Abstract
Planar cell polarity, the orientation of single-cell asymmetries within the plane of a multicellular tissue, is essential to generating the shape and dimensions of organs and organisms. Planar polarity systems align cell behavior with the body axes and orient the cellular processes that lead to tissue elongation. Using Drosophila as a model system, significant progress has been made toward understanding how planar polarity is generated by biochemical and mechanical signals. Recent studies using time-lapse imaging reveal that cells engage in a number of active behaviors whose orientation and dynamics translate planar cell polarity into tissue elongation. Here we review recent progress in understanding the cellular mechanisms that link planar polarity to large-scale changes in tissue structure.
Keywords: Planar polarity, Elongation, Drosophila, Adhesion, Myosin
1. Introduction
In multicellular organisms, epithelial monolayers form barriers between compartments that serve different physiological functions. The three-dimensional structure of epithelial tissues is essential for the development of many organ systems and for the organization of the body plan. To execute these diverse functions, epithelial cells develop apical–basal polarity in which the baso-lateral surface contacts neighboring cells and the apical surface contacts the external environment. In addition, many epithelia display planar cell polarity, the organization of cellular asymmetries with respect to the plane of the tissue, perpendicular to the apical–basal axis. Planar cell polarity is easily visualized in the alignment of external structures such as mammalian hair follicles and the hairs and bristles of the Drosophila cuticle, and is also seen in the oriented cell behaviors that produce tissues of the proper shape and dimensions. Planar polarity requires the establishment of molecular asymmetries within cells and spatial cues that coordinate these polarities with neighboring cells and with the body axes.
Tissue elongation is an evolutionarily conserved outcome of planar polarity systems during development [1–3]. Here we highlight new insights into the cellular and molecular mechanisms by which planar polarity leads to tissue elongation in Drosophila, using examples from the wing, embryo, and egg chamber. These studies reveal new roles for cytoskeletal proteins and the extracellular matrix in generating planar polarity and identify unexpected cell behaviors that lead to large-scale changes in tissue structure.
2. Shaping the Drosophila wing through mechanical forces and spatially regulated microtubule dynamics
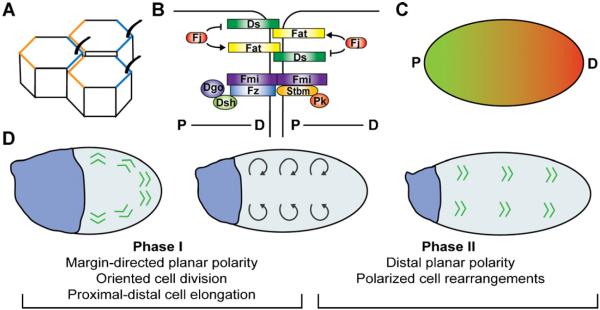
A readily apparent manifestation of planar polarity in Drosophila is the orientation of the external hairs and bristles that populate the adult cuticle. Each of the approximately 30,000 cells in the Drosophila wing generates a single hair that extends distally from the distal cell cortex. Genetic studies have identified proteins that are required for the planar organization of wing hairs, including the Frizzled-dependent planar cell polarity (PCP) pathway and the Fat and Dachsous atypical protocadherins. The PCP pathway is comprised of six core proteins that localize to adherens junctions and define complementary proximal and distal domains (Fig. 1A and B). Frizzled is a seven-pass transmembrane protein that localizes distally, together with the cytosolic proteins Dishevelled and Diego [4–6]. Strabismus (also known as Van Gogh) is a four-pass transmembrane protein that localizes proximally with the cytosolic protein Prickle [7,8]. The atypical cadherin Flamingo (also known as Starry night) localizes to both proximal and distal domains [9,10]. The planar polarized localization of each component requires the activity of all six core proteins. Clones of cells that lack or overexpress Frizzled or Strabismus induce polarity defects in surrounding wild-type cells, demonstrating that planar cell polarity is influenced by local cell interactions [11–13]. Genetic and biochemical studies have suggested several mechanisms for this intercellular signaling [14–17], described in detail in several comprehensive reviews [18–21].
