exist for meniscal tissue. As a next step, the ultrastructure of degenerated meniscal tissue should be evaluated to analyze the influence of an altered gene expression profile on the extracellular matrix of menisci.
In general, pigs are an appropriate animal model in biomedical research because of their similarities to humans in terms of anatomy and metabolism.7,19,53 In meniscus research, goats30 and sheeps are well-established animal models.11,31,58 Nevertheless, the structure of porcine collagen is highly analogous to human collagen.3 This similarity was supported by the results of a comparison of the collagen in the hyaline cartilage among several species. 25 Because pathogenesis for osteoarthritis is similar between human and animal tissue,38 similarities in the pathophysiology of porcine and human meniscal tissue seem likely.
The aim of the current study was to compare the medial menisci isolated from juvenile pigs with the degenerated medial menisci from adult pigs to determine whether differences in their gene expression profiles and ultrastructure are present.
Materials and Methods
Sample collection.
The left or right knee of 8 juvenile (age, 5 mo) and 8 adult (age, 5 y) randomly selected pigs (Sus scrofa domestica) were obtained from a local slaughterhouse. The juvenile pigs were bred for food production, thus providing nearly unlimited availability of juvenile knee joints. The adult sows used for breeding typically are slaughtered at 5 y of age; the limited number of sows somewhat restricted the availability of knee joints from adult pigs, but it remained sufficient. All knees were analyzed for macroscopic signs of osteoarthritis, including a lack of hyaline cartilage and agglomeration of osteophytes. Subsequently, the medial meniscus of each knee was isolated. Immediately after isolation, 2 cross-sections of the pars intermedia were harvested and stored in either formalin (4% formaldehyde solution) or in formalin–glutaraldehyde. The anterior and posterior horns of the meniscus were merged and stored in liquid nitrogen for later processing of RNA.
Histologic procedures.
The formalin-fixed samples of the pars intermedia were machine-dehydrated (TP1020, Leica, Wetzlar, Germany) and subsequently embedded in paraffin by using an embedding station (EG1140C, Leica). Thin (7 µm) sections were cut (RM2165, Leica) and mounted on glass slides (Superfrost-Plus, R Langenbrick, Emmendingen, Germany). Prior to staining, the slides were deparaffinized with xylol and rehydrated in a graded series of ethanol in water. To highlight the overall tissue morphology, the deparaffinized slides underwent standard hematoxylin and eosin staining. In brief, the slides were stained with Mayer hematoxylin (Merck, Darmstadt, Germany) for 10 min and then were washed in tap water for 15 min. The slides were then stained for 3 min with eosin (Carl Roth, Karlsruhe, Germany) and dehydrated in an ascending alcohol series. Prepared slides were preserved by using a mounting medium (Eukitt, O Kindler, Freiburg, Germany).
To highlight proteoglycans, safranin O staining was performed as described previously.48 In brief, deparaffinized, rehydrated slides were stained in Weigert hematoxylin (Carl Roth) for 8 min, washed for 10 min in tap water, stained with fast green (Merck) for 5 min, and dipped in 1% acetic acid (Merck) for 5 s. Safranin O (Sigma–Aldrich Chemie, Munich, Germany) was used as a proteoglycan-specific stain. The slides were then dehydrated in an ascending alcohol series and preserved in mounting medium (Eukitt).
Semiquantitative histologic scoring.
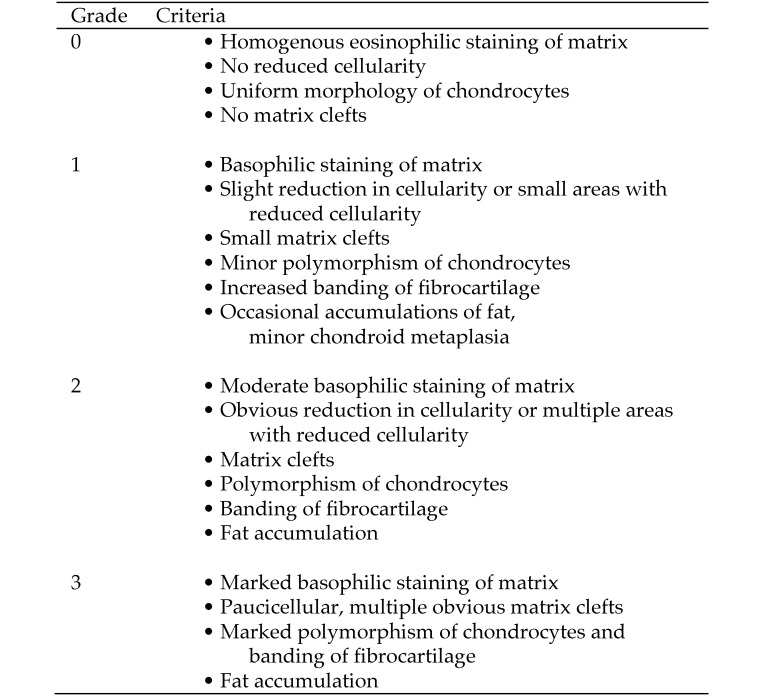
The individual slides stained with hematoxylin and eosin were analyzed by a pathologist (PS) by using a light microscope (Axioskop, Carl Zeiss, Oberkochen, Germany) and graded according to a 4-tier scoring system adapted from published schemes.29, 47 Major criteria were matrix composition, cellularity, size and form of chondrocytes, presence of fat cells, and formation of clefts (Figure 1). In addition, the amount of proteoglycans, visualized through Safranin O staining and known to increase during cartilaginous degenerative processes,8 was assessed. The pathologist was blinded to the age of the animal.
