Abstract
Objectives
To analyze the efficacy of intrarectal ice application as an anesthetic method prior to transrectal ultrasound (TRUS) guided prostate biopsy.
Materials and Methods
A total of 120 consecutive men were included into the study prospectively. Patients were equally randomized as group 1 and 2 with 60 patients each. Ice was applied as an anesthetic method 5 minutes before procedure to the patients in group 1. Patients in group 2 were applied 10 ml of 2% lidocaine gel 10 minutes before procedure. Twelve core biopsy procedure was performed for all patients. The pain level was evaluated using a visual analogue scale (VAS).
Results
Median pain score was 3.5 (1-8) in group 1 and 5 (1-8) in group 2. There is significantly difference between groups regarding the mean sense of pain level during the procedure. (p=0.007) There was also no difference in complications between two groups about presence and duration of macroscopic hematuria and rectal bleeding.
Conclusions
Intrarectal ice application prior to TRUS prostate biopsy has an effect on reducing pain. Development of new techniques about cold effect or ice can make this method more useful and decrease complication rates.
Keywords: Ice, Prostate, Biopsy, Anesthesia
INTRODUCTION
Several studies have shown that 80-96% of patients undergoing transrectal ultrasound (TRUS) guided prostate biopsy reported that they suffered a disturbing pain from this procedure (1, 2). Hence, any anesthetic method is considered to be required prior to TRUS prostate biopsy.
As yet, various anesthetic methods including intrarectal lidocaine gel and periprostatic anesthetic material application have been tried. A method which is easily-applicable, painless and not leading to any additional complication should be chosen as an anesthetic agent prior to TRUS prostate biopsy. Intrarectal lidocaine gel is an easily-applicable method among others. However, factors limiting its efficacy include superficial application, absorption of little fraction of drug into systemic circulation and insufficient penetration to periprostatic tissues. Although periprostatic injection provides an efficient pain control, it has disadvantages including painful application and more frequent complication rates due to additional injections. However, it has currently been reported as a safe, easily-applicable and quite efficient method among all other methods (3).
In this study we aim to analyze the efficacy of intrarectal ice application as an anesthetic method prior to TRUS prostate biopsy. Besides its antinociceptive effect, ice can slow down nerve conduction, reduce muscle spasm and prevent post-traumatic edema (4). Ice has been used for relieving various kinds of pains for many years and its efficacy has also been known.
No study is available in literature regarding intrarectal ice application for TRUS prostate biopsy. However, ice is being used for various muscle-skeletal pathologies and some simple surgical procedures.
It has been known that ice has a vasoconstrictor impact on vessels. We are of the opinion that ice could reduce rates of rectal bleeding and hematuria seen after biopsy.
MATERIALS AND METHODS
The study was designed as a prospective randomized clinical trial comparing two methods during a period of 6 months at the Department of Urology, Faculty of Medicine, University of Kocaeli. Biopsy indication criteria were abnormal digital rectal examination (DRE) and elevated serum PSA levels higher than 4 ng/mL. Inclusion criteria were all patients who were eligible for prostate biopsy. Exclusion criteria for this study included a history of transrectal prostate biopsy; a bleeding diathesis and/or anticoagulant treatment; anal or rectal pathologies such as hemorrhoid, anal fissure or anal stricture; a history of lidocaine allergy; use of analgesic or narcotic drugs; and an inability to rate a visual analogue scale. Oral Ciprofloxacin prophylactic therapy (2x500 mg/day) was used for all the patients 1 day before biopsy and continued for the next 3 days. A Fleet enema was administrated in the morning of biopsy for bowel cleaning. Biopsies were performed with the patient in the left lateral decubitus position using Toshiba SSA-550A ultrasonography machine with 6 Mhz 150º endorectal probe (PVT-651VT) and automatic biopsy gun with 18 gauge biopsy needles by the same urologist.
Ethics committee approved the study and informed consent was obtained from each patient for biopsy and this randomized study. Patients were equally randomized using previously prepared cards in envelopes as group 1 and 2 with 60 patients each. Ice was applied as an analgesic method 5 minutes before procedure to the patients in group 1. Patients in group 2 received 10 ml of 2% lidocaine gel 10 minutes before procedure. No additional analgesia was utilized in any groups. Firstly digital rectal examination was performed, and subsequently analgesic method was administered. Prostate was analyzed in sagittal and coronal sections then diameters of prostate and seminal vesicles were measured. Finally, 12 core biopsies were taken. Blinding could not be possible since all interventions were made by the same urologist and patients were aware of ice application. But the pain scores were assessed by another blinded physician.
