Abstract
With no currently available drug treatment for spinal cord injury, there is a need for additional therapeutic candidates. We took the approach of repositioning existing pharmacological agents to serve as acute treatments for spinal cord injury and previously found imatinib to have positive effects on locomotor and bladder function in experimental spinal cord injury when administered immediately after the injury. However, for imatinib to have translational value, it needs to have sustained beneficial effects with delayed initiation of treatment, as well. Here, we show that imatinib improves hind limb locomotion and bladder recovery when initiation of treatment was delayed until 4 h after injury and that bladder function was improved with a delay of up to 24 h. The treatment did not induce hypersensitivity. Instead, imatinib-treated animals were generally less hypersensitive to either thermal or mechanical stimuli, compared with controls. In an effort to provide potential biomarkers, we found serum levels of three cytokines/chemokines—monocyte chemoattractant protein-1, macrophage inflammatory protein (MIP)-3α, and keratinocyte chemoattractant/growth-regulated oncogene (interleukin 8)—to increase over time with imatinib treatment and to be significantly higher in injured imatinib-treated animals than in controls during the early treatment period. This correlated to macrophage activation and autofluorescence in lymphoid organs. At the site of injury in the spinal cord, macrophage activation was instead reduced by imatinib treatment. Our data strengthen the case for clinical trials of imatinib by showing that initiation of treatment can be delayed and by identifying serum cytokines that may serve as candidate markers of effective imatinib doses.
Key words: : bladder function, chemokines, cytokines, glivec, locomotor function
Introduction
Spinal cord injury affects 12,000 individuals per year in the U.S. and there is no drug treatment in use that may improve functional recovery.1,2 While several experimental drug treatments have had promising effects, few have made it to clinical trials.3 To reposition drugs that are already in clinical use for other indications would circumvent many of the problems associated with drugs for which there is no prior clinical use.4 To determine the reposition potential of the cancer drug imatinib as an acute treatment for spinal cord injury, we previously used a rat spinal cord contusion model and found that the drug improves outcome of both locomotor and bladder function.5
Imatinib was first developed as a treatment of chronic myelogenous leukemia, and its mechanism of action was inhibiting the constitutive activation of tyrosine kinase BCR-Abl caused by a chromosomal translocation. However, imatinib, like most of the receptor tyrosine kinase inhibitors, can inhibit several receptor tyrosine kinases, such as platelet-derived growth factor (PDGFR; α and β), c-Kit, and colony stimulating factor 1 receptor.6 Further, imatinib has been found to have therapeutic potential in several disorders and is today a treatment for such conditions as hypereosinophila and c-Kit–positive gastrointestinal stromal tumors.7,8 Imatinib also has been shown to exert direct effects on the immune system, including mast cells, T-cells, and macrophages, and has experimentally ameliorated such conditions as lung fibrosis, dermal fibrosis, and autoimmune arthritis.9–13
Our work on imatinib as treatment for spinal cord injury was inspired by the finding that imatinib improved blood–brain barrier integrity in an ischemic stroke model through inhibition of PDGF-CC signaling.14 We similarly found a normalizing effect on the vasculature in and around the injury site of the spinal cord and a robust reduction of the inflammatory response. These effects were correlated to improvement of a number of histological parameters, such as lesser astrogliosis, lesser deposition of chondroitin sulphate proteoglycans, confinement of pericytes to the vascular walls, and, importantly, rescue of a larger amount of neurofilament-immunoreactive axons across the site of injury. Moreover, imatinib has been found to have positive effects on bladder function in other studies on spinal cord injury and to be able to reduce inflammation and demyelination of the cord in a rat model of multiple sclerosis.15–18
In contrast to experimental setups, clinical trials of acutely administered drugs for spinal cord injury are associated with many additional difficulties and considerations.19–22 Importantly, the initial dose cannot be administered immediately after injury and the delay until start of treatment will vary between patients. Thus, it needs to be determined for how long treatment can be delayed. It is also important to ensure that treatment does not induce any unwanted side effects, such as pain or allodynia, as that would severely limit the usefulness of any treatment.23 Imatinib has not previously been implicated as promoting allodynia-like symptoms, but since some growth factors do, it may still be of importance to rule out any negative effects on sensory function by potentially compensatory mechanisms.24,25
For drug treatments in acute spinal cord injury, time until treatment may constitute a helpful, but sometimes insufficient, stratification variable for analysis of outcome.5,26,27 Known factors that may reduce imatinib efficacy include drugs that may interact with imatinib, such as cytochrome inducers, human organic cation transporter 1 inhibitors, or St. John's wort.4,28 Further, since imatinib is a substrate for adenosine triphosphate–binding cassette transporters, heterogeneity in patient populations may alter drug availability.29,30 An imatinib-specific biomarker for the effect of drug, preferably present in serum, may thus further support stratification14,19,31 Interestingly, imatinib has been shown to alter the inflammatory cytokine/chemokine response in serum during treatment of chronic myelogenous leukemia.32 Also, inflammatory cytokine responses have been investigated experimentally and clinically in spinal cord injury, and have been shown capable of injury stratification.3,19,20,31,33,34
Here, we investigated if imatinib could improve functional outcome if start of treatment was delayed 4, 8, or 24 h, and continued for 14 d. We also assessed the possible ability of imatinib to alter thermal and mechanical sensitivity after spinal cord contusion injury in rats. Thirdly, we determined if imatinib induced any changes in inflammatory cytokines/chemokines in serum that might serve as biomarkers in the event of clinical application of imatinib for spinal cord injury. Finally, we analyzed spleen, thymus, and bone marrow with respect to macrophage activation responses, due to their involvement in systemic inflammation and roles as immune cell reservoirs.
Methods
Animal work was approved by the Northern Stockholm Animal Ethical Committee and performed in accordance with the Helsinki declaration.
Surgery
Female Sprague–Dawley rats (Scanbur, Germany) weighing 200–275 g were used. Rats were subjected to spinal contusion injury (n=90) using a dedicated instrument (NYU Impactor; Keck Center for Neuroscience,) as previously described.5,35,36 Prior to surgery, animals received analgesics (buprenorphine 0.015 mg/kg, intraperitoneally; Temgesic;). Under anesthesia (isofluorane), the dorsal surface of the spinal cord was exposed by laminectomy of the spinal column at T10 and the caudal half of T9 (sham injury; n=10). By dropping a 10 g weight from a height of 12.5 or 25 mm onto the spinal cord a mild (n=30) or moderate (n=58) contusion injury, respectively, was induced. Mild injury was used in one part of the study, because such animals are able to support their own weight with the hind limbs, which allows sensory testing and automated locomotor assessment. Typically, a moderate injury results in none to a few animals being able to weight support and does not allow the above behavior tests. However, once the experiment with sensory tests had been performed, we returned to using a moderate injury, as used in our original study, because such animals have less intrinsic variability in locomotor recovery and a more robust cytokine response originating from the injured spinal cord.
After surgery, analgesic treatment (buprenorphine 0.015 mg/kg, intraperitoneally; Temgesic) was administered once daily for 3 d and prophylactic antibiotics (0.6 mg/kg trimethoprim; Borgal, Hoechst AG, ) for 7 d. In animals with spinal cord injury, bladders were emptied manually twice daily until the animal regained bladder function. Animals had access to food and water ad libitum and were housed three per cage. The temperature was maintained at 24–26°C and lights were on for 12 h.
Imatinib preparation
Imatinib tablets were grinded and mixed with 0.1 M phosphate-buffered saline (PBS). The suspension was brought to 37°C for 5 min and then spun at 13,000 rcf. The supernatant was transferred to a new tube and later delivered per os at a dose of 250 mg/kg using gavage. Imatinib treatment was begun 4, 8, or 24 h after injury and continued daily for 14 d. Each daily dose was divided into one third in the morning and two thirds in the afternoon. This dose corresponds to 800–1000 mg given once per day in patients37,38 (European medicines Agency Glivec report), and was originally chosen due to being most effective in a mouse stroke model and also later in a rat multiple sclerosis model.14,17
Serum sampling
Rats were put in a cage heated by a heating pad for 15 min prior to blood sampling, performed with the animal in a plastic constrainer. A total of 250 uL was obtained from the tail vein and kept at room temperature for 20–30 min to allow clotting. Subsequently, the tube was spun at 4800 rpm for 10 min at 4°C. The supernatant was collected and transferred to −80°C.
