Abstract
Background
Attention has been paid to cotinine (COT), one of the major metabolites of nicotine (NIC), for its pro-cognitive effects and potential therapeutic activities against Alzheimer's Disease (AD) and other types of cognitive impairment. In order to facilitate pharmacological and toxicological studies on COT for its pro-cognitive activities, we conducted a pharmacokinetic (PK) study of COT in rats, providing important oral and intravenously (IV) PK information.
Methods
In this study, plasma samples were obtained up to 48 hours after COT was dosed to rats orally and IV at a dose of 3 mg/kg. Plasma samples were prepared and analyzed using a sensitive liquid chromatography tandem mass spectrometry (LC-MS/MS) bioanalytical method, providing concentration profiles of COT and metabolites after oral and IV administrations.
Results
The data were fitted into a one-compartment model and a two-compartment model for the oral and IV groups, respectively, providing important PK information for COT including PK profiles, half-life, clearance and bioavailability. The results suggested fast absorption, slow elimination and high bioavailability of COT in rats.
Conclusions
Several important facts about the PK properties in rats suggested COT could be a potential pro-cognitive agent. Information about the pharmacokinetics of COT in rats revealed in this study is of great importance for the future studies on COT or potential COT analogues as agents for improving cognition.
Keywords: Cotinine, hydrophilic interaction chromatography, tandem mass spectrometry, pharmacokinetics, cognition
Introduction
As a result of the unprecedented growth of elderly populations, one of the most significant results of global population aging is the rise in the number of people suffering from age-related forms of dementia, including Alzheimer's disease (AD), schizophrenia, Parkinson's disease and mild cognitive impairment (MCI).[1] AD is a progressive, severe and incurable neurodegenerative disorder, of which the actual cause and pathological mechanism of AD are still not clearly known.[2] Different theories have been published as the possible mechanisms of AD, in which the most accepted one is related to the aggregation of amyloid-β (Aβ) peptides, amyloid angiopathy, and neurofibrillary tangles of phosphorylated tau protein in the brain.[3-5] Current therapeutic agents for AD, including memantine, galantamine and xanomeline can only alleviate the symptoms and may cause significant side effects. [6-9] Similarly, limited therapeutic agents are available for the effective and safe treatment of schizophrenia, Parkinson's disease and mild cognitive impairment (MCI).
The pro-cognitive effects of tobacco have been of great interest to researchers in the past decades.[10-12] Nicotine (NIC) has been demonstrated to have pro-cognitive effects on the central nervous system by acting as an agonist for nicotinic acetylcholine receptors (nAChRs).[13, 14] However, due to the short half-life (2 – 6 hours), high toxicity (mouse oral LD50 = 50 mg/kg) and high addictive potential of NIC, it is unlikely to be developed as an effective and safe therapeutic agent. The major metabolite of NIC, cotinine (COT), has shown pro-cognitive effects in animals.[15-17] COT is a weak agonist of nAChRs, however, its mechanism of action for the pro-cognitive effects is still yet to be fully elucidated.[18] COT was reported to reduce amyloid-β aggregation and improve memory in AD animal models.[19] A recent study published by Gao and coworkers also reported that COT demonstrated neuroprotective effects, which could also contribute to the prevention and treatment of AD.[20] COT also showed significant pro-cognitive effects by attenuating glutamate (NMDA) antagonist-related effects in an animal behavioral study published by Terry et al., which was considered to be valuable for the treatment of schizophrenia.[17] In addition, a study on primate species revealed that COT could selectively activate some nicotinic receptors and showed activities that could be used for the treatment of Parkinson's disease.[21] Last but not least, the longer biological half-life (15–19 h) and lower toxicity (mouse oral LD50 = 1604 mg/kg) of COT make it a more practical prototype drug candidate for the treatment of AD and other mild to severe dementia.
Several studies have been conducted to reveal the pharmacokinetic (PK) properties of NIC.[22-25] NIC was reported to have a low oral factor of bioavailability (about 20%) and short half-life (2 – 6 hours) by these studies. A few PK properties of COT were revealed from some PK studies focused on NIC, providing limited information about the half-life and clearance of COT as a metabolite of NIC.[23, 26] Two studies about the PK of COT in humans were published in 1987 and 1990, respectively, revealing important PK parameters of COT in non-smoking healthy volunteers.[27, 28]
However, no PK studies have been conducted on rodents. Rodents are frequently used as animal models in non-clinical and pre-clinical drug research and development. In most of the current pre-clinical studies on the pre-cognitive effects of COT, rodents are used as test animals for experiments on behavior, pharmacology and toxicology. Information and conclusions about the pro-cognitive effects of COT are primarily based on rodents. Therefore, PK profiles of COT in rodent species are needed as the guidance for the administration of COT in future studies. It is also of great important to have the PK properties of COT available, which could be correlated to the brain distribution and pharmacological activities of COT. Moreover, in humans, COT is primarily metabolized by cytochrome P450 2A6 (CYP2A6), which does not exist in rodents including mice and rats.[29] Therefore, COT may display different metabolism, disposition and PK in rodent species. A PK study of COT in rodents is needed to obtain important PK parameters for future non-clinical and pre-clinical studies on the pharmacology, toxicology and drug delivery of COT. With the PK properties of COT in rats available, such studies can be more specifically conducted, which could be the foundation for further investigations into the pro-cognitive effects of COT on humans.