Figure 1.
Scoring system for meniscal degeneration in pigs. Adapted from references 29 and 47.
Quantitative PCR.
The anterior and posterior horns of each meniscus sample were pooled and homogenized (Polytron PT 3000, Kinematica, Lucerne, Switzerland) in TRIzol reagent (Life Technologies, Darmstadt, Germany), and the RNA was isolated according to the manufacturer's protocol. RNA samples were purified over mini-columns (Mini Kit, Qiagen, Hilden, Germany) and then transcribed into cDNA (Sensiscript RT Kit, Qiagen, Hilden, Germany).
To obtain a standard curve for each target gene, cDNA was PCR-amplified by using specific primers and annealing temperatures (Figure 2); the resulting product was purified through agarose gel electrophoresis followed by gel extraction (QIAquick Gel Extraction Kit, Qiagen). The concentration of the resulting DNA solution was measured, and a series of 10-fold dilutions was made for each gene. Subsequently, quantitative real-time PCR amplification of all genes was performed (Mx3005 P, Stratagene, Waldbronn, Germany) by using gene-specific annealing temperatures and the QuantiTect SYBR-Green PCR Kit (Qiagen) according to the manufacturer´s protocol. A standard curve was created for each gene.
Figure 2.
Primer sequences and annealing temperatures for the genes analyzed.
Each sample of RNA was transcribed and underwent quantitative real-time PCR amplification in duplicate. The housekeeping gene β-actin was chosen as reference, because its expression is very stable in most porcine tissues.45
Electron microscopy.
A small sample of the central meniscus was isolated from the cross-sections of the pars intermedia stored in formalin–glutaraldehyde and processed according to a published protocol.18,36 In brief, slides were washed in cacodylate buffer, incubated in osmium tetroxide, washed in water, dehydrated in an ascending alcohol series, and dried. Samples were then embedded in epoxy resin and polymerized for 48 h. The samples were cut into slices (thickness, 70 to 75 nm) by using an ultramicrotome (EM UC7, Leica), mounted on copper grids, and analyzed under a transmission electron microscope (Tecnai BioTwin 10, FEI, Eindhoven, Netherlands). Two ultra-thin sections of each meniscus were submitted to a blind study and classified according to a published scoring system,17 in which points (0 to 2) are given for maintenance of periodicity and compactness of the collagen fibers. In addition, the variability of the fiber dimension was graded as low (0 points) or high (1 point). The presence of an intrafibrillar edema (1 point) and lack of cross-striation of the collagen fibers (1 point) also influences this score. The total points for each meniscal sample defined the level of degeneration: first-grade degeneration (0 to 2 points), second-grade degeneration (3 to 4 points), or third-grade degeneration (5 to 7 points).
Statistical analysis.
All statistical analysis were completed by using SAS 9.1 for Windows (Microsoft, Redmond, WA). All data are presented as mean ± SD. Groupwise comparisons (juvenile compared with adult) across the various gene entities were performed by using 2-sample t tests. All tests were 2-sided. A P value less than 0.05 is considered significant.
Results
Histologic analysis of meniscal degeneration.
All knee joints of adult pigs showed osteoarthritis-like degenerative changes of the hyaline cartilage, that is, areas with a rough cartilage surface or a lack of hyaline cartilage on the femoral condyle. Furthermore, most knee joints of adult pigs showed agglomerations of osteophytes on the tibial plateau or femoral condyle. In contrast, the knee joints of juvenile pigs did not show any macroscopic degenerative changes. No menisci from either juvenile or adult knee joints showed macroscopic changes or meniscal tears.
