Abstract
Curcumin is the main bioactive ingredient in turmeric extract and widely consumed as part of the spice mix curry or as dietary supplement. Turmeric has a long history of therapeutic application in traditional Asian medicine. Biomedical studies conducted in the past two decades have identified a large number of cellular targets and effects of curcumin. In vitro curcumin rapidly degrades in an autoxidative transformation to diverse chemical species, formation of which has only recently been appreciated. We discuss how degradation and metabolism of curcumin, through products and their mechanism of formation, provide a basis for the interpretation of preclinical data and clinical studies. We suggest that the previously unrecognized diversity of its degradation products could be an important factor in explaining the polypharmacology of curcumin.
Keywords: turmeric, bioactivity, metabolism, curcuminoids, polyphenol, quinone methide, protein adduction, Michael reaction
Introduction
The number of clinical trials testing the therapeutic potential of curcumin has increased exponentially over the last decade. The registry clinicaltrials.gov listed 106 trials in 2014 compared to only four in 2004 for the search term “curcumin” (Fig. 1). Curcumin is evaluated in arthritis, cancer, depression, and neurodegenerative diseases, to list only a few.1-6 This raise in expectation that the dietary agent curcumin can be a remedy for human disease has been fueled by a large number of cell culture-based and animal studies, reflected in a near exponential increase in publications for “curcumin” in the past two decades. With >100 cellular targets reported,7 the number and diversity of biological effects of curcumin in disease models is staggering, ranging from anti-inflammatory, antioxidant, antiviral, to antitumor effects.8, 9
Fig. 1.
Numbers of publications in PubMed and human trials in www.clinicaltrials.gov with the keyword “curcumin” from 1994-2014.
Curcumin is not the only dietary polyphenol that has received increasing attention. Other dietary polyphenols, like resveratrol, quercetin, and epigallocatechin gallate (EGCG) have experienced a similar upward trend, both in preclinical research as well as in human clinical trials.10-13
The biological effects of curcumin in cellular and animal models are surprising considering its chemical and metabolic instability. Little if any curcumin is present unchanged in the systemic circulation.14, 15 Furthermore, curcumin undergoes rapid non-enzymatic degradation in cell culture medium and possibly in vivo as well.16 Chemical transformation does not necessarily mean a loss in activity. Here we argue that understanding the molecular mechanisms of degradation of curcumin is necessary for interpreting in vitro and in vivo studies.
Metabolism of curcumin in vivo
Curcumin is metabolized primarily by reduction and conjugation after oral administration (Fig. 2). Consecutive reduction of the double bonds in the heptadienedione chain results in the formation of di-, tetra-, hexa-, and octahydrocurcumin. Reduction can already occur in the gut by a CurA reductase that has been isolated from intestinal E. coli.17, 18 After systemic absorption, alcohol dehydrogenase reduces curcumin to tetra- and hexahydrocurcumin in the liver whereas formation of di- and octahydrocurcumin required an unidentified microsomal enzyme.19-21 The reduced metabolites, especially tetra- and hexahydrocurcumin, are the largest portion of curcumin metabolites detected.14 With a few exceptions their biological activities are strongly reduced compared to curcumin.22, 23
Fig. 2.
The metabolic and degradation pathways of curcumin. (a) Reduction; (b) conjugation; (c) oxidation; and (d) cleavage.
Curcumin and its reduced metabolites exist almost exclusively as conjugates with glucuronic acid and sulfate in plasma.14, 21 Two additional pathways for the degradation of curcumin in vitro have been described (Fig. 2).16, 24 Whether and how the degradation and oxidation pathways contribute to the in vivo and in vitro biological activities of curcumin will be discussed in the following.
Degradation of curcumin in cell culture
Although the chemical instability of curcumin is widely recognized, this fact is less considered when interpreting studies with cultured cells in vitro. Curcumin degrades quickly at physiological pH but slower when incubated in the presence of serum or with cultured cells.24 Protein increases the half life of curcumin from a few minutes to 1-2 h.24 Nevertheless, there is sufficient time for curcumin to degrade during a typical 4-8 h incubation before cultured cells are harvested and analyzed. When cells have been treated with curcumin for several hours it is impossible to decide whether the observed effects are due to curcumin or its degradation products. Unless interpreted with a consideration of what active or inactive metabolites of curcumin may be formed (or not) during an assay, results can be misleading and give only limited insight into the in vivo biological activity of curcumin.
Similar considerations are relevant for studies claiming that the mechanism of action of curcumin is due to the induction of oxidative stress (“reactive oxygen species”, ROS) in cells. In many instances, the role of curcumin-induced ROS has been shown indirectly, i.e., by addition of a reducing antioxidant, for example, N-acetylcysteine, that abolished the effects of curcumin.25-29 The action of the antioxidant, however, is not only to reduce ROS formation but also to delay or prevent degradation of curcumin. Thus, unless specific probes against reactive oxygen species are used rather than a general antioxidant it is not possible to decide whether effects caused by curcumin are mediated by the induction of oxidative stress or its degradation products.
