Abstract
Objectıve
To investigate the clinical characteristics, prognosis, survival and diagnosis of high-grade primary renal leiomyosarcoma.
Materıals and Methods
From January 2003 to April 2013, 10 cases of high-grade primary renal leiomyosarcoma were retrospectively reviewed. We analyzed clinical manifestations, treatment and prognosis of our group and correlated to the literature.
Results
Ten cases (five male and five female patients; age range 43–77 years, mean=57±std d:12.3 ) were enrolled. The mean diameter of the tumor masses was 9.35±4.5 cm (range 3-18 cm). 40% of the patients were asymptomatic while the major symptom of 60% patients was lumbar pain. Nephrectomy was performed in 90% of patients. Partial nephrectomy surgery was preferred for only one patient. Pleomorphism and necrosis with high-grade, pink spindle cell cytoplasm were viewed in all patients. All patients were high-grade, pink spindle cell cytoplasm and pleomorfism and necrosis were observed in all. In an immunohistochemical examination, vimentin was seen in 100%, desmin in 90% and smooth muscle actin in 80% of the patients. CD117 was negative in all patients. All of the cases were followed-up, and the time of survival varied from 6 to 68 months (mean 23.9±std d:20.1). No patient received adjuvant CTx and/or RTx.
Conclusıon
High-grade primary renal leiomyosarcomas (LMSs) are rare and highly malignant and the prognosis is poor. Early diagnosis and radical nephrectomy can prolong the patient’s life. Surgery is the main treatment modality for renal (leiomyosarcoma) LMS.
Keywords: Sarcoma, Kidney, Leiomyosarcoma, Nephrectomy, Diagnosis, Immunohistochemistry
INTRODUCTION
Sarcomas account for 0.8 to 2.7% of malignant kidney tumors. Similar to leiomyomas, leiomyosarcomas (LMSs) originate from the smooth muscles of the renal capsule, pelvis renalis, calyxes, and blood vessels. In general, LMSs are more common in females and occur in the fourth and sixth decades. LMSs are solitary lesions. The major symptoms include pain, palpable mass, and hematuria. The tumor occurs more frequently on the right side (1). LMSs are generally highly aggressive malignant tumors originating from the smooth muscles of the soft tissues, and they exhibit a high potential for metastasis. LMSs of the kidney can originate from the renal pelvis, calyxes, renal capsule, and blood vessels of the kidneys. However, the tumors most commonly arise from the smooth muscles of the kidney veins (1). LMSs account for 50-60% of kidney sarcomas. The differentiation from the other renal masses is extremely challenging before surgery (2). Among the other urogenital sarcomas, LMSs of the kidney are less frequently encountered compared to sarcomas of the prostate and bladder; however, these tumors are associated with poor prognosis in terms of survival. LMSs of the kidney can reach large sizes, due to the lack of natural barriers for leiomyosarcomas arising from the mesenchymal components. Sarcomas typically possess a pseudocapsule. LMS of the kidney is a rare entity with poor prognosis. LMSs account for only 0.12% of all renal malignancies (3).
MATERIALS AND METHODS
Patients
A total of 10 patients, who underwent surgery due to renal mass between January 2003 and April 2013 and who were diagnosed with high grade primary LMS of the kidney, were included in the present study. During this period, radical nephrectomy was applied to 389 patients. 15(3.8%) of these patients were diagnosed with leimyosarcoma. 10 (2.5%) cases were high-graded. American Joint Committee of Cancer (AJCC) program was used to follow-up the cases. The symptoms at presentation, radiological findings, and immunohistochemical features obtained through pathological examination were evaluated. The therapies (radiotherapy and/or chemotherapy) and survival of the patients were recorded.
