Abstract
Patients vary considerably in their response to treatment of pulmonary tuberculosis. Although several studies have indicated that adverse outcomes are more likely in those patients with delayed sputum sterilization, few tools are available to identify those patients prospectively. In this study, multivariate models were developed to predict the response to therapy in a prospectively recruited cohort of 42 HIV-uninfected subjects with drug-sensitive tuberculosis. The cohort included 2 subjects whose initial response was followed by drug-sensitive relapse. The total duration of culture positivity was best predicted by a model that included sputum M. tuberculosis antigen 85 concentration on Day 14 of therapy, days-to-positive in BACTEC on Day 30, and the baseline radiographic extent of disease (R = 0.63). A model in which quantitative AFB microscopy replaced BACTEC also performed adequately (R = 0.58). Both models predicted delayed clearance of bacilli in both relapses (> 85th percentile of all subjects) using information collected during the first month of therapy. Stratification of patients according to anticipated response to therapy may allow TB treatment to be individualized, potentially offering superior outcomes and greater efficiency in resource utilization, and aiding in the conduct of clinical trials.
There is substantial variability in the response to therapy for pulmonary tuberculosis, even in those patients with fully drug sensitive isolates. In some patients, bacilli are killed rapidly and cleared quickly from sputum. In others, viable organisms persist for many weeks or months, despite multidrug treatment. In yet others, bacilli are cleared, only to reappear after therapy is stopped. These observations form the basis for the definitions of treatment failure and relapse, respectively. The causes of this phenomenon are not well understood, but they may involve mycobacterial and host biologic factors as well as host behavioral factors.
The search for tools to monitor tuberculosis therapy and predict outcome is made particularly complex by the observation that mycobacterial killing is not a single uniform process. Most actively replicating bacilli are killed rapidly during the first 1 to 2 wk of therapy. This phase of treatment can be measured by quantitative sputum culture (early bactericidal activity or EBA) (1–5). There is, however, no known relationship between EBA and the outcome of treatment. Prolonged treatment is required to eradicate persisting organisms with reduced or otherwise altered metabolic activity. Nonreplicating bacilli show reduced susceptibility to the bactericidal activities of antimycobacterial drugs (6, 7). This later, sterilizing phase of therapy appears to be distinct from the first, based in part on the differential activities of antimycobacterial drugs during the two phases.
Two studies indicate that, in contrast to EBA, the time to sterilization is an important determinant of outcome. The first, by Aber and Nunn (8), determined that relapses occurred more frequently in those patients whose sputum cultures remained positive on agar by the third month of therapy. This forms the basis for the WHO recommendation that treatment be prolonged for those patients with delayed sputum sterilization, though this end point is infrequently reached during modern chemotherapy, even in patients who later relapse. In the second, Mitchison (9) reviewed the time to sputum sterilization of subjects enrolled in published comparative tuberculosis chemotherapy trials. He found that regimens with superior sterilizing activity at 2 mo had lower relapse rates, and he suggested that this parameter might be used as an early indicator of the relative efficacy of various regimens, if not to predict outcome in individual patients. These measures have not been widely used clinically, however, because they are primarily retrospective measures. The time to sputum sterilization is usually not known until treatment is essentially complete, particularly when agar cultures are used.
Two recent studies have indicated that earlier measures of the response to tuberculosis therapy might be useful to stratify patients according to risk of adverse outcome. In 1997, Epstein and colleagues (10) reported that failure (sustained culture positivity despite therapy) can be readily identified after 4 to 6 wk of therapy by measuring days-to-positive in mycobacterial growth indicator tube (MGIT) cultures. In 1998, a report from this laboratory indicated that relapse may be predicted by measuring Mycobacterium tuberculosis antigen 85 in sputum after 2 wk of therapy (11). In the present report, the interactions of these and other parameters were examined. The objective was to determine whether a multivariate model could predict the overall risk of failure and relapse in the treatment of drug-sensitive tuberculosis.
