Abstract
Pseudoloma neurophilia (Microsporidia) is a common disease of zebrafish, Danio rerio, including those used as research models. We conducted a study comprised of four separate experiments to determine the effects of husbandry stress on pre-existing and experimental Pseudoloma infections and the subsequent effects on survival, infection onset and intensity, fish growth and reproduction. In fish (AB strain) with pre-existing infections, stress or feeding cortisol significantly increased mortality over 7 wk compared to no stress or cortisol treatment. In contrast, no mortality was observed in fish (TL strain) experimentally-exposed to Pseudoloma over 10 wk. A third experiment involved experimental exposure of AB fish to Pseudoloma and exposure to crowding and handling stressors. No mortality was associated with Pseudoloma regardless of stress treatment over a 20 wk period. However, the onset of infection occurred sooner in stress-treated fish. Stress significantly increased the mean intensity of infection (described as xenoma area/spinal cord area in histological sections) at wk 20 PE (post-exposure). In fish with pre-existing infections, myositis was significantly greater in stressed and cortisol-treated fish than those not stressed. With experimental exposure of AB fish, stressed and infected groups weighed significantly less than the control group at wk 20 PE. Regarding fecundity, the number of larvae hatched at 5 days post fertilization was negatively associated with mean infection intensity among Pseudoloma-infected and stressed AB fish. These experiments are the first to show empirically that Pseudoloma can be associated with reduced weight and fecundity, and that stress can exacerbate the severity of the infection.
Keywords: Pseudoloma neurophilia, Stress, Growth, Reproduction, Mortality, Microsporidia
INTRODUCTION
Microsporidia are spore-producing obligate intracellular parasites with over 150 species infective to fishes (Shaw and Kent 1999; Lom and Nilsen 2003). Microsporidiosis was first reported in zebrafish, Danio rerio, almost 30 years ago (de Kinkelin 1980), and this microsporidium was recently assigned to a new genus and species, Pseudoloma neurophilia (Matthews et al. 2001), which we henceforth refer to in this article as Pseudoloma. Microsporidiosis, the most common disease of laboratory zebrafish, affects the central nervous system and somatic muscle, and is associated with emaciation, spinal deformity, and morbidity (Matthews et al. 2001; Kent and Bishop-Stewart 2003). The spores of Pseudoloma are contained within host-parasite complexes known as xenomas (Chatton 1920; Lom and Dyková 2005). Pseudoloma infections are characterized by multiple xenomas in the hind brain, spinal cord, nerve roots, and occasionally within the somatic muscle. Free spores (probably from ruptured xenoma) are found within phagocytes and are associated with severe, chronic myositis, meningitis, and encephalitis (Matthews et al. 2001; Kent and Bishop-Stewart 2003). Ovarian tissue is often infected and occasionally eggs may harbor Pseudoloma spores indicating potential for vertical transmission (Kent and Bishop-Stewart 2003).
Zebrafish are popular biomedical and environmental research models (Dahm and Geisler 2006; Scholz and Mayer 2008). Over the past 30 years, zebrafish research has increased from a few to thousands of research laboratories worldwide, subsequently increasing the potential for dissemination and exacerbation of diseases such as microsporidiosis (Kent et al. 2009). Control of infectious diseases in zebrafish laboratories typically involves quarantine and chlorine disinfection of eggs (Westerfield 2007). Microsporidian spores are durable and remain infective for long periods (Shaw et al. 2000a) and chlorine treatments used for disinfecting zebrafish eggs have proven to be ineffective for killing Pseudoloma spores (Ferguson et al. 2007). Optimizing rearing conditions may aid in controlling diseases of zebrafish such as microsporidiosis (Lawrence 2007). Infected fish often appear clinically healthy (Matthews 2004; Whipps and Kent 2006) suggesting that another factor, such as rearing environment, may play a key role in the severity of Pseudoloma infections.
Husbandry stress is often implicated in infectious diseases of cultured fishes (Schreck 1996). Stress is an adaptive and dynamic physiological state which occurs after an organism perceives a threat and attempts to restore physiological balance (Schreck et al. 2001). Chronic stress and elevation of cortisol is generally maladaptive resulting in immune suppression and increased susceptibility to infectious diseases (Schreck 1996), and cortisol is typically used as an indicator of both chronic and acute stress in fishes (Barton 2002). In addition, stress has been demonstrated to reduce growth and reproductive fitness of fishes (Campbell et al. 1994; Schreck 2000; Schreck et al. 2001).
Under conditions of chronic stress and elevation of cortisol, immune suppression is typical and often contributes to increased disease prevalence and morbidity in fish populations (Kent and Hedrick 1987; Maule et al. 1989; Saeij et al. 2003). Stress has been suggested to exacerbate microsporidiosis in zebrafish but this has yet to be examined (Matthews et al. 2004). We recently described increases in zebrafish whole-body cortisol following chronic crowding or acute handling stress (Ramsay et al. 2006; Ramsay et al. accepted). In other animal models exposure to corticosteroids generally increases the intensity of microsporidiosis (Feng et al. 2006; Herich et al. 2006; Lovy et al. 2008).
In addition to increasing susceptibility to disease, stress also affects reproduction. Developmental biology is the cornerstone of zebrafish research and a consistent supply of good quality eggs is fundamental to a productive zebrafish laboratory (Lawrence 2007; Westerfield 2007) yet there are no studies examining the effects of stress on zebrafish reproductive fitness. Pathogenic infections may also impair reproductive fitness. Heavy infections of Pleistophora mirandellae (Microsporidia) in the gonads of roach, Rutilus rutilus, were associated with intersex, and infected fish were unable to produce viable offspring (Wiklund et al. 1996). Golden shiners, Notemigonus crysoleucas, infected with Ovipleistophora ovariae had reduced fecundity and failed to spawn due to destruction of the ovaries (Summerfelt and Warner 1970). Additionally, infections with the cestode Ligula intestinalis, which occur outside of the gonad, have been demonstrated to reduce maturation and reproductive output of numerous cyprinids including roach, Rutilus rutilus, and Rastrineobola argentea (Carter et al. 2005; Cowx et al. 2008).
There is currently no effective treatment for Pseudoloma infections in zebrafish. Identifying factors affecting the exacerbation of Pseudoloma infections may aid in controlling outbreaks of disease in this intensively cultured biomedical research model (Reno 1998; Lawrence 2007). Furthermore, elucidating how husbandry-associated factors affect reproductive fitness will aid researchers in optimizing the reproductive output of zebrafish. The overall goal of this study was to understand how Pseudoloma affects the reproduction and growth of zebrafish, and how these effects are modulated by physical husbandry stressors (i.e. crowding and handling) or feeding cortisol. The specific aims of this study, comprised of four separate experiments, were:
To determine the oral dose of cortisol necessary to chronically elevate whole-body cortisol of zebrafish.
To determine if crowding and handling stressors or feeding cortisol affected infection intensity or mortality of adult zebrafish with pre-existing Pseudoloma infections.
To determine if Pseudoloma affected the growth rate of zebrafish (TL strain) over 10 wk.
To determine the sequential developmental of Pseudoloma in zebrafish experimentally infected with the parasite including effects on growth, infection intensity, mortality, and reproduction.
MATERIALS AND METHODS
Fish husbandry and sampling
Experiments were conducted at the Zebrafish Disease Laboratory (ZDL) at Oregon State University, Corvallis OR USA, which used a flow-through system of de-chlorinated, de-gassed city water maintained at 28 °C (ammonia, nitrite, chlorine: 0 ppm; pH: 6.5–7.2). Box aquarium filters with porous lava rock were placed into each tank for biological filtration. Photoperiod was 14 h light: 10 h dark. A 7–14 d acclimation period was used prior to initiating experiments. Fish were fed during the acclimation and experimental periods. Feed was withheld 12 h prior to sampling.
Non-lethal sampling of fish consisted of anesthesia in 150 ppm buffered tricaine methane sulfonate (MS-222; Argent, Redmond WA, USA). Lethal sampling of fish consisted of an overdose of MS-222 (500 ppm). Wet weights (mg) and fork lengths (mm) were measured at various intervals during each experiment (specified below). Condition factor (CF) was calculated using the formula: 100 * weight (mg)/length (mm)3.
Stress treatment
Various stressors, including crowding, net handling with air exposure, and simulated transport, were administered as stress treatments during the experiments. These stressors have all been demonstrated to elevate zebrafish whole-body cortisol (Pottinger and Calder 1995; Ramsay et al. 2006; Ramsay et al. accepted). Low water crowding involved removing the majority of the water from a tank, leaving the fish just enough water to stay upright; fish were typically crowded in this manner for 1–4 h. Fish were also temporarily crowded in a net, by netting all of the fish from a tank, suspending the net out of the water for 10–30 sec, and placing the net containing fish back into the tank; fish were typically crowded in a net for 1–2 h. Acute net handling was also performed in which all of the fish were netted out of the tank and held suspended in the air for various periods ranging from 1–3 min; this procedure was often repeated after short periods of recovery (e.g. in net for 3 min, recover in tank for 3 min, in net for 3 min, etc.). Transport stress was administered by netting all fish out of the tank into a smaller tank. This tank was then transported to another laboratory to simulate transport experienced during sampling. Fish were occasionally, anesthetized with MS-222 (150 ppm) and allowed to recover prior to returning them to their tanks. These stressors were administered a random intervals and durations at least 5 d per wk over the entire experimental periods (specified below) to ensure that the fish did not acclimate to the stressors.
Whole-body cortisol
Whole-body cortisol was measured by the methods of Ramsay et al. (2006). First, whole zebrafish were homogenized and the lipid portion extracted using diethyl ether. The extraction efficiency was determined by adding tritiated cortisol to homogenized samples, extracting the samples and measuring the amount of tritiated cortisol recovered. All samples were corrected for extraction efficiency and weight. Cortisol was measured in the extracted samples using a radioimmunoassay. The average extraction efficiency was 68%, similar to previous studies (Ramsay et al. 2006). Consistency between assays was verified by measuring cortisol in whole-body extracts spiked with known concentrations of cortisol. Intra-assay and inter-assay variation was accepted at no more than 10%.
Histology
Fish were euthanized, placed in Dietrich’s fixative (Gray 1954), and processed for histology. Preserved fish were de-calcified prior to processing using 5% trichloroacetic acid in Dietrich’s fixative. Mid-sagittal sections were cut and stained using a modified Kinyoun’s cold acid-fast method to identify Pseudoloma spores and associated histological changes; acid-fast stains have been demonstrated to be effective at detecting microsporidian spores (Joseph et al. 2006). The method used was similar to that used by the National Institutes of Health (NIH) Zebrafish International Resource Center (ZIRC) diagnostic service at the University of Oregon, Eugene OR, USA (http://zebrafish.org/zirc/health/diseaseManual.php), except de-staining was reduced to less than one minute to enhance red staining of spores.