Diagnosis and staging of Primary renal leiomyosarcoma
The pathological diagnosis and grade of the tumor were evaluated according to the classification of the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC). The histological grade was scored based in the level of differentiation, presence of mitosis, and necrosis in each high power field (4). All patients were found to a have high grade tumor according to this scoring system (Table-1). The immunohistochemical examination included staining for desmin, smooth muscle actin (SMA), vimentin, Bcl-2, CD-34, Ki-67, S-100, and CD117. Markers, which were used for immunohistochemical analysis, were used to distinguish especially from renal cell carcinoma indicating sarcomatoid differentiation, carcisarcoma and other subtypes of sarcoma. Vimentin is not specific for tumor type. Therefore it is not used in the differential diagnosis. However, it is in favor of tumor cells staining with negative desmin with cytokeratin and positive with smooth muscle actin(SMA). Cytokeratin positivity with absence of myoid markers supports a diagnosis of sarcomatoid renal cell carcinoma. Sarcomatoid carcinomas, however, are not uniformly positive for cytokeratins and may express SMA. Some leiomyosarcomas may also express cytokeratin and or epithelial membrane antigen (EMA). In the latter situation, the presence of desmin is diagnostically helpful since it is positive in leiomyosarcoma and not in sarcomatoid carcinoma (5). The staging of the tumor was based on the system developed by the American Joint Cancer Committee (AJCC-2013) on the staging of soft tissue sarcomas.
Table 1. The distribution of the patients according to the French Federation of Cancer Centers Sarcoma Group (FNCLCC).
| Tumor differentiation | ||
|---|---|---|
| Score 1 | n=1 | 10% |
| Score 2 | n=6 | 60% |
| Score 3 | n=3 | 30% |
| Mitotic count | ||
| Score 1 (0-9 mitoses per 10 HPF+) | n=0 | 0% |
| Score 2 (10-19 mitoses per 10 HPF) | n=4 | 40% |
| Score 3 (≥ 20 mitoses per 10 HPF) | n=6 | 60% |
| Tumor necrosis | ||
| Score 0 ( no necrosis) | n=2 | 20% |
| Score 1 (<50% tumor necrosis) | n=6 | 60% |
| Score 2 (≥50% tumor necrosis) | n=2 | 20% |
| Histologic grade | ||
| Grade 1(total score 2,3) | n=3 | 30% |
| Grade 2 ( total score 4,5) | n=3 | 30% |
| Grade 3 ( total score 6,7,8) | n=4 | 40% |
Statistical analysis
SPSS version 16.0 for Windows was used for statistical analysis.
RESULTS
The patients included in the study with the diagnosis of high grade primary LMS of the kidney were followed for 6-68 months (mean; 23.9±std d:20.1, Median; 19.0).
Clinical features
The mean age was 57 years (range: 43-77 years mean=57±std d:12.3) and the male/female ratio was 1/1. The most common symptom was lumbar pain occurring in 60% (n=6) of patients. Of the patients, 40% (n=4) were asymptomatic, and only 10% (n=1) had hematuria, and 20% (n=2) had systemic and gastrointestinal symptoms (Table-2). According to the staging system of the AJCC-2013, 40% (n=4) of the patients had a stage III tumor, 30% (n=3) had a stage IIb tumor, 10% (n=1) had a stage IIa tumor, 10% (n=1) had a stage Ib tumor, and 10% (n=1) had a stage Ia tumor. The mean diameter of the tumor masses was 9.35±4.5 cm (range 3-18 cm) (Figures 1 -3).
Table 2. Age, gender, clinical appearance, stage, and survival of the cases.
| Age | Male / Female | Right / Left | Tumor diameter (cm) | Clinical features | Stage (AJCC-2013) | Treatment | Survival |
|---|---|---|---|---|---|---|---|
| 56 | male | L | 7 (and 3,3,1) | lumbar pain | T2bN0M0 G2 Stage IIB | Radical nephrectomy | 9 months |
| 77 | male | R | 8 | lumbar pain | T2bN0M0 G3 Stage III | Radical nephrectomy | 15 months |
| 43 | female | L | 3.5 | asymptomatic | T1bN0M0 G1 Stage IA | Partial nephrectomy | 44 months |
| 65 | female | L | 3 | asymptomatic | T1bN0M0 G2 Stage IIA | Radical nephrectomy | 68 months |
| 49 | male | R | 13(and 5) | lumbar pain,hematuria | T2bN1M0 G3 Stage III | Radical nephrectomy | 7 months |
| 45 | male | R | 18 | Lumbar pain, nausea, weight loss | T2bN1M0 G3 Stage III | Radical nephrectomy | 6 months |
| 46 | female | L | 10 | asymptomatic | T2bN0M0 G2 Stage Iıb | Radical nephrectomy | 23 months |
| 66 | female | L | 13 | lumbar pain | T2bN1M0 G2 Stage III | Radical nephrectomy | 8 months |
| 73 | male | R | 6 | asymptomatic | T2bN0M0 G1 Stage Ib | Radical nephrectomy | 23 mnoths |
| 50 | female | L | 8 | lumabr pain, dyspepsia | T2bN0M0 G2 Stage IIb | Radical nephrectomy | 36 months |
Figure 1. Magnetic Ressonance Imaging: Leiomyosarcoma in pelvis renalis of left kidney (arrow).