In this study, we used a sensitive, precise and accurate LC-MS/MS method for the quantification of COT and three other major metabolites in rat plasma.[30] Test rats were dosed with a single dose of COT at 3 mg/kg both orally and intravenously, which was the therapeutic dosage level for pro-cognitive effects. Important PK information of COT including PK profiles, half-life, clearance and bioavailability were revealed in this study, which suggested fast absorption, slow elimination and high bioavailability of COT in rats. Moreover, three major metabolites of COT, norcotinine (NCOT), trans-3’-hydroxcotinine (OHCOT) and cotinine (S)-cotinine-N-oxide were also analyzed simultaneously, providing more information about the bio-transformation of COT.
Though much information about the in vivo effects of cotinine is obtained from rodent models, this is the first manuscript designed to specifically focus on cotinine PK specifically in rats. These results about the PK of COT in rats are of great importance for the future studies on COT and its pro-cognitive effects.
Experimental
1. Chemicals and reagents
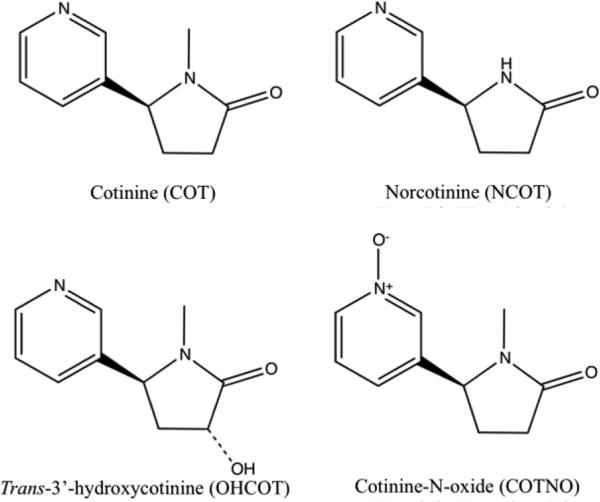
(-)-Cotinine (COT) was purchase from Sigma-Aldrich (St. Louis, MO). Stable isotope labeled internal standard (IS) (±)-Cotinine-D3 solution (1mg/mL in methanol) was obtained from Cerilliant (Round Rock, Texas). (R,S)-norcotinine (NCOT), trans-3’-hydroxcotinine (OHCOT) and cotinine (S)-cotinine-N-oxide, (R,S)-norcotinine-d4 (NCOT-d4), trans-3’-hydroxycotinined3 (OHCOT-d3) and (R,S)-cotinine-N-oxide-d3 (COTNO-d3) were purchased from Toronto Research Chemical (Toronto, Canada). Chemical structures of COT, NCOT, OHCOT and COTNO are shown in Figure 1. Trichloroacetic acid and ammonium acetate were obtained from Baker (Phillipsburg, NJ). LC-MS grade formic acid, acetonitrile (ACN), methanol and water were from Sigma (St. Louis, MO).
Figure 1.
Chemical structures of cotinine (COT), norcotinine (NCOT), trans-3’-hydroxylcotinine (OHCOT) and cotinine-N-oxide (COTNO).
2. Solutions and standards
Individual stock solutions of all the analytes and IS were prepared as 1.0 mg/mL methanol solutions. Combined working solutions were obtained by serial dilution with 90% ACN/water (v/v 9/1). IS working solutions containing COT-d3, NCOT-d4, OHCOT-d3 and COTNO-d3 were prepared at a single concentration of 500.0 ng/mL in the same solvent. Stock solutions were kept at −20 °C when not in use.
Blank rat plasma with sodium EDTA was purchased from Bioreclamation (Westbury, NY). 10 μL of standard or QC working solution was spiked into 100 μL of blank plasma to generate corresponding standard or QC samples. The final concentrations of calibration standards were 20, 50, 100, 200, 500, 1000, 5000 and 10000 ng/mL in plasma while the QC samples were 30, 750 and 7500 ng/mL. Fresh standards and QC samples were prepared on the day of experiments.
3. Dosing and sample collection
Pre-canulated albino Wistar rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN) approximately 2 months old were housed in pairs in a temperature controlled room (25 °C), maintained on a 12:12 h normal light-dark cycle (lights on at 6AM) with free access to water and food until used for PK studies. All procedures employed during this study were reviewed and approved by the Georgia Regents University Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain and discomfort in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996.
To four rats used in the oral group and three in the IV group, a single dose of 3 mg/kg (1.5 mg/mL for oral, 3 mg/mL for IV) COT in saline was administered via intravenous bolus injection through the jugular vein cannula or by oral gavage. Serial blood samples (200 μL) each were collected from the jugular vein cannulae at 0, 15, 30 min and 1, 2, 4, 6, 8, 12, 24 and 48 hours following oral administration of COT. Similarly, blood samples were obtained at 0, 15, 30, 60, 90 min and 2, 3, 4, 5, 6 and 24 hours from the animals in the IV group. The blood samples were placed in centrifuge tubes pretreated with potassium EDTA, followed by centrifugation for 15 min at 2500 × g at 4-5 °C. The separated plasma was frozen at −80 °C until analyzed.
