Abstract
Background
Health categories of elderly patients prior to critical illness may explain differences in mortality during and after admission to intensive care units (ICUs).
Objectives
To estimate the effect of pre-ICU health categories on mortality during and after critical illness, focusing specifically on the effect of pre-ICU frailty on short- and long-term mortality.
Design
Retrospective cohort study using linked Medicare claims data from 2004–2008.
Participants
A nationally representative sample of elderly Medicare beneficiaries who were admitted to an ICU in 2005.
Measurements
Patients were classified into four pre-ICU health categories (Robust; Cancer; Chronic Organ Failure; Frailty) using claims data from the year prior to admission, allowing for assignment to multiple categories. We assessed the association between pre-ICU health categories and hospital and 3-year mortality using multivariable logistic regression and Cox proportional Hazards models.
Results
Among 47,427 elderly ICU patients, 18.8% were Robust; 28.6% had pre-ICU Cancer; 68.1% Chronic Organ Failure and 34.0% Frailty; 41.3% qualified for multiple categories. Overall hospital mortality was 12.6%, with the lowest mortality for Robust patients (9.7%). Patients with pre-ICU Frailty had a higher hospital mortality compared to patients with the same pre-ICU health categories without frailty (adjusted Odds Ratios ranged from 1.27 (95% confidence interval (CI) 1.10–1.47) to 1.52 (95% CI 1.35–1.63)). Robust hospital survivors had the lowest 3-year mortality (24.6%). Pre-ICU Frailty conferred a higher 3-year mortality compared to pre-ICU categories without frailty (adjusted Hazard Ratios ranged from 1.54 (95% CI 1.45–1.64) to 1.84 (95% CI 1.70–1.99).
Conclusion
Critically ill elderly patients can be categorized by Pre-ICU health categories. These categories, particularly pre-ICU Frailty, may be important for understanding risk of death during and after critical illness.
Keywords: Frailty, aged, prognosis, critical illness, Medicare
INTRODUCTION
Elderly people make up a large proportion of the patients cared for in intensive care units (ICUs) worldwide (1). While we have substantial understanding of the impact of acute illness on both short and long-term outcomes for elderly critically ill patients, there is increasing awareness that information on a patient’s status prior to the critical illness may be essential to understanding prognosis (2–4). Four health categories that occur prior to death were previously defined in an elderly Medicare population: sudden death, chronic organ failure, cancer, and frailty (5), each with a distinctive pattern of functional decline in the year before death (5, 6). Such patterns may also be relevant prior to an event such as an episode of critical illness.
Frailty, in particular, may represent a key syndrome that could impact a patient’s resilience from an episode of critical illness. It is a geriatric multi-dimensional syndrome that has been associated with an increased risk of disability, hospitalizations and death in community living elderly patients (7, 8). The presence of frailty may result in decreased physiologic reserve and increase the risk of poor outcomes after illness or surgery (9–11). Prospectively studying the association between pre-ICU frailty and ICU outcomes in elderly patients is particularly difficult due to the low incidence of critical illness in the large cohorts of community dwelling elderly patients where functional status and frailty measures have been captured (12, 13).
Therefore, using a nationally representative sample of elderly Medicare beneficiaries, we sought to use a claims based approach to describe categories of pre-ICU health of elderly patients. We hypothesized that Medicare claims from the year prior to ICU admission could be used to describe distinct groups of elderly patients with clinically significant differences in short- and long-term mortality after critical illness. We, a priori, hypothesized that a pre-ICU frailty would be associated with a substantially increased short and long-term mortality relative to patients in other health categories.
METHODS
Data Source
This was a retrospective cohort study using the Medicare Standard Analytic Files (SAF) from the Center for Medicare and Medicaid Services (CMS). The data set contains all fee-for-service claims, including hospital inpatient, hospital outpatient, skilled nursing facility, “carrier” claims (physician supplier part B files which includes all office visits), home health agency, and durable medical equipment for a random, longitudinal 5% sample of beneficiaries. This was a limited data set with all healthcare encounters identified by the quarter (3-month interval) of the year. We linked data from the years 2004 through 2008 and derived the inception cohort from the 2005 sample. Our cohort consisted of a random 5% sample of Medicare beneficiaries ≥ 66 years old who received intensive care during the year 2005. We excluded patients who were treated only in Intermediate ICU, Coronary Care Units or Psychiatric ICUs.