Bladder recovery
After partial spinal cord injury, rats transiently lose the ability to empty their bladders. We manually emptied the bladders by applying gentle upward pressure on the bladder region of the lower abdomen using the index and middle fingers. Urine volumes were recorded.
Open field locomotion
We used the Basso, Beattie, and Bresnahan (BBB) scoring system to assess hind limb function in an open field. Scores 0-9 span from no hind limb function to extensive movement capability with weight support. Scores of 10–21 define increasing coordination of gait and additional parameters (also included in the BBB subscore) to normal gait (score 21).39 We also used the BBB subscore, which is based on paw position, toe clearance, trunk stability, and tail position.40 Each animal was scored during 4 min/per session (commonly weekly sessions) by two experimenters blinded to treatment. Animals were monitored for 10 weeks in the 4 h delay experiment and for 11 weeks in the 8 and 24 h delay experiment.
Automated locomotion
We used an automated paw placement detection walkway (Noldus Catwalk system; ) Weeks 8, 9, and 10 to evaluate limb coordination, denoted “regularity index.” We also evaluated base of support (width in between paws), stride length (length in between placements of a paw in cm), and paw area. The rats were required to perform three complete runs, defined as continuous locomotion through the measurement area on the walkway. The scores were then averaged and normalized to pre-surgical measurements.
Mechanical sensitivity
We determined hyposensitivity or hypersensitivity to mechanical stimuli from Weeks 2 to 7 by applying von Frey filaments to the hind paws (n=10/group). Prior to surgery, rats were habituated to the testing environment, consisting of a Plexiglas enclosure on top of a wire mesh floor. At the time of testing, rats were allowed to habituate for 30 min and then tested with calibrated von Frey filaments, applying approximately logarithmical incremental forces (0.4, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0, and 15 g), according to the “up-down” method.41 A brisk paw withdrawal counts as a positive response and a 50% probability of withdrawal is calculated based on measures from both hind paws, averaged into one score per animal, and normalized to pre-surgery measurements.
Cold sensitivity
Hypersensitivity to cold stimuli was tested by directing a cold spray to the hind paws (n=10/group). Moving the affected hind limb or a localized transient skin twitch were regarded as normal responses to cold. Hypersensitivity was defined as moving away from the cold spray or moving away and vocalizing.
Tissue preparation
Animals were deeply anesthetized and transcardially perfused via the ascending aorta with 50–75 mL of calcium free Tyrode solution, containing 0.1 mL of heparin, followed by 250 mL 4% paraformaldehyde in 0.1 M PBS. Spinal cord, thymus, spleen, and bone marrow were removed and post-fixed for 1 h in the same fixative. The post-fixed tissues were rinsed and cryoprotected in 0.1 M PBS containing 10% sucrose for 4 d with one exchange of the solution. The spinal cord was divided into segments, embedded in freezing medium (NEG 50; Richard-Allan Scientific, ) and frozen on dry ice. Frozen segments were sectioned on a cryostat to produce 20-μm thick cross-sections for immunohistochemistry. Spleen, thymus, and bone marrow were prepared and sectioned in a similar manner.
Immunohistochemistry and autofluorescence
Primary antibodies against ED1, CD206, and CD8 were used to visualize macrophages, and antibodies against OX-6 to visualize major histocompatibility complex class 2 (MHC-II). Slides were incubated in blocking solution containing 0.1 M PBS and 1.5% serum (same species as the secondary antibody) prior to incubation with a primary antibody in 0.3% Triton-X 100 in 0.1 M PBS overnight at 4°C. The secondary antibody (DyLight™ 555, Jackson Laboratories, ) was diluted in 0.3% Triton-X 100 in 0.1 M PBS and sections were incubated for 1.5 h in room temperature. Mounting medium contained an anti-fading agent (ProLong Gold® [with or without DAPI], Invitrogen, ).
Autofluorescence was noted in control sections from the immunohistochemistry studies in the absence of primary, as well as secondary, antibodies, using epifluorescence microscopy (Nikon Eclipse1000; ; excitation, 450–490; emission, 515–565). Since such autofluorescence was mainly confined to macrophages in imatinib-treatmed animals, this parameter was also quantified.
In spleen and bone marrow, ED1 and autofluorescence intensity was measured from sections originating from spinal cord injured animals with (n=8) and without (n=8) imatinib treatment 7 d after surgery, as well as from uninjured animals at Day 7 with (n=4) and without (n=4) imatinib treatment. The region measured in spleen was the red pulp (areas containing monocytes) and in bone marrow constituted the whole transverse section.
Analysis of immunoreactivity intensity and autofluorescence from the injury site of the spinal cord was measured from sections originating from spinal cord injured animals with (n=8) and without (n=8) imatinib treatment at Day 7 and with (n=7) and without (n=7) treatment at Day 1. The regions measured ED1 (the whole transverse section), Clusters of differentiation (CDxxx; the area of the injury site that displayed immunoreactivity), and autofluorescence (equally sized area boxes). SMI-312 dense area and number of OX6 positive cells, were measured from the same animals used for immunoreactivity measurements. A minimum of three sections, originating from 3 mm of the center of the injury site, was used for all spinal cord injury analysis. Intensity and area measurements were performed using Fiji (www.fiji.sc), the experimenter was blinded to section identity during time of measurement, and background was subtracted from all final measurement values of intensities.
Biochemical biomarker analysis
Levels of cytokines and chemokines were analyzed in rat serum, collected at Days 1, 3, and 7 after surgery using immunoassay kits. The experimental groups analyzed were uninjured animals for baseline measurements (n=6), spinal cord injured animals with (n=9) and without (n=9) imatinib treatment, sham-injured animals (n=10), and uninjured animals with (n=4) and without (n=4) imatinib treatment. The cytokine/chemokine kits were tested and validated following manufacturer's instructions. Rat monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein (MIP)-3α levels were measured using custom made kits and concentrations of INF-□, interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-10, IL-13, keratinocyte chemoattractant (KC)/growth-regulated oncogenes (GRO) and tumor necrosis factor (TNF; Cat No N05044-1) using a multiplex (10-plex) immunoassay kit (MesoScale Discovery, Gaithersburg, MD).
Samples were randomized before assay procedures. Plates pre-coated with antibodies towards the analytes of interest were blocked with the provided diluent for 60 min and washed; thereafter, serum samples diluted 1:4 in assay diluents were added to the wells and the plates were incubated for 2 h. Following washing of the plates, secondary antibody mixtures (MSD Sulfo TAG; Mesoscale Discovery) were added, and the plates were incubated for an additional 1.5 hrs. All incubations were performed at room temperature. After a final washing step, read buffer (2X) was added and the plates analyzed (SECTOR Imager, SI6000, MesoScale Discovery). Lower limits of quantification were 52 pg/mL for IL-1β and IL-2, 42 pg/mL for IL-5 and IL-6, 1 pg/mL for IL-4, 11 pg/mL for KC/GRO, and 4.3–6.1 pg/mL for TNF, INF-□, IL-10, and IL-13. Lower limits for quantification were 39 pg/mL for MIP-3α and 624 pg/mL for MCP-1 using the custom made plate.
Statistical analysis
A two-tailed Student's t-test was used for statistical analysis of immunolabeled sections with respect to intensity and area of labeling, and numbers of cells. Two-way ANOVA with Bonferroni's multiple comparison test was used for statistical analysis of BBB scores,42 regularity index measurements, paw areas, base of support measurements, stride lengths, residual urine measurements, and mechanical hypersensitivity measurements. The Mann-Whitney U test was used to analyze the BBB subscore. Friedman test with Dunn's multiple comparison test was used to analyze cytokine measurements for individual groups over time. Kruskal-Wallis test with Dunn's multiple comparison test was used to analyze cytokine measurements for three groups at one time-point. Linear regression analysis was used to determine correlations between axonal rescue and cytokine measurements, as well as between autofluorecence measurements of cord and spleen to MIP-3α measurements after imatinib treatment.