4. Sample preparation
Sample preparation was carried out by a combined method of protein precipitation (PPT) and solid-phase extraction (SPE). This method was based on a method published by Li et al, while steps with different conditions or parameters were specially noted here.[30]
To each 50 μL of plasma, 10 μL of IS working solution (500.0 ng/mL), 90 μL of 25% (w/v) TCA and 850 μL of water were sequentially added. The mixture was vortexed for 10 min and then centrifuged at 4500 × g for 10 min for PPT. SPE based on mixed-mode cation exchange mechanism was used as the second step of sample preparation, in which Oasis MCX SPE cartridges from Waters (Milford, MA) were used. Processes including conditioning, sample loading, washing and eluting were identical with the published method.[30]
5. LC-MS/MS assay
LC-MS/MS analysis was performed on an Agilent 1100 binary pump HPLC system (Santa Clara, CA) interfaced to a Waters Micromass Quattro Micro triple quadrupole mass spectrometer with an ESI(+) source (Milford, MA). Data acquisition and processing were carried out using Masslynx 4.0 software by Waters (Beverly, MA).
The analytes were separated on a Phenomenex Kinetex™ HILIC column (50 × 2.1 mm ID, 2.6 μm) coupled with a SecurityGuard™ ULTRA HILIC guard column (sub-2 μm, 2.1mm ID). A HILIC method with gradient elution was used with mobile phases A) 10mM ammonium formate aqueous buffer with 0.1% formic acid and B) ACN. The mass spectrometer was operated in positive ion ESI (ESI +) mode. Multiple reaction monitoring (MRM) functions were used for the quantification of analytes. Detailed settings and parameters of the LC-MS/MS method were identical with the previously published method by Li et al.[30]
6. PK analysis
COT plasma concentration versus time data were analyzed by WinNonlin 5.3 software (Pharsight, Moutain View, CA). COT plasma concentrations and corresponding time points were plotted in a semi-logarithmic scale coordinate to obtain the plasma concentration-time curves (PK profiles). To obtain more specific PK parameters, oral and IV data from each test animal were fitted into different compartmental PK models. The area under the plasma concentration-time curves (AUC) ratio between the oral and IV groups were used to determine the absolute factor of bioavailability (F) for orally dosed COT in rats. By comparing the fittings of different PK models, optimal models were chosen to provide the peak plasma concentrations (Cmax), time to reach peak concentration (tmax), half-life (t1/2), clearance (CL) and other PK parameters.
Results and Discussion
1. Sample preparation
Before LC-MS/MS analysis, sample preparation was required to prepare plasma samples into a cleaner injectable sample. In this method development protein precipitation (PPT) and solid-phase extraction (SPE) were combined to achieve sufficient sample clean-up as well as satisfactory analyte recovery. Samples prepared only by PPT still contained impurities, which became more significant when the samples were evaporated and reconstituted at higher concentrations. Based on this, SPE was used following PPT to more effectively remove impurities with high selectivity. MCX (mixed mode cation exchange) SPE provided acceptable recoveries, as all analytes were protonated in acidic solution and bound to cartridges via cation exchange interactions. Cleaner samples and lower matrix effects could be achieved with more specific SPE conditions, which, in the other hand, lowered the analyte recovery. To balance the recovery and matrix effects for all analytes, the strongest washing agent (20% basic methanol) and weakest eluting agent (5% acidic methanol) were optimized to provide acceptable recoveries for all of the analytes. Since stable isotope-labeled internal standards were used for all the analytes, matrix effects were compensated for without affecting the method precision and accuracy.
2. LC-MS/MS analysis
The LC-MS/MS method was originally validated for COT, NCOT, OHCOT and COTNO from 1 to 100 ng/mL in plasma and rat brain tissue homogenate, with intra-day (n = 5) and inter-day (n = 15) precision within 20% for the lower limit of quantitation and 15% for three different QC concentration levels.[30] In order to fit the higher analyte concentrations in this PK study, the linear ranges of COT, NCOT, OHCOT and COTNO were adjusted to be from 20 to 10000 ng/mL in plasma. Accordingly, the following changes were made to the sample preparation and LC-MS/MS method to avoid saturation of the detector. First, in the sample preparation, 50 μL of plasma was used instead of 100 μL, while the volume of reconstitution remained the same. Second, the injection volume was decreased from 10 μL to 5 μL in the LC-MS/MS method. Therefore, the on-column concentrations of analytes were four times lower than samples analyzed by the original method. Good linearity (R2 > 0.99) was observed with the elevated linear range for each analyte. In order to ensure the precision and accuracy of the modified method, a partial method qualification experiments was conducted by analyzing spiked samples (n = 3) at the LLOQ (20 ng/mL) and three QC levels (30, 750 and 7500 ng/mL) together with real PK samples, giving out satisfactory precision (coefficient variation) and accuracy (relative error from the nominal values) within 15%.
3. Intravenous PK of COT
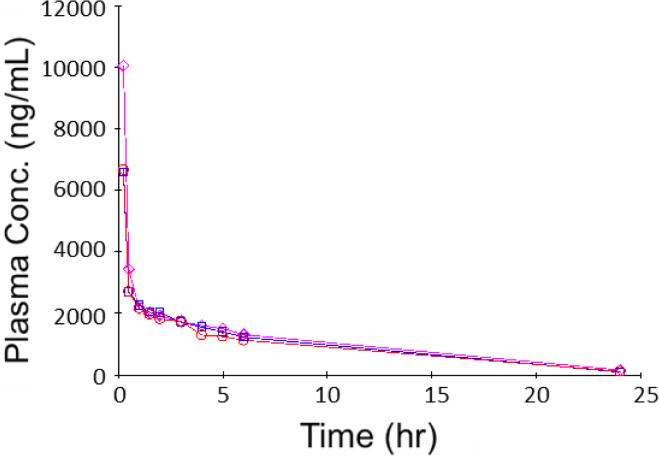
To obtain basic PK parameters, a single dose of 3 mg/kg COT was given to rats (n = 3) via IV administration. Plasma samples obtained up to 24 hours from the administration were analyzed by the LC-MS/MS method described above. All the COT concentrations measured were within the linear range of the analytical method, only except one data point that was slightly over the upper limit of quantitation (ULOQ) by 0.46% and was determined using extrapolation. Plasma COT concentrations were plotted in a regular scale coordinate, as shown in Figure 2. Great agreement was observed among the three tested animals, including the absolute concentrations as well as the trend of concentration changes.