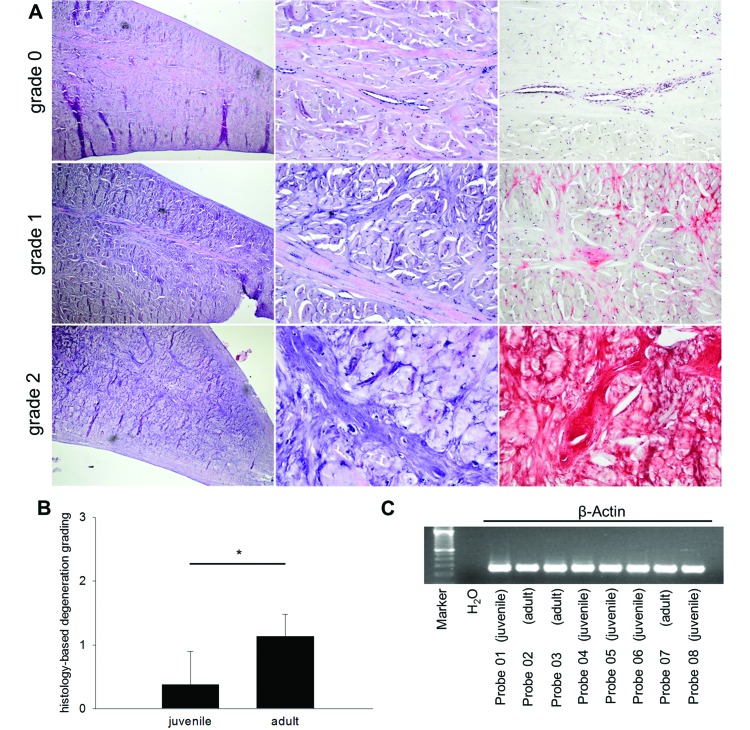
The menisci isolated from juvenile knee joints showed mainly homogenous staining of the matrix and high cellularity with uniform morphology of chondrocytes (Figure 3 A, top row). This presentation is equivalent to normal meniscal tissue (mean degeneration grade 0). In comparison, the meniscal tissue isolated from menisci of adult pigs was primarily characterized by a more basophilic matrix (Figure 3 A), and the histologic analysis of the menisci from adult knee joints revealed a modest reduction in cellularity and variable chondrocyte morphology (mean degeneration grade 1). In addition, some adult menisci demonstrated small areas with markedly decreased cellularity, and several small clefts disrupted the homogeneity of the matrix (Figure 3 A, middle and bottom rows). Therefore, menisci from adult pigs showed significantly (P = 0.0044) greater degeneration (Figure 3 B) than did menisci from juvenile pigs.
Figure 3.
Illustration of histologic findings and semiquantitative scoring of meniscal degeneration in pigs. (A) Grade 0 (top row). Normal meniscus with slender eosinophilic collagenous septae with few capillary blood vessels. Chondrocytes are inconspicuous. Safranin O staining (far right column) is negative. Grade 1 (middle row). Moderate degeneration with some thickening of the fibrous capsule of the meniscus, increase in basophilic fibers, and some demarcation of chondrocytes. Degenerated areas show focal safranin O staining. Grade 2 (bottom row). Marked thickening of the meniscal fibrous capsule, marked increase in basophilic fibers, conspicuous chondrocytes, microcalcifications and mucoid swelling of the collagen. Safranin O is strongly positive. Magnification (left to right): 100×, 400×, 400×. (B) Normal meniscal tissue (grade 0) was found only in juvenile menisci. Grades 1 and 2 were found mainly in adult meniscal tissue, thus showing a significantly higher grade of degeneration. (C) Similar levels of total β-ACTIN RNA expression of 8 different meniscus samples (probes; 5 juvenile and 3 adult).
Analysis of gene expression.
The expression levels of all analyzed genes were normalized relative to that of the housekeeping gene β-ACTIN, which showed a consistent mRNA signal among all meniscal samples (Figure 3 C). The amplification efficiency of the quantitative PCR reactions performed was 90.1% to 105.4%, as calculated from the slopes of the standard curves.
mRNA transcripts for the collagen 1 gene COL1A1 were significantly (P = 0.0052) more abundant in the juvenile menisci than in their adult counterparts (Figure 4 A). COL2 expression was virtually absent in both groups (juvenile, 0.02% ± 0.04%; adult, 0.08% ± 0.14%). mRNA encoding for the proteoglycan ACAN was very highly expressed in both juvenile and adult menisci, with no significant difference between the 2 groups (Figure 4 B). The relative expression levels of the proteoglycan genes DCN and PRG4 were similar between juvenile and adult menisci (Figure 4 C and D).
Figure 4.
Relative expression of genes encoding matrix-component proteins or matrix-associated proteins including (A) COL1A1 (*, P < 0.05), (B) ACAN, (C) DCN, and (D) PRG4 in juvenile and adult porcine medial menisci. n.s., nonsignificant.
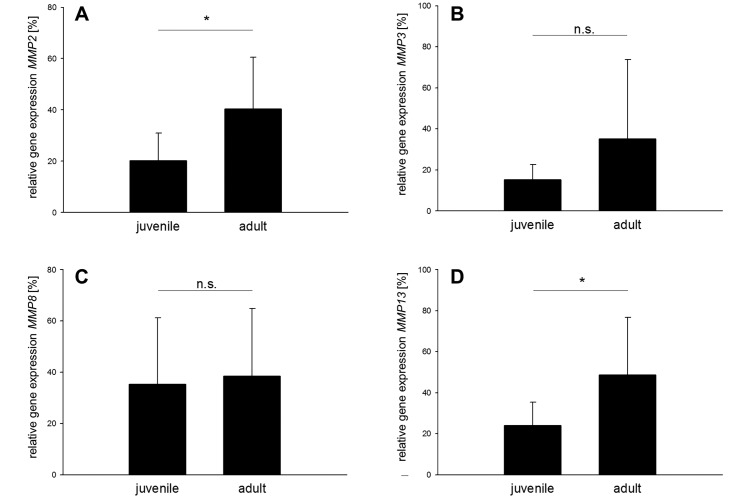
The next group of genes analyzed included 5 catabolic matrix metalloproteinases (MMP): MMP2, MMP3, MMP8, MMP9, and MMP13. Menisci isolated from adult porcine knee joints showed significantly greater expression of MMP2 (P = 0.0254) and MMP13 (P = 0.0365), compared with juvenile menisci (Figure 5 A and D). No significant differences were observed for MMP3 (Figure 5 B) and MMP8 (Figure 5 C). MMP9 expression was virtually absent in both groups (juvenile, 0.02% ± 0.04%; adult, 0.08% ± 0.14%).