Results
4 hour delay
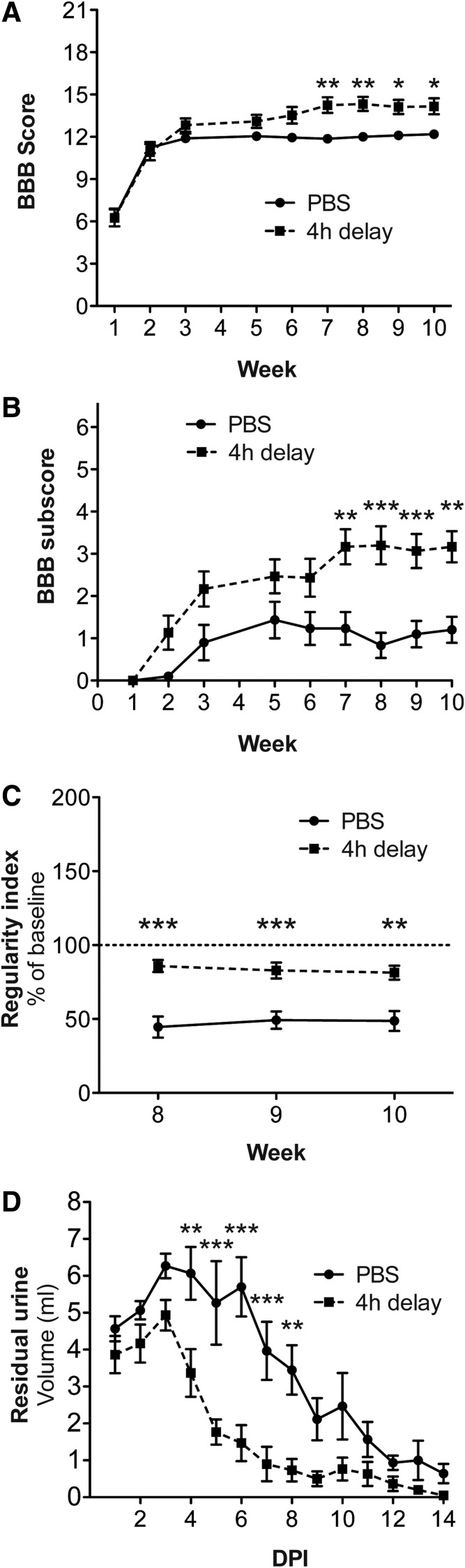
We aimed to determine mechanical and thermal sensitivity, in addition to locomotor function; thus, we induced a mild spinal cord contusion injury that allows weight support of the hind limbs. Four-limb gait also allowed us to perform automated locomotor assessment to confirm and complement observational scoring. We found that treatment with imatinib initiated 4 h after spinal cord injury led to significantly improved hind limb locomotor capability in the open-field test. At Week 10, the imatinib-treated group had a BBB score of 14.2±0.57, compared with 12.2±0.24 for the control group (p<0.05; Fig. 1A). The BBB subscore also was significantly improved by imatinib (3.2±0.37), compared with 1.2±0.31 for the control group (p<0.01; Fig. 1B). The final BBB scores suggest that the imatinib-treated group had improved coordination. This was confirmed by automated measurements of coordination. Thus, the regularity index for imatinib-treated animals revealed significantly better limb coordination during all three weeks of testing. At Week 10, the imatinib-treated animals reached 81.4±4.7% of normal coordination, compared with 48.7±6.7% for control animals (p<0.01; Fig. 1C). Automated assessment also demonstrated that imatinib-treated animals had more normal base of support, stride length, and paw area, compared with control animals (Supplementary Fig. 1; see online supplementary material at www.liebertpub.com).
FIG. 1.
Improved locomotor and bladder recovery with 4 h delay of treatment. Imatinib was administered with a 4 h delay after a mild contusion injury and subsequently administered twice daily for 14 d. Locomotion was assessed during 10 weeks using the Basso, Beattie, and Bresnahan (BBB) score (A) and the BBB subscore (B). (C) Regularity index Weeks 8, 9, and 10. (D) Residual urine during 14 d after injury. Data presented as mean±standard error of the mean. *p<0.05; **p<0.01; ***p<0.001.
We assessed bladder function recovery by measuring residual urine during 14 d after injury and found that imatinib-treated animals regained bladder function faster than control animals. The average volume of expelled urine was less for the imatinib-treated animals, compared with controls, for the whole monitoring period (p<0.001).
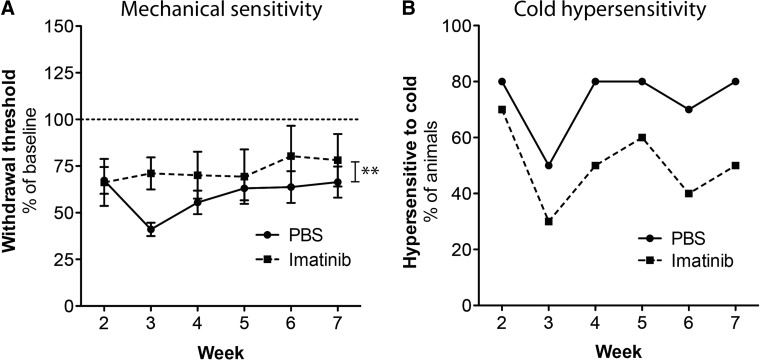
To determine if imatinib had any effect on sensory function after injury, we tested mechanical and thermal sensitivity of the hind paws weekly from Week 2 until Week 7. We found that imatinib did not induce mechanical hypersensitivity; instead imatinib-treated animals were generally less sensitive to mechanical stimulation (p<0.01), compared with control animals (Fig. 2A). At Week 7, hind limb responses were evoked in the imatinib group when applying a force with a strength that was 78.1±14.0% of the strength needed prior to surgery, compared with 66.4±8.4% for controls. We found similar results when assessing sensitivity to cold. Fewer animals in the imatinib group showed a response to the cold spray test, compared with the control group, at all time-points (Fig. 2B). During Week 7, 50% of the animals in the imatinib group displayed a hypersensitive response to cold, compared with 80% of the animals in the control group.
FIG. 2.
Imatinib does not have negative effects on sensory function. Imatinib was administered with a 4 h delay after a mild contusion injury and subsequently administered twice daily for 14 d. Responses to mechanical and thermal stimuli were assessed weekly (Weeks 2–7) (A) Mechanical sensitivity assessed by von Frey filaments and normalized to pre-surgical measurements (dotted line). Data presented as mean±standard error of the mean (SEM). (B) Hypersensitivity to cold assessed using a cold spray and presented as percentage of animals that displayed a hypersensitive response (moved away from cold stimulus). **p<0.01.
Body weight in animals with spinal cord injury reflects general condition and improves as animals recover from the injury. Importantly, the average body weight was higher in the mildly-injured spinal cord group given imatinib, compared with the controls, from Day 10 until the end of the experiment (Supplementary Fig. 2A; see online supplementary material at www.liebertpub.com) resulting in a significant treatment effect for the entire experimental period (p<0.05).
8 h and 24 h delay
When initiation of treatment with imatinib was delayed by 8 or 24 h, BBB scores of imatinib-treated groups of animals with moderate spinal cord injury did not differ from those of untreated injured animals during an 11-week assessment period. At Week 11, the BBB scores were 7.9±0.32, 8.2±1.56, and 8.2±0.30 for the 8 h delay of imatinib, the 24 h delay of imatinib, and the control group, respectively (Fig. 3A). Similarly, there was no sustained weight difference between the imatinib-treated groups and the control group (Supplementary Fig. 2B).
FIG. 3.