Figure 2. Magnetic Ressonance Imaging: Leioyosarcoma in pelvis renalis of left kidney (arrow).

Figure 3. Surgical specimen; Left renal leiomyosarcoma (arrow).

Immunohistopathological Findings
The immunohistochemical examination revealed positive staining for desmin (90%, n=9), SMA (80%, n=8), and vimentin (100%, n=10) (Figures 4 -6). All patients exhibited negative staining for Bcl-2. The Ki-67 proliferation index was positive in 60% (n=6) of the patients. All patients showed negative staining for Bcl-2 and CD117 (Table-3).
Figure 4. Hematoxilyn-eosin staining demostrating high-grade sarcomatoid cells. H&E [100, 200x] Pleomorfism(small figure) and necrosis (arrow).
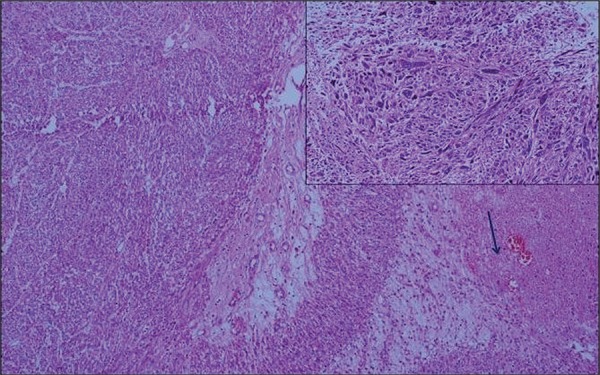
Figure 5. Immunohistochemistry showing Desmin[200x].

Figure 6. Immunohistochemistry showing difuse actin expression in smooth muscle fiber cytoplasm. Actin[200x].

Table 3. The immunohistochemical features of the cases.
| Immunohistochemicalantibody | Positive | Negative |
|---|---|---|
| Desmin | (n=9), 90% | (n=1), 10% |
| Smooth muscle actin (SMA) | (n=8), 80% | (n=2), 20% |
| Vimentin | (n=10), 100% | 0% |
| Bcl-2 | 0% | (n=10), 100% |
| CD-34 | (n=1), 10% | (n=9), 90% |
| *Ki-67 | (n=6), 60% | (n=4), 40% |
| S-100 | (n=2), 20% | (n=8), 80% |
| CD117 | 0% | (n=10), 100% |
*Ki-67 proliferation index ranged between 3% and 70% in the positive cases.
Treatments and Survival
Ninety percent of the patients (n=9) underwent radical nephrectomy, and 10% (n=1) underwent partial nephrectomy. The mean disease-related survival was 23.9±20.1 months (range: 6-68 months), and none of the patients received adjuvant chemotherapy and/or radiotherapy.
DISCUSSION
The incidence of the primary leiomyosarcoma of the kidney increases with age, and this tumor is more common in females than in males and more common in the right kidney than in the left kidney (6). The tumor becomes symptomatic only with the expansion of the tumor size. The symptoms increase with the advancing disease stage. The compression on the neighboring tissues results in lumbar pain and hematuria and the finding of palpable mass on examination; systemic symptoms can also occur, such as nausea, vomiting, and abdominal pain (6).
It is extremely challenging to differentiate LMS of the kidney from renal cell cancer exhibiting sarcomatoid differentiation. These two tumors exhibit similar clinical, radiological, and pathological features. The most prominent features that differentiate sarcomas from renal cell cancer are that sarcomas originate from the capsule or perisinous region and sarcomas expand to large sizes without lymphadenopathy, and the lesion contains components such as liposarcoma and osteosarcoma. These tumors are avascular lesions, with the exception of hemangiosarcomas. The sarcomas should definitely be considered in the differential diagnosis in the presence of fast-growing renal masses. The sarcomas possess a pseudocapsule; however, these capsules do not represent a reliable barrier from the surgical point perspective. The capsule is often found to be infiltrated by the tumor. In general, high grade LMSs show high metastatic potential. Complete surgical resection is the most effective means of therapy (1). Therefore, early diagnosis and early surgical therapy prolong survival in patients with primary LMS. In the present study, the diagnosis and treatment of high grade LMSs and mean survival were discussed together with the literature data.