Figure 5.
Relative expression of genes encoding for catabolic matrix metalloproteinases (MMP). Expression of (A) MMP2 and (D) MMP13 is significantly (*, P < 0.05) higher in adult compared with juvenile porcine medial menisci. No significant differences (n.s.) in gene expression were found for (B) MMP3 and (C) MMP8.
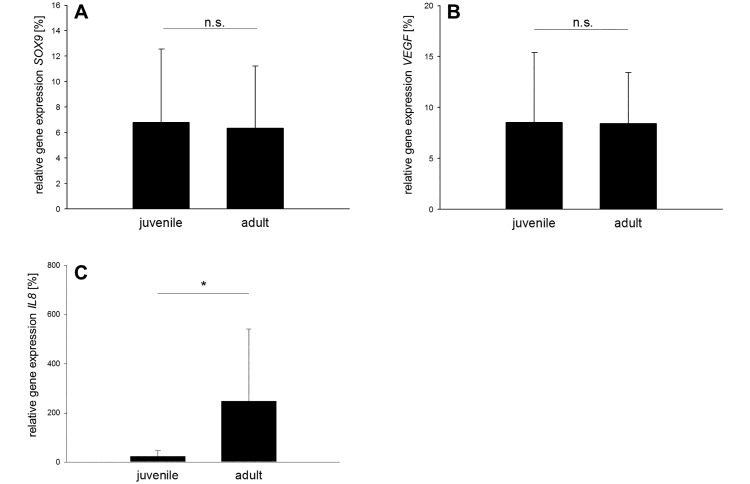
Another group of 4 genes was analyzed and included that for sex-determining region Y box 9 (SOX9), which encodes for a chondrocyte-stabilizing protein, whereas VEGF encodes for vascular endothelial growth factor. The relative expression of these 2 genes was identical in juvenile and adult meniscal tissue (Figure 6 A and B). Finally, 2 genes involved in inflammatory processes were assessed. The IL8 gene showed approximately 10-fold greater expression in adult menisci compared with juvenile menisci (P = 0.0490; Figure 6 C), whereas IL1β expression was virtually absent in both groups and too low for further statistical analysis.
Figure 6.
Relative expression of SOX9, VEGF, and IL8. Similar expression (n.s., nonsignificant) between adult and juvenile menisci was observed for (A) SOX9 and (B) VEGF. (C) The relative expression of IL8 is significantly (*, P < 0.05) higher in adult porcine medial menisci.
Electron microscopic analysis of the collagen extracellular matrix.
The tissue of the juvenile menisci showed collagen fibers organized largely in parallel and at high packing density (Figure 7 A), whereas adult meniscal tissue had severe disruption of the collagen fibers (Figure 7 B). In both groups, fibrils varied greatly in size (Figure 7 C and D). Collagen fibers in the adult meniscal tissue were mainly characterized by interfibrillar edema and weak banding (Figure 7 F) in contrast to collagen fibers of juvenile menisci (Figure 7 E). Semiquantitative scoring of meniscal degeneration revealed a significantly (P = 0.0000) higher grade of collagen matrix degeneration in adult menisci (grade II to III; mean no. of points, 4.38 ± 0.62) compared with juvenile menisci (grade I; mean no. of points: 1.13 ± 0.54).
Figure 7.
Transmission electron microscopy of juvenile and adult porcine menisci. (A) Tightly packed collagen fibers organized in parallel arrangement are characteristic of juvenile meniscal tissue, whereas (B) adult meniscal tissue showed severely disrupted fibers. (C and D) Both groups showed high variability of fibril diameter. Compared with (E) collagen fibers from juvenile meniscal tissue, (F) the adult tissue consists mainly of collagen fibers with intrafibrillar edema and weaker banding.
Discussion
We compared meniscal tissue from juvenile and adult pigs to test the assumption that degenerative processes in terms of osteoarthritis will occur even when an animal has not suffered from an event that is at high risk of leading to osteoarthritis. We documented signs of osteoarthritis in all knee joints from adult pigs but did not score them because, to our knowledge, a scoring system for macroscopic signs of porcine osteoarthritis is not available. Further studies are required to provide a scoring system for porcine osteoarthritis or to analyze the applicability of sheep or goat systems to pigs.33 In the current study, no information on the animals’ history or what events they might have experienced was available, because the knee joints sampled were obtained from a slaughterhouse as they became available. A prospective study observing animals over their entire lifespan would be preferable. However, an observation period of 5 y or more (the approximate age of the adult pigs in the current study) would likely overstrain the resources of most scientific facilities. Nevertheless, the use of porcine knee joints from a slaughterhouse can serve at least for pilot projects or feasibility studies in meniscus research.