Delaying initiation of imatinib treatment 8 or 24 h improves bladder recovery but not locomotor function. Imatinib was administered with an 8 or 24 h delay after a moderate contusion injury and subsequently administered twice daily for 14 d. (A) Locomotor function assessed during 11 weeks using the Basso, Beattie, and Bresnahan score. (B) Bladder recovery assessed by measuring residual urine 21 d after injury. Data presented as mean±standard error of the mean. *p<0.05; **p<0.01; ***p<0.001.
Urinary bladder dysfunction remained responsive to imatinib treatment when treatment was started 8 or even 24 h after injury. Assessing bladder recovery for 21 d after injury, as reflected by residual urine, we found accelerated recovery with both time delays, compared with the control group (Fig. 3B).
Inflammatory cytokines and chemokines in serum
Cytokines and chemokines were monitored in serum from animals in which imatinib treatment was started 4 h after a moderate spinal cord contusion injury (n=9), compared with a control group that received no treatment after the moderate spinal cord contusion injury (n=9), and also to a group subjected to sham surgery (laminectomy; n=10). Baseline measurements were generated from serum of uninjured animals (n=6). Serum samples at 1, 3, and 7 d after injury were analyzed for MCP-1, MIP-3α, INF-□, IL-1β, IL-2, IL-5, IL-4, IL-6, IL-10, IL-13, KC/GRO, and TNF. Three cytokines/chemokines, MCP-1, MIP-3a, and KC/GRO, showed definite potential as biomarkers in serum.
As shown in Figure 4, serum levels of MCP-1 and MIP-3α were increased 1 day after injury or sham surgery. KC/GRO concentrations were instead higher in the sham group, compared with the contusion-injured groups with or without imatinib treatment, making this cytokine a potential serum biomarker for central nervous system (CNS) injury. At 1 day after surgery, there was, however, no robust effect of imatinib treatment of animals with spinal cord injury among the analyzed cytokines. Serum concentrations of MIP-3a and MCP-1 remained elevated in the injured and sham injured group throughout the 7 days in comparison to concentrations in uninjured controls. KC/GRO serum concentrations in the sham and injured group were instead reduced by Day 7 to levels comparable to those of uninjured controls. Strikingly, animals that had received imatinib treatment after surgery displayed a marked increase of serum levels for MCP-1, MIP-3α, and KC/GRO at 7 days. These elevated serum levels were significantly different from those of the injured group that did not receive imatinib, as well as from levels in the sham-operated group (Fig. 4). At Day 7 after injury, concentrations of MCP-1 in the imatinib-treated group reached a median of 25.8 ng/mL (range, 13.1–50.1 ng/mL), compared with a median of 13.0 ng/mL for the control group (range, 7.5–15.8 ng/mL; p<0.001; Fig. 4A). The median serum concentration of MIP-3α in the imatinib-treated group was 457 pg/mL (range, 157–952 pg/mL), while animals in the injury group without imatinib treatment had a median concentration of 159 pg/mL (range, 102–251 pg/mL). The median serum concentrations of KC/GRO in the imatinib-treated group with spinal cord injury was 929 pg/mL (range, 379–1590 pg/mL), while animals with spinal cord injury without imatinib treatment had a median concentration of 361 pg/mL (range, 228–763 pg/mL).
FIG. 4.
Imatinib treatment induces delayed increases of inflammatory cytokine levels in serum after injury. Imatinib was administered with a 4 h delay after a moderate contusion injury and subsequently administered daily for 7 d. Inflammatory cytokines were measured 1, 3, and 7 d after injury in serum of sham injured rats and spinal cord injured rats with and without treatment. (A–C) Serum concentrations of monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-3α, and keratinocyte chemoattractant (KC)/growth-regulated oncogenes (GRO) in sham operated, spinal cord injured, and spinal cord injured imatinib-treated animals (the dotted lines represent baseline measurements from uninjured animals). (D, E) Average serum concentrations of cytokines MIP-3α, MCP-1, and KC/GRO presented as percentage of baseline for each individual rat. Imatinib treatment caused a significant increase of the combined cytokine response in rats with spinal cord injury from Day 1 to Day 7. Data presented as the median±range. *p<0.05; **p<0.01; ***p<0.001.
Analysis of changes over time for individual animals in each experimental group revealed that the imatinib-treated group had significantly elevated concentrations of MCP-1, MIP-3α, and KC/GRO at Day 7, compared with at Day 1, potentially making the need for a control group redundant, when using these cytokines as biomarkers (Supplementary Fig. 3; see online supplementary material at www.liebertpub.com). Analysis of individual cytokine responses over time for each experimental group also identified a potential use for IL-6 as a biomarker. Injured rats that received no treatment displayed reduced serum concentrations of IL-6 when comparing Day 1 with Day 7 (p<0.01), while rats that received treatment were not significantly different at these time-points (Supplementary Fig. 3).
We found no significant correlation between concentrations of MIP-3α, MCP1, and KC/GRO in individual rats in the imatinib-treated group at Day 7. However, we found that combining these three biomarkers, averaging the percentage of baseline (uninjured rats) values of the three cytokines for each individual into a combined cytokine “profile” value, provided a rather robust indicator of imatinib effects. The cytokine-profile values in the imatinib-treated spinal cord injury group were highly significantly different between Day 1 and Day 7 (p<0.001), while this was not the case in the spinal cord injured group that had not received imatinib (Fig. 4D, 4E).
Imatinib induced macrophage activation in lymphoid organs
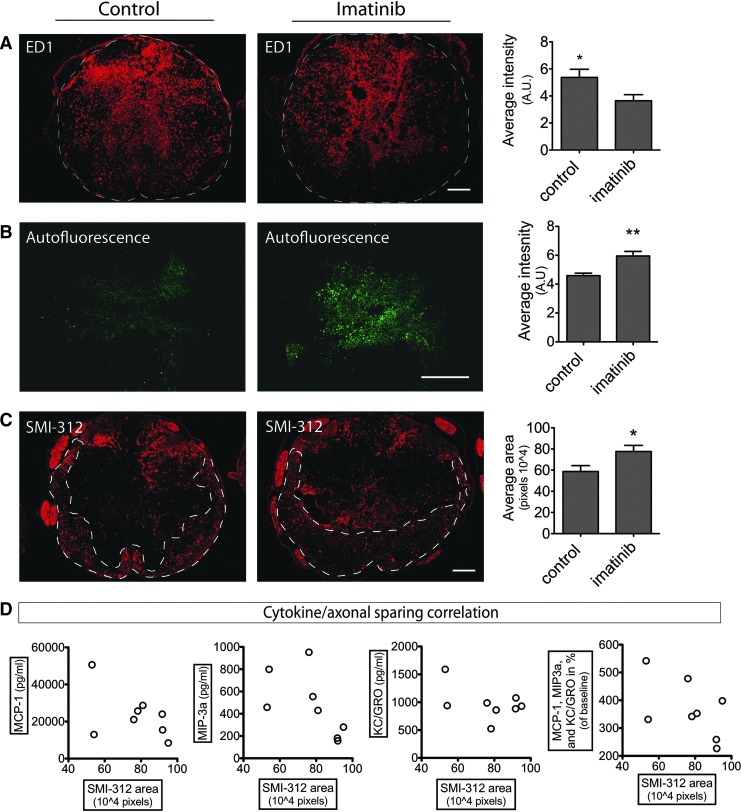
The elevation of inflammatory cytokines/chemokine concentrations in serum after 7 d of imatinib treatment suggested inflammatory activity in the lymphoid organs. MIP-3α and MCP-1 are both directly monocyte/macrophage associated43,44 and both spleen and bone marrow are the origin of macrophages in spinal cord injury, with the spleen being responsible for the major proportion of infiltrating monocytes at Day 7.45 We found splenic monocytes/macrophages to display increased ED1 immunoreactivity in rats that received imatinib, compared with those that did not (p<0.0007; Fig. 5A). The monocytes/macrophages individually displayed greater immunoreactivity and had foamy cell morphology, compared with monocytes/macrophages in controls, although without apparent difference in numbers. We found similarly increased ED1 immunoreactivity of the monocytes/macrophages in bone marrow and thymus (Supplementary Fig. 4; see online supplementary material at www.liebertpub.com). Phagocytosis may cause accumulation of e.g., autofluorescent lipofuscin-like material in macrophages.46,47 Here, we found, in addition to the increase of the scavenger receptor recognized by the ED1 antibody, that the autofluorescence was markedly increased in the monocytes/macrophages of the spleen (p=0.0010; Fig. 5B, 5C) and similarly in thymus and bone marrow (not shown).