Figure 2.
Plasma concentration (ng/mL) versus time (h) data obtained from rats (n = 3) intravenously dosed with 3 mg/kg COT.
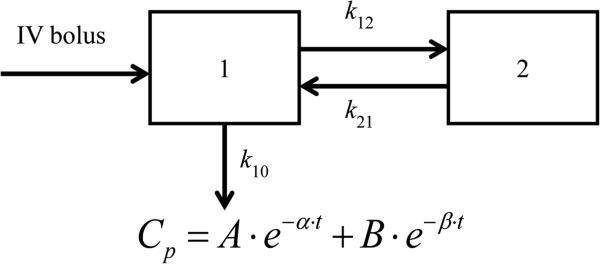
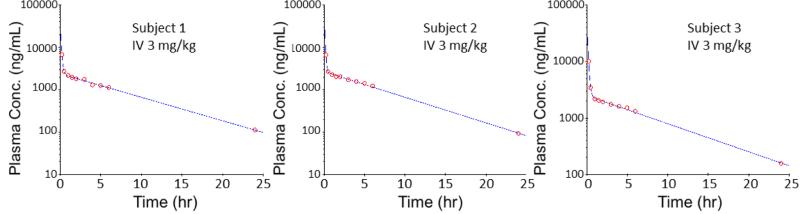
According to the trend of plasma COT concentration changes over the time, the concentration-time curves with a sharp defection point suggested two different first order kinetic phases, the distribution phase and the elimination phase. Therefore, the concentration versus time data were fitted into a two-compartment IV-bolus PK model with first-order elimination, with the schematic diagram and equation shown in Figure 3. By running this PK model with concentration-time data from three tested animals, three individual PK profiles were obtained, as shown in Figure 4. All three PK profiles had good fit with the PK model and great similarity between each other. Different models were tested,including the non-compartment IV model, one-compartment IV model and two-compartment IV model with first-order clearance., Akaike's Information Criteria (AIC) scores were used to determine the fitness of each model. AIC is defined by the equation AIC = N ln Re + 2p, in which N is the number of data points, p is the number of parameters of a PK model and Re is the residual sum of squares. The model with minimum AIC score is considered as the best representation of the time course plots.[31] The two-compartment IV-bolus PK model showed the best fit with the lowest AIC values.
Figure 3.
Schematic diagram and equation of two-compartment IV-bolus PK model with first order elimination. Compartment 1 is the central compartment and compartment 2 is the peripheral compartment. k10, elimination rate constant; k12, distribution rate constant; k21, redistribution rate constant; Cp, plasma concentration; t, time; α, hybrid rate constant for distribution; β, hybrid rat constant for elimination.
Figure 4.
PK profiles in semi-logarithmic scale for rats intravenously dosed with 3 mg/kg COT. Two-compartment IV-bolus PK model with first order elimination was used.
PK parameters for intravenously dosed COT were generated using the two-compartment IV-bolus model, shown in Table 1. After entering the central compartment via an IV bolus injection, COT was rapidly distributed into the peripheral compartment with a high distribution rate constant (k12 = 6.84 h−1) and a very short distribution half-life (t1/2,α = 0.07 h). During the distribution phase, COT plasma concentrations experienced a rapid drop, due to the distribution into the peripheral compartment (tissues) as well as the elimination from the central compartment (circulatory system). A pseudo-equilibrium was reached in a short time (3 to 5 t1/2,α) and the PK of COT entered the elimination phase, where COT was cleared from the central compartment (k10 = 2.34 h−1) while slowly redistributing from the peripheral compartment (k21 = 0.53 h−1). This combination resulted in COT showing very slow elimination from the body with a long elimination half-life (t1/2,β = 5.49 h). Though COT had rapid elimination from the central compartment (k10 half-life = 0.31 h), it still showed a low clearance of 116.67 mL/hr/kg, due to the small volume of the central compartment (V1 = 53.09 mL/kg). Among a large steady state volume of distribution of 712.01 mL/kg, the peripheral compartment accounted for the predominant portion (V2 = 658.92 mL/kg).
Table 1.