Fig. 1.
Planar cell polarity and tissue elongation in the Drosophila wing. (A) The core planar cell polarity (PCP) proteins localize to proximal (orange) and distal (blue) cell surfaces and are required for planar polarized wing hair formation. (B) Four-jointed (Fj) phosphorylates Fat and Dachsous (Ds) and regulates their interaction. The core PCP proteins form molecularly distinct proximal (P) and distal (D) complexes. (C) Graded expression of Ds (green) and Fj (red) may provide spatial information important for planar polarity. (D) Proximal–distal planar polarity develops from an initially margin-directed pattern (indicated by green arrowheads) over a 32-h period during pupal development. In Phase I (early pupal stages), contraction of the proximal wing hinge (blue) is proposed to generate a force that reorients planar polarity (gray arrows), cell rearrangements, cell divisions, and cell elongation to align with the proximal–distal axis, causing the wing blade to narrow and lengthen. In Phase II (mid-pupal stages), new contacts between cells are assembled parallel to the proximal–distal axis, increasing hexagonal packing and the alignment of planar polarity. Diego (Dgo), Dishevelled (Dsh), Four-jointed (Fj), Flamingo (Fmi), Frizzled (Fz), Prickle (Pk), Strabismus (Stbm). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
In addition to local communication between cells, a global input is predicted to be required to align cellular planar asymmetries with the tissue axes. The nature of this global spatial cue is not well understood. The atypical cadherins Fat and Dachsous and the Golgi resident kinase Four-jointed (Fj) are candidates for providing a global directional cue [22,23]. Dachsous and Fj are expressed in opposing transcriptional gradients in the wing, with higher Dachsous expression proximally and higher Fj expression distally (Fig. 1C) [23,24]. Reversing these gradients is sufficient to reverse the direction of wing hair polarity, suggesting that these gradients could provide an instructive directional cue [25,26]. Fat and Dachsous can interact in trans between cells [25]. Phosphorylation of Fat by Fj promotes the ability of Fat to bind Dachsous, whereas phosphorylation of Dachsous by Fj inhibits Dachsous binding to Fat [27–29]. Adjacent cells with different levels of Dachsous and Fj are predicted to have more Dachsous protein at their distal surface and more Fat protein on their proximal surface (Fig. 1B), although these asymmetries have not been visualized directly. The Fat-Ds cadherins are proposed to impart spatial information to the core PCP proteins, as PCP protein localization and activity are disrupted in fat and dachsous mutants [22,23,30]. However, Fat and Dachsous can act through effectors other than Frizzled, and other upstream cues can orient Frizzled-PCP signaling in certain contexts [14,31,32]. Thus the molecular connections between these two systems are currently unclear. Moreover, while flattening the Dachsous and Fj gradients disrupts planar polarity in the Drosophila eye [26], planar polarity in most regions of the wing can be rescued by uniform Dachsous expression [25,26] or by rendering Fat insensitive to the Dachsous gradient by deleting its extracellular domain [33], indicating that other signals contribute to planar polarity in the wing.
Recent studies have provided insight into the cell biological mechanisms of planar polarity. Advances in ex vivo tissue culture approaches, time-lapse imaging, and quantitative analysis reveal that planar polarity does not emerge just prior to wing hair growth, but is dynamically reoriented from a margin-directed distribution present earlier in development (Fig. 1D) [34,35]. The alignment of planar polarity with the proximal–distal axis coincides with contraction of the wing hinge, suggesting that an external force generated by hinge contraction could provide a global mechanical cue that orients planar polarity [35]. Consistent with this idea, severing the hinge from the wing to disrupt their mechanical connection results in planar polarity defects [35].
At the same time as wing hinge contraction, cells in the wing blade engage in dynamic behaviors that promote the lengthening and narrowing of the tissue, including oriented cell divisions, proximal–distal cell elongation, and polarized cell rearrangement (Fig. 1D) [35]. Removing the hinge abolishes the cell elongation and disrupts the orientation of cell divisions and cell rearrangements [35]. These results raise the possibility that wing hinge contraction could promote planar polarity by guiding cell behavior. In support of this idea, a theoretical model indicates that shear forces and oriented cell division are in principle sufficient to convert planar polarity from a margin-directed to a proximodistal pattern, assuming that planar polarity aligns with the long axis of the cell [35]. Proximal–distal cell elongation and oriented cell division, but not polarized cell rearrangements, require Dachsous activity [35,36], perhaps accounting for the defects in planar polarity and wing blade elongation in dachsous mutants.