Another facet of curcumin that is not sufficiently considered is its heterogeneity as a natural product. “Curcumin” is used to describe the chemically pure compound or an extract from the turmeric rhizome that is enriched in curcuminoids, i.e., a mixture curcumin, demethoxy- (DMC) and bisdemethoxycurcumin (BDMC). Their relative abundance in commercial extracts is about 80:15:5. Whether pure curcumin or a mixture of curcuminoids is used in an assay is relevant not only because curcumin, DMC, and BDMC may have distinct activities but also because the stability of the mixture is different from that of the pure compounds.30
The degradation is an autoxidation
For many years cleavage of the heptadienedione chain reported by Wang and co-workers in the late 1990s was considered the prevailing degradation reaction, resulting in vanillin, ferulic acid, and feruloylmethane as products.24 Meanwhile, there is evidence from revisiting the degradation reaction that chain cleavage is only a very minor pathway,31 and thus irrelevant for understanding the biological effects of curcumin in vitro. Interestingly, vanillin, ferulic acid, and feruloylmethane were described to be minor products already in the original paper by Wang and co-workers, and the major product detected was tentatively identified as trans-6-(4’-bydroxy-3’methoxyphenyl)-2,4-dioxo-5-hexenal, the assignment being uncertain because NMR data were not available.24 In hindsight, the identification of the major product was probably incorrect, and the major product detected by Wang and co-workers was likely the bicyclopentadione product of autoxidative transformation of curcumin.16
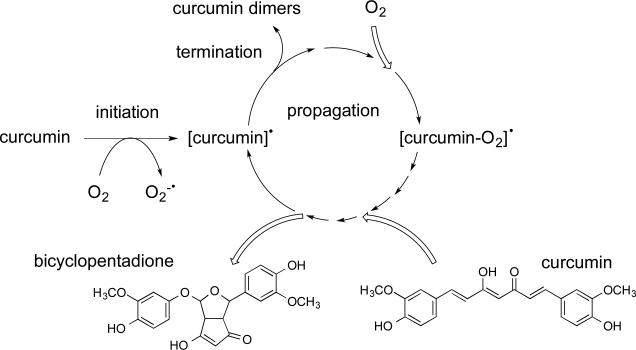
The degradation of curcumin in buffer is a spontaneous, free radical-driven incorporation of O2 that meets the criteria of an autoxidative process (Fig. 3).16 The major product of the autoxidation of curcumin is a bicyclopentadione, formed by oxygenation and double cyclization of the heptadienedione chain connecting the two methoxyphenol rings of curcumin.16 This complex transformation is the result of incorporation of O2 and further rearrangement through several key intermediates.32 Autoxidation is initiated by O2 serving as the initial electron acceptor. The propagation results in a 1:1 molar reaction of curcumin with molecular oxygen: for every molecule of curcumin converted, one molecule of oxygen is consumed.16 Thus, the terms “degradation” and “autoxidation” are equivalent in describing the spontaneous transformation of curcumin in vitro.
Fig. 3.
The degradation of curcumin is an autoxidation to a bicyclopentadione.
Products of oxidative transformation
We analyzed the degradation products of curcumin using [14C2]curcumin as tracer for RP-HPLC analyses.32 We isolated 7 novel compounds and determined their structures using LC-MS, HR-MS, and NMR analyses (Fig. 4). Five products had oxygen substitutions at C-1 and C-7 of the former heptadienedione chain. The oxygen atoms were present in hydroxy, keto, epoxy, ether, and hemiketal moieties. Two products that did not incorporate oxygen were a cyclobutyl cyclopentadione and diguaiacol, an obvious cleavage product. Two of the isolated products, the spiroepoxide and the vinylether cyclopentadiones, were intermediates in the reaction to the bicyclopentadione. The other were end products formed in addition to the bicyclopentadione. Neither vanillin nor ferulic acid were formed in sufficient amount to be detected by the radiodetector.32 Vanillin, however, can be detected as a minor product when a more sensitive UV-diode array detector is used.31
Fig. 4.
Products of autoxidation of curcumin.
An explanation why these novel products have not been described before is probably their minor abundance (e.g., the cyclobutyl cyclopentadiones and diguaiacol) combined with unexpectedly high polarity on RP-HPLC, especially in case of the dihydroxy-, ketohydroxy-, and hemiketal cyclopentadiones and sensitivity to acid. Even brief exposure of the spiroepoxide and vinylether to acidic pH will result in transformation to the bicyclopentadione. Unless precautions are undertaken, the newly identified products undergo further transformation, are not extracted, or elute in the void volume when standard extraction procedures and RP-HPLC conditions are employed.32
Depending on reaction time and sample work-up different product profiles of the curcumin autoxidation reactions were obtained. Reactions conducted for longer than 4 h followed by acidification and extraction (solid-phase or liquid/liquid) gave the bicyclopentadione diastereomers as the almost exclusive products with little to no curcumin remaining.31 Shorter reaction times, between 30 min and 4 h, followed by acidification and extraction also gave the bicyclopentadiones as major products and, in addition, several smaller unidentified peaks were detected.16 If reactions are shortened to 20-45 min and injected directly on RP-HPLC (without acidification and extraction) the chromatograms show prominent products that are more polar than the bicyclopentadiones.32 These peaks comprise the spiroepoxide and vinylether as major, and the dihydroxy-, ketohydroxy-, and hemiketal cyclopentadiones as minor products.