Infection intensity was normalized by measuring the area of parasite (xenomas) occupying visible spinal tissue using SPOT™ Advanced imaging software (Diagnostic Instruments Inc., Sterling Heights, MI, USA). Infection intensity was calculated for each fish with Pseudoloma infections visible by histology. The area of visible spinal tissue was measured followed by the area of xenomas and spores in the spinal tissue. All visible spinal tissue was examined on each histological slide; this involved examining 3–7 fields of view per fish in order to capture all spinal tissue. The percent area occupied by Pseudoloma was then calculated to give xenoma area. Myositis in individual fish was evaluated using a scoring system (0 = no myositis, 1 = one area of myositis, 2 = two areas of myositis, 3 = three or more areas of myositis).
Experiments
Cortisol-dose Experiment (AB Strain)
A preliminary study was performed to determine what dose of cortisol, administered by feeding, would elevate zebrafish whole-body cortisol levels. Adult zebrafish (AB strain; 13 months old; 120 fish) were obtained from a zebrafish facility. We randomly allocated fish to 4 acrylic tanks (30 fish per 10 L tank) and acclimated for one wk. Fish in each tank were exposed to different doses of cortisol by feeding (0, 5, 33, and 100 μg cortisol · g feed−1). Cortisol (hydrocortisone, Sigma 4001) was dissolved in ethanol and sprayed onto a zebrafish diet (Zeigler Bros. Inc., Gardners, PA USA) at the appropriate dose. Cortisol-treated feed was allowed to dry overnight in a fume hood and stored at −20 °C. The dose of cortisol was verified by the methods of Ramsay et al. (2006). Fish were fed to satiation twice daily and feeding behavior was monitored. Whole-body cortisol was measured from 5 fish per tank before beginning the experiment (wk 0) and at wks 1, 2, 4, and 6 post-exposure (PE).
Pre-existing Infection (AB strain)
A population of zebrafish (AB strain; 13 months old; 2/3 male, 1/3 female) with a pre-existing Pseudoloma infection was obtained from a zebrafish facility. Six acrylic tanks (16 L) were each stocked with 50 fish. The following treatments were administered to the tanks over a 7 wk period. STRESS: two tanks received the stress treatment, described above. CORTISOL-FED: Two tanks were fed cortisol-treated zebrafish diet. The dose of cortisol was determined from the Cortisol-dose Experiment which determined changes in whole-body cortisol in zebrafish fed different doses of cortisol over a 7 wk period (Fig. 1A). Cortisol feed was prepared at a dose of 10 μg cortisol · g feed−1 as described in the Cortisol dose Experiment. CONTROL: the remaining two tanks were held at the acclimation conditions (3 fish · L−1). The stressed and control tanks were fed zebrafish diet sprayed with absolute ethanol and air-dried overnight.
Figure 1.
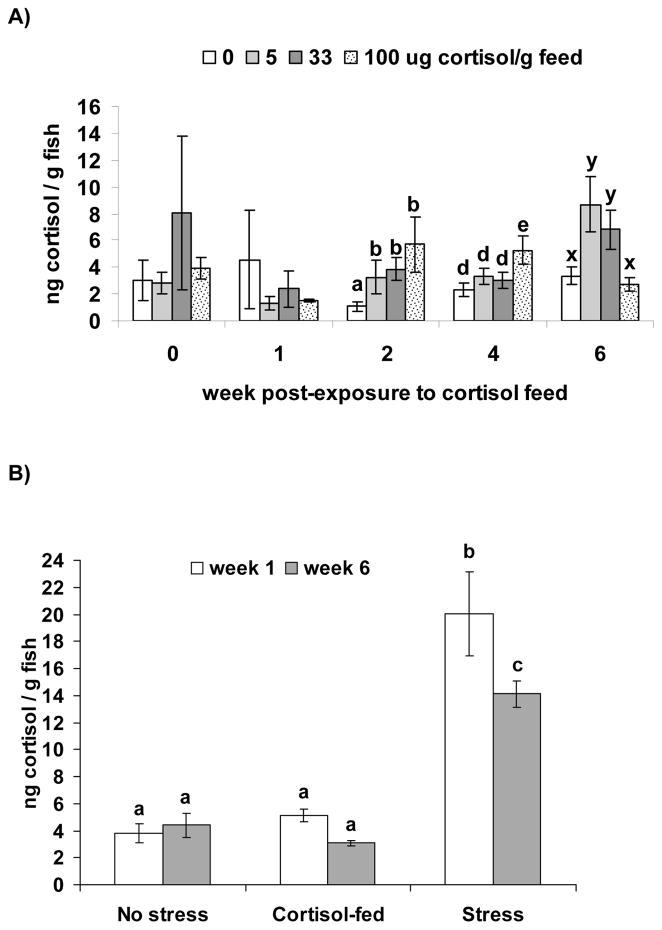
Danio rerio Cortisol-dose Experiment (AB strain) A) Mean whole-body cortisol (ng · g−1; ± SEM) after feeding fish 0, 5, 33 and 100 μg cortisol · g feed−1 for 0, 1, 2, 4 and 6 wk. Different letter over the SEM bars indicate a significant difference between cortisol dose groups at each time post-exposure to cortisol feed (there were not significant differences between cortisol dose groups at wk 0 or 1 PE. B) Mean cortisol (ng · g−1; ± SEM) in groups exposed to no stress, cortisol-fed or exposed to stress for 1 or 6 wks. Different letters over the SEM bars indicate a significant difference between groups (p<0.05).
We evaluated morbidity and mortality over a 7 wk period as well as changes in clinical disease associated with Pseudoloma infection. On Day 1, five fish were lethally sampled from each tank (n=30) and processed for histology to determine the baseline indices of disease for the population (xenoma area and myositis). At wk 1 and 6, whole-body cortisol was measured in 5 fish per tank; in the stressed fish cortisol was measured 15 min following net handling and air exposure in order to measure peak cortisol response (Ramsay et al. in revision). Fish were weighed and measured on Day 1 and at wk 7 of the experiment to determine differences in growth between treatments.
Experimental Exposure and Growth (TL strain)
A preliminary experiment was conducted to evaluate the effects of Pseudoloma infection on body weight. A total of 66 fish (TL strain; 5 months old) were initially divided into two 16 L tanks. One tank of fish was exposed to Pseudoloma by placing the carcasses of 20 Pseudoloma-infected fish (minus viscera) into the tank for 4 d. The second tank was exposed in a similar manner to carcasses from Pseudoloma–free fish. Each tank was divided (2 controls and 2 exposed), and held for 10 wk. Fish were then weighed and the infection determined by screening spinal cords in tissue smears using Fungi-Fluor chitin stain (Polysciences, Warrington PA, USA; Kent and Bishop-Stewart 2003). Length data are not provided as the tails of TL fish are long and variable in length making accurate comparisons difficult.
Experimental Exposure, Stress and Fecundity (AB strain)
Approximately 600 juvenile zebrafish (AB strain; 4 wk old) from Pseudoloma-negative adults were obtained from a zebrafish facility. These fish were randomly allocated to 8 tanks and acclimated for 2 wk. During the acclimation period fish were fed brine shrimp nauplii, Artemia sp., and fed zebrafish larval diet (Westerfield 2007). Diets were changed during the experiment as fish grew. Adult zebrafish diet, in addition to brine shrimp nauplii, was fed to the fish after 3 months of age. Prior to initiating the experiment, 20 fish were lethally sampled from the population, to determine the baseline, weight and length, and identify any existing Pseudoloma by histology. The following 4 treatments were randomly assigned to the tanks: Pseudoloma, stress, Pseudoloma-stress, control. Stress treatment, described above, was administered starting at wk 2 PE to the stress and Pseudoloma-stress tanks and continued at random intervals and durations for the 20 wk experimental period. Control fish were maintained at the acclimation density (4 fish · L−1) and not crowded, handled, or transported, except during spawning (described below).
We exposed fish to Pseudoloma by harvesting spores from zebrafish infected with Pseudoloma and feeding the spores to the infection groups. Forty Pseudoloma-infected fish were euthanized. The brains and spinal cords were removed, minced in sterile deionized water, and passed through sterile needles of decreasing size (18, 23, 26 gauge) to break up the tissue. The tissue was then passed through a 40 μm cell strainer and centrifuged (2000 rpm) for 20 minutes. The pellet, obtained from centrifuging, was re-suspended and the centrifugation repeated. The spores in the re-suspended pellet were counted using a hemocytometer. Fish in the tanks assigned to be infected with Pseudoloma were fed spores at a dose of 10 000 spores · fish−1. During the exposure, water flow into the tanks was turned off and fish were fed brine shrimp to promote ingestion of spores. At this time, control and stress tanks were sham-exposed by feeding brine shrimp with the water to the tanks turned off.
Histology, growth measurement, and spawning
At wk 4 PE, 10 fish were removed from each tank, euthanized, weighed, measured, and processed for histology. Fish were paired spawned at wk 8, 13, and 20 PE prior to being sampled. We evaluated the effects of each treatment on fecundity including the number of eggs spawned, the number of larvae hatched at 5 days post fertilization (dpf), and the percent larvae hatched at 5 dpf. We chose 5 dpf because first feeding occurs at about this time (Westerfield 2007). Feeding is highly variable among larvae and we did not want differences in feeding among larvae to be a factor affecting survival.
Spawning was performed according to protocols used by Westerfield (2007). We spawned five pairs of fish from each treatment tank (10 pairs per treatment) at each 8, 13, and 20 wk PE. From each tank, 5 males and 5 females were randomly selected and paired. In the afternoon (15 00 – 16 00 h) of the day before the spawning was to occur, each pair was placed into a crossing tank with screen insert and divider (Thoren Aquatics, Inc., Hazelton, PA); the male was placed on one side of the divider and the female was placed on the other side of the divider. When the lights came on the next morning (08 00 h), the divider was removed from the crossing cage allowing the pairs to spawn. Pairs were left to spawn for up to 4 h. If fish did not spawn within 2 h, water changes were performed to facilitate spawning (as suggested by Westerfield 2007).
