Abstract
BACKGROUND
Although emotional stress is associated with ischemic heart disease (IHD) and related clinical events, sex-specific differences in the psychobiological response to mental stress have not been clearly identified.
OBJECTIVES
We aimed to study the differential psychological and cardiovascular responses to mental stress between male and female patients with stable IHD.
METHODS
Patients with stable IHD enrolled in the REMIT (Responses of Mental Stress–Induced Myocardial Ischemia to Escitalopram) study underwent psychometric assessments, transthoracic echocardiography, and platelet aggregation studies at baseline and after 3 mental stress tasks. Mental stress–induced myocardial ischemia (MSIMI) was defined as the development or worsening of regional wall motion abnormality, reduction of left ventricular ejection fraction (LVEF) ≥8% by transthoracic echocardiography, and/or ischemic ST-segment change on electrocardiogram during 1 or more of the 3 mental stress tasks.
RESULTS
In the 310 participants with known IHD (18% women, 82% men), most baseline characteristics were similar between women and men (including heart rate, blood pressure, and LVEF), although women were more likely to be nonwhite, living alone (p < 0.001), and unmarried (p < 0.001); they also had higher baseline depression and anxiety (p < 0.05). At rest, women had heightened platelet aggregation responses to serotonin (p = 0.007) and epinephrine (p = 0.004) compared with men. Following mental stress, women had more MSIMI (57% vs. 41%, p < 0.04), expressed more negative (p = 0.02) and less positive emotion (p < 0.001), and demonstrated higher collagen-stimulated platelet aggregation responses (p = 0.04) than men. Men were more likely than women to show changes in traditional physiological measures, such as blood pressure (p < 0.05) and double product.
CONCLUSIONS
In this exploratory analysis, we identified clear, measurable, and differential responses to mental stress in women and men. Further studies should test the association of sex differences in cardiovascular and platelet reactivity in response to mental stress and long-term outcomes. (Responses of Myocardial Ischemia to Escitalopram Treatment [REMIT]; NCT00574847)
Keywords: mental stress, myocardial ischemia, women
Despite therapeutic advances and greater awareness of the risk of cardiovascular disease (CVD) in women, outcomes and prognoses for women with CVD remain worse than those for men (1). This disparity suggests that sex-related differences in disease presentation, mechanisms, risk factors, and treatment may affect prognosis (2). Although some researchers have recently focused on characterizing the sex-specific influence of traditional coronary disease risk factors, these variables failed to completely account for the widely disparate presentations and CVD outcomes between men and women (3,4).
The association of mental stress and CVD prognosis is well established (5), and mental stress is now gaining wide recognition as a potentially modifiable nontraditional CVD risk factor (6). Sex-specific differences in the psychobiological response to mental stress have not been clearly identified (7) and may provide valuable clues towards understanding the differential CVD risk in men and women. Conflicting data exist regarding sex-specific hemodynamic responses to stress, but conclusions are limited by small sample sizes (8–10).
We explored sex differences in response to mental stress across multiple important domains in CVD pathophysiology and prognosis (cardiovascular reactivity [11], platelet aggregation [12–15], and cardiovascular ischemic responses). We conducted this study in patients with clinically stable ischemic heart disease (IHD) who underwent baseline testing in the REMIT (Responses of Mental Stress Induced Myocardial Ischemia to Escitalopram Treatment) trial (16–18).
METHODS
The study cohort examined for this analysis comprised patients who underwent baseline stress testing as a screening procedure for mental stress–induced ischemia (MSIMI) to allow for randomization in the REMIT trial. The REMIT trial (Clinical Trials ID: NCT00574847) study design (18) and primary results (17) were previously described. In brief, the REMIT study was a double-blind clinical trial that randomized subjects with stable coronary disease and MSIMI to either escitalopram treatment or placebo. Patients were invited to participate in screening stress-testing procedures if they had clinically stable IHD defined as the absence of a recent (<3 months) myocardial infarction, coronary artery bypass graft surgery (CABG), or any other revascularization procedures (e.g., percutaneous transluminal coronary angioplasty with or without stenting), unstable angina, and a plan for revascularization procedures. Patients were excluded if they had other significant medical comorbidities (i.e., cardiac, pulmonary, metabolic, renal, hepatic disease, or malignancy) or psychiatric diagnoses (i.e., bipolar spectrum mood disorders, psychotic disorders, current substance abuse/dependence or history within 6 months, and active suicidal ideation) that could interfere with participation in the trial intervention. Patients were also excluded if they had severe psychiatric symptoms, were pregnant, or were using antidepressants, including selective serotonin reuptake inhibitors. The protocol was reviewed and approved by the Duke University Health System Institutional Review Board. All participants provided written, voluntary informed consent before participating in any assessment.
MENTAL STRESS AND EXERCISE TESTING
Study subjects underwent 2 sessions of assessment and testing, which included a psychiatric assessment and psychometric testing on day 1, and mental and exercise stress testing protocols on day 2. Stress testing was conducted between 8 AM and 11 AM at the Duke Cardiac Diagnostic Unit. We chose to withhold only beta-blockers before stress testing. Other anti-angina medications (e.g., calcium-channel blockers, angiotensin-converting enzyme inhibitors, nitrates) were continued per the patient’s home routine through the stress testing. Following a 20-min rest period, participants underwent 3 mental stress tasks in a fixed sequence: mental arithmetic; mirror trace; and anger recall (18). Each stress test was followed by a 6-min rest period to allow any changes to return to baseline. Patients completed the mental stress protocol while in a left lateral position to allow for echocardiography, which was conducted during the final 3 min of each rest period, as well as during each mental stress task. After the mental stress testing, patients completed a treadmill exercise test using the standard Bruce protocol (19). The exercise testing was terminated according to standard guidelines. Transthoracic echocardiography was conducted immediately following the cessation of the exercise. Because studies were read following completion of all stress testing procedures, all patients underwent testing in sequence regardless of the presence or absence of MSIMI.