Time course experiments established that at physiological pH the spiroepoxide has a longer half life than curcumin. Whether this holds true under more physiological conditions is not clear. The spiroepoxide is electrophilic and subject to reaction with thiols.32
Mechanism of oxidative transformation
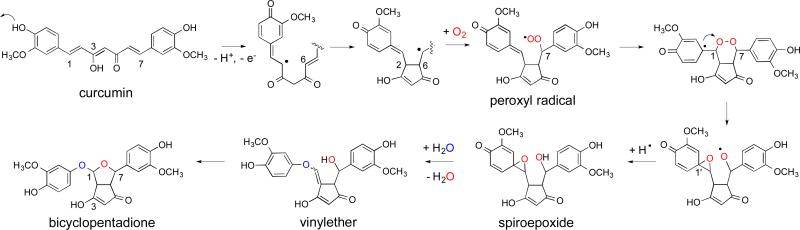
Curcumin and the bicyclopentadione are structurally very different. It is obvious that the degradation must involve a series of discrete reactions resulting in double cyclization and oxygenation of the heptadienedione chain (Fig. 5).32 The two methoxyphenol rings are required for the reaction but participate only indirectly by facilitating certain reaction steps without being changed (see below).30
Fig. 5.
Proposed mechanism for the formation of the bicyclopentadione during autoxidation of curcumin.
Since the m.w. of the bicyclopentadione is increased by 32 mass units compared to curcumin it seemed plausible that O2 would be inserted during the autoxidation and end up in the bicyclopentadione. It was quite surprising then that one 18O from H218O was incorporated into the bicyclopentadione when degradation reactions of curcumin were conducted in labeled buffer.32 Thus, although it is true that O2 is inserted into curcumin during autoxidation, only one of the two oxygen atoms are retained in the final product.
The initiating event of the autoxidation is hydrogen abstraction from one of the phenolic hydroxyls to form a phenoxyl radical.16 The hydrogen abstraction likely occurs as a two-step process of proton loss in the slightly alkaline medium (pH 7.4-8) followed by electron transfer33, 34 from the phenolate anion to molecular oxygen which is reduced to superoxide.16 H-abstraction can also be catalyzed by peroxidases with curcumin acting as a co-substrate, providing an electron to reduce a peroxide. As expected, the addition of H2O2 to the peroxidase-catalyzed transformation of curcumin resulted in a large increase of the enzymatic turnover rate.16
The 1,2-E double bond conjugated to the methoxyphenol ring enables delocalization of the radical to C-2 of the chain leaving the aromatic ring oxidized to a quinone methide. The C-2 radical performs a 5-exo cyclization with the 6E-double bond to form the cyclopentadione ring that is present in all products except for diguaiacol.32 The carbon-centered radical is now localized at C-7 where it reacts with O2 to a peroxyl radical. The peroxyl radical is poised to quench the unstable quinone methide by forming a C-1/C-7 endoperoxide. This brings the radical back to the semiquinone ring. The tertiary radical reacts with the endoperoxide in an intramolecular homolytic substitution reaction (SHi).35, 36 SHi results in cleavage of the endoperoxide O-O bond to give an epoxide (C-1/C-1’-spiroepoxide) and an alkoxyl radical at C-7. Reduction of the alkoxyl radical results in a spiroepoxide cyclopentadione. The reduction may occur as a hydrogen abstraction from curcumin and propagate the free radical chain reaction. The spiroepoxide is the earliest product in the degradation pathway that has been isolated, compatible with being the first non-radical product in the proposed mechanism (Fig. 5).32
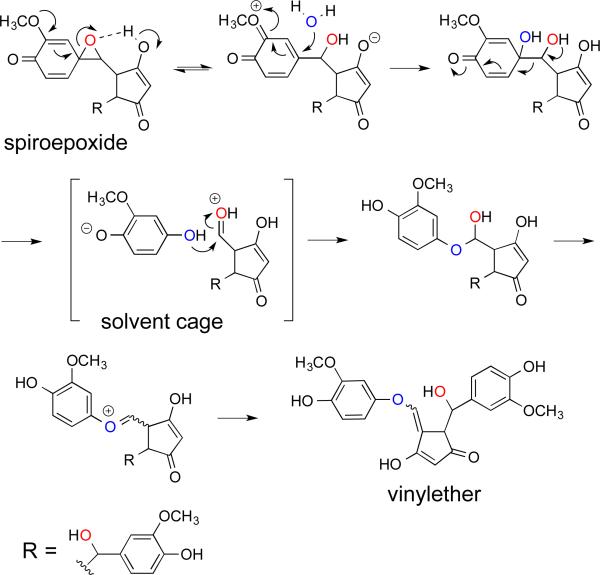
Although hydrolysis of the epoxide by nucleophilic attack of the C-7 hydroxyl would directly lead to the bicyclopentadione,16 this does not occur.32 Instead, the spiroepoxide is transformed to a vinylether as the immediate precursor to the bicyclopentadione. Unexpectedly, the opening of the spiroepoxide also involves the exchange of the epoxide oxygen (that is derived from O2) for an oxygen from H2O. These results can be explained by a mechanism in which the electron-donating effect of the m-methoxy group of the spiroepoxide triggers SN1 opening of the epoxide that is facilitated by hydrogen bonding with the cyclopentadione hydroxyl (Fig. 7).32 Addition of water to the o-quinone intermediate results in a vicinal diol that undergoes C-C bond cleavage, driven by rearomatization of the ring, to an aldehyde and methoxyhydroquinone. The two molecules are in a solvent cage and instantly recombine to a hemiacetal that loses water (containing the original epoxide oxygen) on the way to the vinylether.
