Abstract
National Institute for Occupational Safety and Health (NIOSH)-approved N95 filtering-facepiece respirators (FFR) are currently stockpiled by the U.S. Centers for Disease Control and Prevention (CDC) for emergency deployment to healthcare facilities in the event of a widespread emergency such as an influenza pandemic. This study assessed the fit of N95 FFRs purchased for the CDC Strategic National Stockpile. The study addresses the question of whether the fit achieved by specific respirator sizes relates to facial size categories as defined by two NIOSH fit test panels. Fit test data were analyzed from 229 test subjects who performed a nine-donning fit test on seven N95 FFR models using a quantitative fit test protocol. An initial respirator model selection process was used to determine if the subject could achieve an adequate fit on a particular model; subjects then tested the adequately fitting model for the nine-donning fit test. Only data for models which provided an adequate initial fit (through the model selection process) for a subject were analyzed for this study. For the nine-donning fit test, six of the seven respirator models accommodated the fit of subjects (as indicated by geometric mean fit factor > 100) for not only the intended NIOSH bivariate and PCA panel sizes corresponding to the respirator size, but also for other panel sizes which were tested for each model. The model which showed poor performance may not be accurately represented because only two subjects passed the initial selection criteria to use this model. Findings are supportive of the current selection of facial dimensions for the new NIOSH panels. The various FFR models selected for the CDC Strategic National Stockpile provide a range of sizing options to fit a variety of facial sizes.
Keywords: Respirator Fit, N95 Filtering Facepiece Respirators, Strategic National Stockpile, Respirator Fit Test Panel
INTRODUCTION
In light of the novel H1N1 influenza outbreak in 2009, there has been considerable interest in strategies for maintaining adequate supplies of National Institute for Occupational Safety and Health (NIOSH)-approved N95 filtering-facepiece respirators (FFR) during a widespread emergency. NIOSH-approved N95 FFRs are disposable, tight-fitting, air-purifying respirators that meet or exceed 95% particle filtration efficiency for a standard test aerosol with a mass median aerodynamic diameter particle of approximately 0.3 μm (NIOSH, 1995). This respirator class is used to reduce exposure to airborne respiratory hazards for both biological pathogens and non-oil containing dusts and mists (NIOSH, 1996). N95 FFRs are among the most commonly used types of respirators in U.S. healthcare (Radonovich et al., 2009).
Healthcare personnel are considered to be at risk for exposure to infectious respiratory diseases, including influenza, other viruses (for example, the highly prevalent and seasonal respiratory syncytial virus (RSV)), bacterial pathogens, and emerging diseases (OSHA, 2009; IOM, 2010; IOM, 2015). With 18 million people employed in healthcare settings in the U.S (CDC, 2015a), adequate supplies of N95 FFRs are needed for a response to a widespread disease outbreak. Stockpiling of respirators has been recognized as a strategy to maintain adequate supplies. (CDC, 2015b; OSHA, 2007; IOM, 2008; Radonovich et al.; 2009). During periods of high usage (e.g., a public health emergency such as a widespread influenza outbreak), supplies of FFRs can quickly become depleted. FFR shortages were reported at the hospital level during both the 2004 SARS outbreak and the 2009 H1N1 influenza pandemic (Srinivasan, 2004; Commins, 2013) The U.S. Centers for Disease Control and Prevention (CDC) currently maintains medical supplies and personal protective equipment, including N95 FFRs, in its Strategic National Stockpile (SNS) as a contingency for such events (CDC, 2015b) Supplies in the CDC SNS are stored in strategic locations throughout the U.S. to be ready for deployment to state and local public health departments during large scale emergencies should supplies be needed (Esbitt, 2003).
Facepiece fit (as compared to media filtration efficiency) has been described as the major contributor to particle leakage into FFRs (Qian, et al., 1998; Clayton and Vaughan, 2005; Grinshpun, et al.; 2009). For optimal protection, FFRs require a tight facial seal; thus, individual fit testing is required. The U.S. Occupational Safety and Health Administration (OSHA) Respiratory Protection standard 29 CFR 1910.134 requires every worker who is mandated to wear a tight-fitting respirator, including FFRs, to undergo an annual respirator fit test (OSHA, 1998). Laboratory studies have demonstrated that fit testing is necessary for users to achieve the expected level of protection from FFRs. (Coffey et al., 1999; Coffey, et al.; 2004; Lee et al.; 2004).
New respirator fit test panels have recently been developed by NIOSH to reflect the current anthropometric head/face sizes of the U.S. working population (Zhuang et al., 2007). In 2003, Zhuang and Bradtmiller surveyed the U.S. workforce population who had experience wearing respirators (Zhuang and Bradtmiller, 2005). The data collected from 3,997 subjects (2,543 male and 1,454 female) were analyzed and Zhuang et al. developed criteria for two new fit test panels for both half-mask and full-facepiece respirators (Zhuang et al., 2007). The two panels are named the bivariate panel, which is defined by face length and face width, and the principal component analysis (PCA) panel, which is defined by two principal components which are functions of 10 facial measurements.