Defining Health Categories prior to Admission to an ICU
We used all Medicare claims from the 4 quarters prior to the index hospitalization to provide information on previously existing conditions using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes. We modified a descriptive classification scheme developed for Medicare decedents and grouped patients into four pre-ICU health categories using ICD-9 diagnoses and specific facility claims (see table S1 on the Appendix) (5, 6, 14). Patients with ≥ 1 claim with a diagnosis of cancer (excluding benign neoplasm, carcinoma in-situ or malignancies labeled as “unspecified nature”) were classified into the Cancer group. Patients who had a diagnosis of congestive heart failure, ischemic heart disease, chronic liver disease, chronic obstructive pulmonary disease (excluding patients with a diagnosis of acute bronchitis) were classified in the Chronic Organ Failure group.
We identified patients as being in the Frailty group if they had claims from a nursing facility in the year prior to the index hospitalization or if they had at least one claim in the year prior to ICU hospitalization associated with any of the following diagnoses previously associated with frailty: dementias or dementia from Alzheimer’s disease or senility; sub-acute delirium; Parkinson’s disease pathologic fracture; functional urinary and fecal incontinence; dehydration; debility; pressure ulcer; other unspecified protein-calorie malnutrition, kwashiorkor, nutritional marasmus, severe protein calorie malnutrition or abnormal loss of weight or adult failure to thrive; accidental falls or abnormality of gait or lack of coordination (see table S1) (5, 15, 16). Patients who did not fall into the Cancer, Chronic Organ Failure, or Frailty were classified as Robust. For the patients with pre-ICU Cancer, Chronic Organ Failure and Frailty, we counted the number of ICD-9-CM claims in the year before the index hospitalization that fit the health category criteria to determine whether patients were being categorized primarily based on a single claim, or whether there was substantial redundancy in categorization of patients (see table S3 in the Appendix).
We anticipated substantial overlap among the Cancer, Chronic Organ Failure and Frailty groups (i.e. patients could be classified as fitting into more than one category). Using the four proposed pre-ICU health categories, we also created distinct groups (eight in total) that would classify each individual into mutually exclusive categories: Cancer only; Chronic Organ Failure only; Frailty only; Cancer & Chronic Organ Failure; Cancer & Frailty; Chronic Organ Failure & Frailty; Cancer, Chronic Organ Failure & Frailty.
Data Analyses
We calculated descriptive statistics for demographics, clinical characteristics and outcomes using percentages, means (± standard deviations, SD), and medians (with inter-quartile ranges, IQR) as appropriate for our study cohort. We grouped age into four categories (<70, 70–79, 80–89, >90 years old) and grouped race/ethnicity as: White, non-Hispanic; Black, non-Hispanic; and Other. We categorized each patient as medical or surgical using diagnosis related groups (DRG) for the index hospitalization. We examined specific aspects of the index hospitalization: the frequency of mechanical ventilation (ICD-9-CM codes 96.7x or tracheostomy 31.1 or DRG 483) (17), acute renal failure (ICD-9-CM 584.x) and community-acquired pneumonia (18); we also assessed acute organ dysfunctions (cardiac, respiratory, renal, neurologic, and hepatic) and diagnoses of infection and severe sepsis with ICD-9-CM codes, using standard definitions (19).
We next examined mortality based on pre-ICU health categories. When groups had overlapping patient populations, we did not test for statistical differences between groups. We compared differences in hospital mortality for the eight non-overlapping subgroups using the chi-squared test and logistic regression, with Robust patients as the comparison group. For patients who survived to acute hospital discharge, we assessed 3-year mortality using Kaplan-Meier curves; we used the log-rank test to test for statistical differences in survival curves between non-overlapping pre-ICU health groups and calculated hazard ratios (HRs) using a Cox proportional hazard model after assessing graphically that the proportionality assumption was not violated. Multivariable Cox and logistic regression models were adjusted for age, sex, race/ethnicity and markers of illness severity in the ICU (acute renal failure, severe sepsis, and mechanical ventilation).
We focused on the additive effect of frailty on hospital mortality and on 3-year mortality in hospital survivors by comparing mortality for groups of patients with and without frailty: Frailty only versus Robust; Cancer & Frailty versus Cancer only; Chronic Organ Failure & Frailty versus Chronic Organ Failure only; Cancer, Chronic Organ Failure & Frailty versus Cancer & Chronic Organ Failure. For further assessment of the relationship between pre-ICU frailty and outcomes, we also stratified patients by the individual markers of frailty (dementia diagnoses, other diagnoses, and skilled nursing facility use) and examined hospital mortality and 3- year mortality for these sub-groups.