FIG. 5.
Peripheral inflammatory response to imatinib treatment. Imatinib was administered twice daily for 7 d with a 4 h delay of the initial treatment to animals with and without spinal cord contusion injury, and compared with controls that received no treatment. Spleen and serum was collected at Day 7. (A, B) Representative pictures and quantification of ED1 immunoreactivity and autofluorescence in spleen after moderate spinal cord contusion injury. Scale bar=300 μm. (C) Co-localization of ED1 immunoreactivity and autofluorescence in spleen of spinal cord contusion-injured rats that received imatinib treatment. Scale bar=150 μm. (D, E) Representative pictures and quantification of ED1 immunoreactivity and autofluorescence in spleen of uninjured rats. Scale bar=300 μm. (F) Co-localization of ED1 immunoreactivity and autofluorescence in spleen of uninjured rats that received imatinib treatment. Scale bar=150 μm. (G) Serum concentrations of monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-3α, and keratinocyte chemoattractant (KC)/growth-regulated oncogenes (GRO) in uninjured rats that received imatinib treatment. Data presented as the mean±standard error of the mean. *p<0.05; **p<0.01; ***p<0.001. Autofluorescence intensity has been digitally enhanced to improve visibility.
To determine if imatinib effects on monocytes/macrophages were dependent on injury and thus if our biomarkers MIP-3α, MCP-1, and KC/GRO could be seen as biomarkers of biological activity of imatinib, per se, we next administered imatinib to uninjured rats. We found that imatinib treatment caused an increase of both ED1 immunoreactivity (p=0.028) and autofluorescence (p=0.0009) in the monocytes /macrophages in uninjured rats, compared with controls (n=4/group), that was similar to the effects of imatinib when given to animals with contusion injury of the spinal cord (Fig. 5D, 5E, 5F). Serum levels of MIP-3α, MCP-1, and KC/GRO were affected by imatinib alone and induced a similar temporal pattern of increase as in injured imatinib-treated animals, although the levels were not as high as in imatinib-treated animals with spinal cord injury.
Spinal cord pathology and biomarkers
While imatinib treatment itself induced activation of peripheral monocytes/macrophages in lymphatic organs, the ED1 immunoreactivity of the moderately contused spinal cord was instead significantly reduced with treatment (p=0.039; Fig. 6A). Immunohistochemical analysis of spinal inflammation also revealed a reduction of CD206 immunoreactivity (p<0.01) and OX6 (MHC-II)-positive cell numbers (p<0.01) with treatment, while CD8 and CD45 immunoreactivity remained unchanged (Supplementary Fig. 5; see online supplementary material at www.liebertpub.com). Hence, there was a change in proportions of inflammatory markers and thus the inflammatory response of the spinal cord as a consequence of the imatinib treatment.
FIG. 6.
Delayed initiation of imatinib improves tissue status and increases autofluorescence in the injured spinal cord. Imatinib was administered twice daily for 7 d with a 4 h delay of the initial treatment after moderate spinal contusion injury. Spinal cord tissue was harvested at Day 7 after surgery. (A) ED1 immunoreactivity. Representative pictures and quantification of intensity of ED1 immunoreactivity. (B) Autofluorescence. Representative pictures and quantification of autofluorescence intensity. (C) Area of axon immunoreactivity. Representative pictures and quantification of SMI-312 (pan-axonal marker) immunoreactive areas. Scale bars=150 μm. Data presented as mean±standard error of the mean. *p<0.05 and **p<0.01. (D) Correlation of cytokine levels with axon sparing in imatinib-treated spinal cord injured animals. SMI-312 areas plotted versus monocyte chemoattractant protein (MCP-1), macrophage inflammatory protein (MIP)-3α, keratinocyte chemoattractant (KC)/growth-regulated oncogenes (GRO), as well as all three cytokines combined and presented as % of baseline. None of the cytokines or the combination of cytokines showed significant correlation with axon sparing. Autofluorescence intensity has been digitally enhanced to improve visibility.
As in the spleen, autofluorescence was increased in the macrophage dense center of the injury site of the spinal cords from imatinib-treated animals (p=0.0023; Fig. 6B). A dried droplet of the imatinib solution mixed with 50% serum displayed only minimal or no autofluorescence (not shown). Autofluorescence at the site of injury and MIP-3α were linearly correlated (p=0.027), suggesting potential connection (Supplementary Fig. 6; see online supplementary material at www.liebertpub.com).
Similarly to what had been found with a 30 min delayed imatinib treatment, we found increased axonal sparing (p=0.027) during treatment when administration started 4 h after injury (Fig. 6C). Correlation analysis of cytokine concentrations and axonal sparing at 7 d found no linear correlation and hence we cannot determine from this experiment if cytokine concentrations could be indicators for degree of improved recovery (Fig. 6D).
Discussion
In an effort to determine the translational potential of imatinib for spinal cord injury, we tested if imatinib could improve functional outcome after clinically meaningful delays between injury and onset of drug treatment (Table 1). We also asked if, in a spinal cord contusion injury model, there might be a risk of imatinib aggravating sensory disturbances associated with incomplete spinal cord injury. Additionally, we measured inflammatory cytokines and chemokines in serum in search for biomarkers for effective doses of imatinib. Lymphoid organs, primarily the spleen, were analyzed to provide information on systemic effects of imatinib with regard to inflammatory responses and lymphoid organs as the origin of monocyte/macrophage infiltration into the injury site of the spinal cord.45,48
Table 1.
Experimental Overview
| Experiment (treatment start, main purpose) | Surgery (n) | End point | Assessment | Figures |
|---|---|---|---|---|
| 4 h delay −14 d treatment pilot | Mild injury: SCI (5), SCI+ (4 h)imatinib (5) |
10 weeks | Open field locomotion, bladder recovery, weight |
Fig. 1A, B, D Suppl. Fig. 2A |
| 4 h delay – functional recovery | Mild injury: SCI (10), SCI+ (4 h)imatinib (10) |
10 weeks | Open field locomotion, Automated locomotion, bladder recovery, sensitivity, weight, immunohistochemistry |
Fig. 1 and 2 Suppl. Fig. 1, 2A, and 5 |
| 8 h and 24 h delay – functional recovery | Moderate injury: SCI (9), SCI+ (8 h)imatinib (9), SCI+ (24 h)imatinib (8) |
11 weeks | Open field locomotion, bladder recovery, weight |
Fig. 3 Suppl. Fig. 2B |
| 4 h delay - serum cytokines | Moderate injury: Uninjured (6), Sham (10), SCI (9), SCI+ (4 h) imatinib (9) |
7 d | Cytokine analysis, immunohistochemistry (spinal cord and lymphatic organs) |
Fig. 4, 5 (A–C), and 6 Suppl. Fig. 3, 4, 5, and 6 |
| 4 h delay - serum cytokines | Uninjured (4), Uninjured+ (4 h)imatinib (4) | 7 d | Cytokine analysis, immunohistochemistry (spinal cord and lymphatic organs) | Fig. 5 (D-G) |
| 4 h delay - early (24 h) axonal sparing | Moderate injury: SCI (7), SCI+ (4 h)imatinib (7) |
24 h | Immunohistochemistry (spinal cord) | Suppl. Fig. 7 |
The table displays all included experiments and their resulting figures.
SCI, spinal cord injury.