The 10-point linear Visual Analogue Scala (VAS) was used to assess the pain score. Scores between 1-3 were considered as mild pain, 4-7 as moderate and 8-10 as severe. The patients were asked the question of “How much pain do you feel in a simple blood draw procedure?” and the score obtained was considered as threshold pain value. One and three weeks after biopsy patients were assessed regarding complications. Pain during the anesthesia and probe insertion were expressed by categorical variables (none, mild, moderate and severe) and during TRUS prostate biopsy by VAS score. SPSS® 13.0 software was used for statistical analyses. Student t-test and Mann Whitney U tests were used for continuous variables and Fisher’s Exact Test for categorical variables as statistical methods.
Ice application technique
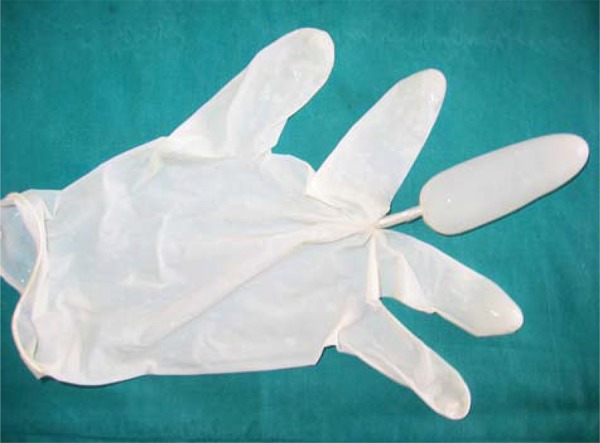
Firstly, middle finger space of the large size glove was filled with water and taped from its bottom part then frozen (Figure-1). During the anesthesia procedure, ice mould lubricated with Vaseline was inserted into the rectum adjacent to the prostate. Five minutes later, ice mould was pulled out of rectum, which was semi-melted.
Figure 1. Ice mould.
RESULTS
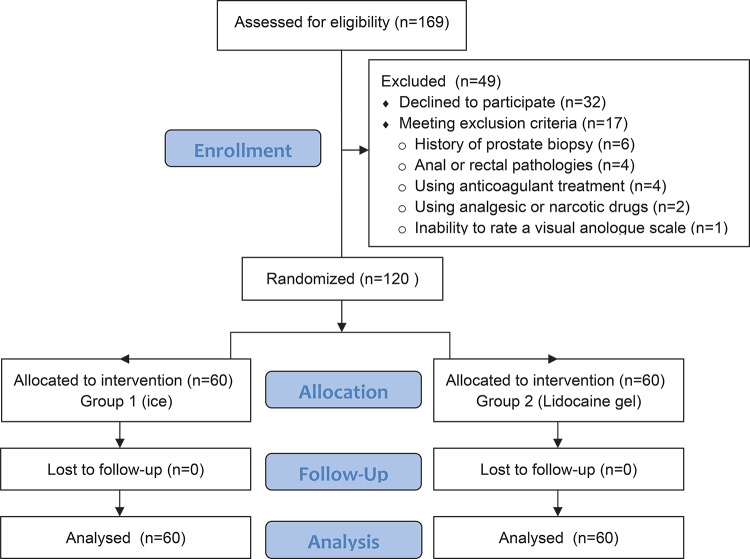
A total of 169 patients were evaluated for eligibility. Seventeen patients were excluded because they met the exclusion criteria and thirty-two patients rejected to sign the written informed consent. Finally 120 patients were included into the study (Figure-2). Both groups were similar in terms of abnormal DRE numbers, age of patients, serum total PSA levels, mean prostate volume, mean duration of procedure and mean threshold levels (Table-1).
Figure 2. Study flow diagram.