We used a two-sided p-value of < 0.05 as our threshold for statistical significance. Due to the large size of the dataset, we also determined a priori that a clinically significant change in mortality would be an absolute difference in mortality of 2% or a change in HR of 0.05 (20). All analyses were done using Stata SE 11.0 (StataCorp, College Station, Texas) and Microsoft Excel 2010 (Microsoft, Redmond, Washington). Because the study involved retrospective analysis of deidentified data, it was reviewed by the Columbia University’s and Montefiore Medical Center’s Institutional Review and was deemed exempt.
RESULTS
Pre-ICU Health Categories
After exclusions (see Figure S1 of the appendix), the study population consisted of 47,427 Medicare recipients admitted to an ICU in 2005. In the cohort, 18.8% (n=8,901) were Robust, 68.1% (n=32,287) had Chronic Organ Failure; 28.6% (n=13,571) had Cancer; and 34.0% (n=16,147) met criteria for Frailty. 41.3% patients were classified into more than one health category (Figure S2 of the appendix). Notably, 95% of patients with pre-ICU Frailty also were classified as either having Chronic Organ Failure and/or Cancer.
The mean age of the cohort was 77.5 ±7.2, with 45.9% between the ages of 70 and 79 years. The median length of stay in the ICU was 3 days (IQR 1–5) with a median hospital length of stay of 6 days (3–11) (see Table 1). The patients with pre-ICU Frailty were oldest (mean age 79.4 ±7.3 vs. 76.4 ±7.2 for Robust patients). A higher proportion of the patients with pre-ICU Frailty were women (57.4%) compared with other categories of patients. Robust patients and those with pre-ICU Cancer were more likely to have surgery during the hospitalization (51.3% and 50.5% respectively) than the patients with pre-ICU Chronic Organ Failure (43.7%) or Frailty (36.5%). (Table 1; see Table S2 in the Appendix for characteristics of the eight non-overlapping subgroups).
Table 1.
Characteristics of the Study Population by pre-ICU health categoriesa
| Characteristics | All (N=47,427) | Robust (N=8,901) | Cancer (N=13,571) | Chronic Organ Failure (N=32,287) | Frailty (N=16,147) |
|---|---|---|---|---|---|
| Male, n (%) | 22,734 (47.9) | 3,950 (44.4) | 7,866 (58.0) | 15,982 (49.5) | 6,883 (42.6) |
| Age, mean (yrs) ± SD | 77.5 ± 7.2 | 76.4 ± 7.2 | 77.6 ± 6.9 | 77.6 ± 7.10 | 79.4 ± 7.3 |
| Race/Ethnicity, n (%) | |||||
| White, non-Hispanic | 41,311 (87.1) | 7,702 (86.5) | 12,215 (90.0) | 28,186 (87.3) | 13,676 (84.7) |
| Black, non-Hispanic | 4,126 (8.7) | 822 (9.2) | 947 (7.0) | 2,740 (8.5) | 1,763 (10.9) |
| Other | 1,990 (4.2) | 377 (4.2) | 405 (3.0) | 1,361 (4.2) | 708 (4.4) |
| Hospitalized in year prior, n (%) | 17,780 (37.5) | 632 (7.1) | 5,865 (43.2) | 15,406 (47.9) | 10,148 (62.9) |
| SNF in year prior, n (%) | 5,016 (10.6) | n/a | 1,459 (10.7) | 4,436 (13.7) | 5,016 (31.1) |
| ICU length of stay, median days (IQR) | 3 (1–5) | 2 (1–5) | 3 (1–5) | 3 (1–6) | 3 (1–6) |
| Hospital length of stay, median days (IQR) | 6 (3–11) | 5 (3–9) | 7 (4–12) | 6 (3–11) | 7 (4–12) |
| Surgical patients, n (%)b | 21,746 (45.9) | 4,568 (51.3) | 6,846 (50.5) | 14,121 (43.7) | 5,898 (36.5) |
| Clinical Diagnoses during Index Hospitalization, n (%) | |||||