Cells of the developing wing also display a planar polarized organization of apical microtubules that align parallel to the proximal–distal axis [37–40]. In addition, microtubules in the proximal wing display a consistent 5–7% enrichment of microtubule plus ends at the distal cortex [40]. In dachsous mutants, microtubules are less aligned with the proximal–distal axis and no longer display distal plus-end asymmetry [40]. Flattening the Dachsous gradient disrupts distal plus-end asymmetry (but not microtubule alignment), and reversing the Dachsous gradient causes microtubule plus ends to become enriched proximally [40]. These results suggest that Dachsous could direct planar cell polarity by regulating microtubule organization [40], perhaps by influencing microtubule-dependent Frizzled transport [39]. Consistent with a role for microtubules in this process, loss of Widerborst, the β′ regulatory subunit of protein phosphatase 2A, or overexpression of the Par-1 serine/threonine kinase both disrupt microtubule organization and planar polarity [40,41].
These studies demonstrate new roles for microtubule organization and mechanical forces in regulating cell behavior and microtubule organization in the Drosophila wing. The mechanisms that convert wing hinge contraction into planar polarized cell behavior, and whether Dachsous acts as part of this mechanotransduction pathway or in parallel, are not understood. How do mechanical forces orient cell behavior, and what determines whether cells respond by rearranging, changing shape, or dividing? How does Dachsous regulate planar cell polarity and microtubule organization, and is the subtle bias in microtubule orientation sufficient to direct vesicle trafficking and protein localization? Finally, cells in the distal wing are planar polarized prior to wing hinge contraction [35] and do not display obvious microtubule plus-end asymmetry [40], indicating that mechanisms that impart spatial information to these cells remain to be discovered.
3. Planar polarized contractility and cell adhesion promote Drosophila axis elongation
One of the earliest morphogenetic feats performed by an embryo is the formation of a body axis of the correct shape and dimensions. During axis elongation in the Drosophila embryo, polarized cell behaviors cause the germband epithelium to double in length along the anterior–posterior (AP) axis and narrow in width along the dorsal–ventral (DV) axis (Fig. 2A). Axis elongation involves the coordination of many cell behaviors, including cell rearrangements, oriented cell divisions, and transient and sustained changes in cell shape [42–45].
Fig. 2.
Axis elongation in the Drosophila embryo. (A) During axis elongation, the germband epithelium (dark gray) lengthens 2.5-fold along the anterior–posterior (AP) axis and narrow along the dorsal–ventral (DV) axis. 80% of this elongation occurs in the first 30–40 min and is driven primarily by cell rearrangement. (B) During neighbor exchange, a single myosin-positive cell–cell interface (red) contracts, forming a 4-cell vertex that resolves through the formation of a new interface (blue) between a dorsal and ventral cell. (C) During rosette formation, the coordinated contraction of several consecutive myosin-positive interfaces (red) generates a multicellular rosette structure that resolves in a perpendicular direction, promoting elongation. Cells are labeled with E-cadherin: GFP. Anterior left, ventral down. Scale bar = 10 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Polarized cell behaviors during Drosophila axis elongation can be explained in large part by the localization, dynamics and activity of nonmuscle myosin II, a contractile force-generating actin motor protein. Myosin II is enriched at interfaces between cells along the AP axis [46], where it promotes cortical contraction leading to local neighbor exchange [47] (Fig. 2B). In addition, the integration of myosin contractility across multiple pairs of cells generates multicellular rosette structures [48] (Fig. 2C). Rosettes form and resolve directionally, promoting axis elongation. Quantitative staging of embryos based on measures of topological disorder [49] revealed that rosettes are most prevalent in stage 8 [48], perhaps explaining why they were overlooked in previous studies that focused on stage 7, when the embryo has only reached one-third of its final length [47,50].