Table 1. Distribution of 2 groups according to various features (Group 1: ice, Group 2: lidocaine gel).
| Group 1 | Group 2 | p Value | |
|---|---|---|---|
| Total patient number (n) | 60 | 60 | |
| Abnormal DRE number, n(%) | 15 (25%) | 19 (31.7%) | 0.544* |
| Median age (IQR) | 64.0 (49-80) | 66.0 (41-82) | 0.507 |
| Median Total PSA (IQR) | 7.7 (1.2-70.0) | 6.8 (0.6-29.6) | 0.152 |
| Number of patients with cancer, n(%) | 24 (40%) | 18 (30%) | 0.339* |
| Median Prostate Volume (IQR) | 53.1 (11.3-153.2) | 47.4 (16.3-122.9) | 0.118 |
| Number of patients developing rectal bleeding n (%) | 16 (26.7%) | 19 (31.7%) | 0.688* |
| Median duration of rectal bleeding (IQR) | 0.0 (0.0-10.0) | 0.0 (0.0-7.0) | 0.884 |
| Number of patients developing hematuria n (%) | 41 (68.3%) | 40 (66.7%) | 1.00* |
| Median duration of hematuria (IQR) | 1.5 (0.0-14.0) | 1.5 (0.0-21) | 0.457 |
| Median threshold level (IQR) | 1.5 (1-4) | 2 (1-4) | 0.525 |
| Median duration (minutes) of procedure (IQR) | 7.3 (5.5-10.0) | 7.0 (5.0-10.5) | 0.244 |
Student t-test, Fisher’s Exact Test*
Median pain score was 3.5 (1-8) in group 1 (ice) and 5 (1-8) in group 2 (lidocaine gel), with significant difference regarding the mean sense of pain during the procedure (p=0.007). Statistical power was calculated as 0.80 with a two-sided 5% significance level and a sample size of 60 patients per group using post hoc analysis (mean±sd group1 and 2 were 3.8±1.5 and 4.6±1.6 respectively).
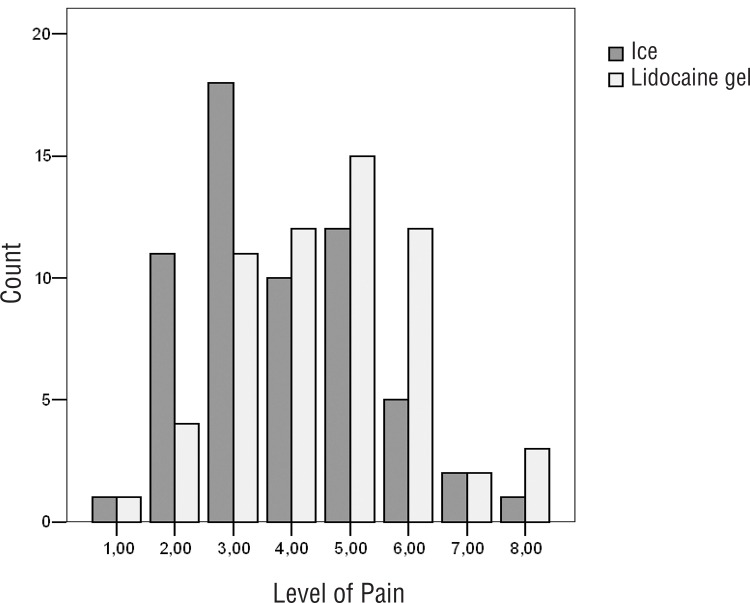
No difference was detected in pain levels during the probe insertion; however, there was an obvious difference in pain levels during anesthesia between two groups (Tables 2 and 3). Distribution of pain level is shown in Figure-3. None of the patients who received lidocaine gel felt pain during the procedure, though 22 of those who received ice felt mild (36.7%), 1 moderate (1.7%) and 2 severe (3.3%) pain (Table-3).
Table 2. Comparison of pains felt during application of anesthetic method, probe insertion and throughout the procedure between the groups (Group 1:ice, Group 2: lidocaine gel).
| Group 1 | Group 2 | p Value | |
|---|---|---|---|
| Total number of patients | 60 | 60 | |
| Number of patients feeling pain during anesthesia, n (%) | 25 (41.7%) | 0 (0%) | 0.000* |
| Number of patients feeling pain during placement of probe, n (%) | 18 (30%) | 27 (45%) | 0.131* |
| Median pain score felt during the procedure (IQR) | 3.50 (1-8) | 5.0 (1-8) | 0.007 |
p: p value, Student t-test, Fisher’s Exact test*
Table 3. Levels of pain felt during application of anesthetic method in both groups (Group 1: ice, Group 2: lidocaine gel).
| Pain during the application of anesthetic method n (%) | |||||
|---|---|---|---|---|---|
| Method | None | Mild | Moderate | Severe | Total |
| Group 1 | 35 (58.3%) | 22 (36.7%) | 1 (1.7%) | 2 (3.3%) | 60 (100.0%) |
| Group 2 | 60 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 60 (100.0%) |
Figure 3. Distribution of pain level during TRUS prostate biopsy. (Group 1:ice, Group 2: lidocaine gel).