| Pneumonia | 2,479 (5.2) | 242 (2.7) | 678 (5.0) | 2,044 (6.3) | 1,098 (6.8) |
| Infection | 15,956 (33.6) | 2,364 (26.6) | 4,549 (33.5) | 11,404 (35.3) | 7234 (44.8) |
| Acute Renal Failure | 5,990 (12.6) | 84.1 (9.5) | 1,732 (12.8) | 4,404 (13.6) | 2,585 (16.0) |
| Severe Sepsisc | 7,579 (16.0) | 978 (11.0) | 2,199 (16.2) | 5,670 (17.6) | 3,604 (22.3) |
| Mechanical Ventilation | 5,151 (10.9) | 808 (9.1) | 1,505 (11.1) | 3,729 (11.6) | 2,085 (12.9) |
| Discharge Destination, n (%) | |||||
| Died in hospital | 5,991 (12.6) | 867 (9.7) | 1,843 (13.6) | 4,322 (13.4) | 2,786 (17.3) |
| Home with self care | 18,315 (38.6) | 4,177 (46.9) | 5,186 (38.2) | 11,950 (37.0) | 3,715 (23.0) |
| Home with health services | 6,407 (13.5) | 1,137 (12.8) | 2,041 (15.0) | 4,444 (13.8) | 1,921 (11.9) |
| Skilled Care Facility/Rehab | 13,058 (27.5) | 2,007 (22.6) | 3,490 (25.7) | 9,127 (28.3) | 6,430 (39.8) |
| Otherd | 9,647 (20.3) | 1,580 (17.8) | 2,853(21.0) | 6,766 (21.0) | 4,081(25.3) |
Notes
Abbreviations: ICU – Intensive Care Unit; SD – Standard Deviation; IQR – interquartile range; SNF – Skilled Nursing Facility; NA – not applicable
Elderly Medicare recipients are grouped based on pre-ICU Medicare claims in the year prior to the index hospitalization during which they received intensive care; patients could be classified into more than one category, thus the groups together add to more than the total cohort.
Diagnosis-related groups were used to classify the index hospitalization as primarily medical or surgical
Severe Sepsis included those patients with infection and acute organ dysfunction (see text for details)
Includes those who left against medical advice or were discharged to other inpatient facility, federal health care facility, hospice or psychiatric hospital
Hospital and 3-year mortality by four overlapping pre-ICU Health Categories
Of the whole study population, 12.6% (n=5,991) died during the index hospitalization. The Robust patients had the lowest hospital mortality (9.7%) and the patients with pre-ICU Frailty had the highest (17.3%). Pre-ICU Cancer patients had a 13.6% hospital mortality and those with pre-ICU Chronic Organ Failure a 13.4% hospital mortality. A similar pattern was observed for 3-year mortality in hospital survivors: Robust patients had the lowest mortality (24.6%) while patients with pre-ICU frailty had the highest (58.8%); 3-year mortality for patients with pre-ICU Cancer (47.6%) was similar to the 3-year mortality for the patients with pre-ICU Chronic Organ Failure (46.4%).
Hospital and 3-year mortality for non-overlapping pre-ICU health categories
Hospital mortality was similar for patients with pre-ICU Cancer only (9.4%) and with pre-ICU Chronic Organ Failure only (10.3%) compared with Robust patients (9.7%) (adjusted odds ratio (aOR) 0.99; 95% CI 0.84–1.16 for Cancer only vs. Robust; aOR 0.99; 95% CI 0.87–1.06 for Chronic Organ Failure vs. Robust) (see Table 2). Hospital mortality was the highest for patients who had pre-ICU Frailty (15.9% for Frailty only up to 19.6% for those with Cancer, Chronic Organ Failure & Frailty: aOR for Frailty only vs. Robust 1.75; 95% CI 1.54–1.99; aOR for Cancer, Chronic Organ Failure & Frailty vs Robust 1.61; 95% CI 1.43–1.81) (Table 2).
Table 2.