Pharmacokinetic parameters of COT obtained from IV bolus experiments (n = 3).
| Parameter | Mean ± SD |
|---|---|
| AUC (hr*ng/mL) | 25900.49 ± 2698.80 |
| k10 (1/hr) | 2.34 ± 0.75 |
| k12 (1/hr) | 6.84 ± 1.46 |
| k21 (1/hr) | 0.53 ± 0.08 |
| k10 half-life (hr) | 0.31 ± 0.09 |
| α (1/hr) | 9.58 ± 2.15 |
| β (1/hr) | 0.13 ± 0.01 |
| t1/2,α (hr) | 0.07 ± 0.02 |
| t1/2, β (hr) | 5.49 ± 0.54 |
| A (ng/mL) | 58182.35 ± 20076.88 |
| B (ng/mL) | 2514.35 ± 143.40 |
| Cmax (ng/mL) | 60696.71 ± 20220.19 |
| Vss (mL/kg) | 712.01 ± 98.47 |
| V1 (mL/kg) | 53.09 ± 16.76 |
| V2 (mL/kg) | 658.92 ± 81.87 |
| CL (mL/hr/kg) | 116.67 ± 12.20 |
| CLD2 (mL/hr/kg) | 352.95 ± 93.09 |
AUC, area under the concentration – time curve; k10, elimination rate constant; k12, distribution rate constant; k21, redistribution rate constant; α, hybrid rate constant for distribution; β, hybrid rate constant for elimination; t1/2,α, distribution half-life; t1/2, β, elimination half-life; Cmax, peak plasma concentration; Vss, volume of distribution at steady state; V1, volume of the central compartment; V2, volume of the peripheral compartment; CL, clearance; CLD2, inter-compartment clearance.
All of the PK parameters for IV dosed COT obtained in this study showed good agreement with previously published PK studies for COT in humans. COT was reported to have a long elimination half-life of 12.2 h and 15.5 h by two studies conducted on humans.[27, 28] COT intravenously dosed to rats showed a shorter elimination half-life of 5.49 h in our study, which was consistent with the plasma half-life of 4.8 to 5.3 h for cotinine studied as a nicotine metabolite in rats, which was a reasonable result, due to the fact that rats usually have faster drug clearance than humans.[32] According to a study based on more than 100 xenobiotics in rats and human, rats tend to have shorter elimination half-lives than humans.[33] This is due to the faster clearance in rats (116.67 mL/hr/kg) than human (63.8 mL/hr/kg), though in vitro microsomal incubation and in vivo renal clearance experiments are still needed to investigate the actual reason of faster clearance.[28] humansCompared to the plasma nicotine half-life ranging 0.9 to 1.1 h in rats revealed by Kyerematen et al., the elimination half-life of 5.49 h of cotinine in rats can be considered significantly longer.[32]
Based on the PK parameters obtained from the IV experiments, COT showed PK properties that agreed with its chemical and physical properties. As COT is a small and polar compound that can easily pass through cellular membranes, the distribution of COT from the circulatory system into the peripheral tissues was fast and thorough. The short distribution phase also suggests that COT tended to enter rapidly equilibrating tissues including red blood cells, liver and kidney. Since COT is a highly polar small-molecule drug, the elimination from the circulatory system is expected to be fast, due to the high water solubility and membrane permeability. However, COT showed a long apparent half-life in PK studies, which was caused by this extensive presence in the peripheral tissues and the sustained redistribution from the peripheral tissues back into the circulatory system. The long half-life of COT explains the observation of the lasting pro-cognitive effects of nicotine, which has a short half-life. The PK properties of COT revealed in these experiments, including the fast distribution into peripheral tissues, lasting redistribution back into the circulatory and long half-life, make COT of great potential to be developed as a therapeutic agent for the treatment of AD and other types of dementia.
4. Oral PK of COT
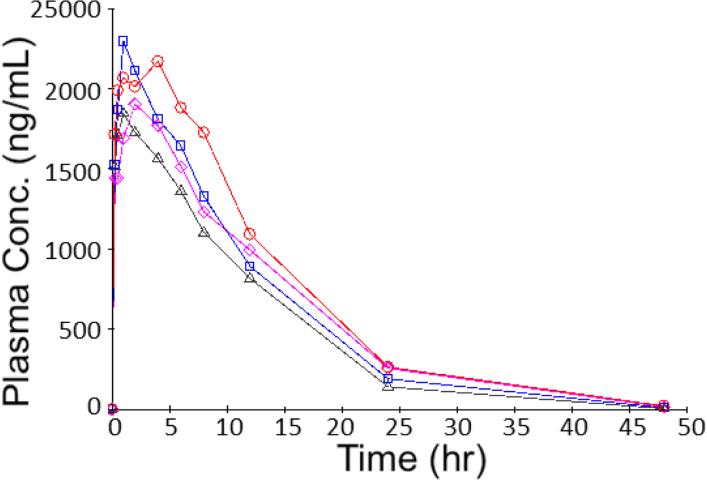
To obtain bioavailability and other additional PK parameters, a single dose of 3 mg/kg COT was orally administered to rats (n = 4). Due to the longer absorption and elimination phases, plasma samples were collected up to 48 hours from the administration and analyzed using LC-MS/MS. All the COT concentrations measured were within the linear range of the analytical method. Plasma COT concentrations versus time were plotted in a regular scale coordinate in Figure 5, in which the four tested animals showed similar concentration changes over time.
Figure 5.
Plasma concentration (ng/mL) versus time (h) data obtained from rats (n = 4) orally dosed with 3 mg/kg COT.
