Abstract
Purpose of review
In this review, we take a combined membrane biologist’s and geneticist’s view of the podocyte, to examine how genetics have informed our understanding of membrane receptors, channels and other signaling molecules affecting podocyte health and disease.
Recent findings
An integral part of the kidney, the glomerulus is responsible for the kidney’s filter function. Within the glomerulus, the podocyte is a unique cell serving a critically important role: it is exposed to signals from the urinary space in Bowman’s capsule, it receives and transmits signals to/from the basement membrane upon which it elaborates, and it receives signals from the vascular space with which it also communicates, thus exposed to toxins, viruses, chemicals, proteins and cellular components or debris that flow in the blood stream. Our understanding of how podocytes perform their important role has been largely informed by human genetics, and the recent revolution afforded by exome sequencing has brought a tremendous wealth of new genetic data to light.
Summary
Genetically defined, rare/orphan podocytopathies, as reviewed here, are critically important to study as they may reveal the next generation targets for precision medicine in nephrology.
Keywords: actin cytoskeleton, calcium, TRPC5, arhgdia, arhgap24
Introduction: The podocyte at the center of glomerular function
The kidney glomerulus works hard with every cardiac cycle to filter blood into an ultrafiltrate that will ultimately become urine. The glomerulus consists of a glomerular tuft and Bowman’s capsule and the basic unit of the glomerular tuft is a single capillary. The glomerular basement membrane (GBM) provides the primary structural scaffold for the glomerular tuft. Endothelial and smooth muscle-like mesangial cells providing capillary support are located inside the GBM, while podocytes are attached to the outer aspect of the GBM. There are therefore four resident cell types in the glomerulus: endothelial cells, mesangial cells, parietal epithelial cells of Bowman’s capsule, and podocytes[1].
Podocytes are pericyte-like cells with a complex cellular organization consisting of a cell body, major processes and foot processes (FPs). Podocyte FPs elaborate into a characteristic interdigitating pattern with foot processes of neighboring podocytes, forming in between the filtration slits that are bridged by the glomerular slit diaphragm (SD)[1]. Podocyte FPs and the interposed SD cover the outer aspect of the GBM and play a major role in establishing the selective permeability of the glomerular filtration barrier, which explains why podocyte injury is typically associated with marked albuminuria associated with the nephrotic syndrome [1].
Podocytes are highly differentiated cells with limited capability to undergo cell division in the adult and the loss of podocytes is a hallmark of progressive kidney disease. The function of podocytes is largely based on the dynamic regulation of their complex cell architecture, in particular the FP structure[1]. Over the past two decades, there has been a growing understanding of the role of specific proteins, which affect critical podocyte functions, a scientific area largely driven by human genetics and the technological advances in the field of genomics [1]. Here we aim to provide insight into the genetics of nephrotic syndrome, which have taught us much of what we know about what regulates podocyte structure and function in health and disease.
Podocyte injury is the hallmark of proteinuria and glomerular disease
The common feature in many forms of human and experimental glomerular disease, such as minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous glomerulopathy (MGN), diabetic nephropathy (DN), and lupus nephritis [2, 3], is podocyte injury. The best-characterized pattern of injury involves a reorganization of the FP actin cytoskeleton, which leads to FP effacement and SD disruption [4, 5]. The transformation of the actin cytoskeleton from parallel contractile bundles [6] into a dense mesh, and loss of the normal interdigitating FP pattern leads to proteinuria [3].