Fig. 7.
Proposed mechanism of the exchange of water during transformation of the spiroepoxide to the vinylether.
The by-products of the autoxidation are formed by alternative reactions of intermediates in the proposed mechanism:32
(i) Cyclobutyl cyclopentadione: The peroxyl radical can undergo β-fragmentation (i.e., the loss of O2 to regain the C-7 carbon radical) at a rate that is fast enough to enable 4-exo cyclization with C-1 to form the cyclobutyl ring. Although the rates for these steps have not been determined, the formation of the cyclobutyl product indicates that the quinone methide intermediates have a considerable lifetime that allows them to undergo alternative reactions, especially with nucleophilic protein or small molecule thiols.
(ii) Dihydroxy-, ketohydroxy-, and hemiketal cyclopentadiones: The endoperoxide radical intermediate is a branching point from which not only the spiroepoxide emerges. Reduction of the tertiary radical gives a covalently complete endoperoxide (Fig. 6). The curcumin endoperoxide is unstable and opens to the ketohydroxy cyclopentadione in a Kornblum-DeLaMare rearrangement.37 The ketohydroxy cyclopentadione is in equilibrium with its hemiketal form. Reduction of the endoperoxide gives the dihydroxy cyclopentadione. These reactions are reminiscent of the reactions of the cyclooxygenase-derived prostaglandin endoperoxide PGH2 and its transformations to the prostaglandins PGD2, PGE2, and PGF2α.38
Fig. 6.
Formation of dihydroxy-, ketohydroxy-, and hemiketal cyclopentadiones from a proposed endoperoxide intermediate.
(iii) Diguaiacol: The mechanism of formation of diguaiacol is uncertain. It obviously involves cleavage of the carbon bond connecting the methoxyphenol ring to the heptadienedione chain. Such a cleavage could be facilitated by, e.g., further oxygenation of the endoperoxide radical as has been described to occur during chain cleavage of lipid hydroperoxides.39, 40
(iv) Vanillin: The trace amounts of vanillin detected in RP-HPLC/UV-diode array analyses might be formed by C-C bond cleavage of the alkoxyl radical intermediate. Cleavage is competing with reduction to the spiroepoxide by H-abstraction from curcumin. This origin of vanillin is compatible with data by Wang and co-workers who noted that vanillin was increased when curcumin was incubated in buffer for an extended time.24 Upon prolonged incubation, the reaction will run out of curcumin as a hydrogen donor, and the alkoxyl radical may be prone to undergo cleavage into vanillin.
Oxidative transformation of DMC and BDMC: role of the m-methoxy group
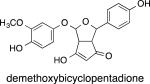
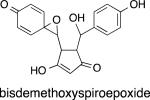
The less abundant curcuminoids DMC and BDMC also can be oxidatively transformed.30 There were, however, two important differences in the oxidation of DMC and BDMC compared with curcumin. First, the rate of autoxidation of DMC was markedly reduced such that its half life is in the range of hours rather than minutes (Table 1). This trend was enhanced in BDMC that did not autoxidize at all. Second, while DMC formed the expected demethoxy bicyclopentadione, BDMC formed a bisdemethoxy spiroepoxide when forced to oxidize. The bisdemethoxy spiroepoxide was stable and did not transform to a bisdemethoxy bicyclopentadione analogue.30
Table 1.
Structures, autoxidation, and products of curcumin and analogues.
| compound/structure | autoxidation (approx. t1/2) | main product |
|---|---|---|
|
|
fast (minutes) |

|

|
slow (hours-days) |
|

|
stable; require enzymatic or chemical oxidation |
|
|
|
slow (hours-days) |

|
|
|
stable | N/A |
The mixture of curcuminoids as it is present in turmeric extract was more stable to autoxidation than predicted based on the relative abundance of curcumin, DMC, and BDMC in the mixture. Thus, not only are DMC and BDMC more stable toward autoxidation, they also appear to protect curcumin from autoxidation.30
These findings point toward a crucial role of the methoxy groups in determining the stability of the curcuminoids toward autoxidation as well as the product profile. The electron donating effect of the methoxy group facilitates transfer of an electron from the phenolate anion to molecular oxygen. The lack of one methoxy group in DMC significantly slows the rate of electron transfer (hydrogen abstraction), although loss of the methoxy group and hydrogen abstraction occur on opposite ends of the molecule. BDMC, lacking both methoxy groups, is stable toward autoxidation. BDMC can be forced to oxidize using horseradish peroxidase/H2O2 or potassium ferricyanide.30
The methoxy group also affects the hydrolysis of the spiroepoxide. The contribution of the methoxy group to SN1 hydrolysis of the epoxide moiety was discussed above.32 It can be deduced that in a DMC spiroepoxide (which is predicted to be formed but has not been isolated) the methoxy group is in the ring that forms the epoxide and thus available to induce hydrolysis of the epoxide. Lack of both methoxy groups in the BDMC spiroepoxide (which has been isolated) considerably increases stability and the compound was stable to acid treatment to pH 3. It is less stable, however, in organic solvent where it quickly decomposes to a mixture of products that have not been identified.30
Manipulation of the phenolic hydroxyl results in similar effects as seen with the methoxy group (Table 1). Substitution of one phenolic hydroxyl by a methoxy group (4’-O-methylcurcumin) leads to greatly enhanced stability although oxidative transformation can be achieved enzymatically (HRP or cyclooxygenase-2/H2O2) or chemically (potassium ferricyanide). The product is a bicyclopentadione with the additional methyl group in the aromatic ring connected to C-7.32 As expected, methylation of both phenolic hydroxyls (4’,4”-O-dimethylcurcumin) abolished oxidative transformation entirely.16
Does autoxidation occur in vivo?