CARDIOVASCULAR REACTIVITY TO STRESS
Blood pressure (BP) was measured and an electrocardiogram was recorded at 1-min intervals during each resting period, mental stress testing period, and during the exercise stress test. Heart rate (HR) was determined from the electrocardiogram, and hemodynamic responses (i.e., changes in systolic and diastolic BP, HR, and rate pressure product) were obtained by subtracting the mean level of recorded values during each mental task and exercise from the baseline resting period. The BP and HR difference between rest and each stress test was considered the cardiovascular reactivity to stress test.
ASSESSMENT OF MYOCARDIAL ISCHEMIA
Transthoracic echocardiography (20) and electrocardiography were used to assess for the presence of ischemia. Images (parasternal long-axis and short-axis views, and apical 4-chamber and 2-chamber views) were acquired using a 3-MHz transducer while in the harmonic imaging mode of the Philips iE33 system (Philips Ultrasound, Bothell, Washington). The American Society of Echocardiography 16-segment model was used to assess left ventricular wall motion (21). Segmental wall motion was graded and scored as follows: 1 = normal or hyperdynamic; 2 = hypokinetic; 3 = akinetic; 4 = dyskinetic; or 5 = aneurysmal wall motion. A wall motion score index was calculated as the sum of the segmental wall motion scores divided by the total number of the scored segments. Separate scores were calculated for the wall motion data collected at rest, during each of the 3 mental stress tests, and during the exercise stress test. The Kappa value for the intraobserver variability and interobserver variability of wall motion analysis in this study ranged between 0.80 and 0.87. Left ventricular ejection fraction (LVEF) was calculated using the biplane Simpson’s method (21). Biplane LVEF was calculated by a single reader and repeated in 15 random subjects by another experienced reader with no systematic difference (bias −1.7, p = 0.6646) and an intraclass coefficient of correlation of 0.90 (95% limits of agreement 0.74 to 0.97).
DEFINITION OF STRESS-INDUCED MYOCARDIAL ISCHEMIA
MSIMI was defined as the development or a worsening of any wall motion abnormality, reduction of LVEF ≥8% by transthoracic echocardiography, and/or ischemic ST-segment change on electrocardiogram occurring during 1 or more of the 3 mental stress tasks, as compared with rest. Exercise stress–induced myocardial ischemia was defined as the presence of any or all of the aforementioned criteria in response to exercise stress testing.
PSYCHOLOGICAL MEASUREMENTS
The Beck Depression Inventory II (BDI-TOT) and the Center for Epidemiologic Studies Depression (CESD) scales were used to assess the severity of depressive symptoms. The BDI-TOT (22) is commonly used to screen for depressive symptoms in cardiac populations and is predictive of cardiovascular outcomes (23). The 21 items of the BDI-TOT span emotional, behavioral, and somatic symptoms.
The CESD (24) is a 20-item questionnaire in which patients report on the frequency of depressive symptoms experienced in the past 2 weeks using a 4-point Likert scale. This assessment was chosen because it was previously used to test the association of depression and MSIMI (25). In addition to the BDI-TOT and the CESD scales, patients were also administered a series of scales to assess levels of hostility, anxiety, and perceived social stress. Hostility was assessed by a 27-item abbreviated version of the Cook-Medley Hostility Scale (26,27) that contained subsets of items identified in a previous study (27) as reflecting cognitive (i.e., cynicism), affective (i.e., hostile affect), and behavioral (i.e., aggressive responding) manifestations of hostility. Anxiety was assessed using the 40-item Spielberger Anxiety Scale (28), which contains two 20-item subscales measuring state and trait manifestations of anxiety. Level of stress was measured via the 10-item Perceived Stress Scale (29), which measures the degree to which situations in one’s life are judged as stressful. These particular scales were selected for use because they measure psychometric constructs that were associated with the development and prognosis of IHD in prior studies (30).
ASSESSMENT OF PLATELET REACTIVITY
Blood samples were collected at rest and at the end of mental stress testing in tubes containing 3.8% sodium citrate solution (9:1) for platelet aggregation studies.
Standard methods employed in our laboratory were used to assess platelet aggregation (31). Briefly, we separated platelet-rich plasma by centrifugation for 15 min at 135 g and adjusted it to 250,000 platelets/μl with autologous platelet-poor plasma for use in a Chronolog 4-channel platelet aggregometer (Chronolog, Havertown, Pennsylvania). Platelet-poor plasma displayed 100% optical transmittance. Because serotonergic and adrenergic stimuli, as well as collagen and adenosine diphosphate (ADP), affect platelet activity in vivo, an assessment of the biological response to multiple stimuli is likely to identify physiological effects that have implications for the pathogenesis of thrombosis in stress (31). Thus, we tested platelet aggregation responses to different agonists. Epinephrine, ADP, and collagen were obtained from Chronolog. Serotonin was obtained from Sigma-Aldrich (St. Louis, Missouri). We evaluated platelet aggregation triggered by epinephrine 10 μmol/l, serotonin 10 μg/ml, collagen 10 μg/ml, and ADP 5 μmol/l, with each agonist combined with serotonin 10 μg/ml, as well as serotonin 10 μg/ml alone. The same qualified technician performed all platelet aggregation studies using a single instrument and an identical lot of agonists. Platelet aggregation was recorded and results reported in terms of the area under the curve, which was derived by % platelet aggregation × time in minutes (31).
STATISTICAL ANALYSIS
Baseline characteristics were described using means, medians, and interquartile ranges for continuous variables and percentages for categorical variables.
Differences between men and women at baseline and their response to stress were studied in 4 domains: 1) psychosocial stress; 2) cardiovascular reactivity; 3) MSIMI; and 4) platelet aggregation. Analysis of variance was used to compare continuous variables between male and female patients. Analyses in which stress-induced changes in variables (i.e., systolic and diastolic BP, HR, platelet aggregation, and measures of state affect) were used as dependent variables included the appropriate pre-stress (i.e., baseline) value as a covariate. To increase the reliability of the measurements obtained during each mental stress test (i.e., systolic BP/diastolic BP/HR and state affect) and reduce the number of statistical tests, we averaged the 3 mental stress measurements. The chi-square test was used to test associations between categorical variables (i.e., sex and MSIMI). We also conducted analyses to see whether background factors, such as race, history of CABG, clopidogrel use (platelet aggregation analysis only), and severity of depressive symptoms, might alter the significance of our findings. The first set of analyses controlled for race, history of CABG, and clopidogrel use (platelet aggregation analysis only). This was followed by a second set of analyses that added the BDI score as an additional covariate. Analyses involving a continuous criterion variable (e.g., blood pressure changes) used analysis of covariance, and analyses involving dichotomous criterion variables (i.e., MSIMI) used logistic regression.