When the ice group is divided into two groups according to the presence of pain during application of ice, no difference was detected in pain level during the procedure between two groups (p=0.088) (Table-4).
Table 4. Comparison of mean pain scores during the procedure in ice group according to presence of pain during the ice application.
| Pain during the application of ice | ||
|---|---|---|
| None | Mild to severe | |
| Number of patients feeling pain during the application of ice, n (%) | 35 (58.3%) | 25 (41.6%) |
| Median pain score during the procedure in ice group (IQR) * | 3.0 (1-8) | 4 (2-7) |
p: 0.088, Mann Whitney U test*
Count
Vasovagal syncope developed in one patient from lidocaine gel group. Urinary retention developed in no patient. No difference was detected between groups about presence and duration of macroscopic hematuria and rectal bleeding (Table-1). No abundant bleeding requiring treatment developed in patients. 100.000 colony/CFU Escherichia coli were detected in one patient with complaint of dysuria in lidocaine gel group and was treated with antibiotics.
DISCUSSION
Prostate biopsy has been considered as gold standard in diagnosis of prostate cancer since Hodge et al. described TRUS guided systematic sextant prostate biopsy technique in 1989 (5). TRUS prostate biopsy has a critical role in urological practice and is generally used in urology clinics as an outpatient procedure.
Although TRUS prostate biopsy is well tolerated in most patients, it also causes a disturbing pain (6). According to the studies, 19-25% of the patients feel moderate or severe pain (7-9). Bastide et al. reported in their study that 80% of patients without local anesthesia felt a significant discomfort and approximately half of them underwent less than 6 core biopsy (2). It was reported that high numbered biopsy protocols increase the possibility of diagnosis of prostate cancer in recent years (10); however, it is considered that high number of biopsies will cause more pain (11). Therefore, the requirement of an anesthetic method during prostate biopsy is accepted by many urologists.
Up to now, many protocols have been developed as anesthetic methods and studies performed. Intrarectal lidocaine gel is an easily-applicable and simple method so it is widely being used by urologists. However, factors limiting its efficacy include topical application, absorption of little fraction of drug into systemic circulation and insufficient penetration to periprostatic tissues. Some studies in literature indicate that the effect of lidocaine gel is suboptimal (12-14).
Periprostatic neural blockage (PNB) is currently the most popular method in recent studies. Various studies have been conducted about PNB regarding the way, number and amount of local anesthetic infiltration and their efficacy has also been shown (6, 15-20). But in one study no difference was detected between the periprostatic injection and lidocaine gel groups (21).
PNB does not ameliorate the pain during the placement of ultrasound probe into the rectum because it is applied afterwards (22). It has been shown that patients feeling pain during placement of probe also felt much more pain during biopsy and their tolerance decreased (23, 24). Besides, injection may also cause pain in proportion to the number of injections (19). In a study, application of PNB and intrarectal lidocaine combination was shown to be more effective than PNB alone and this combination method has been recommended (25).
Numerous studies have been conducted on complications and side effects of PNB, and no statistical difference has been detected between placebo and PNB groups (26-28). In a study conducted by Obek et al., no statistical difference was detected about frequency of urinary infections requiring hospitalization although bacteriuria rates were high (26).
PNB combined with lidocaine gel or alone has been presented as an easily-applicable and safe method, and is currently considered as a gold standard method (3).
General anesthesia may overcome the pain issue during TRUS prostate biopsy but it should be considered that it is not without risk and it could have a significant impact on manpower and financial resources (3). There are several approaches that can be used for this purpose including entonox, propofol, midazolam etc. Barbosa et. al. reported in their study that the intensity of postoperative pain was significantly higher in patients who received only propofol. Fentanyl-propofol had no postoperative pain but two cases of intraoperative respiratory depression, possibly due to the interaction fentanyl-propofol, were observed (29). In consequence of this results, combined local anesthesia is also necessary. Because general analgesia is a complicated treatment due to relatively high costs and the need for operating room conditions, it should only be used in special situations.