Autoxidation is a prominent reaction when curcumin is incubated with cultured cells in vitro. Whether autoxidation also occurs in vivo is a crucial question for deciding whether this process and its products are biologically relevant. Since so far there is no evidence that oxidative transformation of curcumin does indeed occur in vivo, it is worthwhile to consider factors relevant to this question:
-
(i)
Curcumin is usually consumed as turmeric extract, containing also the minor curcuminoids DMC and BDMC. DMC and BDMC contribute to the stability of curcumin in vitro.30 Whether the presence of DMC and BDMC in turmeric extract results in stabilization of curcumin in vivo remains an open question.
-
(ii)
Albumin and protein in general increase the stability of curcumin toward autoxidation.24 There is little doubt that the abundance of cellular protein in vivo enhances the stability of curcumin toward autoxidation.
-
(iii)
Glucuronidation of curcumin at the phenolic hydroxyl is a major phase II metabolic pathway in vivo. Glucuronidation contributes to enhancing the stability of curcumin by blocking the free hydroxyl required for autoxidative transformation (O.N.G., P.B.L., and C.S., unpublished).
-
(iv)
Peroxidases can use curcumin as reducing co-substrate which may results in enzyme-catalyzed oxidative transformation in vivo.16
-
(v)
Oxidative stress at inflammatory sites may contribute to oxidative transformation of curcumin.
-
(vi)
Oxidative transformation to the bicyclopentadione proceeds through several reactive electrophilic intermediates. In vivo, these will be subject to interception by cellular antioxidant defense mechanisms like adduction with glutathione and N-acetylcysteine.32 As long as the cellular antioxidant defense is functional, only a small fraction of reaction intermediates is predicted to “make it” past the antioxidant barrier and form the bicyclopentadione. Thus, any bicyclopentadione detected in vivo might only be the tip of an iceberg, which could indicate that oxidative transformation may actually be an abundant metabolic pathway.
-
(vii)
The glutathione or N-acetylcysteine adducts of the quinone methide and spiroepoxide reaction intermediates could be more appropriate markers of oxidative transformation of curcumin than the bicyclopentadione.
-
(viii)
The reaction intermediates will not only adduct to small soluble thiols but also to reactive cysteine residues in proteins. Covalent adduction to protein may be a major mechanism by which oxidative metabolites of curcumin exert biological effects.
Thus, there is a number of factors that imply that oxidative transformation might or might not occur in vivo. We consider this an open question that we plan to address by careful analysis of in vivo samples.
Putting metabolism, autoxidation, and biological activity together
How does oxidative transformation relate to the structure-function of curcumin? Curcumin can act as a metal chelator, antioxidant, and Michael acceptor (Fig. 8).41-47 The metal chelating activity of the β-diketo moiety is independent of oxidative transformation. It may be disturbed by the formation of the cyclopentadione ring which is more nucleophilic (acidic) than the β-diketo moiety of curcumin.
Fig. 8.
Structure-activity features of curcumin and its quinone methide.
The antioxidant activity of curcumin is dependent on how readily the phenolic hydroxyl can be abstracted by a lipid peroxyl radical and whether the resulting curcumin radical is less prone to initiate a new lipid peroxidation chain in turn.48 The hydrogen abstraction, of course, is the initiation of curcumin autoxidation with the resulting radical reactions as described in Fig. 5. Dimerization of two curcumin quinone methide radicals has been described as a mechanism of termination.49 The same group also identified linoleate peroxyl radical coupling products with curcumin.50 These studies were performed in organic solvent (where curcumin does not autoxidize), and it would be interesting to analyze equivalent reactions in aqueous medium.
The Michael acceptor activity is undoubtedly influenced by autoxidation of curcumin. We hypothesize that it is even dependent on autoxidation.51 The 1,3-enone (α,β-unsaturated carbonyl) in curcumin is only a weak electrophile because it is part of the delocalized system that spans the entire molecule. Oxidative transformation, specifically the initial hydrogen abstraction and delocalization of the radical into the heptadienedione chain, forms a quinone methide as a strong electrophile (Fig. 8). As discussed above, the quinone methide has a considerable lifetime, and because it is present in three distinct intermediates (before and after 5-exo cyclization and in the peroxyl radical; cf. Fig. 5) it possesses a range of reactivities that are anticipated to allow reaction with different strength cellular nucleophiles. Interestingly, in either case (i.e., in curcumin or its quinone methide) the electrophilic carbon is the same, namely C-1.