For the bivariate panel, face length and face width were chosen after a thorough review of the literature on the correlation between respirator fit and facial dimensions. Four of the eight scientific studies in the review found that face width and/or face length were correlated with respirator fit (Zhuang et al., 2007). The NIOSH bivariate panel is composed of 10 cells representing overall face size (Figure 1). Cells 1–3 are considered small face size, cells 4–7 are considered medium face size, and cells 8–10 are considered large face size. A subject's panel cell is determined by his/her face length and face width coordinates on the panel. Face length is defined as the distance from the menton (tip of chin) to the sellion (deepest point in nasal root); face width is defined as the maximum horizontal breadth of the face between the zygomatic arches.
Figure 1.
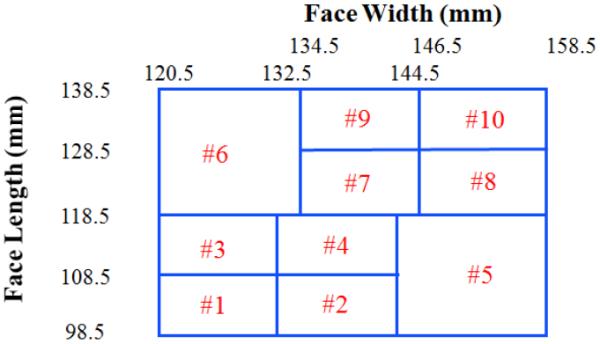
NIOSH Bivariate Panel.
The selection of the 10 dimensions for the PCA panel was also based on literature review, expert opinions, and correlation analyses between all dimensions (Zhuang, et al. 2007). Two principal components represent the axes on the PCA panel. The first principal component (PC1) on the x-axis represents the overall size of the face (small, medium, or large) and the second principal component (PC2) on the y-axis determines the shape of a face, (long/narrow or short/wide). The formulae describing these principal components have been published (Zhuang et al., 2007). The PCA panel is composed of eight cells representing overall face size and shape (Figure 2). Cell 1 is considered small face size, cells 2, 4, 5, and 7 are considered medium face size, cell 8 is considered large face size, cell 3 is considered short/wide, and cell 6 is considered long/narrow. A subject's cell number is determined by their PC1 and PC2 coordinates on the PCA panel. The anthropometric dimensions measured to compute PC1 and PC2 are listed in the Methods section of this paper.
Figure 2.
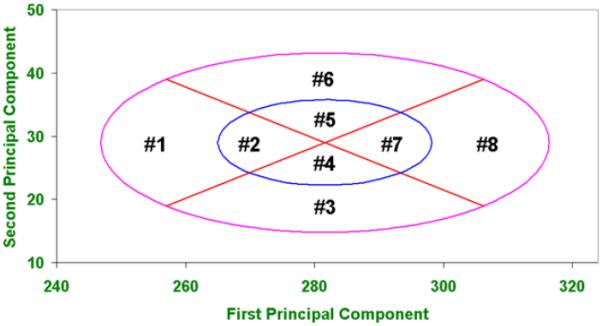
Principal Component Analysis (PCA) Panel.
The question of whether a correlation existed between the NIOSH panel cells and respirator size was addressed in 2008 by Zhuang et al. (2008). Thirty test subjects performed fit testing using P100-rated respirator models (one filtering-facepiece model and three elastomeric half-mask models). For the bivariate panel, respirator size significantly influenced fit within a given panel cell; face size categories also matched the respirator sizing reasonably well. This means that small, medium, and large face size categories achieved the highest geometric mean fit factors in the small, medium, and large respirator sizes, respectively. Face sizes classified by the PCA panel also matched respirator sizing reasonable well, but the relationship was not as strong as compared with the results observed for the bivariate panel.
This study utilizes fit test and anthropometric data from 229 subjects to assess the relationship between specific respirator models purchased for the CDC SNS to faces sizes categories of the NIOSH bivariate panel and head/face size categories of the PCA panel. The data were collected from a separate NIOSH study to investigate factors that impact temporal changes in N95 FFR fit (Zhuang et al., 2014). The study addresses the question of whether the fit achieved by specific respirator sizes relates to facial size categories as defined by the two NIOSH panels.