Ice has been used as an anesthetic agent for pain related to muscle-skeletal system pathologies and simple surgical procedures for a long time, and its efficacy is well-known. In a review, it was reported that the application of intense cold to traumatic injuries and surgical sites were important steps in 18th and 19th century anaesthesia and local tissue temperature could be brought down by the application of ice and salt by the surgeon (30).
There are two studies in the literature about anal hypothermia; one of them is a frozen finger made from a surgical glove for local anal hypothermia to treat acute painful prolapsed piles (31) and the second one is using an anal cooler (a cylindrical plastic device) to reduce pain after rubber band ligation for hemorrhoids (32). The first study reported that the frozen finger reduced the pain and swelling of painful prolapsed piles. In the subsequent study, no additional benefit was found. No study is available in literature regarding intrarectal ice application for TRUS prostate biopsy.
Ice has some impacts such as slowing down neural conductance, reducing muscle spasms and preventing edema secondary to trauma together with antinociceptive effects (4). Because of these effects, we analyzed the efficacy of ice application as an anesthetic method prior to TRUS prostate biopsy in this study. Mean pain scores during the procedure were compared among the groups of intrarectal ice and lidocaine gel in this study, and we found significant differences between two groups (p=0.007). Also, no difference was detected between two groups about feeling pain during the placement of probe. These results indicate that intrarectal ice application prior to TRUS prostate biopsy may have an effect on reducing pain during the procedure.
There are two main disadvantages in use of this method which is performed via intrarectal application of ice mould obtained by glove finger. Firstly, because ice is applied before the procedure, its cold effect on tissues decreases over time. In our study, ice mould was applied during 5 minutes intrarectally and then the procedure begun after it had been taken out. Hence, it is obvious that the anesthetic effect would decrease over time after the ice mould is taken out. Secondly, patients felt pain at the time of the ice application. None of the patients that received lidocaine gel as an anesthetic method felt pain. But in the ice group 22 patients (36.7%) felt mild pain, one felt moderate (1.7%) and two patients (3.3%) felt severe pain while ice was placed through anus. After the ice mould was placed, patients reported a sense of coldness which was not uncomfortable during the 5 minutes. The ice group was divided into two groups as painful and painless application of ice mould to explore the possibility of its effect on the level of pain during the procedure then the main pain levels were compared. No significant difference was determined between both groups (Table-4).
At the beginning of this study, our opinion was that the complication rates of biopsy would be lowered via intrarectal ice application because of its vasoconstrictor effect on vessels. However, we did not detect any significant difference between groups about duration of rectal bleeding and hematuria after biopsy. We think that this result may be related to using the ice at the beginning of the procedure and its effect decreases overtime. If this cold effect can be kept all along the procedure by using new techniques, its combined use with available methods (ice+lidocaine gel or ice+PNB) or using post-procedure ice for biopsy complications, complication rates may decrease and it may provide additional benefits.
CONCLUSIONS
In conclusion, the ice application seems to be effective to reduce the pain during prostate biopsy. The aim of this study was to show that we can have the benefit of cold effect to reduce pain during prostate biopsy. It should be admitted that our method in this present form is difficult to be considered an alternative to easily applicable lidocaine gel or PNB which is quite effective. Development of new techniques about cold effect or ice can make this method more useful and decrease complication rates. Also, its combined use with available methods may provide additional benefit.