To examine the interrelationships between MSIMI and stress reactivity measures in men and women, we used Pearson correlation coefficients to estimate the bivariate relations between continuous variables.
Logistic regression models were used to test the relationship between continuous variables and a dichotomous MSIMI variable. The stress reactivity variables were adjusted for pre-stress values before analysis.
Statistical significance was defined at an alpha level of 0.05. All statistical tests were 2-sided. All statistical calculations were carried out using SAS version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
DISTRIBUTION OF BASELINE CHARACTERISTICS
Of the 310 study participants with stable coronary disease who completed baseline testing, 56 (18%) were women. Although most clinical risk factors were similar between men and women (including HR, systolic and diastolic BP, and LVEF), women were more likely to be nonwhite, living alone, and not married (all p < 0.001) (Table 1). Most study subjects were on a standard cardiac regimen with aspirin, beta-blocker, and statin, though a higher proportion of men reported being on a statin (94% vs. 85%, p = 0.03) and more women reported using an antiplatelet agent other than aspirin (57% vs. 40%, p = 0.02). There were no significant differences in the use of aspirin, beta-blockers, or calcium-channel blockers.
TABLE 1.
Clinical Characteristics
| Females (n = 54) |
Males (n = 256) |
p Value | |
|---|---|---|---|
| Age (yrs) | 62.8 (11.7) | 63.5 (10.3) | 0.65 |
|
| |||
| Race (% nonwhite) | 37.0 | 14.8 | <0.001 |
|
| |||
| Body mass index | 28.9 (4.9) | 29.0 (4.8) | 0.85 |
|
| |||
| Living arrangement (alone) | 35.2 | 11.7 | <0.001 |
|
| |||
| Marital status (not married) | 63.0 | 18.0 | <0.001 |
|
| |||
| Smoking | 0.13 | ||
| Current | 22.2 | 12.1 | |
| Past | 46.3 | 56.3 | |
| Never | 31.5 | 31.6 | |
|
| |||
| ACE inhibitors | 52.8 | 66.4 | 0.06 |
|
| |||
| History of CABG | 27.8 | 46.9 | 0.01 |
|
| |||
| History of PCI | 74.1 | 60.2 | 0.06 |
|
| |||
| History of diabetes | 33.3 | 27.3 | 0.38 |
|
| |||
| History of hypertension | 75.9 | 80.9 | 0.41 |
|
| |||
| History of hyperlipidemia | 88.9 | 95.3 | 0.07 |
|
| |||
| History of myocardial infarction | 0.72 | ||
| Yes | 46.3 | 44.14 | |
| No | 51.9 | 55.1 | |
| NS | 1.9 | 0.8 | |
|
| |||
| NYHA functional class | 0.79 | ||
| I | 88.9 | 92.1 | |
| II | 9.3 | 5.9 | |
| III | 1.9 | 2.0 | |
Values are mean SD or %.
ACE = angiotensin-converting enzyme; CABG = coronary artery bypass graft surgery; PCI = percutaneous coronary intervention; NYHA = New York Heart Association.
Women had a higher prevalence of a history of depression, and scored higher on scale descriptors of sadness, tension, perceived stress, and state and trait anxiety compared with men (p < 0.05). By contrast, men described feeling calmer than women at rest (p < 0.05) (Table 2).
TABLE 2.
Baseline Psychometric, Platelet Aggregation, and Cardiovascular Hemodynamics
| Baseline Psychometric Parameters
| |||
|---|---|---|---|
| Females (n = 52) |
Males (n = 250) |
p Value | |
| H/o depression | 31.48 | 10.16 | <0.001 |
| Baseline sadness | 13.6 (7.7, 19.5) | 6.7 (4.8, 8.6) | 0.007 |
| Baseline tension | 22.7 (15.8, 29.6) | 15.4 (12.9, 17.9) | 0.02 |
| Baseline frustration* | 15.6 (7.8, 23.5) | 7.2 (5.3, 9.2) | 0.18 |
| Baseline calm | 74.8 (67.8, 81.9) | 85.5 (83.1, 88.0) | <0.001 |
| Baseline in-control | 77.4 (70.1, 84.6) | 82.1 (79.1, 85.0) | 0.2 |
| Cook-Medley Hostility score | 9.6 (8.2, 11.0) | 10.4 (9.8, 11.0) | 0.3 |
| State anxiety | 31.4 (28.9, 34.0) | 28.3 (27.2, 29.3) | 0.02 |
| Trait anxiety | 38.6 (35.4, 41.9) | 33.0 (31.8, 34.2) | <0.001 |
| Perceived stress | 26.6 (24.6, 28.5) | 22.1 (21.3, 23.0) | <0.001 |
| BDI II | 10.9 (8.6, 13.1) | 7.9 (7.1, 8.7) | 0.004 |
| CESD score | 33.7 (31.1, 36.6) | 29.4 (28.4, 30.4) | <0.001 |
|
| |||
|
Resting Platelet Aggregation: Area Under the Curve
| |||
|
Females (n = 38) |
Males (n = 238) |
p Value | |
|
| |||
| Epinephrine 10 μm | 191.3 (149.8, 232.8) | 137.9 (125.1, 150.8) | 0.004 |
| Collagen 10 μm | 292.8 (260.0, 325.6) | 273.1 (260.3, 285.9) | 0.26 |
| ADP 5 μm | 239.6 (199.7, 279.5) | 248.9 (234.6, 263.3) | 0.64 |
| 5HT 10 um + epinephrine 10 μm | 264.6 (232.7, 296.5) | 241.5 (230.2, 252.9) | 0.14 |
| 5HT 10 μm + collagen 2μm | 253 (226.9, 279.2) | 238.7 (226.8, 250.7) | 0.37 |
| 5HT 10 μm + ADP 1μm | 229.7 (192.3, 267.2) | 216.2 (202.9, 229.5) | 0.46 |
| 5HT 10 μm | 11.5 (2.8, 20.3) | 4.5 (2.3, 6.0) | 0.007 |
|
| |||
|
Baseline Cardiovascular Hemodynamics
| |||
|
Females (n = 49) |
Males (n = 242) |
p Value | |
|
| |||
| Baseline resting SBP | 128 (122.4, 132.9) | 127 (124.3, 128.9) | 0.73 |
| Baseline resting DBP | 71 (68.0, 74.0) | 73 (71.6, 74.6) | 0.24 |
| Baseline resting HR | 70 (66.7, 73.3) | 68 (66.2, 68.9) | 0.14 |
| Baseline resting HR × SBP | 8,894 (8,313.5, 9,473.5) | 8,563 (8,323.2, 8,801.9) | 0.27 |
Values are % or mean (95% confidence intervals).