In a clinical setting, there will always be a delay before treatment can be started. We had previously found imatinib to improve hind limb locomotor function and bladder function when treatment was initiated within 30 min after injury.5 Here, we found that imatinib improved both hind limb locomotion and bladder function when administered orally with a 4 h delay, and that functional improvements of bladder recovery also were seen with an 8 h or even a 24 h delay. Imatinib did not induce any additional hypersensitivity but rather normalized injury-induced mechanical, as well as temperature hypersensitivity. It should be noted that we also extended the treatment to 14 d in the present studies, compared with 5 d in the original study, to improve chances of detecting effects with delayed onset of treatment protocols. Importantly, we did not observe any negative side effects from the extended treatment period. These observations strengthen the therapeutic potential of imatinib as an acute treatment for spinal cord injury.
Finding improved bladder function recovery even when initiation of treatment was delayed by 24 h after injury suggests that most, if not all, prospective patients could benefit from imatinib treatment. However, it remains to be determined if the imatinib treatment accelerated recovery of bladder function by direct effects on the bladder and its local innervation apparatus or through its improvements of spinal microenvironment around the injury site. Further, since improved locomotor function only was present after a 4 h delay, it seems imperative to administer the treatment as early as possible to gain the greatest benefit from treatment. At 24 h post-injury, when neuronal loss is considered complete, we do find axonal sparing with a 4 h delay of treatment initiation (Supplementary Fig. 7; see online supplementary material at www.liebertpub.com), suggesting that early, meaningful spinal cord tissue rescue has taken place.
These findings are in line with our previous in-depth study, showing neuronal tissue sparing early after acute imatinib treatment.5 Recent reports suggest that axons are in a recoverable state hours after spinal cord contusion injury, and that the number of such axons diminish over time.49 Another reason for part of imatinib's protective effects to be restricted to an early time window could be that most apoptosis or necrosis of neurons and glial cells is happening or has been completed by 9–10 h in rats.50–53 The extent to which imatinib rescues different cell populations from apoptosis and/or necrosis during the course of the acute secondary cell death in the present study remains to be investigated. Notably, reports on cell death and inflammatory responses after spinal cord injury suggest that these events are delayed in humans in comparison to rodents,31,53 potentially allowing extended treatment windows to counter cell loss in humans. Further, since oral administration of imatinib is not fully bioavailable until after 1–2 h,54 intravenous administration of imatinib could allow a longer administration delay. Nevertheless, since determining the effectiveness of an acute therapeutic intervention for spinal cord injury is distinctly difficult,26,27 we find time until initiation of treatment may constitute a useful stratification parameter in the case of imatinib treatment.
Drug efficacy biomarkers have been lacking in previous clinical trials with treatments for spinal cord injury. Here, we suggest serum levels of MCP-1, MIP-3α, and KC/GRO (IL-8) could be used in spinal cord injury to monitor imatinib bioavailability. With a 4 hour delay of the imatinib treatment, we found that these three cytokines were significantly increased from Day 1 to Day 7 after injury in the imatinib group. This suggests that a patient receiving imatinib could be her/his own control using any of these cytokines as an efficacy biomarker for imatinib. Imatinib also elevated serum levels MCP-1, MIP-3α, and KC/GRO similarly in uninjured animals, which supports the conclusion that these cytokines are indeed efficacy biomarkers for the drug.
MCP-1 and IL-8 have previously been found to be increased acutely after spinal cord injury in human cerebrospinal fluid while present in serum to a lower degree (24 h post-injury).31 Importantly, these two cytokines have been found to increase in cancer patients on continuous imatinib treatment32; hence, it seems likely that this would occur also in patients with spinal cord injury receiving imatinib. Previously, MIP-3a was not known to increase after spinal cord injury nor was it known to be affected by imatinib, yet it seems to be the most reliable biomarker of the three selected ones. Nevertheless, we find the combination of all three cytokines to show the most robust temporal response, something that would be particularly important for individuals in a heterogeneous group.
Our results also indicate that early (low) IL-8 levels may serve as a serum biomarker for CNS trauma, as opposed to trauma without CNS injury (high IL-8 levels). However, it remains possible that compromised efferent signaling, due to the spinal injury, could result in a reduced biological response to surgery not displayed by sham injury.55
Spinal cord injury carries with it intrinsic detrimental systemic immunological effects, and systemic immunomodulating treatments may therefore have effects that may be of clinical significance.56–58 Here, the increase of the macrophage-associated cytokines in serum in response to imatinib treatment correlated to an increase of macrophage activation in the lymphoid organs spleen, bone marrow, and thymus. Imatinib has previously been shown to modulate neutrophils, T-cells, mast cells, eosinophils, and macrophages. However, such studies typically report reduced activation of inflammatory cells,8,10,11,59,60 as seen at the injury site of the spinal cord in our model. Interestingly, autofluorescence of macrophages, found in both the lymphoid organs and the injured spinal cord, constitutes an additional histological hallmark of imatinib treatment. Nevertheless, with adverse effects typically being moderate and reversible,61,62 our treatment period being relatively short, and the general health status similar between our treated animals and controls, it appears that the use of imatinib should not necessitate any further precautions than what is appropriate for any systemic treatment for spinal cord injury.63–65
Interestingly, the beneficial effects of imatinib on spinal cord tissue sparing and functional recovery takes place despite, or possibly supported by, an inflammatory activation in lymphatic organs and blood. For example, the early effects on the blood–spinal cord barrier may be responsible for the full protective effect, or it may be aided by the peripheral immune-activation, for example, by restraining macrophage infiltration into the spinal cord by the systemic increase in concentrations of monocyte-attracting chemokines. Further, we found indications that the reduced inflammation of the injured spinal cord also has an altered immune-profile that may have had a role in the improved chronic state of the spinal cord injury.66–69 It is beyond the scope of this study to resolve what effects these delayed immunological changes that occurred as a result of the imatinib treatment had on the spared tissue and its pathological progression.
To conclude, our findings increase the translational value of imatinib repositioning for spinal cord injury by demonstrating that initiation of treatment can be delayed. We also provide stratification variables, further strengthening the case for clinical trials of imatinib in spinal cord injury.
Supplementary Material
Acknowledgments
Supported by grants from the Swedish Brain Foundation, the Swedish Research Council, Swedish Foundation for Strategic Research, Wings for Life, SSMF, the Swedish Agency for Innovation Systems (VINNOVA), The StratNeuro initiative and the Karolinska Institutet DPA program.