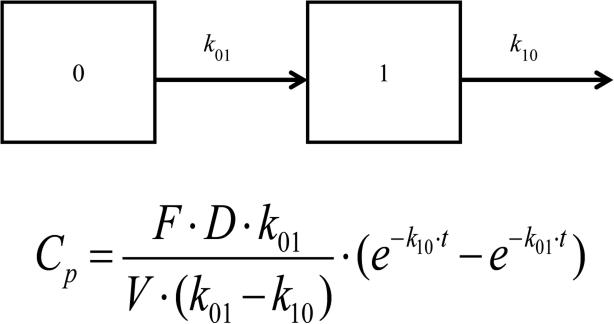
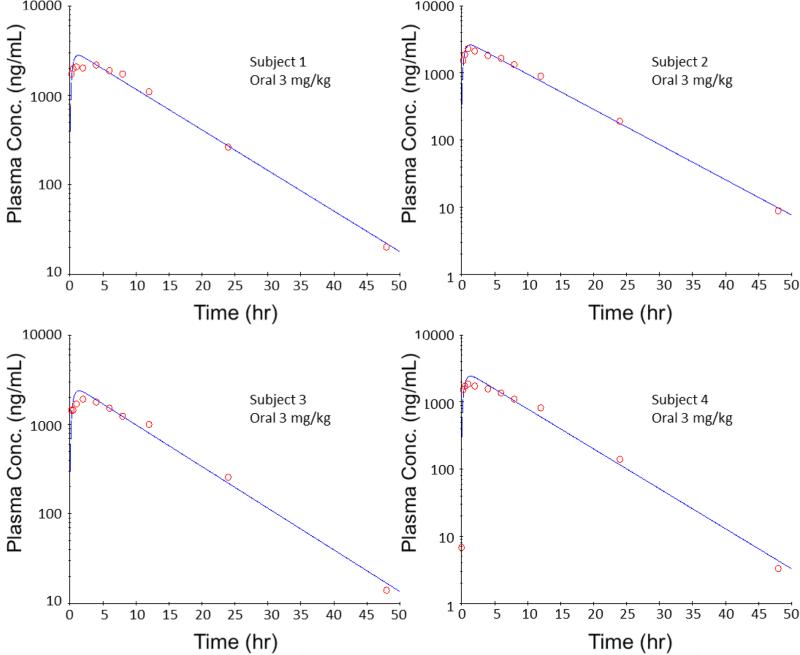
Different PK models were tested, including the non-compartment oral model, one-compartment oral model and two-compartment oral model with first-order clearance. After comparison, the one-compartment oral PK model was chosen as the optimal model, due to the lowest AIC values. The schematic diagram and equation are shown in Figure 6, and the four individual PK profiles are shown in Figure 7. PK profiles from the four tested subjects showed good fitting with the PK model. The PK parameters were obtained by fitting the concentration-time data into the one-compartment oral PK model, as shown in Table 2.
Figure 6.
Schematic diagram and equation of one-compartment oral PK model with first order elimination. Compartment 0 is the GI track and compartment 1 is the central compartment. k10, elimination rate constant; k01, absorption rate constant;; Cp, plasma concentration; F, bioavailability factor; D, dose; V, volume distribution; t, time.
Figure 7.
PK profiles in semi-logarithmic scale for rats orally dosed with 3 mg/kg COT. One-compartment oral PK model with first order elimination was used.
Table 2.
Pharmacokinetic parameters of COT obtained from oral experiments (n = 4).
| Parameter | Mean ± SD |
|---|---|
| AUC (hr*ng/mL) | 24124.74 ± 2430.79 |
| V (mL/kg) | 1030.61 ± 52.08 |
| k01 (1/hr) | 2.33 ± 0.09 |
| k10 (1/hr) | 0.12 ± 0.01 |
| k01 half-life (hr) | 0.30 ± 0.01 |
| k10 half-life (hr) | 5.75 ± 0.70 |
| CL (mL/hr/kg) | 125.25 ± 13.37 |
| Tmax (hr) | 1.34 ± 0.03 |
| Cmax (ng/mL) | 2476.86 ± 116.92 |
AUC, area under the concentration – time curve; V, volume of distribution; k01, absorption rate constant; k10, elimination rate constant; CL, clearance; Tmax, time to reach peak plasma concentration; Cmax, peak plasma concentration.
According to the PK model, orally dosed COT would be rapidly absorbed by the (gastrointestinal) GI tract with a high absorption constant (k01 = 2.33 h−1) and a short absorption half-life (reach the peak plasma concentration (Cmax) at 2476.86 ng/mL in less than two hours (Tmax = 1.34 h). As demonstrated by the PK of IV dosed COT, the elimination of COT followed a two-compartment model with first-order elimination. However, orally dosed COT only demonstrated a absorption phase and a one-compartment elimination phase, without any significant distribution phase. This was due to the rapid distribution of COT, which was even faster than the absorption (k12 > k01). After orally dosed COT was absorbed by the GI tract, it was rapidly distributed from the central compartment into the peripheral compartment. With the short distribution phase overlapped with the absorption phase, the two-compartment model appeared as a one-compartment model, because the concentration changes resulting from the distribution were not as significant and were covered by the absorption. Instead of having multiple PK parameters in a two-compartment model, orally dosed COT demonstrated a low elimination rate constant (k10 = 0.12 h−1) and a long apparent elimination half-life of 5.75 h, even though the theoretical elimination of COT from the central compartment was supposed to be fast (k10 half-life = 0.31 h). The clearance of COT obtained in the oral experiment was 125.25 mL/hr/kg, which was consistent with the clearance obtained in the IV experiment.