Biophysical approaches provide evidence that a mechanical feedback mechanism organizes contractile activity to generate multicellular myosin cables during rosette formation [51]. Consistent with this idea, an ectopic force is sufficient to recruit myosin to the cortex in Drosophila [51,52], as previously shown for single cells in Dictyostelium [53]. Conversely, relieving tension by laser ablation leads to a rapid decrease in cortical myosin [51]. Fluorescence recovery after photobleaching experiments demonstrate that myosin dissociation from the cortex is decreased in regions of high tension, in part through the inhibition of myosin lateral diffusion [51]. These results suggest a positive feedback loop in which myosin generates tension and tension recruits more myosin to the cortex, triggering a wave of myosin localization that propagates contractile activity from cell to cell. The molecular basis of this proposed mechanotransduction mechanism is not well understood. In particular, it is not known if mechanical forces act directly on myosin and its regulatory proteins, or if tension regulates myosin dynamics indirectly through force-dependent changes in cytoskeletal or junctional organization. Tension-dependent changes in myosin localization may serve several functions, including restoring cell shape in response to deformation [53], coordinating the extent and directionality of polarized cell rearrangements [51], and stabilizing supracellular contractile cables involved in epithelial sheet advance [54,55], tube invagination [56], compartment boundary formation [57,58], and wound healing [59,60].
In addition to planar polarized cell rearrangements, intercalating cells also elongate in the direction of tissue elongation, a cell-shape change that is most apparent when intercalation is disrupted [42,61]. This elongation has been proposed to be generated by external forces produced by the ventral mesoderm, the posterior endoderm, or contractions of the dorsal ectoderm [42,61]. In addition, intercalating cells undergo cycles of apical contraction and expansion on a timescale of tens of seconds that are associated with a dynamic actomyosin meshwork at the medial (apical) cell cortex [62–64]. Live imaging studies reveal that medial myosin aggregates flow toward contracting cell interfaces where they merge with junctional myosin [62]. It is not known if the movement of medial myosin toward shrinking junctions is a cause or effect of junctional contraction. Peak levels of medial myosin correlate with the maximum rate of edge contraction in a small sample of cells [62]. However, high levels of junctional myosin are present throughout edge contraction, and contractile activity in either or both structures could contribute to junctional remodeling [50,51]. Methods that selectively target medial or junctional myosin will be necessary to resolve the contributions of these different contractile structures to elongation.
In addition to planar polarized myosin activity, the disassembly of cell–cell junctions is required to translate actomyosin contractility into global tissue reorganization. The adherens junction proteins E-cadherin (also known as shotgun in Drosophila), β-catenin (also known as armadillo), and the multi-PDZ domain protein Par-3 (also known as bazooka) are concentrated at interfaces between neighboring cells along the DV axis in a planar polarized fashion [46,48]. Par-3 activity is required for the correct placement of adherens junctions along the apical–basal axis [65], and for the planar polarized accumulation of adherens junctions at interfaces between dorsal and ventral cells [66]. Rho-kinase, a conserved upstream activator of myosin [67,68], is asymmetrically enriched at shrinking interfaces between anterior and posterior cells, where it is required both to recruit myosin to shrinking edges and to antagonize the cortical localization of Par-3 [66]. Rho-kinase can phosphorylate Par-3 on its C-terminal domain in vitro, and this domain is required for Par-3 planar polarity in vivo [66]. Phosphorylation by Rho-kinase also dissociates Par-3 from the cortex in cultured mammalian cells, although the molecular mechanism may differ [69]. These results indicate that multiple aspects of planar polarity are coordinated by a single kinase that targets substrates involved in contractility and adhesion to different cellular domains. In addition to the stabilization of adherens junctions by Rho-kinase and Par-3, shrinking cell contacts are enriched for the clathrin-dependent endocytic machinery, suggesting that endocytosis of the E-cadherin complex could contribute to junctional disassembly [70].