Since oxidative transformation is abundant under cell culture conditions its products and intermediates need to be considered as mediators of biological effects. We hypothesize that the quinone methide is the functional electrophile in reactions where curcumin is proposed to form a covalent adduct with nucleophilic protein cysteine residues.52-57 The hypothesis is also indirectly supported by the crucial role of quinone methides as the acting principle in many anti-cancer drugs as well as toxic DNA methylating agents.58-60 The requirement for oxidative transformation has clearly been shown for the topoisomerase poisoning activity of curcumin.61 Neither dimethylcurcumin (which does not autoxidize; Table 1) nor reaction conditions that prevented autoxidation of curcumin resulted in poisoning of topoisomerase. The quinone methide intermediates were suggested as the electrophiles binding to active site cysteines, although the spiroepoxide could not be ruled out.61
Another pertinent example of Michael adduction is the anti-inflammatory activity of curcumin, especially when effects on the NF-κB pathway are invoked.62-64 Both NF-κB and its upstream kinase, IKKβ, are targets of curcumin, and both are inhibited by small molecule electrophiles adducting covalently to regulatory cysteines in critical regions of the protein. A Michael type adduction by curcumin via its enone has been invoked as mechanism, whereas we suggest that the quinone methide is the adducting electrophile. This mechanism of adduct formation requires oxidative transformation of curcumin to form the reactive electrophile. This is a crucial difference because the conditions for oxidative transformation may not be met in vivo, which could result in a discrepancy between in vitro and in vivo effects. There is an expanding number of proteins that are redox-regulated through strategically placed cysteines that undergo reversible redox cycling to sulfenic acids and can be adducted by small molecule electrophiles65. Identification which of these might be cellular targets of curcumin will require proteomics approaches.
A second reason why oxidative transformation is relevant for understanding the biological activity of curcumin is the surprising number of products formed. When added to cultured cells, curcumin rapidly changes from a single molecule to a family of diverse chemotypes. It is not unreasonable to suggest that the free radical, quinone methide, endoperoxide, alkoxyl radical, dihydroxy, hemiacetal, ketohydroxy, spiroepoxy, vinylether and other cyclopentadiones each have a distinct set of cellular targets. Together, these products and their biological activities may add up to the “polypharmacology” of curcumin. We are at the beginning of understanding the remarkable transformations of curcumin in vitro and in vivo. Future studies will show which of the many biological effects of curcumin are due to the parent and which are due to its degradation products.
Acknowledgments
Funding sources
This work was supported by awards CA159382, AT006896, and GM076592 by NCI, NCCIH, and NIGMS, respectively, of the National Institutes of Health (NIH) to C.S. O.N.G. acknowledges support by training grant 2T32GM07628 from NIGMS and a predoctoral fellowship award (F31AT007287) from NCCIH of the NIH. The initial phase of this work was supported by pilot awards from the Vanderbilt Institute in Chemical Biology, the Vanderbilt DDRC (P30DK058404), and the NCI SPORE in GI Cancer (5P50CA095103). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Wright LE, Frye JB, Gorti B, Timmermann BN, Funk JL. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Current pharmaceutical design. 2013;19:6218–25. doi: 10.2174/1381612811319340013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. (Phila) 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howells LM, Mahale J, Sale S, McVeigh L, Steward WP, Thomas A, Brown K. Translating curcumin to the clinic for lung cancer prevention: evaluation of the preclinical evidence for its utility in primary, secondary, and tertiary prevention strategies. J. Pharmacol. Exp. Ther. 2014;350:483–94. doi: 10.1124/jpet.114.216333. [DOI] [PubMed] [Google Scholar]
- 4.Lopresti AL, Maes M, Maker GL, Hood SD, Drummond PD. Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014;167:368–75. doi: 10.1016/j.jad.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Brondino N, Re S, Boldrini A, Cuccomarino A, Lanati N, Barale F, Politi P. Curcumin as a therapeutic agent in dementia: a mini systematic review of human studies. TheScientificWorldJournal. 2014;2014:174282. doi: 10.1155/2014/174282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin D, Huebbe P, Pallauf K, Rimbach G. Neuroprotective properties of curcumin in Alzheimer's disease--merits and limitations. Curr. Med. Chem. 2013;20:3955–85. doi: 10.2174/09298673113209990210. [DOI] [PubMed] [Google Scholar]
- 7.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Heger M, van Golen RF, Broekgaarden M, Michel MC. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014;66:222–307. doi: 10.1124/pr.110.004044. [DOI] [PubMed] [Google Scholar]
- 9.Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. Engl. 2012;51:5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 10.Cottart CH, Nivet-Antoine V, Beaudeux JL. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2014;58:7–21. doi: 10.1002/mnfr.201200589. [DOI] [PubMed] [Google Scholar]