METHODS
Data were analyzed from a prospective study conducted in Uganda and Brazil, and reported previously (11). Briefly, patients with initial episodes of pulmonary tuberculosis who had not received prior therapy were prospectively recruited at tuberculosis control clinics in Kampala, Uganda, and Vitória, Brazil. All patients gave informed written consent for HIV testing and study participation. The study protocol was approved by the institutional review boards of Case Western Reserve University (Cleveland), Makerere University (Kampala), Universidade Federal do Espírito Santo (Vitória), Duke University (Durham) and the University of Arkansas (Little Rock). Tuberculosis was presumptively diagnosed by a positive acid-fast smear of sputum and a compatible chest radiograph and was subsequently confirmed by culture. Serology for HIV-1 was performed on all subjects; seropositives were excluded. Subjects were also subsequently excluded from this analysis if their initial isolates were resistant to isoniazid, rifampin, pyrazinamide or ethambutol, or the duration of follow-up was less than 180 d.
Baseline information was collected as to age, body mass, and radiographic extent of disease, using criteria established by the National Tuberculosis and Respiratory Disease Association (12). Subjects were classified as minimal disease (coded with a value of 1) if lesions were noncavitary lesions, of slight to moderate density, and involving a small part of one or both lungs. The total extent was required to be less than the volume of one lung above the second chondrosternal junction and the spine of the fourth or body of the fifth thoracic vertebrae. Subjects were classified as having moderately advanced disease (coded as 2), if they had more than minimal disease but had a total extent of slight or moderately dense lesions limited to the total volume of one lung, and that of dense lesions limited to one-third the volume of one lung. Cavitary lesions were required to be < 4 cm in diameter. Subjects were classified as having far advanced disease (coded as 3) if lesions were more extensive than moderately advanced.
Subjects were treated with daily isoniazid, rifampin, ethambutol, and pyrazinamide at standard doses for 2 mo, followed by daily isoniazid and rifampin for 4 mo. Patients were evaluated on Days 0, 2, 4, 7, 14, 30, monthly until therapy was completed, and then bimonthly. At each evaluation, history and physical examination were performed, and multiple sputum specimens were obtained, which were processed and analyzed as described below. Chest radiography was repeated at regular intervals. Patients were hospitalized for the initial 2 wk of treatment, after which therapy was self-administered. Compliance was assessed at each clinic visit by review of dispensing records, clinic attendance, and by urinary isoniazid metabolite testing (Myco-Dyn Uritec; DynaGen, Inc., Cambridge, MA).
Sputum Processing
Specimens were vortexed with several 4 mm glass beads for 1 min after addition of 1 ml of N-acetylcysteine (NALC) 25 mg/ml in phosphate buffer at pH 6.8. An aliquot was removed and frozen for antigen analysis. The remaining specimen was decontaminated by addition of 2.5 ml of a 1:1 mixture of 4% NaOH and 2.9% sodium citrate for 15 min. The specimen was then buffered, sedimented, resuspended at the original volume, examined by quantitative acid-fast microscopy, and cultured in BACTEC 12B medium. An aliquot was also diluted serially in 0.25% Tween-80 (Sigma Chemical, St. Louis, MO) in 0.9% NaCl, and cultured on Middlebrook Cohn 7H10 and 7H10S (antibiotic containing) agar medium for CFU determination. Cultures were monitored for growth for at least 60 d before being reported as negative. Decontamination of sputum with NaOH has been shown to reduce CFU values by 80% (13). However, this approach was adopted because use of NaOH resulted in reduced contamination rates as compared with antibiotic-containing medium, and because the reduction in CFU was consistent among specimens in a pilot study.