After the fish had spawned, the fish were removed from the crossing tank, euthanized, weighed and measured, and placed into Dietrich’s fixative for histology. Eggs were carefully rinsed with clean water from our fish system, counted, and placed into Petri dishes (polystyrene, 95 × 15 mm; Fisher Scientific, Pittsburg PA, USA) containing embryo media at a density of 50 eggs per dish. Dead eggs were removed daily and the number of hatched larvae was recorded up to 5 dpf. Subsequently, the percent larvae hatched at 5 dpf was calculated.
Group spawning of each tank was performed at wk 6, 10, and 15 PE to ensure fish did not become egg-bound (as suggested by Westerfield 2007). Spawning was set up according the methods of Westerfield (2007). In the afternoon on the day before the spawning was to occur all fish were netted out of their tanks. A tank insert with a false bottom was placed into the treatment tanks which allowed eggs to fall through the bottom. Each group of fish was returned to their respective tanks. Spawning occurred the next day after the lights came on. Fish were then removed from the tank inserts and placed back into the tanks.
Statistics
Statistical analyses were performed using S-PLUS 7 (Insightful Corp., 2005, Seattle WA, USA). In each analysis normal distribution of the data was determined by plotting the residuals and verifying linearity. For the preliminary cortisol-feed dose-response study, we compared mean cortisol values of each dosage group using an analysis of variance (ANOVA) with Fisher’s LSD. For whole-body cortisol, weight, length, xenoma area, and myositis score, duplicate treatment tanks within each study were compared using a Welch’s modified t-test and pooled if there was no significant difference between the duplicates. Pooled cortisol, weight, length, xenoma areas, and myositis scores of different treatments within each study were compared using an ANOVA with Fisher’s LSD. We used Fisher’s exact tests to evaluate the association between treatment group and prevalence of infection, mortality, and, spawning success. We compared the number of eggs produced and the number of 5 dpf larvae produced by each treatment group using an ANOVA. The relationship between the number of eggs laid or the number of hatched larvae or the percent larvae hatched and infection intensity (xenoma area) was determined using regression analyses. The data were explained by fitting a regression model to the data (y=mx+b), where y=eggs laid or hatched larvae or percent hatched and x=xenoma index, m=slope of the line and b=y-intercept. Significant differences were reported at ∀=0.05.
RESULTS
Cortisol-dose Experiment (AB Strain)
Mean whole-body cortisol was highest among fish fed 5 or 33 μg · g cortisol−1 for 6 wk (Fig. 1A).. Feeding activity was reduced in the 100 μg · g−1 cortisol group, and this group had whole-body cortisol levels similar to those of the control dose (0 μg · g−1 cortisol) at wk 6 PE. Therefore, we selected a dose of 10 μg · g−1 cortisol (in between 5 and 33 μg · g−1 cortisol) for the Pre-existing Infection – AB experiment in order to ensure consumption of cortisol-feed and elevation of whole-body cortisol.
Pre-existing Infection – AB Experiment
Whole-body cortisol
Replicate tanks of the same treatment were not significantly different; therefore we pooled the data. Whole-body cortisol was significantly higher in the stressed groups compared to the control and cortisol-fed groups at both wk 1 and 6 PE (Fig. 1B). There were no significant differences between the control and cortisol-fed groups at either wk 1 or 6 PE. Among stressed fish cortisol was significantly lower at wk 6 compared to wk 1 PE.
Weight, Length and Condition Factor
The overall mean initial weight of fish was 509 ± 11 mg and the overall mean final weight of fish was 544 ± 16 mg; with no significant difference between treatment tanks at either wk 1 or 7 PE. Additionally, the initial and final weights did not differ significantly from one another, although there was a trend toward increasing weight over time (p=0.06). The mean length of fish did not differ between treatments. Final length (34 ± 0.3 mm) was significantly greater than initial length (33 ± 0.2 mm). Condition factor did not differ significantly between treatment groups or over time (mean = 1.35 ± 0.01).
Cumulative Mortality, Prevalence and Indices of Infection
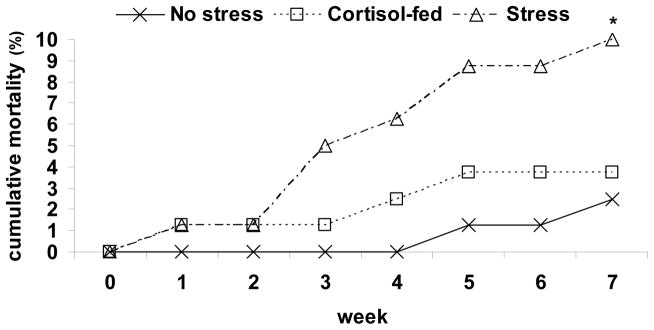
There was some mortality in all tanks (Fig. 2). Mortality occurred earliest among the stressed and cortisol-fed groups during the first week after the treatments were administered. A second wave of mortality occurred in the stress and cortisol-fed groups during the third and fourth weeks of treatment. The final phase of mortality occurred from wk 5 to 7 in all groups including the control. At wk 7, there was a significant association between treatment and mortality with greater mortality occurring among the stressed-treated fish compared to control fish (p=0.018).
Figure 2.
Danio rerio Pre-existing Infection - AB experiment Cumulative mortality of zebrafish exposed to no stress, cortisol-fed (10 μg cortisol · g feed−1) or exposed to random crowding and handling stressors over a 7 wk period. At wk 7, there was a significant association (p=0.018) between mortality and treatment with the stress-treated group having significantly higher mortality (*) than the control group.
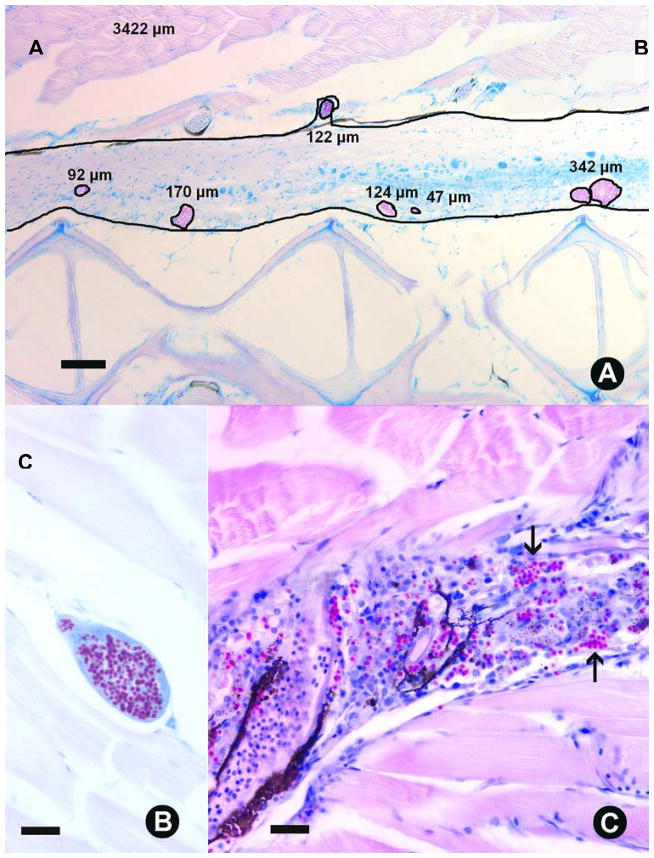
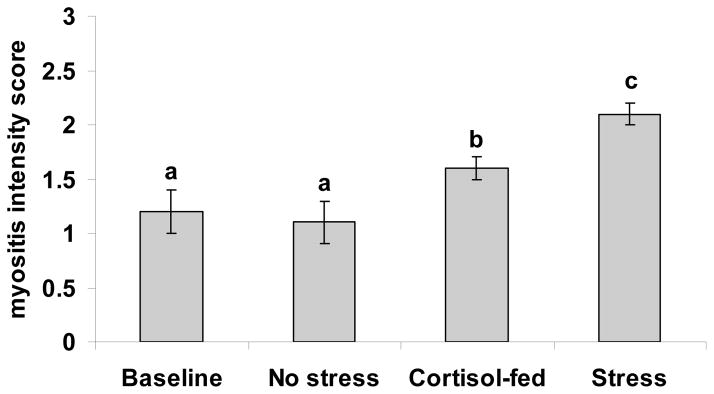
Histological examination of infected fish from the Pre-existing Infection - AB experiment exhibited characteristic microsporidiosis including multiple xenomas in the central nervous system, particularly in the spinal tissue, as well as xenomas in the muscle tissue, along with myositis associated with chronic inflammation of the somatic muscle (Fig 3A, B, C). Additional tissue reactions included meningitis associated with rupturing xenomas. An example of the SPOT analysis used on histological sections to determine the xenoma area occupying visible spinal tissue is provided (Fig 3A). All fish were positive for Pseudoloma, which precluded examination of differences in the prevalence of infection between treatment groups. The xenoma area did not differ between treatment group (overall mean=0.85 ± 0.1%). However, the stress group had a significantly higher mean myositis score than all other groups while the cortisol-fed group had a significantly higher myositis score than the control group. The mean myositis score of the control group was not significantly different from the baseline group (Fig 4).
Figure 3.
Danio rerio Histological sections of zebrafish infected with Pseudoloma neurophilia. Acid-fast. A. Sagittal section of spinal cord, with measurements of xenomas (square μm). Spinal cord area is 3 422 sq. μm. Bar = 100 μm. B. Xenoma in somatic muscle. B = Bar 20 um, Focal, chronic myositis with numerous spores (arrows). Bar = 20 μm.
Figure 4.
Danio rerio Pre-existing Infection - AB experiment. Mean myositis intensity score (± SEM) in fish with existing Pseudoloma infections. Myositis was assessed by sagittal histological sections of whole-zebrafish. Myositis intensity scoring: 1 = one area of myositis, 2 = two areas of myositis, 3 = three or more areas of myositis. The baseline group was sampled prior to initiating treatments. Treatments included no stress, feeding cortisol or stress for 7 wks. Different letters over the SEM bars indicate a significant difference between groups (p<0.05).
Experimental Exposure - TL experiment
Ten weeks after exposure, Pseudoloma-exposed fish were visibly smaller than controls, and females in the control tanks were particularly more rotund than those in the exposed tanks. Pseudoloma spores were observed in the spinal cords or hind brains of all exposed fish whereas no spores were detected in the control fish. No significant difference in weight was found between the replicate treatment tanks so the data were pooled for each treatment. The control group weighed 27% more than the Pseudoloma-exposed group (control= 484 ± 23 mg; Pseudoloma= 354 ± 14 mg).