A nonparametric method of comparison was used.
ADP = adenosine diphosphate; BDI = Beck Depression Inventory; CESD = Center for Epidemiologic Studies Depression Scale; DBP = diastolic blood pressure; H/o = history of; HR = heart rate; SBP = systolic blood pressure; 5HT = serotonin.
A comparison of resting platelet aggregation measures between men and women revealed nonsignificant differences in platelet aggregation response to collagen 10 μg/ml, ADP 5 μmol/l, serotonin (5HT) 10 μg/ml + epinephrine 10 μm, 5HT 10 μg/ml + collagen 2 μg/ml, and 5HT 10 μm + ADP 1μmol/l (Table 2), likely a result of antiplatelet therapy. However, women had a higher resting platelet aggregation response to 5HT (p = 0.007) and epinephrine (p = 0.004). As shown in Table 2, there were no significant differences in resting HR, systolic and diastolic BP, or double product between men and women.
CARDIOVASCULAR AND PSYCHOBIOLOGICAL RESPONSES TO MENTAL STRESS
Following mental stress, more women had MSIMI (57% vs. 41%, p < 0.04) than men, but there were no significant sex differences in the rate of exercise stress–induced myocardial ischemia (Table 3). Women expressed more negative emotion (p = 0.02) and less positive emotion (p < 0.01) (Figure 1), and demonstrated a higher platelet aggregation response to epinephrine, ADP, and collagen (p = 0.04) (Figure 2), whereas men showed a greater increase in traditional physiological measures such as BP and double product (p < 0.05) (Table 3)
TABLE 3.
MSIMI and ESIMI
| Females (n = 53) |
Males (n = 254) |
p Value | |
|---|---|---|---|
| Baseline LVEF | 58.7 (55.6, 61.8) | 56.3 (55.0, 57.6) | 0.14 |
| LVEF change in MS | −1.36 (−2.62, −0.11) | −0.32 (−0.94, 0.30) | 0.16 |
| WMSI change in MS | 0.06 (0.03, 0.08) | 0.04 (0.03,0.06) | 0.40 |
| MSIMI, % | 56.6 | 40.9 | 0.037 |
| ESIMI, % | 39.2 | 32.2 | 0.34 |
Values are presented as means with 95% confidence intervals.
ESIMI = exercise stress–induced myocardial ischemia; LVEF = left ventricular ejection fraction; MS = mental stress; MSIMI = mental stress–induced myocardial ischemia; WMSI = wall motion score index.
FIGURE 1. Sex Differences in Affect Changes in Response to Mental Stress.
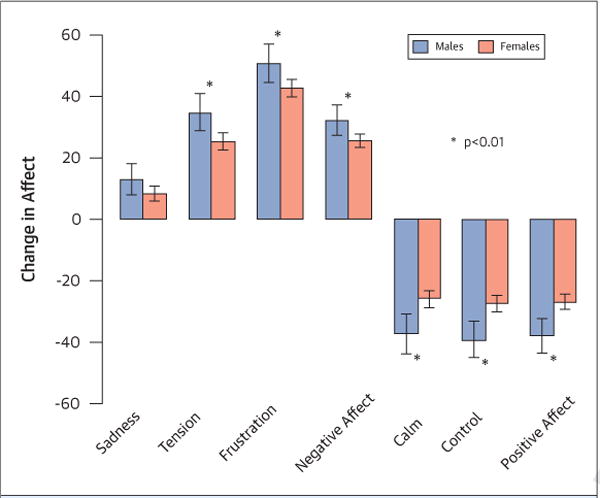
Changes in affect in response to mental stress in men and women.
FIGURE 2. Sex Differences in Platelet Aggregation Changes in Response to Mental Stress.
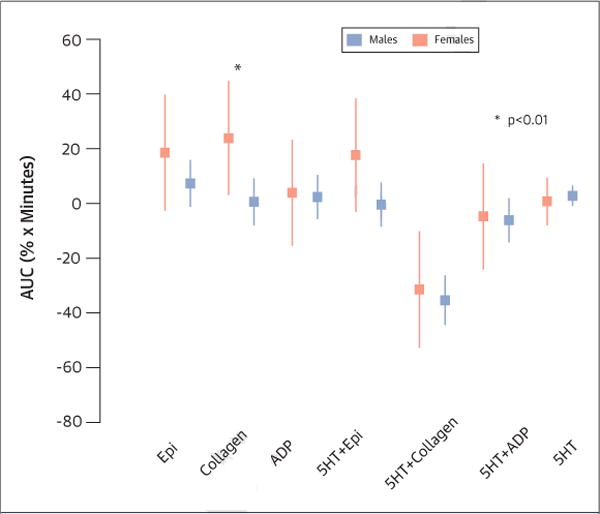
Differences among men and women in platelet aggregation changes stimulated by various agonists in response to mental stress. ADP = adenosine diphosphate; AUC = area under the curve; Epi = epinephrine; 5HT = serotonin.