By integrating the area under the concentration-time curve (AUC), the total exposure of orally dosed 3 mg/kg COT was represented by the AUC of 24124.74 hr*ng/mL. Similarly, the total exposure from IV dosed 3 mg/kg COT was also represented by the AUC of 25900.49 hr*ng/mL. By comparing the oral AUC with the IV AUC at the same dosage level, the factor of bioavailability of orally dosed COT was obtain from the AUC ratio of 93.14 ± 9.39%. This value suggests that almost all orally dosed COT was absorbed, and systemically available, via the GI tract. Results for oral PK of COT obtained in this section also showed good agreement with previously published PK studies of COT in humans. According to De Schepper and coworkers, COT showed that oral factor of bioavailability of COT ranged between 0.84 and 1.11 following 10 mg and between 0.97 and 1.03 following the 20 mg dose in humans.[27]
The PK properties revealed in these experiments, including fast absorption and high bioavailability, were highly consistent with the fact that COT was a small, polar and higher water-soluble chemical. The slow clearance and long apparent half-life observed for the oral dose also agreed with the results from the IV PK experiments.
5. Metabolism of COT
In current literature, there is no specific report of the metabolism of COT as a directly dosed drug in rodent species. Based on the metabolism of NIC in rats, NCOT, OHCOT and COTNO are the major metabolites of COT.[32, 34] In this study, plasma concentrations of NCOT, OHCOT and COTNO were measured simultaneously when COT concentrations from intravenously dosed rats were analyzed by LC-MS/MS. After concentration-time data were obtained for each of these COT metabolites, AUCs were calculated by fitting the data into non-compartment models. NCOT, OHCOT and COTNO demonstrated AUCs of 42.77, 465.78 and 408.84 hr*ng/mL, respectively. By comparing the metabolite AUCs with that of COT, the percentage of COT metabolized into each metabolite was calculated as 0.16% for NCOT, 1.80% for OHCOT and 1.58% for COTNO. These results suggested that only a small fraction of dosed COT was metabolized into these major metabolites. The major fraction of COT was excreted from the body unchanged. According to the previously published studies, only 10 to 12% of COT was excreted unchanged from the body when intravenously dosed to humans.[27, 28] This number was much higher in our study, which might result from the absence of CYP 2A6, the primary enzyme for the metabolism of COT, in rats.[35] There have not been any publications reporting the metabolism of COT as a directly dosed drug. According to a study published by Kyerematen et al., levels of NCOT and COTNO were very low after the dosing of NIC, which is consistent with our study.[32] Since COT was monitored as a metabolite of NIC in all the previous studies, no comparison between the metabolites and COT was available.
Since this study only worked as a rough estimation of the metabolism of COT, there are still limitations for the results and conclusion. The percentage of metabolized COT was estimated based on the concentrations of three major metabolites of COT, which was not based on a direct measurement of metabolized or excreted COT. Other metabolism pathways and metabolites of COT were not considered in this study, which might have lead to an underestimated metabolic rate for COT in rats. However, the conclusion that the majority of COT was excreted unchanged from rats is not unreasonable, since the metabolites studied in this experiment still cover the majority of COT metabolism.[32, 34, 35] Urine collection and renal clearance measurements are still needed for more accurate results.
Conclusions
A PK study of orally and intravenously dosed COT was conducted, using a selective and sensitive LC–MS/MS quantitation method for the simultaneous determination of COT, NCOT, OHCOT and COTNO in rat plasma. Results from this study revealed important PK parameters of COT in rats, including high bioavailability, rapid absorption, fast tissue distribution and a long half-life of COT. All PK parameters obtained in this study were important for COT to effectively enter the tissue and demonstrate its sustained pro-cognitive effects. Such information is of great importance in future pharmacological, toxicological and other pre-clinical studies of COT in rodent species, for the development of COT-based therapeutic agents for the treatment of AD and other types of dementia.
Acknowledgements
The authors would like to acknowledge the National Institute on Aging (AG029617) and the National Institute on Drug Abuse (DA029127) for funding.
Reference
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Van Broeck B, Van Broeckhoven C, Kumar-Singh S. Current insights into molecular mechanisms of Alzheimer disease and their implications for therapeutic approaches. Neurodegener Dis. 2007;4(5):349–65. doi: 10.1159/000105156. [DOI] [PubMed] [Google Scholar]
- 3.Echeverria V, Cuello AC. Intracellular A-beta amyloid, a sign for worse things to come? Mol Neurobiol. 2002;26(2-3):299–316. doi: 10.1385/MN:26:2-3:299. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62(11):1984–9. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 6.Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54(4):465–73. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 7.Coyle J, Kershaw P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: effects on the course of Alzheimer's disease. Biol. Psychiatry. 2001;49(3):289–99. doi: 10.1016/s0006-3223(00)01101-x. [DOI] [PubMed] [Google Scholar]
- 8.van Marum RJ. Update on the use of memantine in Alzheimer's disease. Neuropsychiatric Disease and Treatment. 2009;5:237–247. doi: 10.2147/ndt.s4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Standridge JB. Pharmacotherapeutic approaches to the treatment of Alzheimer's disease. Clin. Ther. 2004;26(5):615–30. doi: 10.1016/s0149-2918(04)90064-1. [DOI] [PubMed] [Google Scholar]