- 11.Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Resveratrol in primary and secondary prevention of cardiovascular disease: a dietary and clinical perspective. Annals of the New York Academy of Sciences. 2013;1290:37–51. doi: 10.1111/nyas.12150. [DOI] [PubMed] [Google Scholar]
- 12.Sak K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer. 2014;66:177–93. doi: 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- 13.Shirakami Y, Shimizu M, Moriwaki H. Cancer chemoprevention with green tea catechins: from bench to bed. Curr. Drug Targets. 2012;13:1842–57. doi: 10.2174/138945012804545506. [DOI] [PubMed] [Google Scholar]
- 14.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug. Metab. Dispos. 1999;27:486–94. [PubMed] [Google Scholar]
- 15.Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008;17:1411–7. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griesser M, Pistis V, Suzuki T, Tejera N, Pratt DA, Schneider C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J. Biol. Chem. 2011;286:1114–24. doi: 10.1074/jbc.M110.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan S, Rupasinghe TW, Tull DL, Boughton B, Oliver C, McSweeny C, Gras SL, Augustin MA. Degradation of curcuminoids by in vitro pure culture fermentation. J. Agric. Food Chem. 2014;62:11005–15. doi: 10.1021/jf5031168. [DOI] [PubMed] [Google Scholar]
- 18.Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6615–20. doi: 10.1073/pnas.1016217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP, Gescher A. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–64. [PubMed] [Google Scholar]
- 20.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomarkers Prev. 2002;11:105–11. [PubMed] [Google Scholar]
- 21.Hoehle SI, Pfeiffer E, Solyom AM, Metzler M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J. Agric. Food Chem. 2006;54:756–64. doi: 10.1021/jf058146a. [DOI] [PubMed] [Google Scholar]
- 22.Aggarwal BB, Deb L, Prasad S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules. 2014;20:185–205. doi: 10.3390/molecules20010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu JC, Tsai ML, Lai CS, Wang YJ, Ho CT, Pan MH. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food & function. 2014;5:12–7. doi: 10.1039/c3fo60370a. [DOI] [PubMed] [Google Scholar]
- 24.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997;15:1867–76. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 25.Das R, Roy A, Dutta N, Majumder HK. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis : an international journal on programmed cell death. 2008;13:867–82. doi: 10.1007/s10495-008-0224-7. [DOI] [PubMed] [Google Scholar]
- 26.Hail N., Jr. Mitochondrial reactive oxygen species affect sensitivity to curcumin-induced apoptosis. Free Radic. Biol. Med. 2008;44:1382–93. doi: 10.1016/j.freeradbiomed.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Choi H, Chun YS, Shin YJ, Ye SK, Kim MS, Park JW. Curcumin attenuates cytochrome P450 induction in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by ROS-dependently degrading AhR and ARNT. Cancer Sci. 2008;99:2518–24. doi: 10.1111/j.1349-7006.2008.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan R, Mo Y, Zhang X, Chien S, Tollerud DJ, Zhang Q. Matrix metalloproteinase-2 and -9 are induced differently by metal nanoparticles in human monocytes: The role of oxidative stress and protein tyrosine kinase activation. Toxicol. Appl. Pharmacol. 2008;233:276–85. doi: 10.1016/j.taap.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan MA, Gahlot S, Majumdar S. Oxidative stress induced by curcumin promotes the death of cutaneous T-cell lymphoma (HuT-78) by disrupting the function of several molecular targets. Mol. Cancer Ther. 2012;11:1873–83. doi: 10.1158/1535-7163.MCT-12-0141. [DOI] [PubMed] [Google Scholar]
- 30.Gordon ON, Luis PB, Ashley RE, Osheroff N, Schneider C. Oxidative transformation of demethoxy- and bisdemethoxycurcumin: Products, mechanism of formation, and poisoning of topoisomerase. doi: 10.1021/acs.chemrestox.5b00009. submitted 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon ON, Schneider C. Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol. Med. 2012;18:361–3. doi: 10.1016/j.molmed.2012.04.011. author reply 363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon ON, Luis PB, Sintim HO, Schneider C. Unraveling curcumin degradation. Autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J. Biol. Chem. 2015 doi: 10.1074/jbc.M114.618785. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litwinienko G, Ingold KU. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004;69:5888–96. doi: 10.1021/jo049254j. [DOI] [PubMed] [Google Scholar]
- 34.Litwinienko G, Ingold KU. Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc. Chem. Res. 2007;40:222–30. doi: 10.1021/ar0682029. [DOI] [PubMed] [Google Scholar]
- 35.Porter NA, Zuraw PJ, Sullivan JA. Peroxymercuration-demercuration of lipid hydroperoxides. Tetrahed. Lett. 1984;25:807–810. [Google Scholar]
- 36.Schneider C, Boeglin WE, Brash AR. Identification of two cyclooxygenase active site residues, leucine-384 and glycine-526, that control carbon ring cyclization in prostaglandin biosynthesis. J. Biol. Chem. 2004;279:4404–4414. doi: 10.1074/jbc.M307431200. [DOI] [PubMed] [Google Scholar]
- 37.Kornblum N, DeLaMare HE. The base catalyzed decomposition of a dialkyl peroxide. J. Am. Chem. Soc. 1951;73:880–81. [Google Scholar]