Controlling for race, history of CABG (odds ratio [OR]: 1.87, 95% confidence interval [CI]: 1.01, 3.48), and clopidogrel use (platelet aggregation analysis only) had minimal impact on the association between sex and stress-induced changes (all p values <0.05). However, additional control for BDI depression scores attenuated the sex effect for MSIMI (OR: 1.68, 95% CI: 0.90, 3.15, p = 0.11), stress-induced change in negative affect (p = 0.14), and to a lesser extent, stress-induced change in systolic BP (p = 0.056).
INTERRELATIONSHIP OF STRESS REACTIVITY MEASURES IN MEN AND WOMEN
We also examined interrelationships of the stress-reactivity measures that had previously shown statistically significant sex-related differences between men and women (i.e., systolic and diastolic BP, positive and negative affect, collagen-stimulated platelet aggregation, and MSIMI). These relationships were examined separately among men and women.
Among women, modest correlations were noted between stress-induced changes in negative and positive affect (r = −0.6, p < 0.001), changes in systolic and diastolic BP (r = 0.5, p < 0.001). In addition, statistically nonsignificant trends were seen in the relationship between changes in collagen-stimulated stress-induced platelet aggregation and MSIMI (r = 0.29, p = 0.08), platelet aggregation and negative affect (r = −0.29, p = 0.09), and platelet aggregation and positive affect (r = 0.3, p = 0.07). All other correlations among reactivity indexes were nonsignificant.
Among men, significant relationships were observed between negative and positive affect (r = −0.7, p < 0.001), and systolic and diastolic BP (r = 0.6, p < 0.001). In addition, nonsignificant trends were noted in the relationship between diastolic BP and positive affect (r = −0.2, p = 0.06), and systolic BP and negative affect (r = 0.11, p = 0.09). There were no significant correlations between affect changes and MSIMI or collagen-stimulated stress-induced platelet aggregation in men.
Logistic regression failed to show any significant associations between any of the continuous stress-reactivity measures and MSIMI.
MEDIATORS OF THE RELATIONSHIP OF SEX AND MSIMI
We next sought to examine whether the relationship between sex and MSIMI might be accounted for by sex-related differences in other reactivity variables. First, we fit a logistic regression model with the sex variable predicting MSIMI. The association between sex and MSIMI was significant (OR: 1.9; 95% CI: 1.02, 3.57, p = 0.043) and remained significant after separately controlling for mental stress–induced changes in systolic BP (OR: 1.96, 95% CI: 1.04, 3.7, p = 0.04), diastolic BP (OR: 1.96, 95% CI: 1.04, 3.7, p = 0.03), positive affect (OR: 2.0, 95% CI: 1.08, 3.8, p = 0.03), negative affect (OR: 2.08, 95% CI: 1.1, 3.9, p = 0.02), collagen-stimulated mental stress–induced platelet aggregation (OR: 2.05, 95% CI: 1.02, 4.15, p = 0.045), and resting epinephrine-stimulated platelet aggregation (OR: 2.2, 95% CI: 1.08, 4.48, p = 0.03).
DISCUSSION
Our study is novel in its exploration of mental stress–related sex-specific differences across multiple, mechanistically important cardiovascular domains (Central Illustration). We report: 1) that there are clear, measurable differences between men and women across multiple areas, including psychological, cardiovascular, and biological response to mental stress; and 2) that compared with men, women expressed more negative emotion, and had a greater rate of MSIMI and collagen-stimulated platelet reactivity to mental stress; conversely, men exhibited greater cardiovascular reactivity following mental stress. Our findings, although exploratory and hypothesis-generating, carry implications for future research in this area.
In the last few decades, the recognition of sex differences in CVD has evolved with regard to presentation, cardiovascular disease mechanisms, and outcomes (2,32). Although recent reports document decreases in CVD mortality for women, it is important to emphasize that the number of cardiovascular deaths for women has exceeded men for the last 20 years (33–35). “Paradoxical sex differences” have been noted in which, compared with men, women have less obstructive coronary disease and normal left ventricular function, but higher rates of angina and death (32,35–38). Microvascular and endothelial dysfunction has been postulated to play a significant role in presentation and pathogenesis of IHD in women, where the influence of mental stress has been previously examined and proven to be important (39).
Our study builds upon prior work by Martin and colleagues (39), who demonstrated reduced cardiovascular reactivity and heightened endothelial dysfunction in response to mental stress in women without IHD; we examined MSIMI responses in men and women with known, stable IHD. We report a significantly higher prevalence of MSIMI among women compared with men. A previous study by York et al. (40), which examined a comparable cohort of females, failed to show a significant difference in the rate of MSIMI between men and women. However, their study employed myocardial perfusion imaging techniques (40), whereas our study utilized real-time stress echocardiography as a means of detecting MSIMI. Differences in our findings may speak to the underlying pathophysiological basis of MSIMI. Perfusion abnormalities report on obstructive coronary disease, whereas wall motion abnormalities are generally considered an end product of ischemia. A higher prevalence of MSIMI in women with IHD in our study, as defined by wall motion changes, may suggest that mechanisms such as microvascular dysfunction, neurogenic stunning of the myocardium, and other catecholamine-related changes, similar to those postulated in Takotsubo or stress-induced cardiomyopathy (a condition predominantly affecting postmenopausal women) may be operational here (41). In the context of its known poorer prognosis, the higher rate of MSIMI in women observed in our study requires further investigation because it may provide a potential explanation for the “ischemic paradox” in women (42).