Although the samples cannot be characterized as degenerated in terms of primary osteoarthritis precisely, we can exclude major traumatic events, such as fractures, given that the pigs would have not reached the age of 5 y in such cases. However, minor injuries including osteochondrosis and damage to the cruciate or collateral ligaments cannot be excluded. In addition, we are unable to exclude the possibility that the meniscal degeneration was a result of degenerative processes in other tissues of the knee joint, such as the synovium or ligaments. Furthermore, the changes in meniscal ultrastructure and gene expression profiles might reflect the aging process or differences in body weight, sex, or multipara. These questions should be addressed in studies comparing meniscal tissue of knee joints from adult pigs with and without osteoarthritis. This investigation is not possible in the current study because all knee joints from adult pigs had macroscopic signs of osteoarthritis.
The histologic analysis and scoring of menisci isolated from adult porcine knee joints revealed a significantly higher degree of degeneration compared with that of menisci isolated from juvenile knee joints. The degenerative alterations seen in the meniscal tissues of adult porcine knee joints likely also reflect variations in the extracellular matrix. Therefore, we analyzed the gene expression pattern of genes encoding for proteins that are thought to be involved in degenerative processes. We thus evaluated 14 genes with presumed roles in the degeneration of hyaline cartilage2,51 or the fibrocartilage of vertebral disc tissue.20
We first analyzed the expression of genes for extracellular matrix proteins (COL1A1 and COL2) and matrix-associated proteins, such as the proteoglycans aggrecan (ACAN), decorin (DCN), and lubricin (PRG4). Collagen I is the main structural protein of meniscal tissue.26 A decrease in collagen I content might be responsible for the changes in the extracellular matrix detected by electron microscopy. In addition to the degenerative processes of the extracellular matrix, other pathologic conditions can lead to downregulation of COL1A1; for example, hypoxic conditions led to decreased expression of COL1A1 in ovine menisci.21 Moreover, changes in the biomechanical load on the meniscus after induced muscle weakness of the hindlimb also resulted in the downregulation of COL1A1.32 In addition, COL1A1 expression is reduced in the presence of MMP2 and MMP13.16 In the present study, MMP2 and MMP13 were significantly upregulated in adult menisci, perhaps indicating a catabolic pathway leading to matrix degradation. Inflammatory mediators such as interleukins are known to increase the mRNA expression of COL2.14 In chondrocytes, the main interleukin is IL1β,15 which showed only very low gene expression in the current study. The lack of this major interleukin could be one reason we did not detect upregulation of collagen I.
We did not detect an age-dependent difference in gene expression for the main meniscal proteoglycan, aggrecan.28 This finding is consistent with previous observations in human meniscal tissue, which showed no significant age-associated changes in ACAN expression levels.10 The mRNA expression of DCN in human menisci is known to increase with age,37 but we found no significant difference in DCN expression in juvenile compared with adult porcine menisci. Lubricin, also known as proteoglycan 4, is another proteoglycan produced by chondrocytes56 and is found on the tibial and femoral meniscal surfaces24 as well as in deeper tissue areas.49 The expression of PRG4 is known to change in response to acute cartilage tears23 as well as in the presence of chronic degenerative changes of hyaline cartilage, such as osteoarthritis.24 In the present study, the expression of PRG4 did not differ in adult compared with juvenile porcine menisci.
The degeneration of hyaline cartilage is caused by an imbalance between anabolic proteins such as collagen and proteoglycans on the one hand and catabolic proteins such as MMP on the other.2 Therefore, an increase in MMP expression might contribute to the degenerative processes that led to the histologic changes that we noted in adult porcine menisci. In the present study, MMP2 showed a significant increase in expression in degenerated adult porcine menisci and thus might have induced the breakdown of the extracellular matrix22 observed in the histopathologic and electron microscopy analyses. This effect has also been observed in chronically inflamed hyaline cartilage, where MMP2 expression was upregulated.44 Whereas the expression of MMP3 and MMP8 did not differ between adult compared with juvenile porcine menisci, that of MMP13, which is upregulated due to degeneration of the hyaline cartilage and meniscus after resection of the anterior cruciate ligament in rabbits, was increased in adult porcine menisci.6 However, the inhibition of MMP13 protected cultured porcine meniscal tissue from further degenerative changes.40
SOX9 encodes the stabilizing protein of chondrocyte metabolism.46 In the case of chronic inflammatory processes in hyaline cartilage, SOX9 is upregulated44 to increase chondrocyte stability. In one study, a laceration created in the anterior horn of the medial menisci of rabbits significantly increased VEGF expression compared with that in an untreated control group.5 In the present study, the expression of VEGF did not differ between adult and juvenile menisci.
We analyzed the expression of IL8 to evaluate the influence of inflammatory processes on the degeneration of meniscal tissue. IL8 is involved in the degenerative reconstruction of cartilage and bone tissue.1 Our results are consistent with this observation: IL8 expression was significantly increased in the menisci isolated from adult porcine knee joints compared with juvenile menisci.
Perhaps gene expression is manifested differently in different sections of the meniscus (the anterior or the posterior horn or the pars intermedia). We included the anterior and posterior horns and observed both up- and downregulation in the expression of the analyzed genes. The pars intermedia served as a histologic reference. However, large portions of both the anterior and posterior horns were needed to obtain sufficient RNA, given that meniscal tissue has low cellularity, and histologic analysis of the same meniscus is required to follow changes in the level of degeneration.