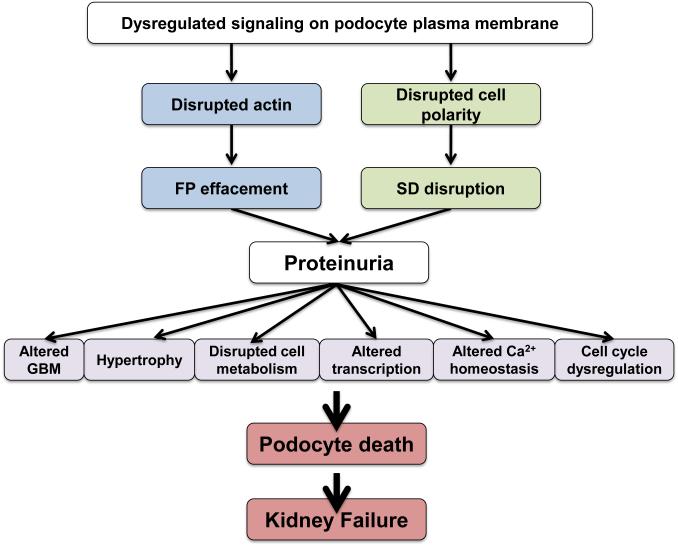
The sequence of events that mediates FP effacement and proteinuria (Fig. 1) follows a canonical pattern, which results over time in further phenotypic changes such as podocyte hypertrophy and ultimately podocyte death (Fig. 1). Many laboratories and many years of hard work have revealed certain patterns of injury including: i) changes in SD structure or function [7, 8], ii) interference with the GBM or the podocyte:GBM interaction [9-15], iii) dysregulation of podocyte calcium homeostasis [16-21] iv) de novo dysfunction of the podocyte actin cytoskeleton [5, 20-27] v) modulation of the negative surface charge of podocytes [28-30], vi) activation of innate immunity pathways such as B7-1/CD80 signaling [31-34], vii) upregulation of CatL-mediated proteolysis [35-39] and viii) disturbances in the transcriptional regulation of podocyte function [40]. Early podocyte injury is reversible, if the actin cytoskeleton is repaired, allowing FPs to ramify once again into their interdigitating pattern (Fig. 1). However, sustained, chronic podocyte injury can lead to the loss of glomerular function and ultimately kidney failure. Therefore strategies to preserve podocytes must be at the center of any and all targeted, precision medicine approaches to treat nephrotic syndrome and other glomerular diseases [41, 42].
Figure 1. Dysregulated podocyte signaling causes proteinuria and ultimately kidney failure.
Dysregulated signaling at the plasma membrane may lead to morphological changes that are reversible. Therefore, proteinuria may arise, with FP effacement, but if the upstream injurious signals are reversed, the cell morphology can revert back to physiologic patterns. Persistence of podocyte injury is manifest in the activation of cellular processes that lead to irreversible changes such as loss of adhesion to the GBM, cell hypertrophy, changes in transcription, disrupted metabolic pathways, aberrant calcium signaling and cell cycle dysregulation. These irreversible changes can cause podocyte death. The resulting loss of podocytes will ultimately lead to irreversible glomerulosclerosis and kidney failure.
The SD: a signaling complex revealed through human genetics
The podocyte SD is a complex signal transduction unit with characteristics of a modified adherens junction spanning the 30-50 nm wide filtration slits [43]. The extracellular portion of the SD is made up of rod-like units, thought to be composed of the extracellular domains of various transmembrane proteins such as nephrin and FAT [44]. These rods are connected by a linear bar, forming a zipper-like pattern, which leaves pores with the same size as or smaller than albumin [44]. The SD’s cytoplasmic portion contains a region of detergent resistant [44] electron dense material, which is reminiscent of the highly insoluble specialization of the submembranous actin cytoskeleton in neurons known as the postsynaptic density (PSD) [45]. The PSD is known to contain multiple receptors and ion channels linked through a multitude of adaptor proteins to the cytoskeletal core, forming a large protein network [46]. Similarly, at the SD, we have now learned that IgG like molecules such as nephrin[47], ion channels such as TRPCs [48], receptors[16], integrins[49] and other membrane proteins are connected to actin via a variety of adaptor and effector proteins[1]. The dysruption of SD structure or function is a common theme in many kidney diseases arising at the level of the podocyte [50]. The SD is thought to function as a key sensor for and regulator of the adaptations in FP shape and length [47, 51]. For example, the movement of each FP needs to be precisely coordinated with the FPs of neighboring podocytes to ensure the integrity of the filtration barrier. This is likely to be achieved through functional coupling of opposed FPs, which generates signaling cascades on both ends of the SD. Simply put, this multiprotein network likely serves a far more complex role as a signaling platform, rather than a simple physical sieve[1].