A prominent psychological response to mental stress of increasing negative emotions and decreasing positive emotions was documented for the entire baseline REMIT cohort, though the magnitude of this change was more pronounced in women than in men. This observation supports the adequacy of the mental stress tasks employed and is consistent with previous data (43). In a study of college students, socially stressful stimuli were associated with more negative emotion in women than in men. Similar to our findings, in that study, a greater elevation in BP in men was observed (43). In another study exploring the sex differences in cardiovascular response and hypothalamic-pituitary-adrenal axis response to stress, men had significantly greater cortisol and diastolic BP responses compared with women (44). Among men, we found a trend towards a correlation between BP and affect changes. Cardiovascular reactivity to mental stress is known to play a role in the development of hypertension, and in younger adults, particularly men, this may provide a novel marker for hypertension and IHD risk (45,46). Our findings validate previous work on sex-specific cardiovascular and psychological responses to stress. It is unclear whether the changes in BP in response to mental stress are mediated through affect changes. It is also not known whether these differential responses to mental stress impact long-term outcomes.
As a possible link between mental stress and myocardial ischemia, the role of abnormal platelet responses has been extensively studied. Previous studies documented increased platelet aggregation and secretion following mental stress tasks in healthy individuals (47,48) and in patients with IHD (49). The REMIT baseline population allowed for better sex-specific characterization of platelet aggregation responses to mental stress. Our finding of a greater increase in platelet aggregation (induced by collagen 10 μmol/l) in response to mental stress in women is novel. It is important to note that although the platelet aggregation to collagen in response to mental stress was statistically different between the sexes, similar trends were seen across other agonists, but they did not reach statistical significance. These findings require further study in larger prospective cohorts. Although there was a trend towards a correlation between platelet reactivity and MSIMI among women, platelet reactivity to collagen or epinephrine did not alter the association of sex and MSIMI. Hence, although these data are thought provoking, we cannot conclude that changes in platelet reactivity accounted for sex differences in the rate of MSIMI. We have established, however, that there are differences in platelet reactivity in response to mental stress between men and women. This association is hypothesis generating for future studies investigating poor cardiovascular outcomes in women.
The implications of our findings are several-fold. Poor cardiovascular outcomes in women may reflect inherent sex-specific differences, several of which were measured in our study. They also underscore the inadequacy of available risk-prediction tools, which currently fail to measure a full spectrum of differential risk across mental stress–induced physiological and psychological changes. We believe that the sex disparity of psychophysiological responses to mental/psychosocial stress needs to be recognized in the cardiovascular risk assessment of patients, tested, and subsequently validated along with standard risk-prediction algorithms. Furthermore, the specific role of MSIMI in the prognosis of IHD, particularly in women, needs to be further elucidated, as do disease-modifying treatment targets addressing MSIMI in men and women.
STUDY LIMITATIONS
Although the REMIT trial enrolled a greater number of women than in past heart–mind studies (8–10), the relatively small sample sizes and lack of platelet data on some patients limit definitive conclusions about interrelationships between sex, stress reactivity measures, and MSIMI. A power estimate on the basis of the current study’s sample size suggests that we had sufficient power to reliably detect medium-sized effects (i.e., Cohen’s D = 0.5 or greater). Assuming a balanced design of equal numbers of males and females, we estimate that we would need a sample size of 360 patients to achieve 0.80 power to detect smaller effect sizes (Cohen’s D = 0.2 to 0.3). We believe that many of the significant differences between men and women observed in this study highlight the robust nature of these sex differences. Despite this limitation, our findings are important and provide guidance for future research designed to determine mechanistic links between sex, MSIMI, and cardiovascular outcomes.
In contrast to some prior heart–mind studies that used myocardial perfusion imaging techniques to describe MSIMI (8,10), we chose wall motion abnormalities and LVEF reductions on echocardiography as a surrogate for ischemia. Myocardial ischemia assessment by echocardiography is an established and well-validated method (50). Echocardiography allowed for real-time assessment of myocardial ischemia and a comprehensive assessment of left ventricular function, while avoiding unnecessary radiation exposure.
CONCLUSIONS
There are clear, measurable, and differential responses to mental stress in women and men across multiple cardiovascular domains. Further studies should determine the association of these sex differences in psychological, cardiovascular, and platelet reactivity responses to mental stress and long-term clinical outcomes.
CENTRAL ILLUSTRATION Mental Stress, Sex, and Cardiovascular Disease.

Effects of mental stress on psychophysiological domains, myocardial ischemia, and outcomes in men and women.
TABLE 4.
Mental Stress–Induced Changes Controlling for Baseline Values
| Mental Stress–Induced Affect Change (Controlling for Resting Affect)*
| |||
|---|---|---|---|
| Females (n = 52) |
Males (n = 250) |
p Value | |
| Change sadness | 13.0 (7.9, 18.0) | 8.3 (6.0, 10.6) | 0.10 |
| Change tension | 34.7 (28.7, 40.8) | 25.3 (22.5, 28.0) | 0.005 |
| Change frustration | 50.6 (44.3, 56.95) | 42.6 (39.7, 45.4) | 0.02 |
| Change calm | −37.1 (−43.6, −30.7) | −26.0 (−28.9, −23.1) | 0.002 |
| Change in-control | −39.1 (−45.0, −33.2) | −27.4 (−30.1, −24.7) | <0.001 |
| Change in negative affect | 32.2 (27.3, 37.1) | 25.5 (23.3, 27.7) | 0.016 |
| Change in positive affect | −37.9 (−43.6, −32.3) | −26.7 (−29.3, −24.2) | <0.001 |
|
| |||
|
Mental Stress-Induced Platelet Aggregation Changes: Area Under the Curve (Controlling for Resting Platelet Aggregation)
| |||
|
Females (n = 38) |
Males (n = 238) |
p Value | |
|
| |||
| Epinephrine 10 μmol/l | 18.2 (−2.3, 38.6) | 7.2 (−0.95,15.2) | 0.33 |
| Collagen 10 μg/ml | 23.7 (3.3, 44.1) | 0.5 (−7.7, 8.6) | 0.039 |
| ADP 5 μmol/l | 3.9 (−14.9, 22.8) | 2.2 (−5.4, 9.7) | 0.87 |
| 5HT 10 μg/ml + Epinephrine 10 μmol/l | 17.6 (−2.8, 37.98) | −0.7 (−8.9, 7.4) | 0.10 |
| 5HT 10 μg/ml +collagen 2 μmol/l | −31.4 (−52.4, −10.4) | −35.6 (−44.0, −27.24) | 0.72 |
| 5HT 10 μmol/l + ADP 1 μmol/l | −5.0 (−23.9,13.9) | −6.5 (−14.0,1.1) | 0.89 |
| 5HT + 10 μmol/l | 0.8 (−7.54, 9.07) | 2.6 (−0.69, 5.88) | 0.69 |
|
| |||
|
Mental Stress-Induced Hemodynamic Changes (Controlling for Resting Values)
| |||
|
Females (n = 49) |
Males (n = 242) |
p Value | |
|
| |||
| SBP change | 22 (18, 25) | 26 (25, 28) | 0.02 |
| DBP change | 11 (9, 13) | 15 (13, 16) | 0.01 |
| HR change | 9 (7, 12) | 9 (8, 10) | 0.99 |
| HR × BP change | 2,882 (2,325, 3,439) | 3,257 (3,008, 3,506) | 0.23 |
Values are least squared mean (95% confidence intervals [CI]), except as noted.