Antigen 85 ELISA
TBC-27 (14) (the monoclonal antibody used for antigen capture) was purified from hybridoma cells grown in serum-free medium. The antibody was biotinylated using sulfo-N-hydroxysuccinimide biotin (Pierce Chemicals, Rockford, IL), and was fixed to avidin-precoated plates (Pierce). Sputum was diluted with an equal volume of buffer and incubated overnight. After washing, bound antigen was detected using rabbit anti-BCG antiserum (DAKO, Carpinteria, CA), which in turn was detected with alkaline phosphatase-conjugated antirabbit Ig (Pierce). After washing, the alkaline phosphatase signal was amplified using a recycling NADH method described by us (11) and by Self (15).
Data Analysis
The DTP value for BACTEC cultures without growth was coded as 99; otherwise, number of days required for GI ≥ 10 was recorded. Specimens that were contaminated with organisms other than M. tuberculosis were excluded from analysis. Specimens for quantitative AFB smear or CFU enumeration without organisms were coded as −1; otherwise, the log10-transformed value was recorded. Groups were compared by Student’s two-tailed t-test or two-tailed chi-square analysis using RS1 (BBN, Boston MA). Correlations were identified by Pearson’s product method, using SigmaStat (SPSS, Chicago IL). Multiple regression analysis was performed by the best subsets method, also using SigmaStat. The Mallows Cp statistic was used as the selection criterion. This approach allows the screening of large numbers of potential variables, and produces a few subsets that include only the relevant variables. The variance inflation factor was used to identify collinear independent variables; subsets with variables whose VIF was 4 or greater were omitted. The threshold for significance was 0.05. The analysis of potential outlying observations was performed using S Plus (MathSoft, Cambridge, MA). The probability of rare events was determined using the Poisson method (16).
RESULTS
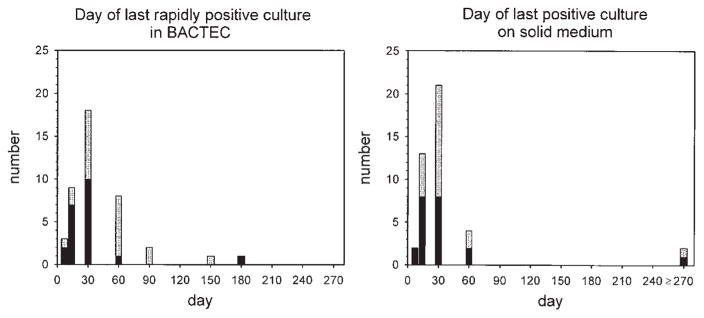
The clinical trial forming the basis of this analysis included 42 evaluable subjects. The frequency distributions of the day of collection of the last sputum specimen to indicate rapid growth of M. tuberculosis in BACTEC medium, or any growth on solid medium, are represented in Figure 1. Rapid growth in BACTEC was defined as that detected within 20 d of inoculation; this threshold was selected based on the report of Epstein and colleagues (10) that this parameter was an early indicator of treatment failure. The distributions of the agar and BACTEC parameters differed, in that once therapy had started, cultures were more likely to be rapidly positive in BACTEC than positive on solid medium (p = 0.049 using Fisher’s exact test). This indicates that agar culture is less sensitive than BACTEC for the detection of viable mycobacteria once therapy has been initiated.
Figure 1.
Frequency distributions of day of last rapidly positive culture in BACTEC (indicating mycobacterial growth within 20 d of inoculation (left panel) and that of the day of last positive culture on solid medium (right panel). Subjects from Uganda are indicated in gray; those from Brazil are indicated in black. The two rightmost subjects in both graphs represent relapses.
Two relapses were identified. Both initially responded, becoming culture negative on agar after Days 14 and 30, and in BACTEC on Days 30 and 90, respectively. Both subjects had recurrence of clinical disease and repeated isolation of drug-sensitive M. tuberculosis on agar after completion of therapy. Nonadherence during the last month of therapy was documented in only one case (by pill-dispensing and clinic-attendance records). In both cases, at least one culture in BACTEC was rapidly positive during the last month of therapy, again indicating that this method may be more sensitive for early detection of recrudescent disease. For the purpose of data analysis, the day of last positive culture (both BACTEC and agar) for these two cases was recorded as 180 (the day of completion of tuberculosis therapy).