Immunohistochemistry was not performed, what might be considered as a limitation of the current study. Thus, we are unable to detect the effects of significant changes in gene expression at the protein level. This is true for proteins that have a role in destructive (MMP2 and MMP13) or inflammatory (IL1β) processes. However, safranin O staining can reveal the presence of proteoglycans. In addition, electron microscopy highlighted the relative involvement of collagen fibers, providing information about both their presence and their arrangement.
The histopathologic analysis showed significant tissue degeneration in the menisci isolated from adult porcine knee joints. Together with the findings of the gene expression analysis for COL1A1, the results indicate an alteration in the collagen network. Although COL1A1 expression did not generally correlate with collagen content, the adult menisci showed marked destruction of the collagen network compared with the situation in the juvenile menisci. Similar alterations of the collagen network have been noted in the degenerated meniscal tissue of humans12 and dogs.50 Because the collagen network in humans becomes more compact with increasing age,41 the changes seen in the present study appear to be due to a degenerative process beyond that associated with aging. This question should be addressed in studies comparing the meniscal tissue of knee joints from geriatric pigs with and without osteoarthritis.
In conclusion, we have demonstrated here that menisci isolated from the knee joints of adult pigs had a significantly higher graded degeneration than did menisci from juvenile knee joints. This degeneration was accompanied by derangement of the collagen network, an effect that potentially was induced by changes in the expression of genes encoding both anabolic and catabolic extracellular matrix proteins. The menisci from adult porcine knee joints are an appropriate model for meniscal degeneration that was not induced surgically. We were able to document the degeneration of the meniscal tissue through multiple methods, including histopathology and the evaluation of gene expression profiles and ultrastructural analysis of the collagen network.
To our knowledge, an animal model for meniscal degeneration that is not surgically induced is currently not available. In addition to insults on the meniscus itself, any manipulation of other structures of the knee joint leads to secondary degeneration of meniscal tissue. The model introduced in the present study offers an inexpensive and almost unlimited opportunity to research meniscal degeneration in a species established in biomedical research.7,19 One drawback of this approach is the lack of information on the housing, feeding, sex, weight, and pregnancy history of the donor pigs. Furthermore, diseases in general and knee diseases in particular are not recorded. Therefore, future studies should be planned in cooperation with swine producers to document this information.
Acknowledgment
We thank Birgit Leis, Norbert Pütz, Elisabeth Seelinger, and Petra Schwarz for technical support in the laboratory.
References
- 1.Aida Y, Maeno M, Suzuki N, Namba A, Motohashi M, Matsumoto M, Makimura M, Matsumura H. 2006. The effect of IL1β on the expression of inflammatory cytokines and their receptors in human chondrocytes. Life Sci 79:764–771. [DOI] [PubMed] [Google Scholar]
- 2.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, Zien A, Obermayr F, Zimmer R, Bartnik E. 2006. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum 54:3533–3544. [DOI] [PubMed] [Google Scholar]
- 3.Aspden RM, Yarker YE, Hukins DW. 1985. Collagen orientations in the meniscus of the knee joint. J Anat 140:371–380. [PMC free article] [PubMed] [Google Scholar]
- 4.Badlani JT, Borrero C, Golla S, Harner CD, Irrgang JJ. 2013. The effects of meniscus injury on the development of knee osteoarthritis: data from the osteoarthritis initiative. Am J Sports Med 41:1238–1244. [DOI] [PubMed] [Google Scholar]
- 5.Becker R, Pufe T, Kulow S, Giessmann N, Neumann W, Mentlein R, Petersen W. 2004. Expression of vascular endothelial growth factor during healing of the meniscus in a rabbit model. J Bone Joint Surg Br 86:1082–1087. [DOI] [PubMed] [Google Scholar]
- 6.Bluteau G, Gouttenoire J, Conrozier T, Mathieu P, Vignon E, Richard M, Herbage D, Mallein-Gerin F. 2002. Differential gene expression analysis in a rabbit model of osteoarthritis induced by anterior cruciate ligament (ACL) section. Biorheology 39:247–258. [PubMed] [Google Scholar]
- 7.Bollen P, Ellegaard L. 1997. The Gottingen minipig in pharmacology and toxicology. Pharmacol Toxicol 80 Suppl 2:3–4. [DOI] [PubMed] [Google Scholar]
- 8.Brandt KD. 1991. Transection of the anterior cruciate ligament in the dog: a model of osteoarthritis. Semin Arthritis Rheum 21:22–32. [DOI] [PubMed] [Google Scholar]
- 9.Bray RC, Smith JA, Eng MK, Leonard CA, Sutherland CA, Salo PT. 2006. Vascular response of the meniscus to injury: effects of immobilization. J Orthop Res 19:384–390. [DOI] [PubMed] [Google Scholar]