Experimental Exposure, Stress and Fecundity – AB experiment
Weight, Length, and Condition Factor
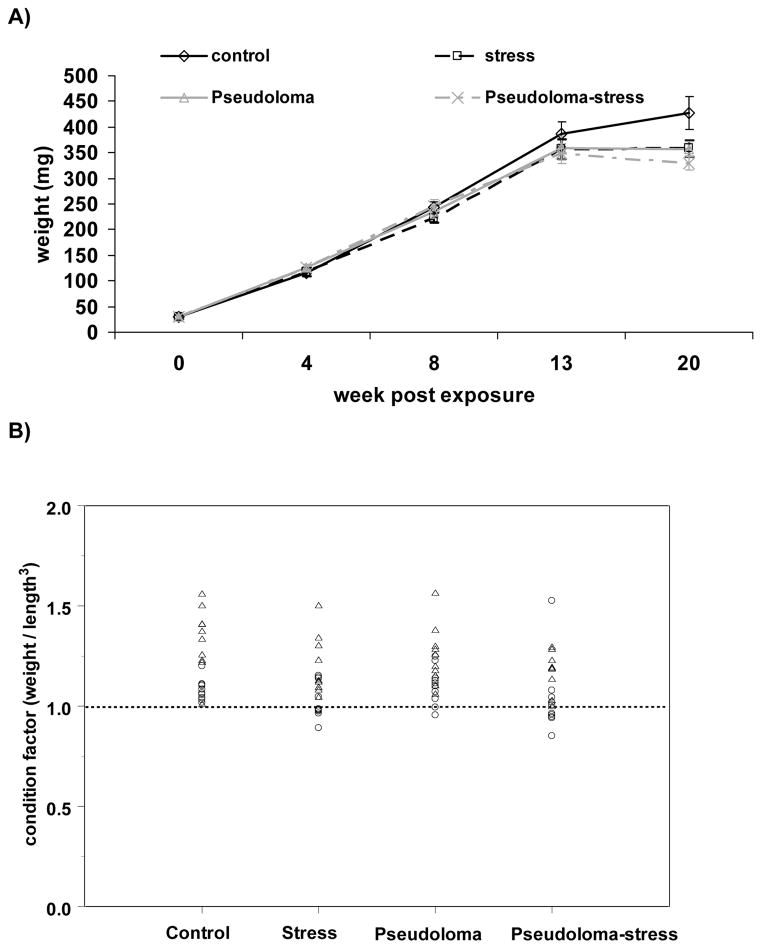
The mean initial weight of zebrafish at the time of infection was 30 ± 0.5 mg. Weight increased over time from wk 4 to 13 PE in all groups (Fig 5A). At wk 20 PE, the control group weighed significantly more than any other group (Fig. 5A). The initial length of zebrafish was 15.1 ± 1.6 mm and the final length was 31.5 ± 0.3 mm, with no significant difference between treatment groups at each time PE. Condition factor increased over time from 1.04 to 1.1 and did not differ between treatments at each time PE. Interestingly, at wk 20 PE, none of the control fish had a CF less than 1 whereas several individuals from the stress, Pseudoloma, and Pseudoloma-stress groups had a CF less than 1 (Fig. 5B).
Figure 5.
Danio rerio Experimental Exposure, Stress and Fecundity – AB experiment. A) Mean weight (mg; ± SEM) over time (wk post-exposure, PE) in groups exposed to stress, Pseudoloma, Pseudoloma and stress, and maintained under control conditions (no stress and no Pseudoloma). B) Condition factors of individual fish in control, stress, Pseudoloma and Pseudoloma-stress groups at wk 20 PE to the Pseudoloma. The dotted line indicates a condition factor of 1.
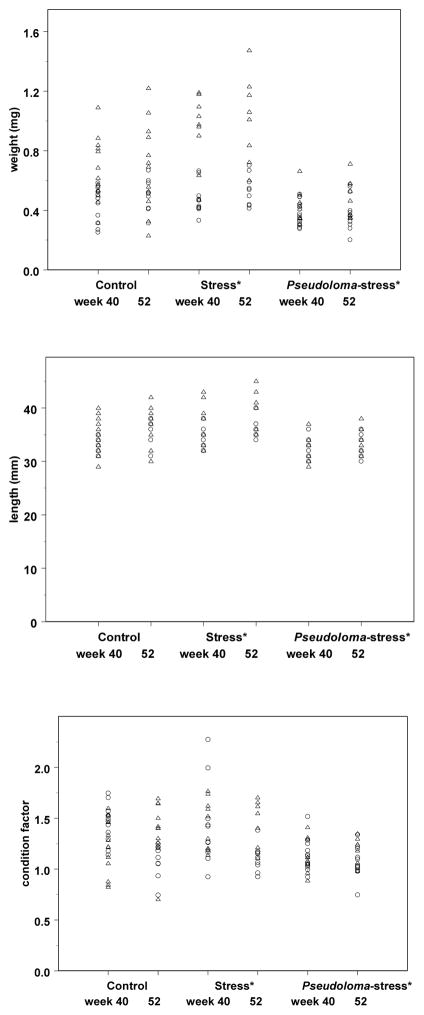
The individual weights, lengths and CFs of the fish remaining at wks 40 and 52 PE are reported in Fig. 6. Generally, fish from the Pseudoloma-infected tank were smaller than that of the uninfected tanks. Statistics were not performed due to lack of replication among treatment tanks (see further explanation below).
Figure 6.
Danio rerio Experimental Exposure, Stress and Feundity – AB experiment. Individual weights (mg), Lengths (mm) and condition factors (100* weight/length3) of fish at week 40 and 52 post-exposure (PE) to Pseudoloma. Circles (○) indicate a male fish and triangles (△) indicate a female fish. *Stress and Pseudoloma-stress groups received stress treatment until wk 23 PE.
Cumulative Mortality, Prevalence and Indices of Infection
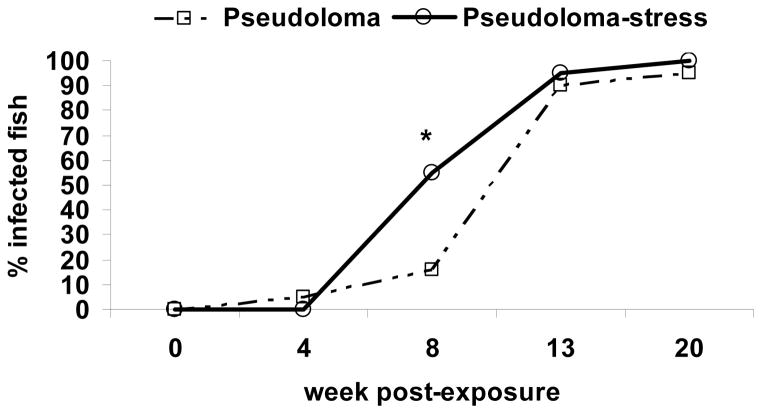
Accidental mortality occurred on occasion during stress protocol or tank cleaning but no Pseudoloma-associated mortality occurred in any group until almost one yr after exposure. No Pseudoloma was identified in any of the fish prior to experimental exposure. Histological presentations of the infection were similar to those seen in the Pre-existing Infection – AB experiment. One fish in the Pseudoloma group was infected at wk 4 PE. At wk 8 PE, there was a significant association between stress treatment and infection prevalence; 55% of the Pseudoloma-stress group and 16% of the Pseudoloma group were positive for the microsporidium. By wk 13 PE, almost 100% of these experimentally infected fish were positive for Pseudoloma regardless of stress treatment. At wk 20 PE, 95% of the Pseudoloma group and 100% of the Pseudoloma-stress group were positive (Fig. 7). None of the control or stress tanks had any infection from wk 4 to 20 PE.
Figure 7.
Danio rerio Experimental Exposure, Stress and Fecundity – AB experiment. Prevalence of Pseudoloma infection (% infected fish) over time (week post-exposure) in fish exposed to Pseudoloma and Pseudoloma and stress. * There was a significant association between prevalence of infection and treatment at wk 8 PE (p<0.05).
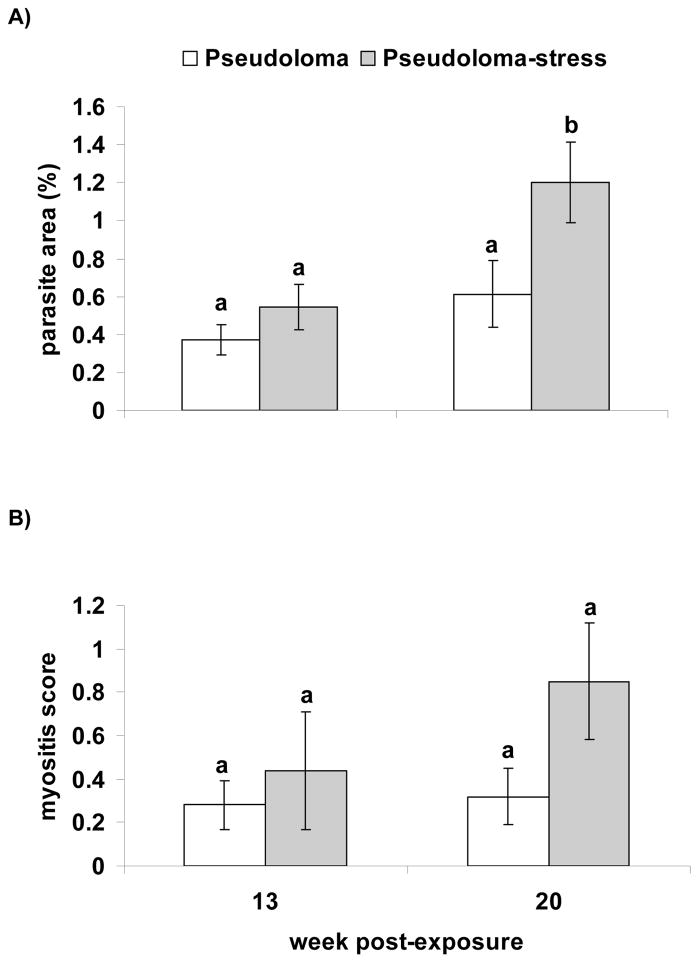
At wk 4 and 8 PE, only a few fish were infected from the Pseudoloma tanks and mean xenoma area was not affected by stress treatment. Among the Pseudoloma tanks, mean xenoma area did not differ between treatment groups at wk 13 PE, but at wk 20 PE the Pseudoloma-stress group had a significantly higher xenoma area than the Pseudoloma group (Fig. 8A). There was a trend toward increased mean myositis score with stress (p=0.09; Fig. 8B). However, due to the large amount of variation in myositis score, there was no significant difference between stressed and non-stressed groups. Among the fish remaining one yr PE in the single remaining Pseudoloma-stress tank, 16 of 18 fish were positive for Pseudoloma with a median xenoma area of 0.5%. Additionally, 3 fish in the control tanks were positive for Pseudoloma.