Author Disclosure Statement
No competing financial interests exist
References
- 1.(2013). Spinal cord injury facts and figures at a glance. J. Spinal Cord Med. 36, 715–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayer F.T., Kronvall E., and Nilsson O.G. (2006). Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 6, 335–343 [DOI] [PubMed] [Google Scholar]
- 3.Steeves J. and Blight A. (2012). Spinal cord injury clinical trials translational process, review of past and proposed acute trials with reference to recommended trial guidelines. Handb. Clin. Neurol. 109, 386–397 [DOI] [PubMed] [Google Scholar]
- 4.Ashburn T.T., and Thor K.B. (2004). Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3, 673–683 [DOI] [PubMed] [Google Scholar]
- 5.Abrams M.B., Nilsson I., Lewandowski S.A., Kjell J., Codeluppi S., Olson L., and Eriksson U. (2012). Imatinib enhances functional outcome after spinal cord injury. PLoS ONE 7, e38760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabian M.A., Biggs W.H., Treiber D.K., Atteridge C.E., Azimioara M.D., Benedetti M.G., Carter T.A., Ciceri P., Edeen P.T., Floyd M., Ford J.M., Galvin M., Gerlach J.L., Grotzfeld R.M., Herrgard S., Insko D.E., Insko M.A., Lai A.G., Lélias J.-M., Mehta S.A., Milanov Z.V., Velasco A.M., Wodicka L.M., Patel H.K., Zarrinkar P.P., and Lockhart D.J. (2005). A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 [DOI] [PubMed] [Google Scholar]
- 7.Siehl J. and Thiel E. (2007). C-kit, GIST, and imatinib. Recent Results Cancer Res. 176, 145–151 [DOI] [PubMed] [Google Scholar]
- 8.Cools J., DeAngelo D.J., Gotlib J., Stover E.H., Legare R.D., Cortes J., Kutok J., Clark J., Galinsky I., Griffin J.D., Cross N.C.P., Tefferi A., Malone J., Alam R., Schrier S.L., Schmid J., Rose M., Vandenberghe P., Verhoef G., Boogaerts M., Wlodarska I., Kantarjian H., Marynen P., Coutre S.E., Stone R., and Gilliland D.G. (2003). A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N. Engl. J. Med. 348, 1201–1214 [DOI] [PubMed] [Google Scholar]
- 9.Daniels C.E., Wilkes M.C., Edens M., Kottom T.J., Murphy S.J., Limper A.H., and Leof E.B. (2004). Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J. Clin. Invest. 114, 1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardanani A., Elliott M., Reeder T., Li C.Y., and Baxter E.J. (2003). Imatinib for systemic mast-cell disease. Lancet. 362, 535–536 [DOI] [PubMed] [Google Scholar]
- 11.Seggewiss R., Loré K., Greiner E., Magnusson M.K., Price D.A., Douek D.C., Dunbar C.E., and Wiestner A. (2005). Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 105, 2473–2479 [DOI] [PubMed] [Google Scholar]
- 12.Dewar A.L., Cambareri A.C., Zannettino A.C.W., Miller B.L., Doherty K.V., Hughes T.P., and Lyons A.B. (2005). Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood 105, 3127–3132 [DOI] [PubMed] [Google Scholar]
- 13.Balachandran V.P., Cavnar M.J., Zeng S., Bamboat Z.M., Ocuin L.M., Obaid H., Sorenson E.C., Popow R., Ariyan C., Rossi F., Besmer P., Guo T., Antonescu C.R., Taguchi T., Yuan J., Wolchok J.D., Allison J.P., and DeMatteo R.P. (2011). Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 17, 1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su E.J., Fredriksson L., Geyer M., Folestad E., Cale J., Andrae J., Gao Y., Pietras K., Mann K., Yepes M., Strickland D.K., Betsholtz C., Eriksson U., and Lawrence D.A. (2008). Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat. Med. 14, 731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp K.G., Yee K.M., and Steward O. (2014). A re-assessment of treatment with a tyrosine kinase inhibitor (imatinib) on tissue sparing and functional recovery after spinal cord injury. Exp. Neurol. 254, 1–11 [DOI] [PubMed] [Google Scholar]
- 16.Abrams M.B., Nilsson I., Kjell J., Lewandowski S., Codeluppi S., Eriksson U., and Olson L. (2014). Response to the report, “A re-assessment of treatment with a tyrosine kinase inhibitor (imatinib) on tissue sparing and functional recovery after spinal cord injury” by Sharp et al. Exp. Neurol. 257, 182–185 [DOI] [PubMed] [Google Scholar]
- 17.Adzemovic M.Z., Zeitelhofer M., Eriksson U., Olsson T., and Nilsson I. (2013). Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response. PLoS One 8, e56586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng J., Zhang Y., Wang L., Zhao J., Song B., and Li L. (2013). The effects of Glivec on the urinary bladder excitation of rats with suprasacral or sacral spinal cord transection. J. Surg. Res. 183, 598–605 [DOI] [PubMed] [Google Scholar]
- 19.Krishna V., Andrews H., Varma A., Mintzer J., Kindy M.S., and Guest J. (2014). Spinal cord injury: how can we improve the classification and quantification of its severity and prognosis? J. Neurotrauma 31, 215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon B.K., Okon E.B., Tsai E., Beattie M.S., Bresnahan J.C., Magnuson D.K., Reier P.J., McTigue D.M., Popovich P.G., Blight A.R., Oudega M., Guest J.D., Weaver L.C., Fehlings M.G., and Tetzlaff W. (2010). A grading system to evaluate objectively the strength of pre-clinical data of acute neuroprotective therapies for clinical translation in spinal cord injury. J. Neurotrauma 28, 1525–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvorak M.F., Noonan V.K., Fallah N., Fisher C.G., Rivers C.S., Ahn H., Tsai E.C., Linassi A.G., Christie S.D., Attabib N., Hurlbert R.J., Fourney D.R., Johnson M.G., Fehlings M.G., Drew B., Bailey C.S., Paquet J., Parent S., Townson A., Ho C., Craven B.C., Gagnon D., Tsui D., Fox R., Mac-Thiong J.-M., and Kwon B.K. (2014). Minimizing errors in acute traumatic spinal cord injury trials by acknowledging the heterogeneity of spinal cord anatomy and injury severity: an observational Canadian cohort analysis. J. Neurotrauma 31, 1540–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filli L. and Schwab M.E. (2012). The rocky road to translation in spinal cord repair. Ann. Neurol. 72, 491–501 [DOI] [PubMed] [Google Scholar]
- 23.Hofstetter C.P., Holmström N.A.V., Lilja J.A., Schweinhardt P., Hao J., Spenger C., Wiesenfeld-Hallin Z., Kurpad S.N., Frisén J., and Olson L. (2005). Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 8, 346–353 [DOI] [PubMed] [Google Scholar]
- 24.Li X., Li G., Xu H., Tang X., Gao Y., Xu C., Liu S., Xie J., Tu G., Peng H., Qiu S., and Liang S. (2012). Effects of anti-rVEGF on the expression of VEGF receptor-2 and P2X(2/3) receptors of the spinal dorsal horn in neuropathic pain rats. Brain Res. Bull. 87, 227–233 [DOI] [PubMed] [Google Scholar]
- 25.Gwak Y.S., Nam T.S., Paik K.S., Hulsebosch C.E., and Leem J.W. (2003). Attenuation of mechanical hyperalgesia following spinal cord injury by administration of antibodies to nerve growth factor in the rat. Neurosci. Lett. 336, 117–120 [DOI] [PubMed] [Google Scholar]
- 26.Lammertse D., Tuszynski M.H., Steeves J.D., and Curt A. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord 45, 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steeves J.D., Lammertse D., Curt A., Fawcett J.W., Tuszynski M.H., Ditunno J.F., Ellaway P.H., Fehlings M.G., Guest J.D., Kleitman N., Bartlett P.F., Blight A.R., Dietz V., Dobkin B.H., Grossman R., Short D., Nakamura M., Coleman W.P., Gaviria M., Privat A., International Campaign for Cures of Spinal Cord Injury Paralysis. (2007). Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 45, 206–221 [DOI] [PubMed] [Google Scholar]
- 28.Haouala A., Widmer N., Duchosal M.A., Montemurro M., Buclin T., and Decosterd L.A. (2011). Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood 117, e75–e87 [DOI] [PubMed] [Google Scholar]
- 29.Gurney H., Wong M., Balleine R.L., Rivory L.P., McLachlan A.J., Hoskins J.M., Wilcken N., Clarke C.L., Mann G.J., Collins M., Delforce S.-E., Lynch K., and Schran H. (2007). Imatinib disposition and ABCB1 (MDR1, P-glycoprotein) genotype. Clin. Pharmacol. Ther. 82, 33–40 [DOI] [PubMed] [Google Scholar]
- 30.Evans W.E. and McLeod H.L. (2003). Pharmacogenomics—drug disposition, drug targets, and side effects. N. Engl. J. Med. 348, 538–549 [DOI] [PubMed] [Google Scholar]