METHODS
Subjects
This study was approved by the NIOSH Institutional Review Board. Individuals who chose to participate signed a consent form. All volunteers received monetary reimbursement for their participation. Subjects were recruited from the NIOSH subject pool for certification testing and physiology study where they maintain a medically cleared status for testing by undergoing an annual physical. Additional subjects were recruited from the general public of the southwestern Pennsylvania region; these subjects were evaluated using only the OSHA Respirator Medical Evaluation Questionnaire (Appendix C to OSHA 29 CFR 1910.134) (OSHA, 1998). Both recruitment efforts were performed because of the need to obtain a large number of subjects (> 200) for a multi-year study assessing the change in respirator fit over time. Subjects made seven visits to the laboratory, each approximately six months apart; however, for this current study, only fit test and anthropometric data from the 229 subjects which participated in the first visit were analyzed. This study did not have predetermined requirements for the numbers of subjects to test individual cells of the NIOSH PCA and bivariate panels; thus, some cells of both panels do not contain any subjects, as will be presented in the Results section. Exclusionary criteria for the study included a history of uncontrolled chronic asthma, pneumonia, and high blood pressure (i.e., systolic > 160 mm Hg, diastolic > 95 mm Hg). On each visit, height, weight, and traditional facial/head anthropometric measurements (described below) were taken. Height was measured using a Seca 242 Digital Stadiometer (Seca, Hanover, MD). Weight was measured using a Seca 882 Digital Scale (Seca, Hanover, MD).
Facial/Head Anthropometric Measurements
Thirteen traditional anthropometric measurements of the linear distance between craniofacial landmarks were collected with spreading calipers, sliding calipers (GPM Instruments, Zurich, Switzerland) and Lufkin steel measuring tape (Cooper Tools, Apex, N.C.). Bony and soft tissue landmarks were indicated with black eyeliner. The dimensions measured were: head breadth, minimal frontal breadth, nasal root breadth, interpupillary breadth, face width, nose breadth, bigonial breadth, lip length, nose length, nose protrusion, face length, menton subnasale length, and head circumference.(Zhuang et al., 2007). Subject facial measurements were used to determine their panel cell placement according to the NIOSH bivariate and PCA panels. All measurements listed above with the exception of lip length, nose length, and head circumference were used to determine a subject's panel cell in the PCA panel. Face length and face width measurements were used to determine a subject's panel cell in the bivariate panel.
Respirators
When this study was initiated in 2007, seven NIOSH-approved N95 FFR models were included based on their purchase at the time for the CDC SNS (Table I). Respirators available in the CDC's SNS were used since they would be representative of models used by healthcare workers. These models also represent a variety of shapes and sizes which could fit a variety of facial sizes. All models were equipped with an adjustable metallic noseband, except for Models A and F which were equipped with a non-adjustable nose-cushion. For anonymous presentation of data in this paper, the individual respirator model names have been assigned a random alphabetical code (Model A – Model G). The sizing system that each model is part of is noted in Table I. For example, Model A is size “medium/large” and is part of a family of models which are available in two sizes: medium/large and small. All models available in 2-size systems had both sizes tested, except for Model E (size “standard”) for which only one size was tested.
Table I.
N95 FFR Model Characteristics
| FFR Model | Size | Shape | Sizing System |
|---|---|---|---|
| A | medium/large | Cup | 2 sizes (medium/large and small) |
| B | standard | tri-fold | 1 size only |
| C | standard | Cup | 1 size only |
| D | regular | Cup | 2 sizes (regular and small) |
| E | standard | Cup | 2 sizes (standard and small) |
| F | small | Cup | 2 sizes (medium/large and small) |
| G | small | Cup | 2 sizes (regular and small) |
Notes:
1. Models A (medium/large) and F (small) are part of the same sizing system.
2. Models D (regular) and G (small) are part of the same sizing system.
3. Model E is part of a 2-size system (standard and small), although only the standard size of this 2-size system was included in the study.
The sizing system information is important for understanding which bivariate and PCA panel cells the respirator model is intended to fit as recommended by Zhuang et al. (2007). For the bivariate panel, a one-size system would be tested in every cell. For a two-size systems, such as small/medium and medium/large, it is recommended that the small/medium is tested on subjects from Cells 1–6 and medium/large is tested with subjects from Cells 5–10. For three-size systems such as small, medium, and large, they are tested with subjects from Cells 1–4 for small, Cells 4–7 for medium, and Cells 7–10 for large. For the PCA panel, a one-size system is tested in every cell. For a two-size system such as small/medium and medium/large, the small/medium is tested on subjects from Cells 1–4 and medium/large is tested with subjects from Cells 5–8. For three-size systems such as small, medium, and large, the small size is tested with subjects from Cells 1–4; Cells 4–7 for medium; and Cells 7–10 for large. In this study, only one-size and two-size systems were evaluated.
Inward Leakage Measurement
The methods for inward leakage measurement have been previously described in the pilot study and found to be feasible for this study (Zhuang et al., 2011). Inward leakage (IL) is the combined leakage of particles entering the facepiece across the interface of the respirator's sealing area and the face (faceseal leakage (FSL)) and through the filter media (filter penetration). The OSHA Respiratory Protection Standard (Appendix A) accepts the use of the TSI PortaCount® (TSI, Inc., Shoreview, MN) for quantitative fit testing (OSHA, 1998). A PortaCount® Plus (Model: 8020A, TSI, Inc.) was used for the IL measurement. Quantitative fit tests were performed using the PortaCount® Plus alone (without the N-95 Companion accessory) to measure inward IL of particles with a detectable size range of 0.02 to > 1 μm (TSI Inc., 2006). By evaluating this wide size range of particles, the maximum achievable FF is > 10,000. The N-95 Companion was not used because doing so would have limited the maximum achievable fit factor to 200. Periodically, when the ambient aerosol concentration fell below the minimum concentration (1,000 particles / cm3) needed to operate the PortaCount®, the ambient aerosol concentration was supplemented with sodium chloride aerosol using a generator (Model: 8026, TSI, Inc.) which was placed centrally in the test room.