A broadly studied membrane protein of the SD, the one which has allowed us to best understand this intricate cellular structure, is nephrin [47]. Mutations in the NPHS1 gene encoding for nephrin have been identified as the cause of congenital nephrotic syndrome of the Finnish type [52]. Nephrin has a single transmembrane domain, a short intracellular tail and a long, immunoglobulin-like extracellular moiety, which is thought to align parallel to the extracellular domains of neighboring nephrin molecules. Through its intracellular domain, nephrin is connected to the actin cytoskeleton by several adapter proteins and plays a pivotal part in the regulation of podocyte actin dynamics [24, 51]. Among others (reviewed in detail in [51]), a recently discovered signaling pathway couples nephrin to the actin cytoskeleton via the adaptor protein Nck [25, 53, 54]. After nephrin phosphorylation by Fyn [8], Nck binds to phospho-nephrin and N-WASP [25, 53], activating the Arp2/3 complex, a major regulator of actin dynamics [24, 25, 51, 53]. Recent work has also shown that Fyn phosphorylation of nephrin promotes activation of phosphoinositide 3 kinase (PI3K) and Rac1 activity [55]. Proteosomal degradation of Nck1 was also shown to regulate RhoA activity in podocytes, thus once again linking nephrin to the actin cytoskeleton[54].
Another essential protein of the SD is podocin, a protein encoded by the gene NPHS2, which is associated with steroid-resistant nephrotic syndrome (SRNS), often with a variable onset of disease ranging from infancy to adulthood, and severe proteinuria resistant to corticosteroids [4, 56-58]. Through immune electron microscopy, it has shown that podocin localizes to the podocyte FP membrane at the insertion site of the slit diaphragm[47]. Podocin interacts with two other important components of the SD, which were revealed by human genetics, namely the adaptor molecule CD2AP and nephrin, thus serving in the structural organization of the SD[47].
Human genetics reveal the causes of podocyte injury leading to disease
Human genetics, and the genomic revolution afforded by deep sequencing technology in recent years in particular, have fueled our progress toward a molecular understanding of the SD and the modulators of FP architecture. In the last fifteen years, human genetic studies revealed that mutations in genes encoding nephrin [52], podocin [58], phospholipase C epsilon [59], coenzyme Q10 biosynthesis monooxygenase 6 (CoQ6) [60], aarF domain containing kinase 4 (ADCK4) [61], or Arhgdia [62] give rise to early onset proteinuria. Due to rapid exome sequencing and analysis, the list at this time is growing at an ever-faster rate. Of note, all these mutations are either direct or indirect regulators of the podocyte actin cytoskeleton, with the exception of CoQ6 and ADCK4, which appear to regulate mitochondrial CoQ10 biosynthesis, but nevertheless appear to be required for proper cytoskeletal dynamics during podocyte migration [60, 61].
The podocyte actin cytoskeleton theme continues with adult-onset familial diseases such as focal segmental glomerulosclerosis (FSGS), which is associated with mutations in genes encoding α-actinin 4 [5], CD2AP [63], INF2 [64], TRPC6 [48, 65, 66], Arhgap24 [67], anillin [68] and synaptopodin [69]. Even mutations in LMX1b, a transcription factor for collagen, result in podocyte cytoskeletal abnormalities due to impaired cell adhesion to the abnormal GBM [70]. Similarly, mutations in Laminin β2, another component of the GBM, lead to podocyte injury and proteinuria [71]. Finally, recent exome sequencing as well as a whole-genome linkage analysis revealed MYO1E mutations, encoding for a mutant form of non-muscle class I myosin, in childhood proteinuric disease and FSGS [72, 73].
Mouse genetic studies have revealed that additional proteins regulating the plasticity of the podocyte actin cytoskeleton such as Rho GDIα [74], podocalyxin [75], FAT1 [76], Nck1/2 [25, 53, 54], synaptopodin [77, 78] and cofilin [79] are also of critical importance for sustained function of the glomerular filtration barrier. Most impressively, all these mutations, whether mouse or human, appear to coalesce to specific and distinct signaling modules or compartments, with podocyte actin regulation rising to the top of all pathways dysregulated by genetic causes of nephrotic syndrome.