REFERENCES
- 1.Zisman A, Leibovici D, Kleinmann J, Siegel YI, Lindner A. The impact of prostate biopsy on patient well-being: a prospective study of pain, anxiety and erectile dysfunction. J Urol. 2001;165:445–454. doi: 10.1097/00005392-200102000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Bastide C, Lechevallier E, Eghazarian C, Ortega JC, Coulange C. Tolerance of pain during transrectal ultrasound-guided biopsy of the prostate: risk factors. Prostate Cancer Prostatic Dis. 2003;6:239–241. doi: 10.1038/sj.pcan.4500664. [DOI] [PubMed] [Google Scholar]
- 3.Autorino R, De Sio M, Di Lorenzo G, Damiano R, Perdonà S, Cindolo L, et al. How to decrease pain during transrectal ultrasound guided prostate biopsy: a look at the literature. J Urol. 2005;174:2091–2097. doi: 10.1097/01.ju.0000181212.51025.06. [DOI] [PubMed] [Google Scholar]
- 4.Ernst E, Fialka V. Ice freezes pain? A review of the clinical effectiveness of analgesic cold therapy. J Pain Symptom Manage. 1994;9:56–59. doi: 10.1016/0885-3924(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 5.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–74. doi: 10.1016/s0022-5347(17)38664-0. discussion 74-5. [DOI] [PubMed] [Google Scholar]
- 6.Soloway MS, Obek C. Periprostatic local anesthesia before ultrasound guided prostate biopsy. J Urol. 2000;163:172–173. [PubMed] [Google Scholar]
- 7.Crundwell MC, Cooke PW, Wallace DM. Patients’ tolerance of transrectal ultrasound-guided prostatic biopsy: an audit of 104 cases. BJU Int. 1999;83:792–795. doi: 10.1046/j.1464-410x.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 8.Irani J, Fournier F, Bon D, Gremmo E, Doré B, Aubert J. Patient tolerance of transrectal ultrasound-guided biopsy of the prostate. Br J Urol. 1997;79:608–610. doi: 10.1046/j.1464-410x.1997.00120.x. [DOI] [PubMed] [Google Scholar]
- 9.Collins GN, Lloyd SN, Hehir M, McKelvie GB. Multiple transrectal ultrasound-guided prostatic biopsies--true morbidity and patient acceptance. Br J Urol. 1993;71:460–463. doi: 10.1111/j.1464-410x.1993.tb15993.x. [DOI] [PubMed] [Google Scholar]
- 10.Presti JC., Jr Prostate biopsy: how many cores are enough? Urol Oncol. 2003;21:135–140. doi: 10.1016/s1078-1439(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 11.Naughton CK, Ornstein DK, Smith DS, Catalona WJ. Pain and morbidity of transrectal ultrasound guided prostate biopsy: a prospective randomized trial of 6 versus 12 cores. J Urol. 2000;163:168–171. [PubMed] [Google Scholar]
- 12.Desgrandchamps F, Meria P, Irani J, Desgrippes A, Teillac P, Le Duc A. The rectal administration of lidocaine gel and tolerance of transrectal ultrasonography-guided biopsy of the prostate: a prospective randomized placebo-controlled study. BJU Int. 1999;83:1007–1009. doi: 10.1046/j.1464-410x.1999.00080.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang SS, Alberts G, Wells N, Smith JA, Jr, Cookson MS. Intrarectal lidocaine during transrectal prostate biopsy: results of a prospective double-blind randomized trial. J Urol. 2001;166:2178–2180. doi: 10.1016/s0022-5347(05)65529-2. [DOI] [PubMed] [Google Scholar]
- 14.Cevik I, Ozveri H, Dillioglugil O, Akdaş A. Lack of effect of intrarectal lidocaine for pain control during transrectal prostate biopsy: a randomized prospective study. Eur Urol. 2002;42:217–220. doi: 10.1016/s0302-2838(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 15.Nash PA, Bruce JE, Indudhara R, Shinohara K. Transrectal ultrasound guided prostatic nerve blockade eases systematic needle biopsy of the prostate. J Urol. 1996;155:607–609. [PubMed] [Google Scholar]
- 16.Pareek G, Armenakas NA, Fracchia JA. Periprostatic nerve blockade for transrectal ultrasound guided biopsy of the prostate: a randomized, double-blind, placebo controlled study. J Urol. 2001;166:894–897. [PubMed] [Google Scholar]