On the first visit of the study (for which this paper only analyzes the data), each subject randomly selected one of the seven respirator models and then watched a training video on how to don the respirator and perform a user seal check (USC). Although respirator manufacturers' instructions for performing USCs vary slightly among respirator models, for most FFRs, a wearer performs a USC by inhaling and/or exhaling sharply while cupping both hands over the entire respirator; during this procedure wearers determine if they can feel air leaking from around the face seal air. If leaks were felt during the USC, the wearer adjusted the respirator on the face and then another USC was performed. Then, while seated and wearing the respirator, subjects waited three minutes to purge the ambient particles inside the respirator (commonly referred to as the “comfort assessment period”). Although the OSHA Respiratory Protection Standard (Appendix A) requires this assessment period to be five minute for fit testing FFRs using the PortaCount®, we chose to decrease this period to three minutes in order to shorten the overall length of the test subject visit (OSHA, 1998).
Subjects then completed a five exercise fit test: normal breathing, deep breathing, breathing while moving their head from side to side, breathing while moving their head up and down, and a return to normal breathing. By only using five one-minute exercises, the overall test subject visit was shortened to save time. If the subject failed to achieve an adequate fit by obtaining a FF ≥ 100 (corresponding to IL ≤ 1 %) on one of three consecutive donnings using the same individual respirator sample, then another model was randomly selected from the remaining models. Data from inadequately fitting models (using this initial selection criterion) were discarded and not analyzed further; thus, this study only contains data for test subject/model combinations for which a good fit could be obtained during this initial respirator model selection process. The first model that provided an adequate fit for the subject was used; therefore, it may not have been the model to provide the best possible fit. The decision for this assignment process was based on time restraints of the study. Because a one donning fit test has been shown to have a beta error of 9 % (the error of passing a respirator that should fail) (Coffey et al., 2002; Coffey et al., 2006), we chose to utilize a multi-donning study design in an attempt to reduce beta error. Using the respirator model determined to provide an adequate fit, the subject completed a nine-donning fit test (composed of 3 fit tests performed on each of 3 individual respirator samples). The respirator was doffed and redonned between each fit test. Following each fit test, the sample was given to the test operator to readjust the adjustable nosepiece (if equipped) to its original position.
Filter Penetration Measurement and Faceseal Leakage Calculation
Filter penetration for each respirator sample worn for a fit test was measured separately from the IL leakage test performed by the subject. Following the subject's visit, each sample was sealed to an acrylic plate using melted beeswax. The plate had a 3.5 cm diameter hole drilled into its center. The plate was then inserted into a test fixture and air was drawn through the respirator by means of a vacuum line at a constant flowrate of approximately 10.3 L/min to simulate the breathing minute volume of a person while seated (Silverman et al., 1952). Filter penetration was measured by the TSI PortaCount® Plus using the same test duration that was employed for the subject fit test. Each respirator was tested three times.
Mean filter penetration for each respirator sample was subtracted from IL for each donning of the corresponding sample to calculate faceseal (FSL) for each donning. The reciprocal of the FSL values were taken to compute the FF values used for data analysis. For both the IL and filter penetration measurements, ambient aerosol was used as the test agent. Periodically, when the ambient aerosol concentration fell below the minimum concentration (1,000 particles / cm3) needed to operate the PortaCount®, the ambient aerosol concentration was supplemented with sodium chloride aerosol using a generator (Model: 8026, TSI, Inc.) which was placed centrally in the test room.
Data Analysis
Geometric mean (GM) FFs and geometric standard deviations (GSD) by FFR model were calculated by panel size for both the bivariate and PCA panels. For each subject/respirator model/ panel size combination, the nine FFs were log-transformed and then averaged because FFs are highly variable and are usually log-normally or near log-normally distributed (Nicas and Neuhaus, 2004). These mean log-transformed fit factors were then averaged for all subjects testing a particular panel size; this new average was used for the GM FF calculation of each FFR model/panel size combination. A general linear model procedure followed by a Duncan's Multiple Range test for post-hoc analysis was used to analyze differences in GM FFs by panel size for each FFR model (a significance level of 0.05 was selected to test the null hypothesis that means are not different between panel sizes within an FFR model). Passing rates for each respirator model/ panel size combination were calculated using GM FF ≥ 100 as the passing criterion for the GM of the nine fit tests performed by each test subject. The passing rate (presented as a percentage) is the percentage of subjects within each panel cell which achieving a GM FF ≥ 100. SAS version 9.3 (SAS Institute, Inc., Cary, NC) was used for all calculations and analyses.