The spatial cues that control planar cell polarity during axis elongation in Drosophila are independent of several components of the Frizzled-PCP pathway [46], but instead require the striped pattern of cell fates along the AP axis [42,46,71]. The striped expression of the Even-skipped (Eve) and Runt transcriptional regulators along the AP axis is necessary for planar polarity and axis elongation [42,46], and clones of cells misexpressing Eve or Runt are sufficient to reorient planar polarity along the clone boundary [46]. These results suggest that myosin is recruited to the cortex by local differences in gene expression. Identification of the targets that link gene expression to planar polarity would fill a critical gap in understanding how cell–cell communication generates tissue organization.
4. Tissue elongation through whole-tissue rotations in the Drosophila egg chamber
The Drosophila egg chamber is emerging as another excellent system for studying how planar cell polarity influences tissue structure [72]. The egg chamber consists of fifteen supporting nurse cells that share a continuous cytoplasm with the oocyte, all of them encapsulated by a follicular epithelium. After budding from the germarium, the egg chamber undergoes substantial growth and a 2.5-fold increase in aspect ratio, elongating along its anterior–posterior axis. Unlike the wing and embryo, elongation of the egg chamber does not appear to involve cell rearrangements, cell-shape changes, or oriented cell division.
During egg elongation, follicle cells display planar polarized basal actin filaments that align parallel to the dorsal–ventral axis, perpendicular to the direction of elongation [73]. Several mutants that fail to properly localize actin have defects in egg elongation [74–78]. Based on these observations, it has been proposed that actin filaments encircling the egg generate a molecular corset that promotes AP egg elongation by constraining growth along the DV axis [73,75,76]. However, this model has been difficult to reconcile with pharmacological studies showing that drugs that disrupt actin filaments do not cause eggs to round up, as would be predicted if actin were the source of the constrictive force [79].
It has long been appreciated that interactions between the follicular epithelium and the surrounding extracellular matrix (ECM) are required for proper egg elongation [72]. The ECM components laminin A [74], Perlecan [80] and collagen IV [81] assemble into fibrils that lie parallel to the DV axis of the egg, similar to the orientation of basal actin filaments in the adjacent follicle cells. The ECM is necessary for egg elongation, as mutants for collagen IV and the Drosophila integrin βPS subunit (also known as myospheroid) fail to fully elongate [76,81], and egg elongation defects are observed in mutants for several components involved in cell–matrix interactions [77,80,82,83].
Recent studies have used time-lapse live imaging to directly investigate follicle cell behavior during egg chamber elongation. Strikingly, the entire follicle epithelium was found to undergo multiple rotations around its long (AP) axis during elongation, with each egg chamber completing three rotations on average (Fig. 3) [81]. Egg chamber rotations coincide with a 1.8-fold increase in aspect ratio, raising the possibility that egg rotation and elongation are mechanistically linked. The ECM is necessary for egg rotation to occur, as eggs lacking collagen IV or βPS integrin fail to sustain rotation and eventually lose their elongated shape [81]. These results demonstrate that egg rotation behaviors require the extracellular matrix, and that defects in egg rotation correlate with a disruption in egg elongation.
Fig. 3.
Elongation of the Drosophila egg chamber. (A) During egg chamber elongation, the Drosophila egg undergoes a 2.5-fold increase in aspect ratio in a process involving multiple rotations (blue arrow) of the entire egg chamber around its long axis in stages 5–9 (~20 h) and periodic contractions of a planar polarized basal actomyosin network in stages 9–10 (~16 h) that produce transient oscillations in basal area during a period of rapid egg chamber growth. (B) During rotation, actin filaments at the basal surface of the follicle cells (red) and collagen IV fibrils in the ECM (green) become aligned parallel to the DV axis. Anterior left, ventral down. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
What is the purpose of egg chamber rotation and how does it influence egg shape? Genetic and live imaging studies are consistent with a model in which egg rotation is required for the establishment of a planar polarized ECM [79,81]. Collagen IV alignment coincides with the onset of rotation, and collagenase treatment of eggs that have completed rotation causes them to round up [79,81]. In cultured mammalian cells, forces generated by actin-based cell migration or cell traction lead to conformational changes in the ECM [84] and the alignment of collagen fibrils [85,86]. These results suggest that whole-tissue rotations of the egg chamber about its AP axis could impart planar polarity to the ECM, and that collagen IV, and not actin, could provide the molecular corset's restrictive force. However, eggs lacking collagen IV are not only defective for rotation, but also display defects in actin planar polarity [81], and defects in actin planar polarity are characteristic of several mutants that disrupt cell–matrix interactions [76,82,83]. It is likely that coordination between egg chamber rotations, cell–matrix interactions, and actin organization is necessary to establish and maintain egg chamber structure.