- 10.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. 2012. Molecular analysis of age- and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am 94:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevrier A, Nelea M, Hurtig MB, Hoemann CD, Buschmann MD. 2009. Meniscus structure in human, sheep, and rabbit for animal models of meniscus repair. J Orthop Res 27:1197–1203. [DOI] [PubMed] [Google Scholar]
- 12.Dahmen G. 1962. [Electron microscopic findings in meniscus degeneration] Arch Orthop Unfallchir 53:620–632. [Article in German] [DOI] [PubMed] [Google Scholar]
- 13.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, Torner J, Nevitt MC, Sack B, Felson DT. 2009. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum 60:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Z, Bau B, Yang H, Soeder S, Aigner T. 2005. Freshly isolated osteoarthritic chondrocytes are catabolically more active than normal chondrocytes but less responsive to catabolic stimulation with interleukin 1β. Arthritis Rheum 52:136–143. [DOI] [PubMed] [Google Scholar]
- 15.Fithian DC, Kelly MA, Mow VC. 1990. Material properties and structure–function relationships in the menisci. Clin Orthop Relat Res 252:19–31. [PubMed] [Google Scholar]
- 16.Fuller ES, Smith MM, Little CB, Melrose J. 2012. Zonal differences in meniscus matrix turnover and cytokine response. Osteoarthritis Cartilage 20:49–59. [DOI] [PubMed] [Google Scholar]
- 17.Gelber PE, Gonzalez G, Lloreta JL, Reina F, Caceres E, Monllau JC. 2007. Freezing causes changes in the meniscus collagen net: a new ultrastructural meniscus disarray scale. Knee Surg Sports Traumatol Arthrosc 16:353–359. [DOI] [PubMed] [Google Scholar]
- 18.Glauert AM. 1974. The high-voltage electron microscope in biology. J Cell Biol 63:717–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotterbarm T, Breusch SJ, Schneider U, Jung M. 2008. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: retrospective analysis of 180 defects. Lab Anim 42:71–82. [DOI] [PubMed] [Google Scholar]
- 20.Guehring T, Omlor GW, Lorenz H, Bertram H, Steck E, Richter W, Carstens C, Kroeber M. 2005. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration—an in vivo animal study. Spine 30:2510–2515. [DOI] [PubMed] [Google Scholar]
- 21.Hofstaetter JG, Wunderlich L, Samuel RE, Saad FA, Choi YH, Glimcher MJ. 2005. Systemic hypoxia alters gene expression levels of structural proteins and growth factors in knee joint cartilage. Biochem Biophys Res Commun 330:386–394. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh YS, Yang SF, Chu SC, Chen PN, Chou MC, Hsu MC, Lu KH. 2004. Expression changes of gelatinases in human osteoarthritic knees and arthroscopic debridement. Arthroscopy 20:482–488. [DOI] [PubMed] [Google Scholar]
- 23.Jones AR, Chen S, Chai DH, Stevens AL, Gleghorn JP, Bonassar LJ, Grodzinsky AJ, Flannery CR. 2009. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum 60:133–142. [DOI] [PubMed] [Google Scholar]
- 24.Jones AR, Flannery CR. 2007. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater 13:40–45, discussion 45. [DOI] [PubMed] [Google Scholar]
- 25.Kaab MJ, Gwynn IA, Notzli HP. 1998. Collagen fiber arrangement in the tibial plateau articular cartilage of man and other mammalian species. J Anat 193:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambic HE, McDevitt CA. 2006. Spatial organization of types I and II collagen in the canine meniscus. J Orthop Res 23:142–149. [DOI] [PubMed] [Google Scholar]
- 27.Katz JN, Martin SD. 2009. Meniscus—friend or foe: epidemiologic observations and surgical implications. Arthritis Rheum 60:633–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krenn V, Knoss P, Ruther W, Jakobs M, Otto M, Krukemeyer MG, Heine A, Mollenhoff G, Kurz B. 2010. [Meniscal degeneration score and NITEGE expression: immunohistochemical detection of NITEGE in advanced meniscal degeneration] Orthopade 39:475–485. [Article in German] [DOI] [PubMed] [Google Scholar]
- 29.Krenn V, Kurz B, Krukemeyer MG, Knoess P, Jakobs M, Poremba C, Mollenhoff G. 2010. [Histopathological degeneration score of fibrous cartilage. Low- and high-grade meniscal degeneration] Z Rheumatol 69:644–652. [Article in German] [DOI] [PubMed] [Google Scholar]
- 30.Laurent D, O'Byrne E, Wasvary J, Pellas TC. 2006. In vivo MRI of cartilage pathogenesis in surgical models of osteoarthritis. Skeletal Radiol 35:555–564. [DOI] [PubMed] [Google Scholar]
- 31.Lazovic D, Wirth CJ, Sieg A, Gosse F, Maschek HG. 1997. Influence of the operative technique on meniscus transplants—an experimental investigation. Unfallchirurg 100:541–546. [Article in German] [DOI] [PubMed] [Google Scholar]
- 32.Leumann A, Longino D, Fortuna R, Leonard T, Vaz MA, Hart DA, Herzog W. 2011. Altered cell metabolism in tissues of the knee joint in a rabbit model of Botulinum toxin A-induced quadriceps muscle weakness. Scand J Med Sci Sports 22: 776–782. [DOI] [PubMed] [Google Scholar]