RESULTS AND DISCUSSION
Geometric mean FFs by FFR model are illustrated in Figure 3. Respirator Model B achieved the highest GM FF of 192 (GSD = 2.4). The GM FFs for all models were > 100 with the exception of respirator Model C, which achieved a GM FF of 76 (GSD = 2.3), although it should be noted that only two subjects tested Model C; the number of subjects testing the other six respirator models ranged from 22 to 57.
Figure 3. Geometric Mean Fit Factors by FFR Model.

Note: One geometric standard deviation from the mean is shown for each bar.
This study did not have predetermined requirements for the numbers of subjects to test individual cells of the NIOSH PCA and bivariate panels; thus, some cells of both panels do not contain any subjects. Table II presents GM IL, GM filter penetration, and GM FSL results by respirator model. The lowest GM FSL was achieved with Model B (GM FSL = 0.53%; GSD = 2.4%). The highest GM FSL occurred with Model C (GM FSL = 1.32%; GSD = 2.3%). All respirator models achieved GM FSL < 1% (corresponding to FF > 100), with the exception of Model C; however, given that only two subjects tested this model, its performance could have been different if the sample size was larger.
Table II.
Geometric Mean Inward Leakage (IL), Filter Penetration, and Faceseal Leakage (FSL) by Model
| Model | Test Subjects (n) | GM IL (GSD) (%) | Mean Filter Pen (GSD) (%) | GM FSL (GSD) (%) |
|---|---|---|---|---|
| A | 50 | 0.57(2.1) | 0.02 (0.03) | 0.54 (2.1) |
| B | 57 | 0.53 (2.4) | <0.01 (<0.01) | 0.52 (2.4) |
| C | 2 | 1.37 (2.3) | 0.03 (0.02) | 1.32 (2.3) |
| D | 34 | 0.80 (1.9) | 0.02 (0.02) | 0.78 (1.9) |
| E | 22 | 0.80 (1.82) | 0.02 (0.02) | 0.78 (1.9) |
| F | 37 | 0.59 (2.3) | 0.03 (0.03) | 0.55 (2.3) |
| G | 27 | 0.62 (1.8) | 0.04 (0.04) | 0.58 (1.9) |
Geometric mean FFs by FFR model and bivariate panel size were calculated (Table III). Only one of the tested FFR model / panel size combinations (Model C, panel size small) resulted in a GM FF < 100 (shown in red font), although only two subjects were tested in this model/panel size combination. All other tested model/panel size combinations resulted in GM FF > 100 indicating an overall good fit performance among the various FFR models in their respective tested cells. Models B and E (both size standard) were the only models tested in all three panel sizes and achieved GM FF > 100 for all panel sizes, suggesting that these models can accommodate a good fit for wide variation of face sizes. Models F and G (both size small) each had similar GM FF results for panel sizes small and medium. In the case of Model G, only six subjects tested the medium size, thus results may have been different for this this panel size if more subjects were tested. Model E was the only model to show significant differences in GM FF between panel sizes (size small (n=3 subjects, GM FF 197, GSD 1.9) and size large (n=4 subjects, GM 141, GSD 1.3). Models B and F each had one subject whose facial dimensions placed them outside the limits of the panel.
Table III.
Geometric Mean Fit Factors by Respirator Model and Bivariate Panel Size
| Respirator Model | |||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |
| Respirator Size | med/large (n=50) | standard (n=57) | standard (n=2) | regular (n=34) | standard (n=22) | small (n=37) | small (n=27) |
| Bivariate Panel Size | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n |
| small (Cells 1, 2, 3) | NT | 157 (1.8) 14 | 76 (1.2) 2 | NT | 197 (1.9) 3 A | 175 (2.0) 15 | 177 (1.6) 21 |
| medium (Cells 4, 5, 6, 7) | 177 (1.6) 34 | 191 (1.7) 33 | NT | 125 (1.5) 26 | 122 (1.4) 15 | 176 (1.6) 21 | 166 (1.6) 6 |
| large (Cells 8, 9, 10) | 194 (2.0) 16 | 259 (1.6) 9 | NT | 141 (1.3) 8 | 141 (1.3) 4 B | NT | NT |
| **Out of Limits** | NT | 275 (2.5) 1* | NT | NT | NT | 78 (1.7) 1* | NT |
Notes:
1. n indicates the number of test subjects.
2. NT (not tested) indicates no tests were performed for this size.
3. “Out of Limits” refers to subjects whose anthropometric measurements placed them outside of the boundaries of the panel.
4. * for panel sizes with only 1 test subject, the GM and GSD are calculated using only the nine fit tests for that subject.