At later stages of oogenesis, actomyosin contractility at the apical [87] or basal [88] cortex of the follicular epithelium has been proposed to resist the pressure from a nearly 10-fold increase in egg chamber volume, channeling oocyte growth into further AP elongation. Time-lapse imaging studies reveal that follicle cells undergo cycles of basal contraction and relaxation that produce transient and anisotropic changes in basal cell area, with most of the changes occurring along the dorsal–ventral axis [88]. Treatments that enhance basal myosin activity lead to hyperelongation, strongly suggesting that myosin contractility promotes egg elongation [88]. Similar myosin-driven oscillatory cell behaviors occur in cells of the elongating germband [63,64], as well as in other epithelial tissues that do not elongate, such as apically constricting cells of the Drosophila mesoderm and amnioserosa [89–92]. These oscillations are reminiscent of focal contractile behaviors in cells engaged in distinct behaviors in Caenorhabditis elegans [93] and Xenopus [94,95], suggesting that they may represent an intrinsic feature of contractile networks. Further work will be required to determine whether the oscillatory nature of these shape changes influences the extent or dynamics of tissue elongation.
5. Conclusions
The transmission of mechanical forces between cells in epithelial tissues may be particularly well suited to providing planar information. The three examples of tissue elongation described in this review illustrate how cells use biochemical and mechanical signals to acquire spatial information about their environment and highlight the range of strategies that organize cell polarity and behavior. These include the effect of wing contraction and the Dachsous cadherin on polarized cell behavior in the Drosophila wing, the role of spatially regulated contractility and adhesion in polarized cell rearrangements in the embryo, and the interplay between cell–matrix interactions, the cytoskeleton, and whole-tissue rotations during elongation of the egg chamber. While the molecular mechanisms of junctional and cytoskeletal regulation are beginning to be understood, the upstream spatial cues that break planar symmetry and set planar polarized morphogenetic events in motion are still largely unknown. Identification and characterization of the global spatial cues that orient planar cell polarity will be essential to understanding how biochemical and mechanical interactions at the level of cells and molecules are integrated to produce large-scale tissue structure.
Acknowledgments
We thank the members of the Zallen lab and Richard Zallen for comments on the review. Work in the authors' laboratory is supported by a Burroughs Wellcome Fund Career Award in the Biomedical Sciences, a W.M. Keck Foundation Distinguished Young Scholar in Medical Research Award, and NIH/NIGMS R01 grant GM079340 to JAZ. AV was supported by an NIH T32 grant. JAZ is an Early Career Scientist of the Howard Hughes Medical Institute.