Models of the association between pre-ICU health categories and hospital- and 3-year mortality. All statistical testing is comparing each group with the Robust category
| Pre-ICU health categories | Number Hospital Deaths/Total at Risk (%) | Hospital mortalitya AOR (95% CI)b |
Died within 3- years/Survived to Hospital Discharge (%) | 3-year mortalitya AHR (95% CI)b |
|---|---|---|---|---|
| Robust | 867/8,901 (9.7) | ____ | 1,973/8,034 (24.6) | ____ |
| Cancer only | 274/2,902 (9.4) | 0.99 (0.84, 1.16) | 884/2,628 (33.6) | 1.43 (1.32, 1.55) |
| Chronic Organ Failure only | 1,396/13,578 (10.3) | 0.99 (0.87, 1.06) | 4,240/12,182 (34.8) | 1.47 (1.39, 1.54) |
| Cancer & Chronic Organ Failure | 668/5,899 (11.3) | 1.11 (0.99, 1.25) | 2,281/5,231 (43.6) | 1.93 (1.82, 2.06) |
| Frailty only | 389/2,499 (15.9) | 1.27 (1.10, 1.47) | 987/2,060 (47.9) | 1.84 (1.70, 1.99) |
| Cancer & Frailty | 139/888 (15.7) | 1.48 (1.19, 1.85) | 409/749 (54.6) | 2.27 (2.04, 2.53) |
| Chronic Organ Failure & Frailty | 1,496/8,928 (16.8) | 1.37 (1.24, 1.51) | 4,488/7,432 (60.4) | 2.66 (2.52, 2.81) |
| Cancer, Chronic Organ Failure & Frailty | 762/3,882 (19.6) | 1.61 (1.43, 1.81) | 1,974/3,120 (63.3) | 2.83 (2.65, 3.02) |
Notes
Abbreviations: ICU – Intensive Care Unit; AOR – Adjusted Odds Ratio; AHR – Adjusted Hazard Ratio
For the outcome of hospital mortality we used logistic regression models and for 3-year mortality Cox proportional hazard models
Multivariable models were adjusted for age, sex, race/ethnicity, acute renal failure, severe sepsis and mechanical ventilation (see text for details)
Among hospital survivors, all other pre-ICU health categories were associated with significantly higher 3-year mortality than the Robust patients (Table 2). Differences in comparison with Robust patients remained significant after multivariable adjustment (Table 2), with the largest difference in mortality for patients with Cancer, Chronic Organ Failure & Frailty compared with the Robust population (adjusted Hazard Ratio (aHR) 2.83; 95% CI 2.65–3.02).
Additional impact of Frailty on hospital and 3-year mortality
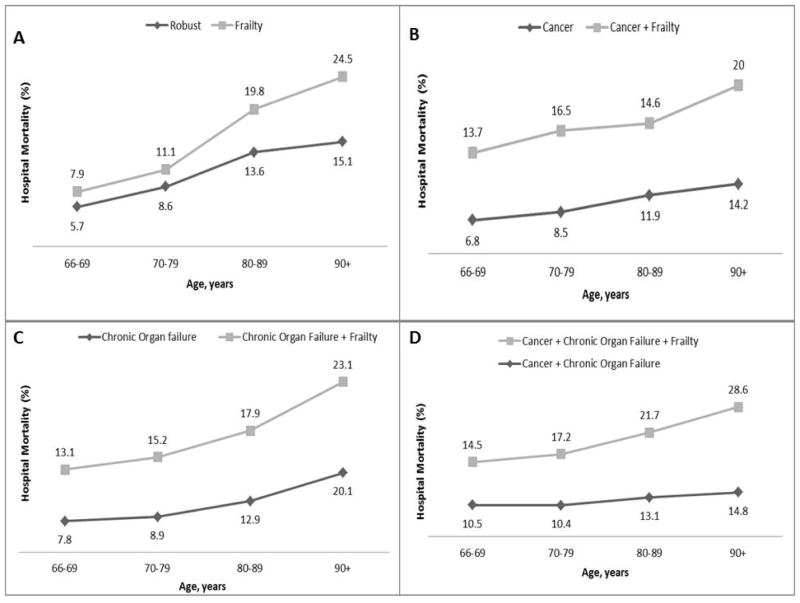
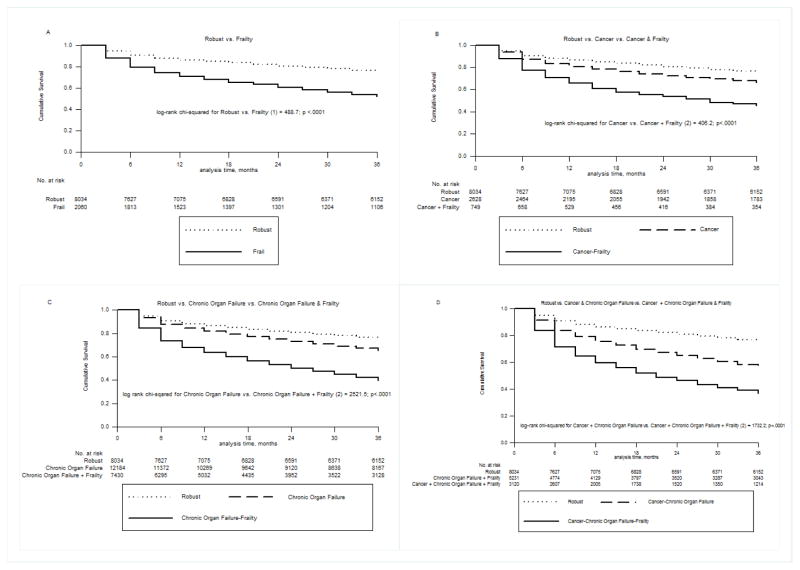
The prevalence of pre-ICU Frailty among elderly patients admitted to ICU increased with age (22.8% of those age 66 to 69 years; 42.0% of those between 80 and 89 years; 52.7% for those over age 90). Comparing patients with each pre-ICU health category with and without frailty, there was a consistent increased hospital mortality with frailty (aOR 1.27; 95% CI 1.10–1.47 for Frailty only vs. Robust up to an aOR of 1.52; 95% CI 1.35–1.63 for Cancer, Chronic Organ Failure & Frailty vs. Cancer & Chronic Organ Failure). Adjusted hazard ratios (aHRs) for 3-year mortality showed an even larger effect, with the highest additional mortality for those who were Frail only vs. Robust (aHR 1.84; 95% CI 1.70–1.99). Pre-ICU Frailty was also consistently associated with an increased risk of hospital mortality and 3-year mortality across all age-groups (Figures 1&2).