Life expectancy is lower with the sarcomas of the kidney compared to the other sarcomas of the urinary tract. The 5-year survival is 82% in patients with retroperitoneal sarcoma, 73% in patients with the sarcomas of the bladder, 44% in patients with prostate sarcoma, and 39% in patients with the sarcomas of the kidney (7). According to Geonseok et al. the 5-year survival was 51.4% in all of the urogenital sarcomas (7). There are two studies in the literature supporting these findings. These studies reported a mean survival around 50% (8, 9). The surgical resection was the most significant prognostic factor in these patients. Lewis et al. reported that the presence of unresectable disease and incomplete surgical resection were the most significant factors predictive of disease-specific death (10). In a study by van Dalen et al., of 143 patients treated in the Netherlands, complete tumor resection was correlated with better overall survival in the multivariate analysis (11). The common notion in the literature is that surgical excision was nearly the only prognostic factor in LMSs. There was no correlation with the subtypes of the sarcomas and the survival, although statistical evaluation provided limited data due to small number of patients (7). Complete surgical resection with wide margins is the recommended means of therapy in patients with renal LMS. However, the literature also reported cases with renal LMS treated with partial nephrectomy (12). In our series, one patient was treated with partial nephrectomy and this patient survived for 44 months, which is higher than the mean survival time. The tumor size being lower than 5 cm is a good prognostic feature (3). In our series, 20% (n=2) of the patients had a tumor measuring less than 5 cm. These cases achieved the highest survival rate (44 and 68 months). These findings suggest that small tumor diameter was associated with a good prognosis. In LMS, metastasis to the lungs, liver, and colon point to poor prognosis (1). The 5-year survival in patients with sarcoma is around 50% according to the statistical records of the National Cancer Institute (NCI) and AJCC. Vallery et al. reported a survival between 17.9 and 25 months in patients with leiomyosarcoma of the kidney (3). The survival rate of 23.9% in our series was also consistent with the literature data. Although the 5-year mean survival is around 50% in LMS, this rate drops by half in patients with high grade primary LMS of the kidney. This finding suggests that the presence of a high grade tumor decreases life expectancy by half, even if the number of patients was limited in the current cross-sectional study.
In general, it is known that adjuvant/neoadjuvant chemotherapy/radiotherapy in LMS does not provide survival advantage (1). However, Raut et al. reported that adjuvant chemotherapy could be used on partially resected tumors (13). Nagumo et al. administered systemic chemotherapy with gemcitabine and docetaxel in a 64-year-old patient with renal LMS and lung metastasis and obtained an incomplete response in the lung metastasis. The patient developed new metastases in the lungs and pancreas at the end of 29 months. This study is one of the rare reports in the literature the demonstrated the survival benefit of chemotherapy, particularly therapy with gemcitabine and docetaxel (14). There are sporadic reports of cases with primary LMS of the kidney that achieved long-term survival up to 70 months after being submitted to radical nephrectomy and concurrent adjuvant chemotherapy and radiotherapy (15). However, surgery may have provided a complete cure in this patient. It is therefore likely that adjuvant chemotherapy and radiotherapy may have been an unnecessary procedure. There are reports in the literature demonstrating the survival benefits of neoadjuvant chemotherapy in LMSs. Kamba et al. showed that LMS could become resectable with the administration of neoadjuvant chemotherapy with CYVADIC (cyclophosphamide, vincristine, adriamycin, and dacarbazine) (16). There are reports on cases with LMS that achieved an incomplete response with the combination of anti-metabolite gemcitabine and mTOR blocker rapamycin (17). In addition, phase II studies reported the possibility of treating LMS with tyrosine kinase inhibitors such as sunitinib (18).