Values are mean (95% CI).
BP = blood pressure; other abbreviations as in Table 2.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Sex-based differences in response to mental stress are related to pathogenesis and outcomes in cardiovascular disease and may be mediated, in part, by changes in platelet reactivity.
COMPETENCY IN PATIENT CARE
Physiological differences between men and women in response to mental or psychosocial stress should be recognized during cardiovascular risk assessment.
TRANSLATIONAL OUTLOOK
Increased platelet reactivity in response to mental stress, even in patients receiving antiplatelet medications, has potential implications for concurrent therapy that should be investigated in future studies.
Acknowledgments
National Heart, Lung, and Blood Institute grant RO1HL085704 supported this work, including salary support for Drs. Samad, Becker, Williams, Kuhn, Ortel, Rogers, O’Connor, Velazquez, and Jiang. Dr. Samad has received research funding from Boston Scientific–Duke University Strategic Alliance for Research and the American Society of Echocardiography. Dr. Vora has received research funding from Boston Scientific–Duke University Strategic Alliance for Research. Dr. Becker received research grant support from Baxter, Bristol-Myers Squibb, Johnson & Johnson, and Regado Biosciences; and received consulting/lecture fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Merck, Regado Biosciences, and Sanofi-Aventis. Dr. Williams holds a U.S. patent on the 5HTTLPR L allele for use as a marker of increased cardiovascular risk in stressed persons and is a founder and major stockholder of Williams LifeSkills Inc. Dr. Ortel is a consultant for Instrumentation Laboratory, Boehringer Ingelheim, and Bayer; and has received or has grants pending from GlaxoSmithKline, Eisai, Pfizer, Daiichi Sankyo, Instrumentation Laboratory, and Stago. Dr. Rogers received funding from Boston Scientific Corporation, HeartWare, and Thoratec Corporation. Dr. O’Connor received funding from Actelion Pharmaceuticals Ltd., Amgen Inc., Biscardia LLC, Faculty Connection, GE Healthcare, Ikaria, Novella Clinical Inc., Pfizer Inc., Pozen, and Roche Diagnostics; serves as a consultant for Novartis, HeartWare, ResMed, Johnson & Johnson, Gilead, Critical Diagnostics, BG Medicine, Otsuka, Astellas, Cytokinetics, and Capricor; holds stock or stock options in Neurotronik/Interventional Autonomics Corporation; and is named on a patent application to Duke University related to selective serotonin reuptake inhibitor (SSRI) treatment for mental stress-induced myocardial ischemia (MSIMI). Dr. Velazquez received research grants from Abbott Laboratories, Evalve, and Ikaria; received consulting fees from Boehringer Ingelheim, Gilead, and Novartis; and is named on a patent application to Duke University related to SSRI treatment for MSIMI. Dr. Jiang is named on a patent application to Duke University related to SSRI treatment for MSIMI.
ABBREVIATIONS AND ACRONYMS
- 5HT
serotonin
- ADP
adenosine diphosphate
- BDI-TOT
Beck Depression Inventory II scale
- BP
blood pressure
- CABG
coronary artery bypass graft surgery
- CESD
Center for Epidemiologic Studies Depression scale
- CI
confidence interval
- CVD
cardiovascular disease
- HR
heart rate
- IHD
ischemic heart disease
- LVEF
left ventricular ejection fraction
- MSIMI
mental stress-induced myocardial ischemia
- OR
odds ratio
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Carl J. Pepine, MD, MACC, served as Guest Editor for this paper.