Figure 1.
Additive effect of pre-ICU frailty on hospital mortality for elderly Medicare beneficiaries stratified by age for A) Frailty only versus Robust, B) pre-ICU Cancer & Frailty versus pre-ICU Cancer only, C) pre-ICU Chronic Organ Failure & Frailty versus pre-ICU Chronic Organ Failure only D) pre-ICU Cancer, Chronic Organ Failure & Frailty versus pre-ICU Cancer & Chronic Organ Failure
Figure 2.
Kaplan-Meier curves of 3-year mortality for elderly Medicare beneficiaries who survived the index hospitalization with critical illness, comparing patients with and without pre-ICU Frailty: A) Robust versus Frailty only B) Robust versus pre-ICU Cancer & Frailty versus pre-ICU Cancer only, C) Robust versus pre-ICU Chronic Organ Failure and Frailty versus pre-ICU Chronic Organ Failure only, D) Robust versus pre-ICU Cancer, Chronic Organ Failure & Frailty versus pre-ICU Cancer & Chronic Organ Failure
For patients categorized as having pre-ICU Frailty, we examined the hospital- and 3-year mortality based on the reason they qualified as having pre-ICU Frailty (e.g. diagnoses of dementia, other frailty diagnoses or skilled nursing facility use in the year prior to ICU admission) (Table S4 in the appendix). Hospital- and 3-year mortality were similarly high for patients who qualified for pre-ICU Frailty by either a diagnosis of dementia, other frailty diagnoses or the use of a skilled nursing home in the year prior to the ICU; the presence of multiple frailty markers in the year prior was associated with a higher mortality (table S4).
DISCUSSION
Using Medicare claims data from the year prior to critical illness we were able to categorize pre-ICU health of elderly patients using previously described categories used for decedents (5, 6). Notably, among elderly ICU patients, 70% had Chronic Organ Failure prior to admission, 35% were Frail, and only 19% were Robust. Patients with pre-ICU Frailty had a substantially higher hospital- and 3-year mortality than other groups of patients. When pre-ICU Frailty overlapped with other pre-ICU categories, it was associated with an increase in mortality above and beyond other pre-ICU health categories and across all age-groups. These results will help policy makers, caregivers and clinicians to better understand the impact of pre-ICU health on mortality outcomes after critical illness for elderly patients.
In this study we modified a claims-based approach to classifying health of Medicare decedents and applied it to the “black box” of the year prior to a critical illness. Diagnoses were informed by previous work on the classification of Medicare decedents (5, 6, 14) and the definition of frailty was supplemented by an extensive review of the geriatric literature to include such multifactorial geriatric conditions like debility, accidental falls, pathologic fractures, malnutrition and weight loss, and pressure ulcers, that have all been associated with disability and decreased quality of life in the elderly (21, 22). The face validity of this approach is reflected in the fact that our patients with pre-ICU Frailty were older, were more likely to be female and less likely to be admitted to ICU for surgical treatment, consistent with much of the literature (8, 11, 21). Most patients qualified for pre-ICU Frailty by more than one ICD-9 claim making spurious or erroneous classification less likely (see table S3 in the Appendix).
Our study is novel for its focus on elderly patients with critical illness, and we departed from previous work in this area by acknowledging that the pre-ICU Frailty may occur with, rather than independent from, other health categories such as chronic organ dysfunction. The idea of concomitant frailty is important as elderly patients commonly have multiple medical problems supported by our finding that 19,597 (41.3%) of patients were classified into more than one health category.