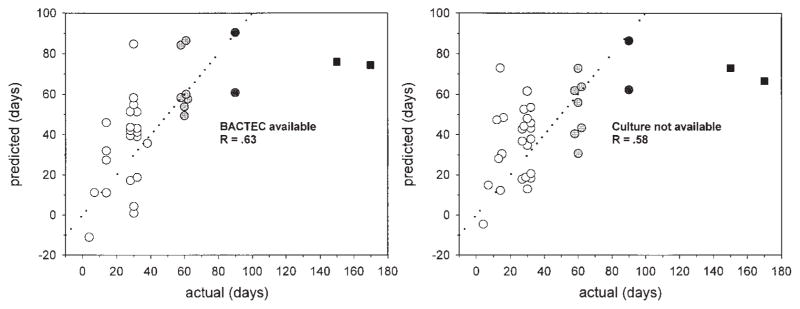
Multiple linear regression analysis was performed to identify early predictors of the duration of culture positivity, using the best subsets method. The analysis included the Day 7 to 30 values of all four microbiologic measures, as well as all baseline clinical parameters (including study site). As indicated in Table 1, antigen 85 on Day 14 and BACTEC DTP on Day 30 were strong independent predictors of the duration of culture positivity. The synergy between these two parameters appeared to contribute substantially to the accuracy of the model. Radiographic extent of disease entered the model but was of marginal statistical significance. The overall significance value of the regression model was p = 0.005, R = 0.63. The relationship between observed and predicted values is shown in the left panel of Figure 2. The predicted values of the relapsed cases were 74 and 76 d, representing the 85th and 88th percentiles, respectively, of the predicted values of all subjects.
TABLE 1.
LINEAR REGRESSION MODEL TO PREDICT THE DAY OF LAST RAPIDLY POSITVE SPUTUM CULTURE, BASED ON MEASURES OBTAINED DURING THE FIRST MONTH OF THERAPY, INCLUDING DAYS TO POSITIVE IN BACTEC*
| Variable | Coefficient | Standard Error | p Value |
|---|---|---|---|
| Constant | −3.985 | 25.691 | |
| Antigen 85, Day 14 | 0.459 | 0.146 | 0.004 |
| BACTEC DTP, Day 30 | −0.564 | 0.217 | 0.016 |
| Extent of disease | 17.879 | 8.501 | 0.046 |
The overall significance of the regression model is 0.005, R = 0.63.
Figure 2.
The relationship between the predicted and observed day of last rapidly positive BACTEC culture, using the multivariate models shown in Table 1. Two relapses are indicated as black squares. The dotted lines indicate equality. The right hand model does not require mycobacterial culture. Predicted values are not shown for those subjects with incomplete data.
Regression analysis can occasionally be unduly influenced by variables that are not normally distributed, or by a small number of outlying observations. Several tests were performed to examine these concerns. After log transformation, extent was no longer a significant predictor of outcome, but Day 14 antigen 85 and Day 30 DTP remained highly significant. To determine the influence of each single observation on the regression coefficients, the Cook’s distance value for each data point was calculated (17). This parameter measures the extent to which regression coefficients change when a single observation is deleted from the data set. Cook and Weisberg (18) suggest that points with values > 1 should be evaluated in more detail. The greatest Cook’s distance identified in this data set was 0.24. These findings suggest that the model is statistically sound.
Most cases of tuberculosis occur in regions of the world where laboratory facilities are limited. The question therefore arises whether low-cost alternative models can be developed that do not require ELISA plate readers or BACTEC. Although Day 14 antigen 85 values correlated highly with those on Day 7, they did not correlate with any of the other quantitative microbiologic parameters. As a consequence, it was not possible to develop satisfactory models that did not include antigen 85. In contrast, BACTEC DTP, quantitative AFB smear, and CFU were very highly collinear (p ranging from 10−4 to 10−11). Therefore, there were many alternatives to BACTEC. The model with greatest potential application in low-income regions is shown in Table 2. This model does not require mycobacterial culture but instead substitutes quantitative acid fast microscopy. The correlation coefficient (R) of the model was 0.58. The relationship between observed and predicted values is indicated in the right panel of Figure 2. Additionally, no models could be developed to predict the last day of positive culture on solid medium; this likely reflected the increased skewness and kurtosis of that data.