In their study, Deyrup et al. demonstrated the relationship between increasing histological grade of the renal LMS and survival. The histological grade was defined as a poor prognostic factor (19). Compared to the subtypes of other renal malignancies, primary LMS of the kidney is associated with considerably poor prognosis. The correct diagnosis and proper management of the disease requires detailed morphological analysis and careful interpretation of the immunohistochemical markers (20). In the literature, the Ki-67 proliferation index ranged from 6% to 50% in LMS (21). The Ki-67 proliferation index in our series of patients is parallel to that reported in the literature. The studies in the literature found that p16 and p53 tumor suppressor proteins are over-expressed in LMS, and therefore could be used as a prognostic marker (22, 23). Unlike many other soft tissue tumors, the genetic basis of LMSs has been poorly understood. LMSs are known to be genetically complex, often showing ‘chaotic’ karyotypes including aneuploidy or polyploidy, and no recurrent tumor-specific translocations have been detected (24). Some soft tissue sarcomas are also encountered in patients with EBV, AIDS, and post-transplant patients (24).
CONCLUSIONS
Primary LMS of the kidney is a smooth muscle tumor with complex genetic and molecular basis exhibiting aggressive biological behavior and poor prognosis. The life expectancy in high-grade primary LMS of the kidney is shorter compared to low-grade tumors. It must be kept in mind that mean survival is shorter in patients with high-grade primary LMS, and these patients will die within two years. The elucidation of the biological behavior, molecular and genetic basis of this tumor, and mutagenic factors triggering the disease will increase the understanding of this cancer. Currently, the genetic basis of this tumor is described as “chaotic”. However, the primary goal should be the elucidation of the molecular and genetic basis of the cancer. The discovery of new agents other than gemcitabine and docetaxel is required in order to prolong mean survival in metastatic disease. The data presented in this study contribute to the understanding of high-grade primary renal leiomyosarcoma and may help in the development of an optimal therapeutic strategy to treat high-grade primary renal leiomyosarcoma .The only effective means of therapy is the complete resection of the tumor with wide surgical margins. Other than the surgical complete resection, tumor grade and diameter are the important prognostic factors in primary LMS of the kidney.
ABREVATIONS
LMS = Leiomyosarcoma
LMSs = Leiomyosarcomas
CTx = Chemotherapy
RTx = radiotherapy
CK = Cytokeratin
EMA = Epithelial membrane antigen
SMA = smooth muscle actin
Bcl-2 = B-cell lymphoma-2
HMB45 = Mouse anti Human melenoma antibody-45
EBV = Epstein–Barr virüs
FH = fumarate hydratase
RCC = Renal cell carcinoma
NCI = National Cancer Institute
FNCLCC = French Federation of Cancer Centers Sarcoma Group
AJCC = American Journal of Climate Change
CYVADIC = cyclosphosphamide, vincristine, adriamycin and dacarbazine
REFERENCES
- 1.Venkatesh K, Lamba Saini M, Niveditha SR, Krishnagiri C, Babu S. Primary leiomyosarcoma of the kidney. 652398Patholog Res Int. 2010;2010 doi: 10.4061/2010/652398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-Cornelio A, Ramos-Salgado F, Hernández-Ramírez D, García-Álvarez KG, Alvarado-Cabrero I, Hernández-Toriz N. Leiomyosarcoma of the kidney: case report. Cir Cir. 2011;79:260–263. 282–285. [PubMed] [Google Scholar]
- 3.Valery JR, Tan W, Cortese C. Renal leiomyosarcoma: a diagnostic challenge. 459282Case Rep Oncol Med. 2013;2013 doi: 10.1155/2013/459282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, Le Doussal V, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14:869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 5.Iwata J, Fletcher CD. Immunohistochemical detection of cytokeratin and epithelial membrane antigen in leiomyosarcoma: a systematic study of 100 cases. Pathol Int. 2000;50:7–14. doi: 10.1046/j.1440-1827.2000.01001.x. [DOI] [PubMed] [Google Scholar]