References
- [1].Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–63. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- [2].Skoglund P, Keller R. Integration of planar cell polarity and ECM signaling in elongation of the vertebrate body plan. Curr Opin Cell Biol. 2010;22:589–96. doi: 10.1016/j.ceb.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–33. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–7. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–75. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- [6].Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–76. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- [7].Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–81. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- [8].Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–14. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- [9].Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–95. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- [10].Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–63. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- [11].Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–51. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- [12].Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–9. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- [13].Taylor J, Abramova N, Charlton J, Adler PN, Van Gogh. a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–72. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, et al. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–64. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–9. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–63. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964–71. doi: 10.1016/j.semcdb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [20].Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–92. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–88. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- [23].Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–7. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- [24].Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–96. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- [25].Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–94. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- [26].Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–84. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- [27].Ishikawa HO, Takeuchi H, Haltiwanger RS, Irvine KD. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–4. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–10. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of Fat: Dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–7. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–68. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- [31].Repiso A, Saavedra P, Casal J, Lawrence PA. Planar cell polarity: the orientation of larval denticles in Drosophila appears to depend on gradients of Dachsous and Fat. Development. 2010;137:3411–5. doi: 10.1242/dev.047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Donoughe S, DiNardo S. dachsous and frizzled contribute separately to planar polarity in the Drosophila ventral epidermis. Development. 2011;138:2751–9. doi: 10.1242/dev.063024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–24. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- [34].Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–17. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- [35].Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–86. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- [36].Baena-Lopez LA, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol. 2005;15:1640–4. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- [37].Eaton S, Wepf R, Simons K. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J Cell Biol. 1996;135:1277–89. doi: 10.1083/jcb.135.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Turner CM, Adler PN. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech Dev. 1998;70:181–92. doi: 10.1016/s0925-4773(97)00194-9. [DOI] [PubMed] [Google Scholar]
- [39].Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–22. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- [40].Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, et al. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hannus M, Feiguin F, Heisenberg CP, Eaton S. Planar cell polarization requires Widerborst, a B' regulatory subunit of protein phosphatase 2A. Development. 2002;129:3493–503. doi: 10.1242/dev.129.14.3493. [DOI] [PubMed] [Google Scholar]
- [42].Irvine KD, Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development. 1994;120:827–41. doi: 10.1242/dev.120.4.827. [DOI] [PubMed] [Google Scholar]
- [43].Zallen JA, Blankenship JT. Multicellular dynamics during epithelial elongation. Semin Cell Dev Biol. 2008;19:263–70. doi: 10.1016/j.semcdb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lye CM, Sanson B. Tension and epithelial morphogenesis in Drosophila early embryos. Curr Top Dev Biol. 2011;95:145–87. doi: 10.1016/B978-0-12-385065-2.00005-0. [DOI] [PubMed] [Google Scholar]
- [45].Lecuit T, Lenne PF, Munro E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu Rev Cell Dev Biol. 2011;27(15):1–28. doi: 10.1146/annurev-cellbio-100109-104027. [DOI] [PubMed] [Google Scholar]
- [46].Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–55. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- [47].Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- [48].Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–70. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- [49].Zallen JA, Zallen R. Cell-pattern disordering during convergent extension in Drosophila. J Phys-Condens Matter. 2004;16:S5073–80. doi: 10.1088/0953-8984/16/44/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol. 2008;10:1401–10. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- [51].Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–43. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- [53].Effler JC, Kee YS, Berk JM, Tran MN, Iglesias PA, Robinson DN. Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr Biol. 2006;16:1962–7. doi: 10.1016/j.cub.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–90. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–21. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- [56].Nishimura M, Inoue Y, Hayashi S. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development. 2007;134:4273–82. doi: 10.1242/dev.010397. [DOI] [PubMed] [Google Scholar]