- 31.Kwon B.K., Stammers A.M.T., Belanger L.M., Bernardo A., Chan D., Bishop C.M., Slobogean G.P., Zhang H., Umedaly H., Giffin M., Street J., Boyd M.C., Paquette S.J., Fisher C.G., and Dvorak M.F. (2010). Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma 27, 669–682 [DOI] [PubMed] [Google Scholar]
- 32.Hayashi Y., Nakamae H., Katayama T., Nakane T., Koh H., Nakamae M., Hirose A., Hagihara K., Terada Y., Nakao Y., and Hino M. (2012). Different immunoprofiles in patients with chronic myeloid leukemia treated with imatinib, nilotinib or dasatinib. Leuk Lymphoma 53, 1084–1089 [DOI] [PubMed] [Google Scholar]
- 33.Lubieniecka J.M., Streijger F., Lee J.H.T., Stoynov N., Liu J., Mottus R., Pfeifer T., Kwon B.K., Coorssen J.R., Foster L.J., Grigliatti T.A., and Tetzlaff W. (2011). Biomarkers for severity of spinal cord injury in the cerebrospinal fluid of rats. PLoS One 6, e19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stammers A.T., Liu J., and Kwon B.K. (2012). Expression of inflammatory cytokines following acute spinal cord injury in a rodent model. J. Neurosci. Res. 90, 782–790 [DOI] [PubMed] [Google Scholar]
- 35.Gruner J.A. (1992). A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 9, 123–128 [DOI] [PubMed] [Google Scholar]
- 36.Kjell J., Josephson A., Abrams M.B., Sandor K., and Svensson C.I. (2013). Rat substrains differ in the magnitude of spontaneous locomotor recovery and in the development of mechanical hypersensitivity after experimental spinal cord injury. J. Neurotrauma. 30, 1805–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petain A., Kattygnarath D., Azard J., and Chatelut E. (2008). Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin. Cancer Res. 14, 7102–7109 [DOI] [PubMed] [Google Scholar]
- 38.Hoshino-Yoshino A., Kato M., Nakano K., Ishigai M., Kudo T., and Ito K. (2011). Bridging from preclinical to clinical studies for tyrosine kinase inhibitors based on pharmacokinetics/pharmacodynamics and toxicokinetics/toxicodynamics. Drug Metab. Pharmacokinet. 26, 612–620 [DOI] [PubMed] [Google Scholar]
- 39.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 40.Lankhorst A.J., Verzijl M.R., and Hamers F.P.T. (1999). Experimental spinal cord contusion injury: comparison of different outcome parameters. Neurosci. Res. Commun. 24, 135–148 [Google Scholar]
- 41.Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., and Yaksh T.L. (1994). Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 42.Scheff S.W., Saucier D.A., and Cain M.E. (2002). A statistical method for analyzing rating scale data: the BBB locomotor score. J. Neurotrauma 19, 1251–1260 [DOI] [PubMed] [Google Scholar]
- 43.Matsui T., Akahoshi T., and Namai R. (2001). Selective recruitment of CCR6‐expressing cells by increased production of MIP-3α in rheumatoid arthritis. Clin. Exper. Immunol. 125, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker L.C. (2005). Yin and yang of MCP-1. Circ. Res. 96, 812–814 [DOI] [PubMed] [Google Scholar]
- 45.Blomster L.V., Brennan F.H., Lao H.W., and Harle D.W. (2013). Mobilisation of the splenic monocyte reservoir and peripheral CX 3 CR1 deficiency adversely affects recovery from spinal cord injury. Exper. Neurol. 247:226–240 [DOI] [PubMed] [Google Scholar]
- 46.Brunk U.T. and Terman A. (2002). Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 33, 611–619 [DOI] [PubMed] [Google Scholar]
- 47.Greenhalgh A.D. and David S. (2014). Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J. Neurosci. 34, 6316–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donnelly D.J., Longbrake E.E., and Shawler T.M. (2011). Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J. Neurosci. 31, 9910–9922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams P.R., Marincu B.-N., Sorbara C.D., Mahler C.F., Schumacher A.M., Griesbeck O., Kerschensteiner M., and Misgeld T. (2014). A recoverable state of axon injury persists for hours after spinal cord contusion in vivo. Nat Commun 5, 5683. [DOI] [PubMed] [Google Scholar]
- 50.Lou J., Lenke L.G., Ludwig F.J., and O'Brien M.F. (1998). Apoptosis as a mechanism of neuronal cell death following acute experimental spinal cord injury. Spinal Cord 36, 683–690 [DOI] [PubMed] [Google Scholar]
- 51.Crowe M.J., Bresnahan J.C., Shuman S.L., Masters J.N., and Beattie M.S. (1997). Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 3, 73–76 [DOI] [PubMed] [Google Scholar]
- 52.Shuman S.L., Bresnahan J.C., and Beattie M.S. (1997). Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J. Neurosci. Res. 50, 798–808 [DOI] [PubMed] [Google Scholar]
- 53.Emery E., Aldana P., Bunge M.B., Puckett W., Srinivasan A., Keane R.W., Bethea J., and Levi A.D. (1998). Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 89, 911–920 [DOI] [PubMed] [Google Scholar]
- 54.Peng B. (2004). Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J. Clin. Oncol. 22, 935–942 [DOI] [PubMed] [Google Scholar]
- 55.Paulson T.A.W., Goosey-Tolfrey V.L., Lenton J.P., Leicht C.A., and Bishop N.C. (2013). Spinal cord injury level and the circulating cytokine response to strenuous exercise. Med. Sci. Sports. Exerc. 45, 1649–1655 [DOI] [PubMed] [Google Scholar]
- 56.Schwab J.M., Zhang Y., Kopp M.A., Brommer B., and Popovich P.G. (2014). The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 258, 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gris D., Hamilton E.F., and Weaver L.C. (2008). The systemic inflammatory response after spinal cord injury damages lungs and kidneys. Exp. Neurol. 211, 259–270 [DOI] [PubMed] [Google Scholar]
- 58.Sauerbeck A.D., Laws J.L., Bandaru V.V.R., Popovich P.G., Haughey N.J., and McTigue D.M. (2015). Spinal cord injury causes chronic liver pathology in rats. J. Neurotrauma 32, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adzemovic M.V., Zeitelhofer M., Eriksson U., Olsson T., and Nilsson I. (2013). Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response. PLoS One 8, e56586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi S., Kimura F., Ikeda T., Osawa Y., Torikai H., Kobayashi A., Sato K., and Motoyoshi K. (2009). BCR-ABL promotes neutrophil differentiation in the chronic phase of chronic myeloid leukemia by downregulating c-Jun expression. Leukemia 23, 1622–1627 [DOI] [PubMed] [Google Scholar]
- 61.Veneri D., Franchini M., and Bonora E. (2005). Imatinib and regression of type 2 diabetes. N. Engl. J. Med. 352, 1049–1050 [DOI] [PubMed] [Google Scholar]
- 62.Paniagua R.T., Sharpe O., Ho P.P., Chan S.M., Chang A., Higgins J.P., Tomooka B.H., Thomas F.M., Song J.J., Goodman S.B., Lee D.M., Genovese M.C., Utz P.J., Steinman L., and Robinson W.H. (2006). Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J. Clin. Invest. 116, 2633–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mughal T.I., and Schrieber A. (2010). Principal long-term adverse effects of imatinib in patients with chronic myeloid leukemia in chronic phase. Biologics 4, 315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Widakowich C., de Castro G., and De Azambuja E. (2007). Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 12, 1443–1455 [DOI] [PubMed] [Google Scholar]
- 65.Tsao A.S., Kantarjian H., Cortes J., O'Brien S., and Talpaz M. (2003). Imatinib mesylate causes hypopigmentation in the skin. Cancer. 98, 2483–2487 [DOI] [PubMed] [Google Scholar]
- 66.Kigerl K.A., Gensel J.C., Ankeny D.P., Alexander J.K., Donnelly D.J., and Popovich P.G. (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 29, 13435–13444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rapalino O., Lazarov-Spiegler O., Agranov E., Velan G.J., Yoles E., Fraidakis M., Solomon A., Gepstein R., Katz A., Belkin M., Hadani M., and Schwartz M. (1998). Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat. Med. 4, 814–821 [DOI] [PubMed] [Google Scholar]
- 68.David S. and Kroner A. (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12, 388–399 [DOI] [PubMed] [Google Scholar]
- 69.Silver J., Schwab M.E., and Popovich P.G. (2014). Central nervous system regenerative Failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 7, a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.