- 17.Leibovici D, Zisman A, Siegel YI, Sella A, Kleinmann J, Lindner A. Local anesthesia for prostate biopsy by periprostatic lidocaine injection: a double-blind placebo controlled study. J Urol. 2002;167:563–565. doi: 10.1016/S0022-5347(01)69086-4. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez A, Kyriakou G, Leray E, Lobel B, Guillé F. Prospective study comparing two methods of anaesthesia for prostate biopsies: apex periprostatic nerve block versus intrarectal lidocaine gel: review of the literature. Eur Urol. 2003;44:195–200. doi: 10.1016/s0302-2838(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 19.Schostak M, Christoph F, Müller M, Heicappell R, Goessl G, Staehler M, et al. Optimizing local anesthesia during 10-core biopsy of the prostate. Urology. 2002;60:253–257. doi: 10.1016/s0090-4295(02)01730-2. [DOI] [PubMed] [Google Scholar]
- 20.Taverna G, Maffezzini M, Benetti A, Seveso M, Giusti G, Graziotti P. A single injection of lidocaine as local anesthesia for ultrasound guided needle biopsy of the prostate. J Urol. 2002;167:222–223. [PubMed] [Google Scholar]
- 21.Mallick S, Humbert M, Braud F, Fofana M, Blanchet P. Local anesthesia before transrectal ultrasound guided prostate biopsy: comparison of 2 methods in a prospective, randomized clinical trial. J Urol. 2004;171:730–733. doi: 10.1097/01.ju.0000108125.96072.ff. [DOI] [PubMed] [Google Scholar]
- 22.Zisman A, Leibovici D, Kleinmann J, Cooper A, Siegel Y, Lindner A. The impact of prostate biopsy on patient well-being: a prospective study of voiding impairment. J Urol. 2001;166:2242–2246. [PubMed] [Google Scholar]
- 23.Philip J, McCabe JE, Roy SD, Samsudin A, Campbell IM, Javlé P. Site of local anaesthesia in transrectal ultrasonography-guided 12-core prostate biopsy: does it make a difference? BJU Int. 2006;97:263–265. doi: 10.1111/j.1464-410X.2006.05957.x. [DOI] [PubMed] [Google Scholar]
- 24.Stirling BN, Shockley KF, Carothers GG, Maatman TJ. Comparison of local anesthesia techniques during transrectal ultrasound-guided biopsies. Urology. 2002;60:89–92. doi: 10.1016/s0090-4295(02)01671-0. [DOI] [PubMed] [Google Scholar]
- 25.Obek C, Ozkan B, Tunc B, Can G, Yalcin V, Solok V. Comparison of 3 different methods of anesthesia before transrectal prostate biopsy: a prospective randomized trial. J Urol. 2004;172:502–505. doi: 10.1097/01.ju.0000131601.06286.26. [DOI] [PubMed] [Google Scholar]
- 26.Obek C, Onal B, Ozkan B, Onder AU, Yalçin V, Solok V. Is periprostatic local anesthesia for transrectal ultrasound guided prostate biopsy associated with increased infectious or hemorrhagic complications? A prospective randomized trial. J Urol. 2002;168:558–561. [PubMed] [Google Scholar]
- 27.Seymour H, Perry MJ, Lee-Elliot C, Dundas D, Patel U. Pain after transrectal ultrasonography-guided prostate biopsy: the advantages of periprostatic local anaesthesia. BJU Int. 2001;88:540–544. doi: 10.1046/j.1464-410x.2001.02324.x. [DOI] [PubMed] [Google Scholar]
- 28.Rabets JC, Jones JS, Patel AR, Zippe CD. Bupivacaine provides rapid, effective periprostatic anaesthesia for transrectal prostate biopsy. BJU Int. 2004;93:1216–1217. doi: 10.1111/j.1464-410X.2004.04843.x. [DOI] [PubMed] [Google Scholar]
- 29.Barbosa RA, da Silva CD, Torniziello MY, Cerri LM, Carmona MJ, Malbouisson LM. A comparative study among three techniques of general anesthesia for ultrasound-guided transrectal prostate biopsy. Rev Bras Anestesiol. 2010;60:457–465. doi: 10.1016/S0034-7094(10)70057-X. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer M. History of anaesthesia: early forms of local anaesthesia. Eur J Anaesthesiol. 2014;31:1–12. doi: 10.1097/EJA.0b013e328365ad7c. [DOI] [PubMed] [Google Scholar]
- 31.El Ashaal Y, Chandran VP, Siddiqui MN, Sim AJ. Local anal hypothermia with a frozen finger: a treatment for acute painful prolapsed piles. 520Br J Surg. 1998;85 doi: 10.1046/j.1365-2168.1998.00647.x. [DOI] [PubMed] [Google Scholar]
- 32.Lam TJ, Felt-Bersma RJ. A novel device reduces anal pain after rubber band ligation: a randomized controlled trial. Tech Coloproctol. 2012;16:221–226. doi: 10.1007/s10151-012-0824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]