References
- 1.Mosca L, Manson JE, Sutherland SE, Langer RD, Manolio T, Barrett-Connor E, Writing Group Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association. Circulation. 1997;96:2468–82. doi: 10.1161/01.cir.96.7.2468. [DOI] [PubMed] [Google Scholar]
- 2.Merz CN, Kelsey SF, Pepine CJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–61. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 3.Matthan NR, Zhu L, Pencina M, et al. Sex-specific differences in the predictive value of cholesterol homeostasis markers and 10-year cardiovascular disease event rate in Framingham Offspring Study participants. J Am Heart Assoc. 2013;2:e005066. doi: 10.1161/JAHA.112.005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tulloch-Reid MK, Younger NO, Ferguson TS, et al. Excess cardiovascular risk burden in Jamaican women does not influence predicted 10-year CVD risk profiles of Jamaica adults: an analysis of the 2007/08 Jamaica Health and Lifestyle Survey. PloS One. 2013;8:e66625. doi: 10.1371/journal.pone.0066625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J. 2003;24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 6.Rozanski A, Blumenthal JA, Davidson KW, et al. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–4. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 8.Akinboboye O, Krantz DS, Kop WJ, et al. Comparison of mental stress-induced myocardial ischemia in coronary artery disease patients with versus without left ventricular dysfunction. Am J Cardiol. 2005;95:322–6. doi: 10.1016/j.amjcard.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI) Circulation. 1996;94:2402–9. doi: 10.1161/01.cir.94.10.2402. [DOI] [PubMed] [Google Scholar]
- 10.Krantz DS, Santiago HT, Kop WJ, et al. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292–7. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 11.Manuck SB, Olsson G, Hjemdahl P, et al. Does cardiovascular reactivity to mental stress have prognostic value in postinfarction patients? A pilot study Psychosom Med. 1992;54:102–8. doi: 10.1097/00006842-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–94. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 13.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–34. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 14.Trip MD, Cats VM, van Capelle FJ, et al. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322:1549–54. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 15.Vorchheimer DA, Becker R. Platelets in atherothrombosis. Mayo Clin Proc. 2006;81:59–68. doi: 10.4065/81.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Samad Z, Boyle S, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol. 2013;61:714–22. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W, Velazquez EJ, Kuchibhatla M, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309:2139–49. doi: 10.1001/jama.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W, Velazquez EJ, Samad Z, et al. Responses of mental stress-induced myocardial ischemia to escitalopram treatment: background, design, and method for the Responses of Mental Stress Induced Myocardial Ischemia to Escitalopram Treatment trial. Am Heart J. 2012;163:20–6. doi: 10.1016/j.ahj.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce R, Lovejoy FW, Jr, Pearson R, et al. Normal respiratory and circulatory pathways of adaptation in exercise. J Clin Invest. 1949;28:1423–30. doi: 10.1172/JCI102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottdiener JS, Livengood SV, Meyer PS, et al. Should echocardiography be performed to assess effects of antihypertensive therapy? Test-retest reliability of echocardiography for measurement of left-ventricular mass and function. J Am Coll Cardiol. 1995;25:424–30. doi: 10.1016/0735-1097(94)00375-z. [DOI] [PubMed] [Google Scholar]
- 21.Schiller N, Shah P, Crawford M, et al. Recommendation for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiog. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 23.Jiang W, Krishnan RR, O’Connor CM. Depression and heart disease: evidence of a link, and its therapeutic implications. CNS Drugs. 2002;16:111–27. doi: 10.2165/00023210-200216020-00004. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Jiang W, Babyak MA, Rozanski A, et al. Depression and increased myocardial ischemic activity in patients with ischemic heart disease. Am Heart J. 2003;146:55–61. doi: 10.1016/S0002-8703(03)00152-2. [DOI] [PubMed] [Google Scholar]
- 26.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–8. [Google Scholar]
- 27.Barefoot JC, Dodge KA, Peterson BL, et al. The Cook-Medley hostility scale: item content and ability to predict survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Speilberger CD, Gorsuch R, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists; 1983. [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 30.Jiang W, Krishnan RR, O’Connor CM. Depression and heart disease: evidence of a link, and its therapeutic implications. CNS Drugs. 2002;16:111–27. doi: 10.2165/00023210-200216020-00004. [DOI] [PubMed] [Google Scholar]
- 31.Berger JS, Becker RC, Kuhn C, et al. Hyperreactive platelet phenotypes: relationship to altered serotonin transporter number, transport kinetics and intrinsic response to adrenergic co-stimulation. Thromb Haemost. 2013;109:85–92. doi: 10.1160/TH12-03-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–75. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–32. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 35.Bairey Merz CN, Shaw LJ, Reis SE, et al. WISE Investigators Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 36.Moriel M, Rozanski A, Klein J, et al. The limited efficacy of exercise radionuclide ventriculography in assessing prognosis of women with coronary artery disease. Am J Cardiol. 1995;76:1030–5. doi: 10.1016/s0002-9149(99)80290-2. [DOI] [PubMed] [Google Scholar]
- 37.Shaw LJ, Bairey Merz CN, Pepine CJ, et al. WISE Investigators Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(Suppl):S4–20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 38.Shaw LJ, Shaw RE, Merz CN, et al. American College of Cardiology–National Cardiovascular Data Registry Investigators Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology–National Cardiovascular Data Registry. Circulation. 2008;117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 39.Martin EA, Tan SL, MacBride LR, Lavi S, Lerman LO, Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res. 2008;18:339–45. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.York KM, Hassan M, Li Q, et al. Do men and women differ on measures of mental stress-induced ischemia? Psychosom Med. 2007;69:918–22. doi: 10.1097/PSY.0b013e31815a9245. [DOI] [PubMed] [Google Scholar]
- 41.Akashi YJ, Goldstein DS, Barbaro G, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754–62. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bairey Merz CN. Women and ischemic heart disease paradox and pathophysiology. J Am Coll Cardiol Img. 2011;4:74–7. doi: 10.1016/j.jcmg.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Morris-Prather CE, Harrell JP, Collins R, et al. Gender differences in mood and cardiovascular responses to socially stressful stimuli. Ethn Dis. 1996;6:123–31. [PubMed] [Google Scholar]
- 44.Traustadottir T, Bosch PR, Matt KS. Gender differences in cardiovascular and hypothalamic-pituitary-adrenal axis responses to psychological stress in healthy older adult men and women. Stress. 2003;6:133–40. doi: 10.1080/1025389031000111302. [DOI] [PubMed] [Google Scholar]
- 45.Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension. 1993;22:479–85. doi: 10.1161/01.hyp.22.4.479. [DOI] [PubMed] [Google Scholar]
- 46.Treiber FA, Kamarck T, Schneiderman N, et al. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Malkoff SB, Muldoon MF, Zeigler ZR, et al. Blood platelet responsivity to acute mental stress. Psychosom Med. 1993;55:477–82. doi: 10.1097/00006842-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Levine SP, Towell BL, Suarez AM, et al. Platelet activation and secretion associated with emotional stress. Circulation. 1985;71:1129–34. doi: 10.1161/01.cir.71.6.1129. [DOI] [PubMed] [Google Scholar]
- 49.Grignani G, Pacchiarini L, Zucchella M, et al. Effect of mental stress on platelet function in normal subjects and in patients with coronary artery disease. Haemostasis. 1992;22:138–46. doi: 10.1159/000216310. [DOI] [PubMed] [Google Scholar]
- 50.Pellikka PA, Nagueh SF, Elhendy AA, et al. American Society of Echocardiography American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–41. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]