In this retrospective cohort of elderly patients with three years of follow-up, pre-ICU Frailty was associated with a 50% or greater increase in the risk of death over 3 years of follow-up when compared to patients without pre-ICU Frailty. These results are consistent with those from the first prospective evaluation of the prevalence and associated outcomes of frailty among critically ill patients which found that frailty, as diagnosed by a subjective bedside assessment tool, was associated with higher hospital and 1-year mortality (23).
This association between pre-hospital frailty and risk of death is consistent with the growing literature to suggest that the baseline functional status and pre-existing comorbid illness have prognostic value in critically ill patients (2–4). The concept of pre-ICU Frailty may more easily capture the decreased physiologic reserve that is associated with poorer outcomes after acute illness. Many of the operational definitions of frailty described in the geriatric literature currently include performance and questionnaire measures that are impractical for use in the acutely ill patient (24,25). As such, the strong association seen in this study between the pre-ICU Frailty (easily defined by medical history) and mortality is a step towards developing more practical approaches to assessing pre-ICU frailty of patients who are often unable to provide detailed medical history.
The association between pre-ICU health categories and outcomes has important methodological implications for studies focused on the long-term effects of critical illness in elderly patients. Many studies of long-term outcomes have found that older survivors of critical illness have limitations and deficits – muscle weakness, cognitive impairment, muscle wasting -- that mirror the frailty syndrome (26–30). Recent work using the Health and Retirement Study demonstrated that it was important to adjust accurately for the pre-diagnosis health trajectory in order to avoid spurious inferences about the effect of critical illness on long-term outcomes (13). A “progeric hypothesis” has been proposed whereby critical illness facilitates a more rapid development of accumulated deficits which would have taken years to develop in the outpatient geriatric population without critical illness (13, 31). The relationship we found between pre-ICU health categories and outcomes after critical illness in a representative population of elderly patients, most of whom had multiple chronic co-morbidities, is further evidence to suggest that understanding long-term outcomes of critical illness will require a longitudinal perspective that carefully assesses pre-ICU status (32).
This study has important limitations. First, we used billing claims to describe pre-ICU health categories, which cannot fully reflect the true clinical picture. Our approach has been validated in previous studies with Medicare decedents but never used to describe a population of ICU patients. Nevertheless, the use of administrative data is an important option to consider given the difficulty of obtaining full pre-ICU history (and in particular, information on frailty) for many patients. Second, although we adjusted for identifiable markers of the critical illness, we could not fully assess the severity of illness of patients on admission to hospital or ICU using data available from Medicare. However, the substantial associations we found between pre-ICU health categories and mortality suggest that important effects would remain even with further adjustment for other potential confounders.
In conclusion, we were able to classify elderly critically ill patients into four pre-ICU health categories and some of these categories are associated with an increase in both short and long-term mortality after intensive care. Pre-ICU Frailty was associated with the greatest increase in mortality. Future research may focus on both assessment strategies for pre-ICU Frailty in the critically ill elderly population and on specific approaches to modifying ICU treatment to improve post-ICU outcomes in this high-risk patient population.
Supplementary Material
Table 3.
Comparison of hospital- and 3-year mortality for patients categorized into pre-ICU health categories with or without frailty
| Pre-ICU Health Categories | Hospital Mortalitya | 3-year mortalitya | |
|---|---|---|---|
|
| |||
| Comparison group | Reference group | AOR (95% CI)b | AHR (95% CI)b |
| Frailty only | Robust | 1.27 (1.10, 1.47) | 1.84 (1.70, 1.99) |
| Cancer & Frailty | Cancer only | 1.52 (1.19, 1.95) | 1.69 (1.50, 1.91) |
| Chronic Organ Failure & Frailty | Chronic Organ Failure | 1.42 (1.30, 1.54) | 1.82 (1.74, 1.90) |
| Cancer, Chronic Organ Failure & Frailty | Cancer & Chronic Organ Failure | 1.52 (1.35, 1.72) | 1.54 (1.45, 1.64) |
Notes
Abbreviations: ICU – Intensive Care Unit; AOR – Adjusted Odds Ratio; AHR – Adjusted Hazard Ratio
For the outcome of hospital mortality we used logistic regression models and for 3-year mortality Cox proportional hazard models
Multivariable models were adjusted for age, sex, race/ethnicity, acute renal failure, severe sepsis and mechanical ventilation (see text for details)
Acknowledgments
This study was supported by the Division of Critical Care Medicine at Albert Einstein College of Medicine, grant U01 HL10871203 from the National Heart, Lung and Blood Institute to Dr. Gong and K08AG038477 from the National Institute on Aging to Dr. Wunsch.
| Elements of Financial/Personal Conflicts | Aluko Hope | Michelle Gong | Carmen Guerra | Hannah Wunsch | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
Footnotes
Author Contributions: A.A.H. contributed to the study design, data preparation, data analysis and interpretation, writing and review of the manuscript. M.N.G. contributed to the study design, data analysis and interpretation, writing and review of the manuscript. C.G. contributed to the study design, data preparation, data analysis, interpretation of the data, writing and review of the manuscript. H.W. contributed to the study design, acquisition of the data, data analysis, interpretation of the data, writing and review of the manuscript.