TABLE 2.
LINEAR REGRESSION MODELS TO PREDICT THE DAY OF LAST RAPIDLY POSITIVE SPUTUM CULTURE, BASED ON MEASURES OBTAINED DURING THE FIRST MONTH OF THERAPY EXCLUDING CULTURE*
| Variable | Coefficient | Standard Error | p Value |
|---|---|---|---|
| Constant | −41.909 | 27.048 | |
| Antigen 85, Day 14 | 0.400 | 0.140 | 0.008 |
| qAFB, Day 30 | 23.415 | 10.036 | 0.027 |
| (qAFB Day 30)2 | −2.907 | 1.551 | 0.071 |
| Extent of disease | 13.004 | 7.455 | 0.091 |
The results of quantitative AFB microscopy (qAFB) were best described using a second-order equation, with box x and x2 components. Extent of disease was forced into this model to allow comparison with that in Table 1. The overall significance of the regression model is 0.002, R = 0.58.
Two of the three parameters included in the models described above were collected prior to the initiation of treatment or during its in-hospital phase. These parameters were therefore unaffected by patient adherence. The third parameter was obtained on Day 30, 2 wk after discharge from hospital. To objectively assess patient adherence during the outpatient phase of therapy (which was not directly observed), urine was collected at each clinic visit, and was tested for a metabolite of isoniazid. All Day 30 specimens were collected; of these, all but one (98%) were positive. Of the 210 specimens to be collected during Months 2 to 6, 198 (94%) were actually collected, and of these, all but 16 (92%) were positive. This trend toward reduced adherence in Months 2 to 6 did not reach statistical significance (p = 0.16 by Poisson analysis). There was no correlation between the proportion of positive tests and the day of last rapidly positive culture (p = 0.9).
DISCUSSION
The eradication of tuberculosis has proven to be an elusive goal. Strategies for eradication based on treatment of active cases require highly effective regimens for success. However, high cure rates often are not achieved in the clinical setting. For example, the WHO recently reported cure rates of 78% in countries that used directly observed short course therapy (DOTS), and 45% in those that did not (19). True rates may be lower, as the WHO classification system does not require culture evidence of cure and does not reclassify “cures” that subsequently relapse. Despite its potential advantages, implementation of DOTS has been limited by its cost, which may be more than three times that of self-administered therapy (20, 21). Other strategies may not be widely implemented either, unless they demonstrate reduced costs as well as improved outcomes.
The predictive models developed in this study may help to address this need. This report indicates that parameters measured during the first month of treatment may be used to predict the microbiology of specimens obtained many months later. Larger trials will be required to determine the extent to which the predicted parameter—the day of last rapidly positive culture—correlates with true risk of treatment failure or relapse, as these clinical outcomes occurred in only two patients in this study. If this approach were validated, prospective trials would be indicated in which the intensity or duration of treatment was increased for those at high risk. Because the models can predict rapid as well as delayed bacillary clearance, trials in which the duration of therapy was reduced for selected subjects might also be indicated. Studies of such “ultra-short” tuberculosis therapy indicate that many patients can be cured with as little as 3 or 4 mo of treatment (22). Stratification according to risk may thus potentially offer increased efficiency in resource utilization as well as improved outcomes.