5. Bold letters indicate significantly different GM FFs (P < 0.05) within a model.
Geometric Mean FFs by FFR model and PCA panel size were calculated (Table IV). All model / panel size combinations for six of the seven models (Model C being the exception) resulted in a GM FF > 100, indicating good fitting performance for these six models. Models B (standard size, a one-size system) and D (regular size, part of a two-size system) were the only models tested in all facial sizes and were able to provide good fitting performance for all facial sizes. The two small size models (Models F and G) provided good performance for facial sizes small, medium, and short/wide. Model C (standard size) achieved GM FFs < 100 for both the short/wide and small facial sizes, although these results are based on only one test subject in each of these panel sizes and therefore may not be representative of a larger sample of subjects testing this model. Model E (standard size, part of a two-size system) showed a significance difference in GM FF between the small panel size and all other panel sizes; however, only one test subject tested in the small panel size, thus results may have been different given more subjects testing in this panel size. Model B showed a significant difference between GM FFs of the long/narrow and small sizes.
Table IV.
Geometric Mean Fit Factors by Model and PCA Panel Size
| Respirator Model | |||||||
|---|---|---|---|---|---|---|---|
| A | B | c | D | E | F | G | |
| Respirator size | med/large (n=50) | standard (n=57) | standard (n=2) | regular (n=34) | standard (n=22) | small (n=37) | small (n=27) |
| PCA Panel Size | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n | GM FF (GSD) n |
| **Out of Limits** | 145 (1.2) 3 | NT | NT | 196 (1.5) 2 | 160 (2.0) 1* B | NT | NT |
| long/narrow | 145 (1.9) 6 | 322 (1.5) 5 A | NT | 175 (1.2) 3 | NT | NT | NT |
| large | 162 (2.1) 8 | 169 (1.4) 3 | NT | 122 (1.0) 2 | 143 (1.2) 1* B | NT | NT |
| medium | 202 (1.7) 27 | 201 (1.6) 32 | NT | 115 (1.4) 20 | 118 (1.4) 15 B | 194 (1.8) 19 | 157 (1.6) 9 |
| short/wide | 191 (1.4) 6 | 170 (1.6) 6 | 69 (1.7) 1* | 133 (1.5) 5 | 127 (1.3) 4 B | 135 (2.0) 7 | 230 (1.7) 6 |
| small | NT | 148 (2.0) 11 B | 84 (3.0) 1* | 156 (1.0) 2 | 401 (1.6) 1* A | 163 (1.5) 11 | 164 (1.6) 12 |
Notes:
1. n indicates the number of test subjects.
2. NT (not tested) indicates no tests were performed for this size.
3. “Out of Limits” refers to subjects whose anthropometric measurements placed them outside of the boundaries of the panel.
4. * for panel sizes with only 1 test subject, the GM and GSD are calculated using only the nine fit tests for that subject.
5. Bold letters indicate significantly different GM FFs (P < 0.05) within a model.
This study found that six of the seven respirator models purchased for the CDC SNS accommodated the fit of subjects (as indicated by GM FF > 100) for not only the intended NIOSH bivariate and PCA panel sizes corresponding to the respirator size, but also for other panel sizes which were tested for each model. The various respirator sizes (small, standard, regular, and medium/large) were capable of fitting subjects well in all tested panel sizes with the exception of one model (Model C). Models A, D, and E (sizes med/large, regular, and standard, respectively) achieved GM FF > 100 for all tested sizes of both the bivariate and PCA panels; note that these models (A, D, and E) are each part of a two-size respirator system, and being the larger size of the two-size system, achieved good FF performance (as expected) in the larger panel sizes of both NIOSH panels (the large size of the bivariate panel and the large and medium sizes of the PCA panel. Models F and G (both size small), also achieved a GM FF > 100 in all panel sizes tested, including the small panel sizes of both NIOSH panels. Because Models B and C are each a one-size system, they would be expected to fit subjects well in all bivariate panel and PCA panel sizes. In fact, Model B achieved a GM FF > 100 in all bivariate and PCA panel sizes. Model C (only having two subjects) failed to achieve GM FF > 100 in any of the tested bivariate and PCA panel sizes, although these results may not accurately represent this model's performance due to only one subject being evaluated per tested bivariate size or PCA panel size. It must be re-emphasized that all data reported are from test subject/respirator model combinations for which the test subject was able to achieve an adequate fit during the initial model selection process; thus no data are reported here for which subjects could not achieve an initial adequate fit on a particular model. This means that the GM FFs reported here could have been lower if subjects went on to test models for the nine donning fit test which did not initially fit them well during the initial model selection process.