Sponsor’s Role: The sponsors played no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
References
- 1.Carson SS. The epidemiology of critical illness in the elderly. Crit Care Clin. 2003;19:605–617. doi: 10.1016/s0749-0704(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 2.Orwelius L, Nordlund A, Nordlund P, Simonsson E, Backman C, Samuelsson A, Sjoberg F. Pre-existing disease: The most important factor for health related quality of life long-term after critical illness: A prospective, longitudinal, multicentre trial. Crit Care. 2010;14:R67. doi: 10.1186/cc8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sligl WI, Eurich DT, Marrie TJ, Majumdar SR. Only severely limited, premorbid functional status is associated with short- and long-term mortality in patients with pneumonia who are critically ill: A prospective observational study. Chest. 2011;139:88–94. doi: 10.1378/chest.10-1054. [DOI] [PubMed] [Google Scholar]
- 4.Roch A, Wiramus S, Pauly V, Forel JM, Guervilly C, Gainnier M, Papazian L. Long-term outcome in medical patients aged 80 or over following admission to an intensive care unit. Crit Care. 2011;15:R36. doi: 10.1186/cc9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. J Am Geriatr Soc. 2002;50:1108–1112. doi: 10.1046/j.1532-5415.2002.50268.x. [DOI] [PubMed] [Google Scholar]
- 6.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 7.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: Emergence and consequences in women aged 65 and older in the women’s health initiative observational study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM, Sharp TJ, Buckley MJ, Moss M. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213:37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202:511–514. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: The case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnato AE, Labor RE, Freeborne NE, Jayes RL, Campbell DE, Lynn J. Qualitative analysis of medicare claims in the last 3 years of life: A pilot study. J Am Geriatr Soc. 2005;53:66–73. doi: 10.1111/j.1532-5415.2005.53012.x. [DOI] [PubMed] [Google Scholar]
- 15.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: A systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 16.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: A systematic review. Ageing Res Rev. 2011;10:104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Kahn JM, Carson SS, Angus DC, Linde-Zwirble WT, Iwashyna TJ. Development and validation of an algorithm for identifying prolonged mechanical ventilation in administrative data. Health Serv Outcomes Res Method. 2009;9:117–132. [Google Scholar]
- 18.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: Age- and sex-related patterns of care and outcome in the united states. Am J Respir Crit Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 19.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the united states: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the united states. Crit Care Med. 2010;38:1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 21.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: The health and retirement study. J Am Geriatr Soc. 2009;57:830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 22.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagshaw SM, Stelfox T, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, Artiuch B, Ibrahim Q, Stollery DE, Rokosh E, Majumdar SR. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2013;10:1503. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: A novel concept. Crit Care. 2011;15:301. doi: 10.1186/cc9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilmer SN, Perera V, Mitchell S, Murnion BP, Dent J, Bajorek B, Matthews S, Rolfson DB. The assessment of frailty in older people in acute care. Australas J Ageing. 2009;28:182–188. doi: 10.1111/j.1741-6612.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 26.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 27.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 28.de Jonghe B, Lacherade JC, Sharshar T, Outin H. Intensive care unit-acquired weakness: Risk factors and prevention. Crit Care Med. 2009;37:S309–15. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 29.van der Schaaf M, Beelen A, Dongelmans DA, Vroom MB, Nollet F. Poor functional recovery after a critical illness: A longitudinal study. J Rehabil Med. 1041;41:1041–1048. doi: 10.2340/16501977-0443. [DOI] [PubMed] [Google Scholar]
- 30.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenfeld GD. Does the hospital make you older faster? Am J Respir Crit Care Med. 2012;185:796–798. doi: 10.1164/rccm.201202-0267ED. [DOI] [PubMed] [Google Scholar]
- 32.Iwashyna TJ, Prescott HC. When is critical illness not like an asteroid strike? Am J Respir Crit Care Med. 2013;188:525–527. doi: 10.1164/rccm.201306-1092ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.