The most serious potential limitation to these models here is the extent to which they can be implemented where the need is greatest. The analysis indicates that the requirement for culture can be replaced by the use of quantitative acid-fast smear, but the need for ELISA remains. ELISA for antigen 85 was performed in this study using enzyme signal amplification. This method increases the sensitivity of the assay, but also increases its complexity. In retrospect, such sensitivity may not be required for analysis of these specimens, as the critical threshold for antigen 85 appears to be approximately 50 pg/ml. This level may be detected readily by conventional ELISA. It may also be detectable by methods based on agglutination or through the use of antibody-impregnated paper matrices. Utilization of such a test would depend on the extent to which it reduced the costs of therapy in those patients at low risk for relapse. Like most ELISAs, the cost of the antigen 85 assay is between $5 and $10 per specimen. One possible strategy for its use is in the selection of patients for directly observed therapy. Zwarenstein and colleagues (21) reported a cost differential in South Africa between self-administered and observed therapy of approximately £315 ($529). This would indicate that testing of all patients may be cost effective even if only a small proportion were shifted to self-administered therapy.
Predictive models such as these may also assist in the evaluation of new drugs in early clinical trials. Conventional trials, in which one new drug is added to an existing regimen may fail to demonstrate an additive effect unless large numbers of subjects are studied. An alternative strategy for phase II evaluation of promising but unproven compounds has been suggested in which brief treatment with an experimental regimen is followed by full treatment with a standard regimen. The value of such a trial is dependent on the accuracy of early surrogate markers as predictors of long-term outcome. Such trials may be greatly enhanced by the incorporation of the models described here.
In summary, a simple model was developed to predict the duration of culture positivity in pulmonary tuberculosis. Given the relationship between this parameter and clinical outcome, the model may be of value in the evaluation of new tuberculosis therapeutics, as well as in the care of individual patients.
Acknowledgments
Supported by Grants AI45244 and AI41911, from the National Institutes of Health, USA.
References
- 1.Jindani AJ, V, Aber R, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 2.Chan SL, Yew WW, Ma WK, Girling DJ, Aber VR, Felmingham D, Allen BW, Mitchison DA. The early bactericidal activity of rifabutin measured by sputum viable counts in Hong Kong patients with pulmonary tuberculosis. Tuber Lung Dis. 1992;73:33–38. doi: 10.1016/0962-8479(92)90077-W. [DOI] [PubMed] [Google Scholar]
- 3.Allen BW, Mitchison DA. Counts of viable tubercle bacilli in sputum related to smear and culture gradings. Med Lab Sci. 1992;49:94–98. [PubMed] [Google Scholar]
- 4.Sirgel FA, Botha FJ, Parkin DP, Van de Wal BW, Donald PR, Clark PK, Mitchison DA. The early bactericidal activity of rifabutin in patients with pulmonary tuberculosis measured by sputum viable counts: a new method of drug assessment. J Antimicrob Chemother. 1993;32:867–875. doi: 10.1093/jac/32.6.867. [DOI] [PubMed] [Google Scholar]
- 5.Donald PR, Sirgel FA, Botha FJ, Seifart HI, Parkin DP, Vandenplas ML, Van de Wal BW, Maritz JS, Mitchison DA. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:895–900. doi: 10.1164/ajrccm.156.3.9609132. [DOI] [PubMed] [Google Scholar]
- 6.Heifets L, Lindholm-Levy P. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am Rev Respir Dis. 1992;145:1223–1225. doi: 10.1164/ajrccm/145.5.1223. [DOI] [PubMed] [Google Scholar]