Beyond familial causes of nephrotic syndrome, human genomics applied to large population studies have revealed common variations in a number of genes that predispose or confer susceptibility to proteinuric kidney disease. These gene polymorphisms are not yet directly linked to podocyte-specific defects, but this is an area of active research. A large locus containing numerous genes was recently identified in African American populations [80, 81]. Initial studies revealed MYH9 as a likely gene candidate in this locus. This was an attractive hypothesis given previous work showing that MYH9 is responsible for two genetic causes of proteinuria, known as Epstein and Fechtner syndromes [82]. Interestingly, further work revealed that the likely candidate gene conferring risk for kidney disease is APOL1. The gene encodes Apolipoprotein L1, a molecule previously known for its trypanolytic properties, which confer an evolutionary advantage for its prevalence in an African American population [83]. More recent work has begun to unravel the role of APOL1 in kidney disease progression [84-86], in what may be the first example of a sophisticated Genome Wide Association Study (GWAS) approach for the identification of a tractable target for kidney disease therapeutics. Furthermore, common variations in GPC5 encoding glypican 5 also associate with acquired nephrotic syndrome [87]. Further work is likely to reveal how we can best utilize this knowledge to diagnose and treat patients with nephrotic syndrome.
Conclusions
A central mission for modern medicine is the development of precision therapeutics. In recent years, cancer therapies have been clearly at the leading edge of this effort, while nephrology has unfortunately lagged behind most other fields on the path to precision medicine [41]. As reviewed here, rather than defining diseases based on symptoms (nephrotic syndrome), we can now use genomics to provide molecular definitions for diseases (for example, Rac1-activating mutation-mediated nephrotic syndrome as in patients with Arhgdia [62] or Arhgap24 [67] mutations), which can in turn guide our therapies, hoping to avoid unnecessary toxicities and complications. Much work lies ahead, however, as we attempt to develop precise, genetically inspired therapies, which will allow us in nephrology to fulfill a timely quest for precision medicine.
Key Points.
- Human genetics have revealed the podocyte as essential to filter barrier function in the kidney
- The orthogonal convergence of genetics and cell biology provide the best available rationale for targeted treatments for proteinuric kidney disease.
- Human mutations reveal the regulation of the podocyte actin cytoskeleton as a top priority target for future precision medicines for nephrotic syndrome
Acknowledgements
I apologize to my colleagues whose work I was not able to cite in this review due to space limitations. Reviews were often quoted at the expense of original work. I thank Dr. Peter Mundel and Dr. Joseph Bonventre for helpful discussions. I would like to dedicate this review to my friend and colleague Dr. Michelle Winn, a world-class nephrotic syndrome geneticist, whose untimely loss marks a significant loss for our field.
Financial Support and Sponsorship
A.G. is funded by NIH grants DK095045, DK099465, DK103658 and DK057683.
Footnotes
Conflict of interest
Dr. Greka declares consultation services for Bristol Myers Squibb, Merck, Astellas and Third Rock Ventures.
References
- 1.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24(4):333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 3.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108(11):1583–7. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tryggvason K, Wartiovaara J. Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens. 2001;10(4):543–9. doi: 10.1097/00041552-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan JM, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24(3):251–6. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 6.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59(5):673–82. [PubMed] [Google Scholar]
- 7.Simons M, et al. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol. 2001;159(3):1069–77. doi: 10.1016/S0002-9440(10)61782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma R, et al. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278(23):20716–23. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 9.Regele HM, et al. Glomerular expression of dystroglycans is reduced in minimal change nephrosis but not in focal segmental glomerulosclerosis. J Am Soc Nephrol. 2000;11(3):403–12. doi: 10.1681/ASN.V113403. [DOI] [PubMed] [Google Scholar]
- 10.Raats CJ, et al. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272(42):26734–41. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- 11.Kreidberg JA, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122(11):3537–47. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 12.Noakes PG, et al. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet. 1995;10(4):400–6. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- 13.Kretzler M, et al. Integrin-linked kinase as a candidate downstream effector in proteinuria. Faseb J. 2001;15(10):1843–5. doi: 10.1096/fj.00-0832fje. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzen J, et al. The role of osteopontin in the development of albuminuria. J Am Soc Nephrol. 2008;19(5):884–90. doi: 10.1681/ASN.2007040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachs N, et al. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006;175(1):33–9. doi: 10.1083/jcb.200603073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian D, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3(145):ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassiliadis J, et al. Calcium mediates glomerular filtration through calcineurin and mTORC2/Akt signaling. J Am Soc Nephrol. 