- 33.Little CB, Smith MM, Cake MA, Read RA, Murphy MJ, Barry FP. 2010. The osteoarthritisRSI histopathology initiative— recommendations for histological assessments of osteoarthritis in sheep and goats.. Osteoarthritis Cartilage 18 Suppl 3:S80–S92. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz H, Wenz W, Ivancic M, Steck E, Richter W. 2005. Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res Ther 7:R156–R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubowitz JH, Verdonk PC, Reid JB, 3rd, Verdonk R. 2007. Meniscus allograft transplantation: a current concepts review. Knee Surg Sports Traumatol Arthrosc 15:476–492. [DOI] [PubMed] [Google Scholar]
- 36.Luft JH. 1961. Improvements in epoxy-resin embedding methods. J Biophys Biochem Cytol 9:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAlinden A, Dudhia J, Bolton MC, Lorenzo P, Heinegard D, Bayliss MT. 2001. Age-related changes in the synthesis and mRNA expression of decorin and aggrecan in human meniscus and articular cartilage. Osteoarthritis Cartilage 9:33–41. [DOI] [PubMed] [Google Scholar]
- 38.McCoy AM, Toth F, Dolvik NI, Ekman S, Ellermann J, Olstad K, Ytrehus B, Carlson CS. 2013. Articular osteochondrosis: a comparison of naturally occurring human and animal disease. Osteoarthritis Cartilage 21:1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGonagle D, Tan AL, Carey J, Benjamin M. 2010. The anatomical basis for a novel classification of osteoarthritis and allied disorders. J Anat 216:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNulty AL, Weinberg JB, Guilak F. 2008. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res 467:1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merkel KH. 1980. The surface of human menisci and its aging alterations during age. A combined scanning and transmission electron microscopic examination (SEM, TEM).. Arch Orthop Trauma Surg 97:185–191. [DOI] [PubMed] [Google Scholar]
- 42.Messner K, Gao J. 1998. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J Anat 193:161–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mine T, Ihara K, Kawamura H, Date R, Umehara K. 2013. Collagen expression in various degenerative meniscal changes: an immunohistological study. J Orthop Surg (Hong Kong) 21:216–220. [DOI] [PubMed] [Google Scholar]
- 44.Nam J, Perera P, Liu J, Rath B, Deschner J, Gassner R, Butterfield TA, Agarwal S. 2011. Sequential alterations in catabolic and anabolic gene expression parallel pathological changes during progression of monoiodoacetate-induced arthritis. PLoS One 6:e24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nygard AB, Jorgensen CB, Cirera S, Fredholm M. 2007. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol Biol 8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochi K, Daigo Y, Katagiri T, Saito-Hisaminato A, Tsunoda T, Toyama Y, Matsumoto H, Nakamura Y. 2003. Expression profiles of 2 types of human knee-joint cartilage. J Hum Genet 48:177–182. [DOI] [PubMed] [Google Scholar]
- 47.Raunest J, Hotzinger H, Burrig KF. 1994. Magnetic resonance imaging (MRI) and arthroscopy in the detection of meniscal degenerations: correlation of arthroscopy and MRI with histology findings. Arthroscopy 10:634–640. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg L. 1971. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am 53:69–82. [PubMed] [Google Scholar]
- 49.Schumacher BL, Schmidt TA, Voegtline MS, Chen AC, Sah RL. 2006. Proteoglycan 4 (PRG4) synthesis and immunolocalization in bovine meniscus. J Orthop Res 23:562–568. [DOI] [PubMed] [Google Scholar]
- 50.Stockwell RA, Billingham ME. 1984. Early response of cartilage to abnormal factors as seen in the meniscus of the dog knee after cruciate ligament section. Acta Biol Hung 35:281–291. [PubMed] [Google Scholar]
- 51.Stoker AM, Cook JL, Kuroki K, Fox DB. 2006. Site-specific analysis of gene expression in early osteoarthritis using the Pond–Nuki model in dogs. J Orthop Surg Res 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton JH, Hanley EN, Jr, Gruber HE. 2010. Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet Disord 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomsen M, von Strachwitz B, Loew M, Cotta H, Kirsch S, Schunk O, Kubein-Meesenburg D. 1997. [The Gottinger minipig as an animal model in hip endoprosthesis. Anatomy, anesthesia, operation results] Z Orthop Ihre Grenzgeb 135:58–62. [Article in German] [DOI] [PubMed] [Google Scholar]
- 54.Walker PS, Erkman MJ. 1975. The role of the menisci in force transmission across the knee. ClinOrthop Relat Res 109:184–192. [DOI] [PubMed] [Google Scholar]
- 55.Wang QG, Magnay JL, Nguyen B, Thomas CR, Zhang Z, El Haj AJ, Kuiper NJ. 2009. Gene expression profiles of dynamically compressed single chondrocytes and chondrons. Biochem Biophys Res Commun 379:738–742. [DOI] [PubMed] [Google Scholar]
- 56.Wimmer MA, Schmid TM, Jacobs JJ. 2007. Tribology: a portal to understand joint failure? Arthritis Rheum 56:3511–3513. [DOI] [PubMed] [Google Scholar]
- 57.Wojtys EM, Chan DB. 2005. Meniscus structure and function. Instr Course Lect 54:323–330. [PubMed] [Google Scholar]
- 58.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, Read RA, Melrose J, Sonnabend DH, Flannery CR, Little CB. 2006. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther 8:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]