- 7.Yamori S, Ichiyama S, Shimokata K, Tsukamura M. Bacteriostatic and bactericidal activity of antituberculosis drugs against Mycobacterium tuberculosis, Mycobacterium avium-Mycobacterium intracellulare complex and Mycobacterium kansasii in different growth phases. Microbiol Immunol. 1992;36:361–368. doi: 10.1111/j.1348-0421.1992.tb02035.x. [DOI] [PubMed] [Google Scholar]
- 8.Aber VR, Nunn AJ. Short term chemotherapy of tuberculosis: factors affecting relapse following short term chemotherapy. Bull Int Union Tuberc. 1978;53:276–280. [PubMed] [Google Scholar]
- 9.Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months (letter) Am Rev Respir Dis. 1993;147:1062–1063. doi: 10.1164/ajrccm/147.4.1062. [DOI] [PubMed] [Google Scholar]
- 10.Epstein MD, Schluger NW, Davidow AL, Bonk SB, Rom WN, Hanna B. Time to detection of M. tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest. 1997;113:379–386. doi: 10.1378/chest.113.2.379. [DOI] [PubMed] [Google Scholar]
- 11.Wallis RS, Perkins M, Phillips M, Joloba M, Demchuk B, Namale A, Johnson JL, Williams D, Wolski K, Teixeira L, Dietze R, Mugerwa RD, Eisenach KD, Ellner JJ. Induction of the antigen 85 complex of M. tuberculosis in sputum: a determinant of outcome in pulmonary tuberculosis. J Infect Dis. 1998;178:1115–1121. doi: 10.1086/515701. [DOI] [PubMed] [Google Scholar]
- 12.Falk A, O’Connor JB, Pratt PC, Webb WR, Wier JA, Wolinsky E. Diagnostic Standards and Classification of Tuberculosis. 12. National Tuberculosis and Respiratory Disease Association; New York: 1969. Classification of pulmonary tuberculosis; p. 68. [Google Scholar]
- 13.Yajko DM, Wagner C, Tevere VJ, Kocagoz T, Hadley WK, Chambers HF. Quantitative culture of Mycobacterium tuberculosis from clinical sputum specimens and dilution endpoint of its detection by the Amplicor PCR assay. J Clin Microbiol. 1995;33:1944–1947. doi: 10.1128/jcm.33.7.1944-1947.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salata RA, Sanson AJ, Malhotra IJ, Wiker HG, Harboe M, Phillips NB, Daniel TM. Purification and characterization of the 30,000 dalton native antigen of Mycobacterium tuberculosis and characterization of six monoclonal antibodies reactive with a major epitope of this antigen. J Lab Clin Med. 1991;118:589–598. [PubMed] [Google Scholar]
- 15.Self CH. Enzyme amplification: a general method applied to provide an immunoassisted assay for placental alkaline phosphatase. J Immunol Methods. 1985;76:389–393. doi: 10.1016/0022-1759(85)90316-3. [DOI] [PubMed] [Google Scholar]
- 16.Ott L. An Introduction to Statistical Methods and Data Analysis. 3. PSW-Kent; Boston: 1988. Categorical data; p. 217. [Google Scholar]
- 17.Kleinbaum DG, Kupper LL, Muller KE. Applied Regression Analysis and Other Multivariable Methods. PWS-KENT Publishing Co; Boston: 1987. p. 200. [Google Scholar]
- 18.Cook RD, Weisberg S. Residuals and Influence in Regression. Chapman and Hall; New York: 1982. [Google Scholar]
- 19.Global Tuberculosis Programme. Annual Report. World Health Organization; Geneva: 1998. http://www.who.int/gtb/publications/globrep/index.htm. [Google Scholar]
- 20.Global Tuberculosis Programme. Progress against TB stalled in key countries (press release) World Health Organization; Geneva: 1998. http://www.who.int/gtb/press/WTBD98Release-progress.htm. [Google Scholar]
- 21.Zwarenstein M, Schoeman JH, Vundule C, Lombard CJ, Tatley M. Randomised controlled trial of self-supervised and directly observed treatment of tuberculosis. Lancet. 1998;352:1340–1343. doi: 10.1016/S0140-6736(98)04022-7. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramanian R, Sivasubramanian S, Vijayan VK, Ramachandran R, Jawahar MS, Paramasivan CN, Selvakumar N, Somasundaram PR. Five year results of a 3-month and two 5-month regimens for the treatment of sputum-positive pulmonary tuberculosis in south India. Tubercle. 1990;71:253–258. doi: 10.1016/0041-3879(90)90037-9. [DOI] [PubMed] [Google Scholar]