Figure 8.
Danio rerio Experimental Exposure, Stress and Fecundity – AB experiment. A) Mean spinal parasite area (%; ± SEM) and B) mean myositis score (± SEM) with and without stress at wk 13 and 20 PE (n=12 Pseudoloma parasite area at wk 13 PE; n=13 Pseudoloma-stress area at wk 13 PE) (wk 20 PE: n=28 Pseudoloma parasite area and n=32: Pseudoloma-stress area). Different letters over the SEM bars indicate a significant difference between treatment groups.
Fecundity and its relationship to infection indices
Fecundity data are reported in Table 1. There was no effect of treatment (control, stress, Pseudoloma, Pseudoloma-stress) on the number of pairs that successfully spawned at any week PE. We did not find any effects of treatment on the mean or median number of eggs laid. We did not see any effects on the number or percent larvae hatched at 5 dpf. Within each treatment group, there was no effect of time (wk 8, 13, 20 PE) on egg production or larvae hatched.
Table 1.
Danio rerio Experimental Exposure, Stress and Fecundity - AB experiment. The number of pairs of zebrafish spawned, eggs laid and hatched and the percent viable larvae surviving to 5 days post-fertilization (dpf) at wks 8, 13, and 20 post-exposure (PE) to Stress, Pseudoloma, Pseudoloma and stress and control conditions (no stress and no Pseudoloma). Tank A and B indicate replicate tanks within the same treatment.
| Week 8 PE | Week 13 PE | Week 20 PE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Treatment | Tank | Number of pairs spawned | Number of eggs laid (hatched) | % viable larvae (5 dpf) | Number of pairs spawned | Number of eggs laid (hatched) | % viable larvae (5 dpf) | Number of pairs spawned | Number of eggs laid (hatched) | % viable larvae (5 dpf) |
| Control | A | 5/5 | 89 (47) | 52 | 5/5 | 199 (162) | 74 | 2/5 | 263 (223) | 85 |
| Control | B | 4/5 | 102 (80) | 84 | 3/5 | 136 (67) | 41 | 3/5 | 63 (39) | 70 |
| Stress | A | 4/5 | 72 (31) | 45 | 5/5 | 193 (91) | 48 | 5/5 | 135 (124) | 91 |
| Stress | B | 4/5 | 83 (64) | 71 | 5/5 | 151 (65) | 32 | 3/5 | 53 (32) | 56 |
| Pseudoloma | A | 4/5 | 98 (23) | 33 | 3/5 | 105 (81) | 78 | 5/5 | 181 (143) | 65 |
| Pseudoloma | B | 5/5 | 118 (101) | 78 | 4/5 | 167 (125) | 72 | 3/5 | 250 (181) | 71 |
| Pseudoloma stress | A | 4/5 | 151 (90) | 56 | 3/5 | 220 (187) | 70 | 5/5 | 54 (32) | 56 |
| Pseudoloma stress | B | 4/5 | 129 (123) | 94 | 5/5 | 193 (105) | 49 | 3/5 | 216 (192) | 86 |
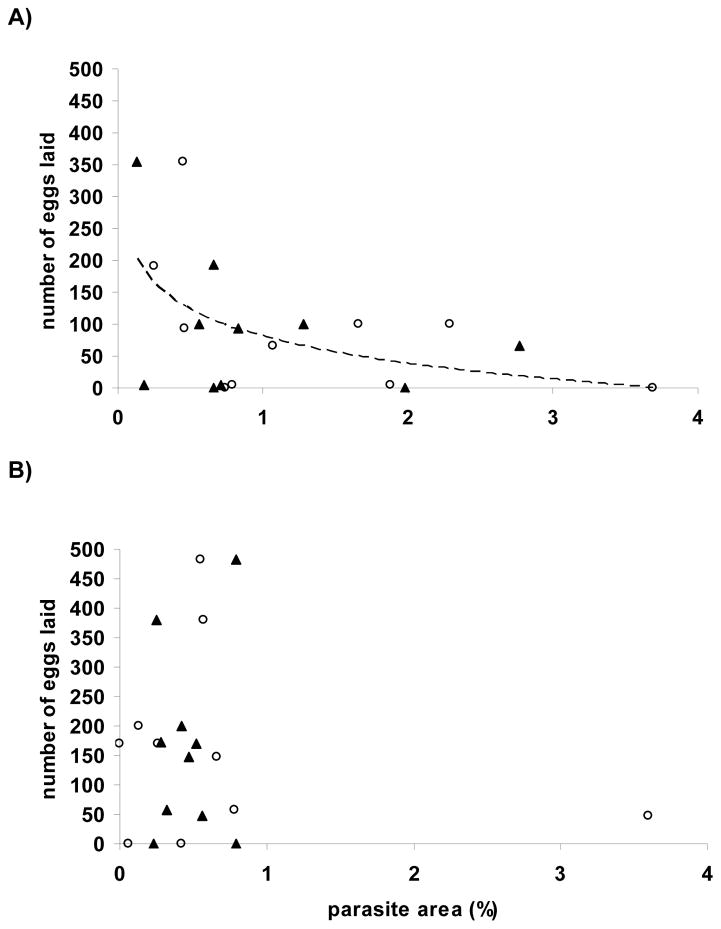
At wk 20 PE, there was a negative natural logarithmic relationship between the number of eggs laid and xenoma area in the Pseudoloma-stress group, with males and females combined, described by the following equation: number of eggs laid = 80 – 61 ln (xenoma area); R2 = 0.25; p=0.025 (Fig. 9A). There was a similar relationship between the number of larvae hatched at 5 dpf described by the following equation: 61 – 63 ln (xenoma area); R2 = 0.3; p=0.01. At wk 20 PE fish from the Pseudoloma-stress group with a xenoma area greater than 0.8% produced fewer than 75 larvae. Although there was no significant relationship between the percent larvae hatched at 5 dpf, there was a trend toward a negative natural logarithmic relationship (p=0.06). Among the Pseudoloma group (no stress) at wk 20 PE, there were no significant relationship between fecundity and xenoma area (Fig. 9B).
Figure 9.
Danio rerio Experimental Exposure, Stress and Fecundity – AB experiment. Number of eggs laid vs. parasite (xenoma) area (%) at wk 20 post-exposure to Pseudoloma. A) Among the Pseudoloma-infected-stressed fish the relationship was described by the following equation: eggs laid = 80 – 61 ln (xenoma area); R2 = 0.25; p=0.025. B) There was no significant relationship between the number of eggs laid and parasite area among the Pseudoloma-infected group in the absence of stress (p=0.62). Male fish are indicated by an open circle (○). Female fish are indicated by a closed triangle (▲).
Fish Remaining after Week 20 PE
Due to a lack of mortality during the experimental period, a number of fish remained in each tank after the wk 20 PE sampling. We continued to monitor these groups for infection. However, at wk 23 PE, all fish in 5 of the 8 tanks died from an unexpected failure of the tank water system. We continued to monitor fish from the surviving tanks (one control tank, one stress tank, one Pseudoloma-stress tank) but no longer administered stress treatment. Fish from these tanks were all weighed and measured at wk 40 PE. Fish from the Pseudoloma-infected tank started to die between wks 40 and 52 PE; all fish were euthanized, weighed and measured and examined by histology at wk 52 PE. Weight, length, and CF data are reported, including median values, but the treatments were not compared using statistical tests due to a lack of tank replication
Background infection with Mycobacterium spp
A small number of fish in both the studies were positive for Mycobacterium spp. by histology. The majority of mycobacteria were found in the swim bladder and ovaries. In the Pre-existing Infection – AB experiment, 22 out of 202 fish (11%) were positive for mycobacteria. With Experimental Exposure, Stress and Fecundity – AB experiment, a single fish was positive for mycobacteria out of over 400 sampled.
DISCUSSION
Pseudoloma is the most prevalent disease of laboratory zebrafish. The infection is often associated with morbidity (De Kinkelin 1980; Matthews et al. 2001), but infected fish are often asymptomatic. This is based on examination of hundreds of zebrafish from many laboratories submitted to the ZIRC diagnostic service (http://zebrafish.org/zirc/health/index.php) over the past 10 years, which included apparently healthy fish for routine health checks (Matthews 2004).
Size and Infection
Disease tends to limit the growth potential and condition of fishes. Pseudoloma-infected zebrafish are often referred to as having “skinny disease” (Matthews et al. 2001) suggesting that microsporidiosis is associated with weight loss or poor growth. Here, we present the first empirical data supporting this observation. Our two laboratory transmission experiments with TL and AB zebrafish demonstrated that Pseudoloma is associated with reduced size. There are many examples of reduced growth associated with chronic parasite infections (Crompton 1986; Beamish et al. 1996, Barber and Svensson 2003). Specifically, Speare et al. (1998) showed that infections by L. salmonae in rainbow trout caused reduced growth, but growth was compensated following recovery. Furthermore, L. salmonae-associated reductions in growth were associated with xenoma onset and accompanied by a reduction in feed intake (Ramsay et al. 2004). It is not known whether zebrafish recover from Pseudoloma or if auto-infection occurs as is believed to occur for some fish-infecting microsporidia (Rodriguez-Tovar et al. 2003; Matos et al. 2003; Kent and Speare 2005). With Pseudoloma infections, zebrafish did not recover over the duration of the study and they were smaller in size at wk 20 PE than the control group. Perhaps the most analogous infection to Pseudoloma is Encephalitozoon cuniculi, which infects rabbits and other mammals (Wasson and Peper 2000). Both species of microsporidia are wide spread in their respective hosts, including within research laboratories. Both cause encephalitis and meningitis, but many animals are asymptomatic. Infections by E. cuniculi are associated with stunted growth, particularly in young dogs, accompanied by other clinical changes (Wasson and Peper 2000). Although data were derived from two separate experiments, Pseudoloma had a greater impact on reducing the size of the TL strain than the AB strain. This is consistent with observations in our laboratory with other exposure studies and from the ZIRC diagnostic service. Although not investigated in zebrafish, there are numerous examples of differences in susceptibility to parasites amongst strains of the same species of fish (Jones et al. 2001). With fish microsporidia, different strains of Chinook salmon, O. tshawytscha, have shown differences in susceptibility to L. salmonae (Shaw et al. 2000b).