- 10.Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 2007;17(3):259–73. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 11.Leibovici D, Ritchie K, Ledesert B, Touchon J. The effects of wine and tobacco consumption on cognitive performance in the elderly: a longitudinal study of relative risk. Int. J. Epidemiol. 1999;28(1):77–81. doi: 10.1093/ije/28.1.77. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol. Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Seidl R, Tiefenthaler M, Hauser E, Lubec G. Effects of transdermal nicotine on cognitive performance in Down's syndrome. Lancet. 2000;356(9239):1409–10. doi: 10.1016/S0140-6736(00)02848-8. [DOI] [PubMed] [Google Scholar]
- 14.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr. Opin. Pharmacol. 2004;4(1):36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Buccafusco JJ, Terry AV. The potential role of cotinine in the cognitive and neuroprotective actions of nicotine. Life Sciences. 2003;72(26):2931–2942. doi: 10.1016/s0024-3205(03)00226-1. [DOI] [PubMed] [Google Scholar]
- 16.Terry AV, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. Cotinine, a neuroactive metabolite of nicotine: Potential for treating disorders of impaired cognition. CNS Drug Reviews. 2005;11(3):229–252. doi: 10.1111/j.1527-3458.2005.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terry AV, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, Hutchings EJ, Chapman JM, Li P, Bartlett MG. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem. Pharmacol. 2012;83(7):941–951. doi: 10.1016/j.bcp.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buccafusco JJ, Beach JW, Terry AV. Desensitization of Nicotinic Acetylcholine Receptors as a Strategy for Drug Development. J. Pharmacol. Exp. Ther. 2009;328(2):364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, Mamcarz M, Wang L, Sattelle DB, Kirshner DA, Mori T, Leblanc RM, Prabhakar R, Arendash GW. Cotinine reduces amyloid-beta aggregation and improves memory in Alzheimer's disease mice. J. Alzheimers Dis. 2011;24(4):817–35. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- 20.Gao J, Adam B-L, Terry AV. Evaluation of nicotine and cotinine analogs as potential neuroprotective agents for Alzheimer's disease. Bioorg. Med. Chem. Lett. 2014;24(6):1472–1478. doi: 10.1016/j.bmcl.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Leary K, Parameswaran N, McIntosh JM, Quik M. Cotinine selectively activates a subpopulation of alpha3/alpha6beta2 nicotinic receptors in monkey striatum. J. Pharmacol. Exp. Ther. 2008;325(2):646–654. doi: 10.1124/jpet.108.136838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson CK. Clinical pharmacokinetics of nicotine. Clin. Pharmacokinet. 1987;12(1):30–40. doi: 10.2165/00003088-198712010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL, Jacob P. Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin. Pharmacol. Ther. 1993;53(3):316–323. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- 24.Zins BJ, Sandborn WJ, Mays DC, Lawson GM, McKinney JA, Tremaine WJ, Mahoney DW, Zinsmeister AR, Hurt RD, Offord KP, Lipsky JJ. Pharmacokinetics of nicotine tartrate after single-dose liquid enema, oral, and intravenous administration. J. Clin. Pharmacol. 1997;37(5):426–436. doi: 10.1002/j.1552-4604.1997.tb04320.x. [DOI] [PubMed] [Google Scholar]
- 25.Molander L, Hansson A, Lunell E. Pharmacokinetics of nicotine in healthy elderly people. Clin. Pharmacol. Ther. 2001;69(1):57–65. doi: 10.1067/mcp.2001.113181. [DOI] [PubMed] [Google Scholar]
- 26.Scherer G, Jarczyk L, Heller WD, Biber A, Neurath GB, Adlkofer F. Pharmacokinetics of nicotine, cotinine, and 3′-hydroxycotinine in cigarette smokers. Klin. Wochenschr. 1988;66(S11):5–11. [PubMed] [Google Scholar]
- 27.De Schepper PJ, Van Hecken A, Daenens P, Van Rossum JM. Kinetics of cotinine after oral and intravenous administration to man. Eur. J. Clin. Pharmacol. 1987;31(5):583–588. doi: 10.1007/BF00606635. [DOI] [PubMed] [Google Scholar]
- 28.Curvall M, Elwin CE, Kazemivala E, Warholm C, Enzell CR. The pharmacokinetics of cotinine in plasma and saliva from non-smoking healthy volunteers. Eur. J. Clin. Pharmacol. 1990;38(3):281–287. doi: 10.1007/BF00315031. [DOI] [PubMed] [Google Scholar]
- 29.Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Beck WD, Callahan PM, Terry AV, Bartlett MG. Quantitation of cotinine and its metabolites in rat plasma and brain tissue by hydrophilic interaction chromatography tandem mass spectrometry (HILIC-MS/MS). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012;907:117–125. doi: 10.1016/j.jchromb.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 1978;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- 32.Kyerematen GA, Taylor LH, Debethizy JD, Vesell ES. Pharmacokinetics of nicotine and 12 metabolites in the rat. Application of a new radiometric high performance liquid chromatography assay. Drug Metab. Dispos. 1988;16(1):125–129. [PubMed] [Google Scholar]
- 33.Sarver JG, White D, Erhardt P, Bachmann K. Estimating xenobiotic half-lives in humans from rat data: influence of log P. Environ Health Perspect. 1997;105(11):1204–1209. doi: 10.1289/ehp.971051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sastry BVR, Chance MB, Singh G, Horn JL, Janson VE. Distribution and retention of nicotine and its metabolite, cotinine, in the rat as a function of time. Pharmacology. 1995;50(2):128–136. doi: 10.1159/000139274. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama H, Okuda H, Nakashima T, Imaoka S, Funae Y. Nicotine metabolism by rat hepatic cytochrome P450s. Biochem. Pharmacol. 1993;45(12):2554–2556. doi: 10.1016/0006-2952(93)90238-r. [DOI] [PubMed] [Google Scholar]