The results of this study support an earlier NIOSH study that found face size categories matched respirator sizing reasonably well for one P100 FFR and three P100 elastomeric half-mask respirators. (Zhuang et al., 2008). One disadvantage of this study was that the sample size for each respirator model/panel size combination was not uniform and, in one case, only two subjects tested Model C. Fit performance for Model C may not be accurately represented due to the small sample size; thus, further testing should be performed with this model to better assess its performance. Additionally, some of the respirator model/panel size test combinations had few subjects (n < 10); thus, the GM FFs and passing rates for these test combinations may have been different given a larger number of test subjects. A limitation of this study is that our test method of calculating FF by separately determining IL and filter penetration to calculate FSL (FF being calculated as the inverse of FSL) differs from the OSHA ambient aerosol condensation nuclei counter (CNC) quantitative fit test protocol (described in the OSHA Respiratory Protection Standard, 29 CFR 1910.134) in which fit factors are calculated directly from a person's fit test using the PortaCount®; thus, fit factors measured in this study using the methods described may not be representative of those measured with this OSHA ambient aerosol CNC quantitative protocol. Additionally, three of the fit test exercises used in the OSHA protocol were omitted from this study's fit test protocol: talking, grimace, and bending in place; as a result, the FFs obtained in this study may not represent FFs obtained using the full set of eight exercises. It is important to note that respirator users falling under OSHA's jurisdiction in the U.S. are required to pass an OSHA-accepted fit test and be included in a managed respiratory protection program meeting the requirements of 29 CFR 1910.134 in order to wear a particular respirator model.
CONCLUSIONS
Six of the seven respirator models accommodated the fit of subjects (as indicated by GM FF > 100) for not only the intended NIOSH bivariate and PCA panel sizes corresponding to the respirator size, but also for other panel sizes which were tested for each model. One model (Model B, a one-size system) was capable of fitting subjects well (GM FF > 100) in all panel sizes of both NIOSH panels. The other models were capable of fitting the subjects well in the tested panel sizes of both panels, with the exception of one model (Model C). Only two subjects met the initial selection criteria to use Model C, so results may not accurately represent this model's performance. Under the test methods presented in this study, the various sized FFRs tested were capable of fitting test subjects having a wide variety of facial sizes as defined by the NIOSH panels. Findings are supportive of the selection of the seven FFR models chosen for the CDC SNS given that these models provide a range of sizing options for a variety of facial sizes.
Footnotes
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of any product name does not imply endorsement by the National Institute for Occupational Safety and Health.
REFERENCES
- [accessed December 14, 2015];Centers for Disease Control (CDC) Workplace Safety and Health Topics: Healthcare Workers. 2015a http://www.cdc.gov/niosh/topics/healthcare.
- [accessed December 14, 2015];Centers for Disease Control and Prevention (CDC) Emergency Preparedness & Response: Strategic National Stockpile. 2015b http://www.bt.cdc.gov/stockpile/
- Clayton M, Vaughan N. Fit for Purpose? The Role of Fit Testing in Respiratory Protection. Ann. Occup. Hyg. 2005;49(7):545–548. doi: 10.1093/annhyg/mei046. [DOI] [PubMed] [Google Scholar]
- Coffey CC, Campbell DL, Zhuang Z. Simulated Workplace Performance of N95 Respirators. Am. Ind. Hyg. Assoc. J. 1999;60(5):618–624. doi: 10.1080/00028899908984481. [DOI] [PubMed] [Google Scholar]
- Coffey CC, Lawrence RB, Campbell DL, Zhuang Z, Calvert CA, Jensen PA. Fitting Characteristics of Eighteen N95 Filtering-Facepiece Respirators. J. Occup. Environ. Hyg. 2004;1(4):262–271. doi: 10.1080/15459620490433799. [DOI] [PubMed] [Google Scholar]
- Coffey CC, Lawrence RB, Zhuang Z, Campbell DL, Jensen PA, Myers WR. Comparison of Five Methods for Fit-Testing N95 Filtering-Facepiece Respirators. Appl. Occup. Environ. Hyg. 2002;17(10):723–730. doi: 10.1080/10473220290107002. [DOI] [PubMed] [Google Scholar]
- Coffey CC, Lawrence RB, Zhuang Z, Duling MG, Campbell DL. Errors Associated with Three Methods of Assessing Respirator Fit. J. Occup. Environ. Hyg. 2006;3(1):44–52. doi: 10.1080/15459620500455398. [DOI] [PubMed] [Google Scholar]
- Commins J. [accessed December 14, 2015];Nurses Protest H1N1 Respirator Mask Shortage. 2013 http://www.healthleadersmedia.com/content/NRS-241429/Nurses-Protest-H1N1-Respirator-Mask-Shortage.html.