- 38.Hamberg M, Samuelsson B. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 1967;242:5344–5354. [PubMed] [Google Scholar]
- 39.Schneider C, Boeglin WE, Yin H, Porter NA, Brash AR. Intermolecular peroxyl radical reactions during autoxidation of hydroxy and hydroperoxy arachidonic acids generate a novel series of epoxidized products. Chem. Res. Toxicol. 2008;21:895–903. doi: 10.1021/tx700357u. [DOI] [PubMed] [Google Scholar]
- 40.Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J. Biol. Chem. 2008;283:15539–43. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minear S, O'Donnell AF, Ballew A, Giaever G, Nislow C, Stearns T, Cyert MS. Curcumin inhibits growth of Saccharomyces cerevisiae through iron chelation. Eukaryot. Cell. 2011;10:1574–81. doi: 10.1128/EC.05163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiao Y, Wilkinson J. t., Christine Pietsch E, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006;40:1152–60. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Jiao Y, Wilkinson J. t., Di X, Wang W, Hatcher H, Kock ND, D'Agostino R, Jr., Knovich MA, Torti FM, Torti SV. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113:462–9. doi: 10.1182/blood-2008-05-155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuda T, Hidaka K, Shinohara A, Maekawa T, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J. Agric. Food Chem. 1999;47:71–77. doi: 10.1021/jf9805348. [DOI] [PubMed] [Google Scholar]
- 46.Amolins MW, Peterson LB, Blagg BS. Synthesis and evaluation of electron-rich curcumin analogues. Bioorg. Med. Chem. 2009;17:360–7. doi: 10.1016/j.bmc.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinkova-Kostova AT, Abeygunawardana C, Talalay P. Chemoprotective properties of phenylpropenoids, bis(benzylidene)cycloalkanones, and related Michael reaction acceptors: correlation of potencies as phase 2 enzyme inducers and radical scavengers. J. Med. Chem. 1998;41:5287–96. doi: 10.1021/jm980424s. [DOI] [PubMed] [Google Scholar]
- 48.Schneider C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 49.Masuda T, Toi Y, Bando H, Maekawa T, Takeda Y, Yamaguchi H. Structural identification of new curcumin dimers and their contribution to the antioxidant mechanism of curcumin. J. Agric. Food Chem. 2002;50:2524–30. doi: 10.1021/jf011601s. [DOI] [PubMed] [Google Scholar]
- 50.Masuda T, Maekawa T, Hidaka K, Bando H, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcumin: analysis of oxidative coupling products from curcumin and linoleate. J. Agric. Food Chem. 2001;49:2539–47. doi: 10.1021/jf001442x. [DOI] [PubMed] [Google Scholar]
- 51.Gordon O, Schneider C. Spice of Life. Chem Ind-London. 2014;78:36–39. [Google Scholar]
- 52.van Iersel ML, Ploemen JP, Lo Bello M, Federici G, van Bladeren PJ. Interactions of alpha, beta-unsaturated aldehydes and ketones with human glutathione S-transferase P1-1. Chem. Biol. Interact. 1997;108:67–78. doi: 10.1016/s0009-2797(97)00096-3. [DOI] [PubMed] [Google Scholar]
- 53.Jurrmann N, Brigelius-Flohe R, Bol GF. Curcumin blocks interleukin-1 (IL-1) signaling by inhibiting the recruitment of the IL-1 receptor-associated kinase IRAK in murine thymoma EL-4 cells. J. Nutr. 2005;135:1859–64. doi: 10.1093/jn/135.8.1859. [DOI] [PubMed] [Google Scholar]
- 54.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 55.Jung Y, Xu W, Kim H, Ha N, Neckers L. Curcumin-induced degradation of ErbB2: A role for the E3 ubiquitin ligase CHIP and the Michael reaction acceptor activity of curcumin. Biochim. Biophys. Acta. 2007;1773:383–90. doi: 10.1016/j.bbamcr.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Pullakhandam R, Srinivas PN, Nair MK, Reddy GB. Binding and stabilization of transthyretin by curcumin. Arch. Biochem. Biophys. 2009;485:115–9. doi: 10.1016/j.abb.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, Neckers L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2006;2:169–74. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- 58.Tomasz M, Lipman R, Chowdary D, Pawlak J, Verdine GL, Nakanishi K. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science. 1987;235:1204–8. doi: 10.1126/science.3103215. [DOI] [PubMed] [Google Scholar]
- 59.Thompson DC, Thompson JA, Sugumaran M, Moldeus P. Biological and toxicological consequences of quinone methide formation. Chem. Biol. Interact. 1993;86:129–62. doi: 10.1016/0009-2797(93)90117-h. [DOI] [PubMed] [Google Scholar]
- 60.Awad HM, Boersma MG, Boeren S, Van Bladeren PJ, Vervoort J, Rietjens IM. Quenching of quercetin quinone/quinone methides by different thiolate scavengers: stability and reversibility of conjugate formation. Chem. Res. Toxicol. 2003;16:822–31. doi: 10.1021/tx020079g. [DOI] [PubMed] [Google Scholar]
- 61.Ketron AC, Gordon ON, Schneider C, Osheroff N. Oxidative metabolites of curcumin poison human type II topoisomerases. Biochemistry. 2013;52:221–7. doi: 10.1021/bi3014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J. Immunol. 1999;163:3474–83. [PubMed] [Google Scholar]
- 63.Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-κB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 64.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IκB kinase and NFκB activation in macrophages. Biochem. Pharmacol. 2000;60:1665–1676. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 65.Lo Conte M, Carroll KS. The redox biochemistry of protein sulfenylation and sulfinylation. J. Biol. Chem. 2013;288:26480–8. doi: 10.1074/jbc.R113.467738. [DOI] [PMC free article] [PubMed] [Google Scholar]