Stress and growth
We also investigated the effects of stress on growth and Pseudoloma infection, but first we had to evaluate our stressors. In our preliminary cortisol dose experiment, we determined a dose between 5 and 33 μg · g cortisol−1 was best for increasing whole-body cortisol for the 7 wk duration of the experiment. Fish fed the highest dose of cortisol (100 μg · g−1) had reduced feed intake and failed to elevate whole-body cortisol. Stress and exogenous cortisol has been demonstrated to reduce feed intake (Bernier et al. 2004; Peterson and Small 2005) which may explain why feed intake was reduced at the high cortisol doses used in our study.
Whole-body cortisol was significantly elevated among stressed fish compared to the control and cortisol-fed groups at both wk 1 and 6 PE, confirming our earlier studies (Ramsay et al. 2006; Ramsay et al. accepted) and indicating that Pseudoloma infections were impacted by persistent crowding coupled with handling inducing chronic stress and elevated cortisol levels. Interestingly, cortisol was significantly lower at wk 6 than wk 1 PE among the stressed group, suggesting that fish were acclimating to the random stressors we were administering. Whole-body cortisol was not significantly elevated among the cortisol-fed groups compared to the control groups despite the fact that the dose we used (10 μg · g cortisol−1) was within the dose range (5 and 33 μg · g−1) which elevated whole-body cortisol in our preliminary cortisol-dose experiment. A possible explanation for this lack of effect include an increased clearance rate of cortisol as has been demonstrated for salmonids exposed to chronic stress or exogenous cortisol (Redding et al. 1984). Negative feedback loops suppress cortisol secretion in the presence of exogenous cortisol (Bradford et al. 1992), which may also explain why whole-body cortisol among cortisol-fed fish was not elevated. The lack of increase in whole-body cortisol may also have been a reflection of variation in the feeding and digestive rates between fish of differing social hierarchies as has been suggested for channel catfish, Ictalurus punctatus (Davis et al. 2003).
Size and Stress
Both disease and stress alter metabolism and oxygen consumption, often resulting in less energy available for both growth and reproduction, and may explain the smaller size of the Pseudoloma-infected group (Barton and Schreck 1987; Mommsen et al. 1999; Heins and Baker 2003; Leef et al. 2007). We did not see any effect of stress or feeding cortisol on changes in weight, length, or condition factor among fish in the Pre-existing Infection – AB experiment, perhaps because the fish were near fully grown when the treatments were initiated. Accordingly, it has been suggested that the finite size of zebrafish may limit their use as a model to study growth (Mommsen 2001). Interestingly, we did see a reduction in weight associated with both stress and infection in the Experimental Exposure, Stress and Fecundity - AB experiment, but here infection was initiated in juvenile fish (6 wk old). Young zebrafish are particularly susceptible to Pseudoloma (Ferguson et al. 2007), and perhaps the impact of the infection on growth is most evident if the infection is initiated when fish are young. Weights at wk 20 PE were significantly lower among the stress, Pseudoloma, and Pseudoloma-stress groups compared to the control group. Stressed and Pseudoloma-infected groups had a number of individuals with a condition factor below 1, while all of the control fish had condition factors above 1. Stress and elevated cortisol are associated with metabolic costs that have been demonstrated to reduce growth and condition factor in fishes (Barton and Schreck 1987; Gregory and Wood 1999; Bernier et al. 2004) which may explain our results.
Mortality and Stress
Mortality in the Pre-existing Infection – AB experiment was greatest amongst the stressed group followed by the cortisol-fed and then the control groups. Shipping stress has been demonstrated to increase mortality in parasitized fishes (Goulding et al. 2004; Kåll et al. 2004), and handling stress tends to increase mortality of diseased fishes (Saeij et al. 2003; Dror et al. 2006). Additionally, rainbow trout, infected with the myxosporean PKX and implanted with cortisol, experienced increased mortality compared to parasitized fish not exposed to cortisol (Kent and Hedrick 1987).
Mortality was not related to Pseudoloma infection in both experiments with laboratory exposure. Juvenile fish were used for the Experimental Exposure, Stress and Fecundity – AB experiment, whereas adult fish were used for the Pre-existing Infection – AB experiment. Life history stage is important in determining stress and immune responsiveness (Schreck 1996) and may have influenced the stress-related mortality in Pseudoloma-infected fish. While young fish may be more susceptible, this chronic infection may require many months before clinical disease is evident. We began to see mortality between week 40 and 52 PE. Although not validated by statistics due to lack of tank replication for these time points, this indicates that Pseudoloma-associated mortality tends to occur later after infection, which is consistent with observations from the Pre-existing Infection – AB experiment in that it is probable that fish from this population had Pseudoloma for many months before we initiated our experiment.
Histology and prevalence of infection
We observed some differences in the infection associated with stress at a histological level. Stress was associated with earlier detection of infection in experimentally exposed fish subjected to stress. At wk 8 PE, 16% of fish from the Pseudoloma group and 55% of fish from the Pseudoloma-stress group were positive. Among catfish, Ictalurus punctatus, infected with Edwardsiella ictaluri, stress increased the overall percentage of infected fish (Small and Bilodeau 2005). By wk 13 and 20 PE almost all fish from both Pseudoloma groups were positive in this experiment. This concurs with Kent and Bishop-Stewart (2003) where 30% of fish were positive for Pseudoloma at wk 8 PE and 100% were positive at wk 20 PE.
Husbandry stress or feeding cortisol exacerbated pre-existing Pseudoloma infections in adult zebrafish. Although stress did not affect the size of fish in the Pre-existing Infection – AB experiment, it was associated with increased myositis. Indeed, most histological changes occur as the infection progresses from the central nervous system to the muscle, where the most prominent inflammatory changes are observed. This is consistent with observations from diagnostic cases; when microsporidiosis is the diagnosis for the cause of morbidity, it is most often associated with chronic myositis (Matthews 2004). The sequence of infection and progression of lesions with Pseudoloma in zebrafish is similar to gill infections by Loma salmonae in salmonids (Kent and Speare 2005). In this case, intact xenomas in the gills are associated with little inflammation and clinical disease. This is followed by rupture of the xenomas and severe chronic branchitis, with accumulation of released spores within phagocytes.
Infection intensity: xenoma area and myositis
Husbandry stress increased the intensity of infection during the Experimental Exposure, Stress and Fecundity – AB experiment. Mean xenoma area was greater among stressed fish experimentally infected with Pseudoloma compared to non-stressed fish at wk 20 PE. Stress and cortisol-treatment has been demonstrated to increase the intensity of infection in diseased fish. In Northern pike, Esox lucius, the number of gill arteries infected with nematodes increased after transport stress (Kåll et al. 2004), and cortisol-treated rainbow trout had an increase in the density of PKX spores in the interstitium of the kidney (Kent and Hedrick 1987). Interestingly, the mean myositis score was not significantly affected by stress treatment, although there was a trend toward increasing myositis among stressed fish at wk 20 PE. The large amount of variation in the data may explain why we did not see any difference. Additionally, differences in myositis between stressed and non-stressed groups may have been more apparent past wk 20 PE as the infection progressed, but with lack of replicates beyond 20 wk PE we could not evaluate this.
There was no effect of stress or feeding cortisol on the mean xenoma area of fish with pre-existing Pseudoloma infections. The fish used in the Pre-existing Infection – AB experiment had a baseline mean parasite area of 0.91%, whereas during the Experimental Exposure, Stress and Fecundity – AB experiment, the mean parasite area was 0.61% for the Pseudoloma group and 1.2% for the Pseudoloma-stress group. In the former experiment, fish were stressed long after the infection was established. There may be a maximum area of tissue that can be occupied by Pseudoloma, after which the host either resists the parasite or succumbs to disease. Alternatively, the short duration of exposure to stressors or cortisol (7 wk) may not have been sufficient to change the mean parasite area in fish with existing Pseudoloma infections.
At wk 52 PE, a few fish from the control and previously stressed groups were positive for Pseudoloma. Inadvertent infection of these Pseudoloma-control groups may have been due to aerosol transmission of the parasite from adjacent tanks containing Pseudoloma-infected fish. Control tanks were intermixed with the infected tanks on the same racks to randomize differences in extraneous stressors such as light and the position of the tank in the room. Aerosol transmission has been reported for fish pathogens including Aeromonas salmonicida and Ichthyophthirius multifiliis (Wooster and Bowser 1996). Microsporidian spores are capable of surviving relatively long periods outside the host (Shaw et al. 2000a) and this may have facilitated transmission of Pseudoloma to the control and stress tanks.
Fecundity
In fish infected with Pseudoloma, increasing infection intensity was associated with decreasing reproductive fitness as evidenced by a negative relationship between the number of eggs laid and parasite area. Although stressed and infected fish may have produced fewer eggs, this was not verified statistically as the number of eggs spawned and larvae hatched at 5 dpf was extremely variable within groups. This is typical for zebrafish; females produced eggs and spawn many times throughout the year, but their egg production can be highly variable (Paull et al. 2008). There are relatively few studies examining the effects of environmental parameters on zebrafish reproductive output (Eaton and Farely 1974; Laale 1977), and no studies that have examined the effects of stress or Pseudoloma on reproduction in zebrafish. The lack of any direct effects on fecundity may reflect the need to better understand the dynamics of reproduction with respect to husbandry (Lawrence 2007).
Whereas stress and infectious diseases have been documented to reduce fecundity (Barber et al. 2000; Schreck et al. 2001), there are examples where the reverse occurs (Gowaty et al. 2007). Candolin and Voigt (2001) reported an inhibitory effect of cestode tapeworms on reproduction in stickleback males in the natural environment but not in the laboratory, suggesting that the favorable laboratory environment, free of predation risk and fluctuating environment, mitigates the negative effects parasitism has on reproduction. We observed a negative natural logarithmic relationship between the number of eggs laid or the number of larvae and parasite area at wk 20 PE among the Pseudoloma-stress group. Only stressed fish had heavy infections (> 1% xenoma area), and these fish consistently spawned poorly. Infection level, not stress treatment, determined spawning success which was highly variable in lightly infected or uninfected fish and poor in heavily infected fish. This was even the case for the non-stressed fish; the one fish in this group with a heavy infection (>1% xenoma area) spawned poorly (<50 eggs). Interestingly fecundity was similarly reduced whether males or females were infected. Our results suggest a possible effect of the parasite on spawning behavior or the neurological control of sperm release as Pseudoloma affects the CNS and nerve roots (Matthews et al. 2001; Barber and Poulin 2002).