2011;22(8):1453–61. doi: 10.1681/ASN.2010080878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrasquillo R, et al. SNF8, a member of the ESCRT-II complex, interacts with TRPC6 and enhances its channel activity. BMC Cell Biol. 2012;13:33. doi: 10.1186/1471-2121-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greka A, Mundel P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J Am Soc Nephrol. 2011;22(11):1969–80. doi: 10.1681/ASN.2011040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greka A, Mundel P. Calcium regulates podocyte actin dynamics. Semin Nephrol. 2012;32(4):319–26. doi: 10.1016/j.semnephrol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaldecker T, et al. Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest. 2013;123(12):5298–309. doi: 10.1172/JCI71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smoyer WE, Mundel P. Regulation of podocyte structure during the development of nephrotic syndrome. J Mol Med. 1998;76(3-4):172–83. doi: 10.1007/s001090050206. [DOI] [PubMed] [Google Scholar]
- 23.Kos CH, et al. Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest. 2003;111(11):1683–90. doi: 10.1172/JCI17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tryggvason K, Pikkarainen T, Patrakka J. Nck links nephrin to actin in kidney podocytes. Cell. 2006;125(2):221–4. doi: 10.1016/j.cell.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Verma R, et al. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116(5):1346–59. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata S, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14(12):1370–6. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 27.Lu TC, et al. HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem. 2008;283(13):8173–82. doi: 10.1074/jbc.M708920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlando RA, et al. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 2001;12(8):1589–98. doi: 10.1681/ASN.V1281589. [DOI] [PubMed] [Google Scholar]
- 29.Takeda T, et al. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108(2):289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galeano B, et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117(6):1585–94. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagase M, et al. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47(6):1084–93. doi: 10.1161/01.HYP.0000222003.28517.99. [DOI] [PubMed] [Google Scholar]
- 32.Navarro-Munoz M, et al. Messenger RNA expression of B7-1 and NPHS1 in urinary sediment could be useful to differentiate between minimal change disease and focal segmental glomerulosclerosis in adult patients. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr128. [DOI] [PubMed] [Google Scholar]
- 33.Reiser J, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113(10):1390–7. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu CC, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369(25):2416–23. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asanuma K, et al. Selective modulation of the secretion of proteinases and their inhibitors by growth factors in cultured differentiated podocytes. Kidney Int. 2002;62(3):822–31. doi: 10.1046/j.1523-1755.2002.00539.x. [DOI] [PubMed] [Google Scholar]
- 36.Faul C, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14(9):931–8. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiser J, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279(33):34827–32. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 38.Ronco P. Proteinuria: is it all in the foot? J Clin Invest. 2007;117(8):2079–82. doi: 10.1172/JCI32966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sever S, et al. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest. 2007;117(8):2095–2104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quaggin SE. Transcriptional regulation of podocyte specification and differentiation. Microsc Res Tech. 2002;57(4):208–11. doi: 10.1002/jemt.10076. [DOI] [PubMed] [Google Scholar]
- * 41.Mundel P, Greka A. Developing therapeutic 'arrows' with the precision of William Tell: the time has come for targeted therapies in kidney disease. Curr Opin Nephrol Hypertens. 2015;24(4):388–92. doi: 10.1097/MNH.0000000000000137. This recent review put forward the concept of precision medicine in nephrology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wieder N, Greka A. Calcium, TRPC channels, and regulation of the actin cytoskeleton in podocytes: towards a future of targeted therapies. Pediatr Nephrol. 2015 doi: 10.1007/s00467-015-3224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiser J, et al. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11(1):1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 44.Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192(5):385–97. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy MB. The postsynaptic density. Curr Opin Neurobiol. 1993;3(5):732–7. doi: 10.1016/0959-4388(93)90145-o. [DOI] [PubMed] [Google Scholar]
- 46.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5(10):771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 47.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354(13):1387–401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 48.Reiser J, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37(7):739–44. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, et al. Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol. 2004;165(2):617–30. doi: 10.1016/s0002-9440(10)63326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15(1):1–7. doi: 10.1097/01.mnh.0000199012.79670.0b. [DOI] [PubMed] [Google Scholar]