- 6.Beardo P, José Ledo M, Jose Luis RC. Renal leiomyosarcoma. Rare Tumors. 2013;5: doi: 10.4081/rt.2013.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee G, Lee SY, Seo S, Jeon S, Lee H, Choi H, et al. Prognostic factors and clinical outcomes of urological soft tissue sarcomas. Korean J Urol. 2011;52:669–673. doi: 10.4111/kju.2011.52.10.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez JC, Perez EA, Franceschi D, Moffat FL, Jr, Livingstone AS, Koniaris. et al. Outcomes for soft-tissue sarcoma in 8249 cases from a large state câncer registry. J Surg Res. 2007;141:105–114. doi: 10.1016/j.jss.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Perez EA, Gutierrez JC, Moffat FL, Jr, Franceschi D, Livingstone AS, Spector SA, et al. Retroperitoneal and truncal sarcomas: prognosis depends upon type not location. Ann Surg Oncol. 2007;14:1114–1122. doi: 10.1245/s10434-006-9255-x. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dalen T, Plooij JM, van Coevorden F, van Geel AN, Hoekstra HJ, Ch Albus-Lutter, et al. Dutch Soft Tissue Sarcoma Group. Long-term prognosis of primary retroperitoneal soft tissue sarcoma. Eur J Surg Oncol. 2007;33:234–238. doi: 10.1016/j.ejso.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Cocuzza M, Arap S, Lucon AM, Saldanha LB. Renal leiomyosarcoma treated with partial nephrectomy. Clinics (Sao Paulo) 2005;60:345–346. doi: 10.1590/s1807-59322005000400013. [DOI] [PubMed] [Google Scholar]
- 13.Raut CP, Pisters PW. Retroperitoneal sarcomas: Combined-modality treatment approaches. J Surg Oncol. 2006;94:81–87. doi: 10.1002/jso.20543. [DOI] [PubMed] [Google Scholar]
- 14.Nagumo Y, Kimura T, Ichioka D, Uchida M, Oikawa T, Suetomi T, et al. Long-term survival with gemcitabine and docetaxel for renal leiomyosarcoma : a case report. Hinyokika Kiyo. 2013;59:497–501. [PubMed] [Google Scholar]
- 15.Sharma D, Pradhan S, Aryya NC, Shukla VK. Leiomyosarcoma of kidney: a case report with long term result after radiotherapy and chemotherapy. Int Urol Nephrol. 2007;39:397–400. doi: 10.1007/s11255-006-9022-8. [DOI] [PubMed] [Google Scholar]
- 16.Kamba T, Kawakita M, Noguchi T, Kamoto T, Okabe T, Takeuchi E, et al. Neoadjuvant CYVADIC (cyclophosphamide, vincristine, adriamycin and dacarbazine) therapy for retroperitoneal leiomyosarcoma: a case report. Hinyokika Kiyo. 1997;43:577–580. [PubMed] [Google Scholar]
- 17.Merimsky O. Targeting metastatic leiomyosarcoma by rapamycin plus gemcitabine: an intriguing clinical observation. Int J Mol Med. 2004;14:931–935. [PubMed] [Google Scholar]
- 18.Mahmood ST, Agresta S, Vigil CE, Zhao X, Han G, D’Amato G, et al. Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int J Cancer. 2011;129:1963–1969. doi: 10.1002/ijc.25843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deyrup AT, Montgomery E, Fisher C. Leiomyosarcoma of the kidney: a clinicopathologic study. Am J Surg Pathol. 2004;28:178–182. doi: 10.1097/00000478-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Dhawan S, Chopra P, Dhawan S. Primary renal leiomyosarcoma: A diagnostic challenge. Urol Ann. 2012;4:48–50. doi: 10.4103/0974-7796.91623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills AM, Ly A, Balzer BL, Hendrickson MR, Kempson RL, McKenney JK, et al. Cell cycle regulatory markers in uterine atypical leiomyoma and leiomyosarcoma: immunohistochemical study of 68 cases with clinical follow-up. Am J Surg Pathol. 2013;37:634–642. doi: 10.1097/PAS.0b013e318287779c. [DOI] [PubMed] [Google Scholar]
- 22.Hakverdi S, Güngören A, Yaldiz M, Hakverdi AU, Toprak S. Immunohistochemical analysis of p16 expression in uterine smooth muscle tumors. Eur J Gynaecol Oncol. 2011;32:513–515. [PubMed] [Google Scholar]
- 23.Hewedi IH, Radwan NA, Shash LS. Diagnostic value of progesterone receptor and p53 expression in uterine smooth muscle tumors. 1Diagn Pathol. 2012;7 doi: 10.1186/1746-1596-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miettinen M. Smooth muscle tumors of soft tissue and non-uterine viscera: biology and prognosis. Mod Pathol. 2014;27(Suppl 1):S17–S29. doi: 10.1038/modpathol.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]