Fish with severe Pseudoloma infections are typically emaciated and the females lack large numbers of eggs typical of zebrafish (Matthews et al. 2001). Pseudoloma is a chronic persistent disease, and perhaps examining reproduction in fish with higher infection intensities later in the infection may have resulted in more significant effects of the parasite on fecundity and offspring survival.
Mycobacteria
A small number of fish were infected with Mycobacterium spp. Mycobacteria are common pathogens of zebrafish (Astrofsky et al. 2000; Kent et al. 2004) and have been found at background levels in previous studies without clinical signs of disease (Beran et al. 2006; Harriff et al. 2007; Zanoni et al. 2008; Whipps et al. 2008). We have shown that stress exacerbates mycobacterial infections in zebrafish (Ramsay et al. in press). Fortunately, only a few experimental fish had concurrent mycobacteriosis, and thus it is unlikely that this compromised our experiment.
Conclusion
Pseudoloma is the most common pathogen of laboratory zebrafish, yet little is known about husbandry factors that affect the development and progression of clinical disease. Our study is an initial step in examining the dynamic interactions of stress, reproduction, and Pseudoloma in laboratory zebrafish. We have described the sequential development of Pseudoloma in zebrafish, showing that it persists for well over 6 months after exposure. Furthermore, we have demonstrated that stress exacerbates clinical disease associated with Pseudoloma infections with direct effects on fish size.
Fecundity, rather than growth, is usually the most important criterion in zebrafish used for research, particularly when used in developmental genetics research. Reproductive output was negatively related to the intensity of Pseudoloma infection in stressed fish with heavy infections. Therefore, we recommend that if avoidance or eradication of the parasite is not an option in a given research laboratory, then particular care should be taken to minimize stress if fecundity is an important laboratory endpoint. Moreover, it should be recognized that spores of Pseudoloma are resistant to chlorine at the levels used in research laboratories (Ferguson et al. 2007), and there is a risk of transmission of Pseudoloma to the next generation or even true vertical transmission within eggs (Kent et al. 2009). Further investigation on the interactions of Pseudoloma infection and stress and the subsequent effects on survival, growth, and reproductive fitness will aid in controlling outbreaks and optimizing zebrafish health and reproduction in order to ensure the continued success of this important biomedical research model.
Acknowledgments
We thank B. Barr and T. Eng for assistance with fish care and R. Chitwood for assistance in the construction of the ZDL flow-through water system. We thank M. Westerfield and Z. Varga as well as the ZIRC staff for their advice in designing this study. The manuscript was critically reviewed by J. Leatherland, M. Westerfield, and D. Landers. J.M.R. was supported by the Departments of Fisheries and Wildlife and Microbiology at Oregon State University and the ZIRC at the University of Oregon. This study was supported by grants from the National Institutes of Health (NIH NCRR 5R24RR017386-02 and NIH NCRR P40 RR12546-03S1). Mention of a brand name does not imply endorsement of the product by the U.S. Federal Government.
LITERATURE CITED
- Astrofsky KM, Schrenzel MD, Bullis RA, Smolowitz RM, Fox JG. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comp Med. 2000;50:666–672. [PubMed] [Google Scholar]
- Barber I, Hoare D, Krause J. Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev Fish Biol Fish. 2000;10:131–165. [Google Scholar]
- Barber I, Poulin R. Interactions between fish, parasites and disease. In: Hart PJB, Reynolds JD, editors. Handbook of Fish Biology and Fisheries Vol. 1 Fish Biology. Blackwell Publishing; Malden MA USA: 2002. pp. 359–389. [Google Scholar]
- Barber I, Svensson PA. Effects of experimental Schistocephalus solidus infections on growth, morphology and sexual development of female three-spined sticklebacks, Gasterosteus aculeatus. Parasitology. 2003;126:359–367. doi: 10.1017/s0031182002002925. [DOI] [PubMed] [Google Scholar]
- Barton BA. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol. 2002;42:517–525. doi: 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- Barton BA, Schreck CB. Metabolic cost of acute physical stress in juvenile steelhead. Trans Am Fish Soc. 1987;116:257–263. [Google Scholar]
- Beamish FWH, Sitja-Bobadilla A, Jebbink JA, Woo PTK. Bioenergetic cost of cryptobiosis in fish: rainbow trout Oncorhynchus mykiss infected with Cryptobia salmositica and with an attenuated live vaccine. Dis Aquat Org. 1996;25:1–8. [Google Scholar]
- Beran V, Matlova L, Dvorska L, Svastova P, Pavlik I. Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment. J Fish Dis. 2006;29:383–393. doi: 10.1111/j.1365-2761.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- Bernier NJ, Bedard N, Peter RE. Effects of cortisol on food intake, growth, and forebrain neuropeptide Y and corticotropin-releasing factor gene expression in goldfish. Gen Comp Endocrinol. 2004;135:230–240. doi: 10.1016/j.ygcen.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Bradford CS, Fitzpatrick MS, Schreck CB. Evidence for ultra-short-loop feedback in ACTH-induced interrenal steroidogenesis in coho salmon: acute self suppression of cortisol secretion in vitro. Gen Comp Endocrinol. 1992;87:292–299. doi: 10.1016/0016-6480(92)90034-h. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Pottinger TG, Sumpter JP. Preliminary evidence that chronic confinement stress reduces the quality of gametes produced by brown and rainbow trout. Aquaculture. 1994;120:151–169. [Google Scholar]
- Candolin U, Voigt HR. No effect of a parasite on reproduction in stickleback males: a laboratory artefact? Parasitology. 2001;122:457–464. doi: 10.1017/s0031182001007600. [DOI] [PubMed] [Google Scholar]
- Carter V, Pierce R, Dufour S, Arme C, Hoole D. The tapeworm Ligula intestinalis (Cestoda: Pseudophyllidea) inhibits LH expression and puberty in its teleost host, Rutilus rutilus. Reproduction. 2005;130:939–945. doi: 10.1530/rep.1.00742. [DOI] [PubMed] [Google Scholar]
- Chatton E. Un complexe xéno-parasitaire morphologique et physiologique Neresheimeria paradoxa chez Fritillaria pellucida. C. R. Acad. Sci. Paris. 1920;171:55–57. [Google Scholar]
- Cowx IG, Rollins D, Tumwebaze R. Effect of Ligula intestinalis on the reproductive capacity of Rastrineobola argentea in Lake Victoria. J Fish Biol. 2008;73:2249–2260. [Google Scholar]
- Crompton DW. Nutritional aspects of infection. Trans R Soc Trop Med Hyg. 1986;80:697–705. doi: 10.1016/0035-9203(86)90368-8. [DOI] [PubMed] [Google Scholar]
- Dahm R, Geisler R. Learning from small fry: the zebrafish as a genetic model organism for aquaculture fish species. Mar Biotechnol. 2006;8:329–345. doi: 10.1007/s10126-006-5139-0. [DOI] [PubMed] [Google Scholar]
- Davis KB, Griffin BR, Gray WL. Effect of dietary cortisol on resistance of channel catfish to infection by Ichthyopthirius multifiliis and channel catfish virus disease. Aquaculture. 2003;218:121–130. [Google Scholar]
- de Kinkelin PD. Occurrence of a microsporidian infection in zebra danio Brachydanio rerio (Hamilton-Buchanan) J Fish Dis. 1980;3:71–73. [Google Scholar]
- Dror M, Sinyakov MS, Okun E, Dym M, Sredni B, Avtalion RR. Experimental handling stress as infection-facilitating factor for the goldfish ulcerative disease. Vet Immunol Immunopath. 2006;109:279–287. doi: 10.1016/j.vetimm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Farley RD. Spawning cycle and egg production of zebrafish, Brachydanio rerio, in the laboratory. Copeia. 1974;1974:195–204. [Google Scholar]
- Feng X, Akiyoshi DE, Sheoran A, Singh I, Hanawalt J, Zhang Q, Widmer G, Tzipor S. Serial propagation of the microsporidian Enterocytozoon bieneusi of human origin in immunocompromised rodents. Infect Immun. 2006;74:4424–4429. doi: 10.1128/IAI.00456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J, Watral V, Schwindt A, Kent ML. Spores of two fish Microsporidia (Pseudoloma neurophilia and Glugea anomola) are highly resistant to chlorine. Dis Aquat Org. 2007;76:205–214. doi: 10.3354/dao076205. [DOI] [PubMed] [Google Scholar]
- Gregory TR, Wood CM. The effects of chronic plasma cortisol elevation on the feeding behaviour, growth, competitive ability, and swimming performance of juvenile rainbow trout. Physiol Biochem Zool. 1999;72:286–295. doi: 10.1086/316673. [DOI] [PubMed] [Google Scholar]
- Goulding DR, Blankenship-Paris TL, Lewbart GA, Myers PH, Demianenko TK, Clark JA, Forsythe DB. Gill trematodes (flukes) in wild-caught killifish (Fundulus heteroclitus) Contemp Top Lab Anim Sci. 2004;43:32–34. [PubMed] [Google Scholar]
- Gowaty PA, Anderson WW, Bluhm CK, Drickamer LC, Kim YK, Moore AJ. The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc Natl Acad Sci. 2007;104:15023–15027. doi: 10.1073/pnas.0706622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. The Microtomist’s Guide and Formulary. The Blakiston Company Inc; New York: 1954. [Google Scholar]
- Harriff MJ, Bermudez LE, Kent ML. Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: a potential model for environmental mycobacterial infection. J Fish Dis. 2007;30:587–600. doi: 10.1111/j.1365-2761.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- Heins DC, Baker JA. Reduction of egg size in natural populations of threespine stickleback infected with a cestode macroparasite. J Parasitol. 2003;89:1–6. doi: 10.1645/0022-3395(2003)089[0001:ROESIN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Herich R, Levkutová M, Kokinčáková T, Reiterová K, Hipíková V, Levkut M. Diagnosis and manifestation of encephalitozoonosis in mice after experimental infection with different species and application of dexamethasone. J Vet Med Assoc. 2006;53:340–345. doi: 10.1111/j.1439-0442.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- Jones SRM. The occurrence and mechanisms of innate immunity against parasites in fish. Dev Comp Immunol. 2001;25:841–952. doi: 10.1016/s0145-305x(01)00039-8. [DOI] [PubMed] [Google Scholar]