- 51.Faul C, et al. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17(9):428–37. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Kestila M, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1(4):575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 53.Jones N, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440(7085):818–23. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 54.Buvall L, et al. Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics. Nat Commun. 2013;4:2863. doi: 10.1038/ncomms3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73(5):556–66. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 56.Tsukaguchi H, et al. NPHS2 mutations in late-onset focal segmental glomerulosclerosis: R229Q is a common disease-associated allele. J Clin Invest. 2002;110(11):1659–66. doi: 10.1172/JCI16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winn MP. Not all in the family: mutations of podocin in sporadic steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2002;13(2):577–9. doi: 10.1681/ASN.V132577. [DOI] [PubMed] [Google Scholar]
- 58.Boute N, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24(4):349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 59.Hinkes B, et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet. 2006;38(12):1397–405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 60.Heeringa SF, et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121(5):2013–24. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashraf S, et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J Clin Invest. 2013;123(12):5179–89. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gee HY, et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013;123(8):3243–53. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JM, et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300(5623):1298–300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 64.Brown EJ, et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 42(1):72–6. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winn MP, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308(5729):1801–4. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 66.Heeringa SF, et al. A novel TRPC6 mutation that causes childhood FSGS. PLoS ONE. 2009;4(11):e7771. doi: 10.1371/journal.pone.0007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akilesh S, et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest. 2011;121(10):4127–37. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 68.Gbadegesin RA, et al. Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. J Am Soc Nephrol. 2014;25(9):1991–2002. doi: 10.1681/ASN.2013090976. This recent paper is important because it once again reinforces on a genetic basis the theme of how centrally important the actin cytoskeleton is for podocyte health and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai S, et al. Functional analysis of promoter mutations in the ACTN4 and SYNPO genes in focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2010;25(3):824–35. doi: 10.1093/ndt/gfp394. [DOI] [PubMed] [Google Scholar]
- 70.Morello R, et al. Regulation of glomerular basement membrane collagen expression by LMX1B contributes to renal disease in nail patella syndrome. Nat Genet. 2001;27(2):205–8. doi: 10.1038/84853. [DOI] [PubMed] [Google Scholar]
- 71.Zenker M, et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13(21):2625–32. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 72.Mele C, et al. MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med. 2011;365(4):295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanna-Cherchi S, et al. Exome sequencing identified MYO1E and NEIL1 as candidate genes for human autosomal recessive steroid-resistant nephrotic syndrome. Kidney Int. 2011;80(4):389–96. doi: 10.1038/ki.2011.148. [DOI] [PubMed] [Google Scholar]
- 74.Togawa A, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18(39):5373–80. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 75.Schmieder S, et al. Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J Am Soc Nephrol. 2004;15(9):2289–98. doi: 10.1097/01.ASN.0000135968.49899.E8. [DOI] [PubMed] [Google Scholar]
- 76.Moeller MJ, et al. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. Embo J. 2004;23(19):3769–79. doi: 10.1038/sj.emboj.7600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asanuma K, et al. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115(5):1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asanuma K, et al. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8(5):485–91. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 79.Garg P, et al. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem. 2010;285(29):22676–88. doi: 10.1074/jbc.M110.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kao WH, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40(10):1185–92. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kopp JB, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40(10):1175–84. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arrondel C, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13(1):65–74. doi: 10.1681/ASN.V13165. [DOI] [PubMed] [Google Scholar]
- 83.Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–5. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruchi R, et al. Copy Number Variation at the APOL1 Locus. PLoS One. 2015;10(5):e0125410. doi: 10.1371/journal.pone.0125410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 85.Ma L, et al. Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol. 2015;26(2):339–48. doi: 10.1681/ASN.2013091017. This recent paper began to explore the very important question of how the genomic data related to APOL1 may translate into kidney biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 86.Thomson R, et al. Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci U S A. 2014;111(20):E2130–9. doi: 10.1073/pnas.1400699111. This recent paper explored how evolutionary pressure may have affected the emergence of APOL1 as a major risk factor for kidney disease in African Americans, by analogy to the sickle cell disease/malaria story. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okamoto K, et al. Common variation in GPC5 is associated with acquired nephrotic syndrome. Nat Genet. 2011;43(5):459–63. doi: 10.1038/ng.792. [DOI] [PubMed] [Google Scholar]