- Esbitt D. The Strategic National Stockpile: Roles and Responsibilities of Health Care Professionals for Receiving the Stockpile Assets. Dis. Manag. Resp. 2003;1(3):68–70. doi: 10.1016/s1540-2487(03)00044-0. [DOI] [PubMed] [Google Scholar]
- Grinshpun SA, Haruta H, Eninger RM, Reponen T, McKay RT, Lee SA. Performance of An N95 Filtering Facepiece Particulate Respirator and a Surgical Mask During Human Breathing: Two Pathways for Particle Penetration. J. Occup. Environ. Hyg. 2009;6(10):593–603. doi: 10.1080/15459620903120086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preparing For an Influenza Pandemic. Personal Protective Equipment for Healthcare Workers. DC: The National Academies Press; Washington: 2008. Institute of Medicine (IOM) [Google Scholar]
- Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Workers: Update 2010. DC: The National Academies Press; Washington: 2010. Institute of Medicine (IOM) [PubMed] [Google Scholar]
- The Use and Effectiveness of Powered Air Purifying Respirators in Health Care: Workshop Summary. DC: The National Academies Press. 2015; Washington: 2015. Institute of Medicine (IOM) [PubMed] [Google Scholar]
- Lee K, Slavcev A, Nicas M. Respiratory Protection Against Mycobacterium Tuberculosis: Quantitative Fit Test Outcomes for Five Types of N95 Filtering-Facepiece Respirators. J. Occup. Environ. Hyg. 2004;1(1):22–28. doi: 10.1080/15459620490250026. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) Respiratory Protective Devices. US Government Printing Office; Office of the Federal Register; Washington, DC: 1995. Title 42, Code of Federal Regulations, Part 84. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) NIOSH Guide to the Selection and Use of Particulate Respirators Certified Under 42 CFR 84. 1996:96–101. DHHS (NIOSH) Publication No. [Google Scholar]
- Nicas M, Neuhaus J. Variability in Respiratory Protection and the Assigned Protection Factor. J. Occup. Environ. Hyg. 2004;1(2):99–109. doi: 10.1080/15459620490275821. [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration (OSHA) Respiratory Protection. US Government Printing Office; Office of the Federal Register; Washington, DC: 1998. Title 29, Code of Federal Regulations, Part 1910.134. [Google Scholar]
- Guidance on Preparing Workplaces for an Influenza Pandemic. May, 2009. Occupational Safety and Health Administration (OSHA) 2009. OSHA 3327-05R. [Google Scholar]
- Qian Y, Willeke K, Grinshpun SA, Donnelly J, Coffey CC. Performance of N95 Respirators: Filtration Efficiency for Airborne Microbial and Inert Particles. Am. Ind. Hyg. Assoc. J. 1998;59:128–132. doi: 10.1080/15428119891010389. [DOI] [PubMed] [Google Scholar]
- Radonovich LJ, Cheng J, Shenal BV, Hodgson M, Bender BS. Respirator Tolerance in Health Care Workers. JAMA. 2009;301(1):36–38. doi: 10.1001/jama.2008.894. [DOI] [PubMed] [Google Scholar]
- Radonovich LJ, Magalian PD, Hollingsworth MK, Baracco G. [Accessed December 14, 2015];Stockpiling Supplies For The Next Influenza Pandemic [online report] 2009 doi: 10.3201/eid1506.081196. Available from http://wwwnc.cdc.gov/eid/article/15/6/08-1196. Emerg. Infect. Dis. [serial on the Internet] [DOI] [PMC free article] [PubMed]
- Silverman LG, Plotkin LT, Sawyers LA, Yancey AR. Airflow Measurements on Human Subjects With and Without Respiratory Resistance. Arch. Ind. Hyg. Occup. Med. 1952;3:461–478. [PubMed] [Google Scholar]
- Srinivasan A, Jernign DB, Liedtke LL, Strausbaugh LL. Hospital Preparedness For Severe Acute Respiratory Syndrome in the United States: Views From a National Survey of Infectious Diseases Sonsultants. Clin. Infect. Dis. 2004;39(2):272–274. doi: 10.1086/421777. [DOI] [PubMed] [Google Scholar]
- TSI, Inc . PORTACOUNT® Plus Model 8020 Operation and Service Manual P/N 1980092, Revision. 2006. [Google Scholar]
- Zhuang Z, Benson S, Lynch S, Palmiero A, Roberge R. Laboratory Study to Assess Causative Factors Affecting Temporal Changes in Filtering Facepiece Respirator Fit: Part I – Pilot Study. J. Occup. Environ. Hyg. 2011;8(12):729–739. doi: 10.1080/15459624.2011.627294. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Bergman M, Palmiero A, Niezgoda G, He K, Roberge R. Temporal Changes In Filtering-Facepiece Respirator Fit. International Society for Respiratory Protection (ISRP) Conference; Prague, Czech Republic. 2014. [Google Scholar]
- Zhuang Z, Bradtmiller B, Shaffer RE. New Respirator Fit Test Panels Representing the Current U.S. Civilian Workforce. J. Occup. Environ. Hyg. 2007;4(9):647–659. doi: 10.1080/15459620701497538. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Bradtmiller B. Head-and-Face Anthropometric Survey of US Respirator Users. J. Occup. Environ. Hyg. 2005;2(11):567–576. doi: 10.1080/15459620500324727. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Groce D, Ahlers H, Iskander W, Landsittel D, Guffey S, Benson S, Viscusi D, Shaffer RE. Correlation Between Respirator Fit and Respirator Fit Test Panel Cells by Respirator Size. J. Occup. Environ. Hyg. 2008;5(10):617–628. doi: 10.1080/15459620802293810. [DOI] [PubMed] [Google Scholar]