- Joseph J, Murthy S, Garg P, Sharma S. Use of different stains for microscopic evaluation of corneal scrapings for diagnosis of microsporidial keratitis. J Clin Mircobiol. 2006;44:583–585. doi: 10.1128/JCM.44.2.583-585.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kåll S, Fagerholm HP, Sarvala J. Pathogenicity of the gill artery worm Philometra obturans (Nematoda) in northern pike (Esox lucius) in southwest Finland. J Parasitol. 2004;90:177–181. doi: 10.1645/GE-2979RN. [DOI] [PubMed] [Google Scholar]
- Kent ML, Hedrick RP. Effects of cortisol implants on the PKX myxosporean causing proliferative kidney disease in rainbow trout, Salmo gairdneri. J Parasitol. 1987;73:455–461. [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish, Danio rerio (Hamilton) J Fish Dis. 2003;26:423–426. doi: 10.1046/j.1365-2761.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Kent ML, Speare DJ. Review of the sequential development of Loma salmonae (Microsporidia) based on experimental infections of rainbow trout (Oncorhynchus mykiss) and Chinook salmon (O. tshawytscha) Folia Parasitol. 2005;52:63–68. doi: 10.14411/fp.2005.009. [DOI] [PubMed] [Google Scholar]
- Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp Biochem Physiol Part C. 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sánchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comp Biochem Physiol Part C. 2009;140:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laale HW. The biology and use of zebrafish, Brachydanio rerio in fisheries research: a literature review. J Fish Biol. 1977;10:121–173. [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture. 2007;269:1–20. [Google Scholar]
- Leef MJ, Harris JO, Powell MD. Metabolic effects of amoebic gill disease (AGD) and chloramine-T exposure in seawater-acclimated Atlantic salmon Salmo salar. Dis Aquat Organ. 2007;78:37–44. doi: 10.3354/dao01853. [DOI] [PubMed] [Google Scholar]
- Lom J, Nilsen F. Fish microsporidia: fine structural diversity and phylogeny. Int J Parasitol. 2003;33:107–127. doi: 10.1016/s0020-7519(02)00252-7. [DOI] [PubMed] [Google Scholar]
- Lom J, Dyková I. Microsporidian xenomas in fish seen in wider perspective. Folia Parasitol. 2005;52:69–81. [PubMed] [Google Scholar]
- Lovy J, Speare DJ, Stryhn H, Wright GM. Effects of dexamethasone on host innate and adaptive immune responses and parasite development in rainbow trout Oncorhynchus mykiss infected with Loma salmonae. Fish Shellfish Immunol. 2008;24:649–658. doi: 10.1016/j.fsi.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Matos E, Corral L, Azevedo C. Ultrastructural details of the xenoma of Loma myrophis (phylum Microsporidia) and extrusion of the polar tube during autoinfection. Dis Aquat Organ. 2003;54:203–07. doi: 10.3354/dao054203. [DOI] [PubMed] [Google Scholar]
- Matthews JL, Brown AMV, Larison K, Bishop-Stewart JK, Rogers P, Kent ML. Pseudoloma neurophilia n.g., n.sp., a new genus and species of Microsporidia from the central nervous system of the zebrafish (Danio rerio) J Eukaryot Microbiol. 2001;48:229–235. doi: 10.1111/j.1550-7408.2001.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Matthews JL. Common diseases of laboratory fishes. Meth Cell Biol. 2004;77:617–637. doi: 10.1016/s0091-679x(04)77033-8. [DOI] [PubMed] [Google Scholar]
- Maule AG, Tripp RA, Kaattari SL, Schreck CB. Stress alters immune function and disease resistance in Chinook salmon (Oncorhynchus tshawytscha) J Endocrinol. 1989;120:135–142. doi: 10.1677/joe.0.1200135. [DOI] [PubMed] [Google Scholar]
- Mommsen TP, Vijayan MM, Moon TW. Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fisher. 1999;9:211–268. [Google Scholar]
- Mommsen TP. Paradigms of growth in fish. Comp Biochem Physiol B. 2001;129:207–219. doi: 10.1016/s1096-4959(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Paull GC, Van Look KJ, Santos EM, Filby AL, Gray DM, Nash JP, Tyler CR. Variability in measures of reproductive success in laboratory-kept colonies of zebrafish and implications for studies addressing population-level effects of environmental chemicals. Aquat Tox. 2008;87:115–126. doi: 10.1016/j.aquatox.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Peterson BC, Small BC. Effects of exogenous cortisol on the GH/IGF-I/IGFBP network in channel catfish. Domest Anim Endocrinol. 2005;28:391–404. doi: 10.1016/j.domaniend.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Pottinger TG, Calder GM. Physiological stress in fish during toxicological procedures: a potentially confounding factor. Environ Toxicol Water Qual. 1995;10:135–146. [Google Scholar]
- Ramsay JM, Speare DJ, Daley J. Timing of changes in growth rate, feed intake and feed conversion in rainbow trout, Oncorhynchus mykiss (Walbaum), experimentally infected with Loma salmonae (Microspora) J Fish Dis. 2004;27:425–429. doi: 10.1111/j.1365-2761.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture. 2006;258:565–574. [Google Scholar]
- Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. Rapid cortisol response of zebrafish to acute net handling stress. Aquaculture. doi: 10.1016/j.aquaculture.2009.08.035. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay JM, Watral V, Schreck CB, Kent ML. Husbandry stress exacerbates mycobacterial infections in adult zebrafish, Danio rerio Hamilton. J Fish Dis. doi: 10.1111/j.1365-2761.2009.01074.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding JM, Patiño R, Schreck CB. Clearance of corticosteroids in yearling coho salmon, Oncorhynchus kisutch, in freshwater and seawater after stress. Gen Comp Endocrinol. 1984;54:433–443. doi: 10.1016/0016-6480(84)90159-x. [DOI] [PubMed] [Google Scholar]
- Reno PW. Factors involved in the dissemination of disease in fish populations. J Aquat An Health. 1998;10:160–171. [Google Scholar]
- Rodriguez-Tovar LE, Wadowska DW, Wright GM, Groman DB, Speare DJ, Whelan DS. Ultrastructural evidence of autoinfection in the gills of Atlantic cod Gadus morhua infected with Loma sp. (phylum Microsporidia) Dis Aquat Organ. 2003;57:227–230. doi: 10.3354/dao057227. [DOI] [PubMed] [Google Scholar]
- Saeij JP, Verburg-van Kemenade LB, van Muiswinkel WB, Wiegertjes GF. Daily handling stress reduces resistance of carp to Trypanoplasma borreli: in vitro modulatory effects of cortisol on leukocyte function and apoptosis. Dev Comp Immunol. 2003;27:233–245. doi: 10.1016/s0145-305x(02)00093-9. [DOI] [PubMed] [Google Scholar]
- Schreck CB. Immunomodulation: endogenous factors. In: Iwama G, Nakanishi T, editors. The Fish Immune System: Organism, Pathogen, and Environment. Academic Press; London: 1996. pp. 311–337. [Google Scholar]
- Schreck CB. Accumulation and long-term effects of stress in fish. In: Moberg GP, Mench JA, editors. The Biology of Animal Stress: Assessment and Implications for Animal Welfare. CAB International; Wallingford: 2000. pp. 147–158. [Google Scholar]
- Schreck CB, Contreras-Sánchez W, Fitzpatrick MS. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture. 2001;197:3–24. [Google Scholar]
- Scholz S, Mayer I. Molecular biomarkers of endocrine disruption in small model fish. Molecul Cell Endocrinol. 2008;293:57–70. doi: 10.1016/j.mce.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Shaw RW, Kent ML. Fish Microsporidia. In: Wittner M, Weiss L, editors. Microsporidia and Microsporidiosis. American Society of Microbiology Press; Washington, DC: 1999. pp. 418–44. [Google Scholar]
- Shaw RW, Kent ML, Adamson ML. Viability of Loma salmonae (Microsporidia) under laboratory conditions. Parasitol Res. 2000a;86:978–981. doi: 10.1007/pl00008529. [DOI] [PubMed] [Google Scholar]
- Shaw RW, Kent ML, Adamson ML. Innate susceptibility differences in Chinook salmon Oncorhynchus tshawytscha to Loma salmonae (Microsporidia) Dis Aquat Org. 2000b;43:49–53. doi: 10.3354/dao043049. [DOI] [PubMed] [Google Scholar]
- Small BC, Bilodeau AL. Effects of cortisol and stress on channel catfish (Ictalurus punctatus) pathogen susceptibility and lysozyme activity following exposure to Edwardsiella ictaluri. Gen Comp Endocrinol. 2005;142:256–262. doi: 10.1016/j.ygcen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Speare DJ, Daley J, Markham RJF, Sheppard J, Beaman HJ, Sanchez JG. Loma salmonae-associated growth rate suppression in rainbow trout, Oncorhynchus mykiss (Walbaum), occurs during early onset xenoma dissolution as determined by in situ hybridization and immunohistochemistry. J Fish Dis. 1998;21:345–354. [Google Scholar]
- Summerfelt RC, Warner MC. Geographical distribution and host parasite relationships of Pleistophora ovariae (Microsporida, Nosematidae) in Notemigonus crysoleucas. J Wildl Dis. 1970;6:457–465. doi: 10.7589/0090-3558-6.4.457. [DOI] [PubMed] [Google Scholar]
- Wasson K, Peper RL. Mammalian microsporidiosis. Vet Pathol. 2000;37:113–128. doi: 10.1354/vp.37-2-113. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio) 4. University of Oregon Press; Eugene, OR: 2007. [Google Scholar]
- Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish (Danio rerio) Dis Aquat Org. 2008;82:45–54. doi: 10.3354/dao01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipps CM, Kent ML. Polymerase chain reaction detection of Pseudoloma neurophilia, a common microsporidian of zebrafish (Danio rerio) reared in research laboratories. Contemp Top Lab Anim Sci. 2006;45:13–16. [PMC free article] [PubMed] [Google Scholar]
- Wiklund T, Lounasheimo L, Lom J, Bylund G. Gonadal impairment in roach Rutilus rutilus from Finnish coastal areas of the northern Baltic sea. Dis Aquat Org. 1996;26:163–171. [Google Scholar]
- Wooster GA, Bowser PR. The aerobiological pathway of a fish pathogen: survival and dissemination of Aeromonas salmonicida in aerosols and its implications in fish health management. J World Aquacult Soc. 1996;27:7–14. [Google Scholar]
- Zanoni RG, Florio D, Fioravanti ML, Rossi M, Prearo M. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433–441. doi: 10.1111/j.1365-2761.2008.00924.x. [DOI] [PubMed] [Google Scholar]