- [57].Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, et al. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–5. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- [58].Monier B, Pelissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat Cell Biol. 2010;12:60–5. doi: 10.1038/ncb2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–83. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- [60].Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121:565–78. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, et al. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat Cell Biol. 2009;11:859–64. doi: 10.1038/ncb1894. [DOI] [PubMed] [Google Scholar]
- [62].Rauzi M, Lenne PF, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468:1110–4. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- [63].Fernandez-Gonzalez R, Zallen JA. Oscillatory behaviors and hierarchical assembly of contractile structures in intercalating cells. Phys Biol. 2011;8:045005. doi: 10.1088/1478-3975/8/4/045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sawyer JK, Choi W, Jung KC, He L, Harris NJ, Peifer M. A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension. Mol Biol Cell. 2011;22:2491–508. doi: 10.1091/mbc.E11-05-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Harris TJ, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol. 2004;167:135–47. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Simoes Sde M, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, et al. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell. 2010;19:377–88. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- [68].Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–54. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, et al. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–15. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- [70].Levayer R, Pelissier-Monier A, Lecuit T. Spatial regulation of Dia and myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol. 2011;13:529–40. doi: 10.1038/ncb2224. [DOI] [PubMed] [Google Scholar]
- [71].da Silva SM, Vincent JP. Oriented cell divisions in the extending germband of Drosophila. Development. 2007;134:3049–54. doi: 10.1242/dev.004911. [DOI] [PubMed] [Google Scholar]
- [72].Horne-Badovinac S, Bilder D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232:559–74. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- [73].Gutzeit HO. The microfilament pattern in the somatic follicle cells of midvitellogenic ovarian follicles of Drosophila. Eur J Cell Biol. 1990;53:349–56. [PubMed] [Google Scholar]
- [74].Gutzeit HO, Eberhardt W, Gratwohl E. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J Cell Sci. 1991;100(Pt 4):781–8. doi: 10.1242/jcs.100.4.781. [DOI] [PubMed] [Google Scholar]
- [75].Frydman HM, Spradling AC. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development. 2001;128:3209–20. doi: 10.1242/dev.128.16.3209. [DOI] [PubMed] [Google Scholar]
- [76].Bateman J, Reddy RS, Saito H, Van Vactor D. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr Biol. 2001;11:1317–27. doi: 10.1016/s0960-9822(01)00420-1. [DOI] [PubMed] [Google Scholar]
- [77].Conder R, Yu H, Zahedi B, Harden N. The serine/threonine kinase dPak is required for polarized assembly of F-actin bundles and apical-basal polarity in the Drosophila follicular epithelium. Dev Biol. 2007;305:470–82. doi: 10.1016/j.ydbio.2007.02.034. [DOI] [PubMed] [Google Scholar]
- [78].Viktorinova I, Konig T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–32. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- [79].Gutzeit HO, Haas-Assenbaum A. The somatic envelopes around the germ-line cells of polytrophic insect follicles: structural and functional aspects. Tissue Cell. 1991;23:853–65. doi: 10.1016/0040-8166(91)90035-r. [DOI] [PubMed] [Google Scholar]
- [80].Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, et al. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–15. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–4. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, et al. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–84. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- [83].Mirouse V, Christoforou CP, Fritsch C, St Johnston D, Ray RP. Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev Cell. 2009;16:83–92. doi: 10.1016/j.devcel.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [84].Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA. 2002;99:5139–43. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Friedrichs J, Taubenberger A, Franz CM, Muller DJ. Cellular remodelling of individual collagen fibrils visualized by time-lapse AFM. J Mol Biol. 2007;372:594–607. doi: 10.1016/j.jmb.2007.06.078. [DOI] [PubMed] [Google Scholar]
- [86].Staple DB, Loparic M, Kreuzer HJ, Kreplak L. Stretching, unfolding, and deforming protein filaments adsorbed at solid-liquid interfaces using the tip of an atomic-force microscope. Phys Rev Lett. 2009;102:128302. doi: 10.1103/PhysRevLett.102.128302. [DOI] [PubMed] [Google Scholar]
- [87].Wang Y, Riechmann V. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol. 2007;17:1349–55. doi: 10.1016/j.cub.2007.06.067. [DOI] [PubMed] [Google Scholar]
- [88].He L, Wang X, Tang HL, Montell DJ. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol. 2010;12:1133–42. doi: 10.1038/ncb2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin–myosin network drive apical constriction. Nature. 2009;457:495–9. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–42. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- [91].David DJ, Tishkina A, Harris TJ. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development. 2010;137:1645–55. doi: 10.1242/dev.044107. [DOI] [PubMed] [Google Scholar]
- [92].Blanchard GB, Murugesu S, Adams RJ, Martinez-Arias A, Gorfinkiel N. Cytoskeletal dynamics and supracellular organisation of cell shape fluctuations during dorsal closure. Development. 2010;137:2743–52. doi: 10.1242/dev.045872. [DOI] [PubMed] [Google Scholar]
- [93].Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior–posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–24. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- [94].Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–44. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kim HY, Davidson LA. Punctuated actin contractions during convergent extension and their permissive regulation by the non-canonical Wnt-signaling pathway. J Cell Sci. 2011;124:635–46. doi: 10.1242/jcs.067579. [DOI] [PMC free article] [PubMed] [Google Scholar]