Abstract
Purpose
To investigate the relationship between various characteristics of a normal population and laser speckle flowgraphy (LSFG) measurements of mean blur rate (MBR) in the optic nerve head (ONH).
Methods
A total of 189 eyes of 189 normal subjects (93 male, 96 female, mean age 45 ± 14 years old, age range: 20–72) without any history of hypertension, hyperlipidemia or diabetes were enrolled. ONH microcirculation was measured with LSFG and overall MBR (MA), vessel-area MBR (MV), and tissue-area MBR (MT) were derived from these measurements. The statistical association of these measurements with characteristics such as sex, age, intraocular pressure (IOP) and systolic blood pressure (SBP) was then determined.
Results
There was a trend towards decreased IOP and MV and increased SBP with age (P = 0.002, P = 0.035, and P = 0.006, respectively). Furthermore, IOP, MV and SBP were correlated with age (r = -0.23, P = 0.011; r = -0.24, P < 0.001; and r = 0.30, P < 0.001, respectively). Separate multiple regression analyses of independent contributing factors revealed that sex and IOP contributed to MA (P < 0.001 and P = 0.002, respectively), sex, IOP, and age contributed to MV (P < 0.001, P = 0.003, and P = 0.024, respectively), while only IOP contributed to MT (P = 0.003).
Conclusion
In a normal population, MBR was affected by IOP in both the large vessel and capillary areas of the ONH, but not by SBP. MV was also affected by age and sex, while MT was stable independent of age or sex.
Introduction
Ocular diseases related to ischemia, such as diabetic retinopathy, and ocular diseases related to circulatory disturbances and axonal damage, such as glaucoma, are the main causes of blindness worldwide.[1,2,3,4] They are particularly common in industrialized countries, and their successful treatment is a goal of public health. However, the pathogenesis of these blood flow-related ocular diseases remains unclear. It is therefore important to find new ways of studying these diseases and identifying them in patients. This calls for the development of new, non-invasive methods to measure ocular blood flow in living eyes.
Laser speckle flowgraphy (LSFG) is a promising candidate for such a method. It uses the laser speckle phenomenon to detect and analyze ocular blood flow, and can quantify ocular circulation in vivo. A recently developed LSFG measurement parameter, mean blur rate (MBR), can serve as a quantitative index of retinal blood cell speed. An earlier study showed that MBR was closely correlated to hydrogen gas clearance-based measurements of capillary blood flow (CBF) in the optic nerve head (ONH) of albino rabbits.[5] Another study found that MBR in the ONH of rhesus monkeys, with or without experimentally induced glaucoma, was closely correlated with microsphere-based measurements of CBF.[6] Clinically, MBR has been shown to be a highly reproducible, inter-individually comparable parameter, useful in evaluating the effect of medical interventions, grading the severity of glaucoma, and confirming the presence of ocular ischemic diseases.[7,8,9,10,11,12,13] However, the question still remains as to what effect individual characteristics such as age, sex, intraocular pressure (IOP) and blood pressure have on measurements of MBR. Additionally, the impact on MBR of age-related alterations in blood flow in the vascular and tissue regions of the ONH has not yet been determined.[14]
Vascular changes such as arterial stiffening are an inevitable part of aging that can affect many clinical biomarkers. In order to determine the precise effect of these changes on LSFG measurements of MBR, we were careful to select normal subjects who ranged widely in age. We divided these subjects into groups by decade of life, analyzed how MBR varied between each group, and determined the potential of MBR as a normative value for inter-individual and inter-group comparisons.
Materials and Methods
Setting and Patients
This retrospective study included 189 eyes of 189 healthy Japanese individuals (93 male, 96 female; mean age 45.4 ± 13.8 years old (YO); age range 20–74 YO) (Table 1) recruited from patients undergoing standard medical check-ups, i.e., internal medical check-ups, at Sendai Open Hospital. The exclusion criteria were: clinical evidence or history of ocular disease other than mild cataract or refractive error; evidence from optical coherence tomography or fundus photography suggesting glaucoma, i.e., abnormal loss of circumpapillary retinal nerve fiber layer thickness (cpRNFLT) or a cup-to-disc ratio of more than 0.4, respectively; history of intraocular surgery; presence of blood flow-affecting systemic disease requiring medical treatment; IOP more than 21 mmHg; and hypertension, hyperlipidemia, or diabetes. This study followed the tenets of the Declaration of Helsinki. All subjects gave written informed consent for their participation. The Ethics Committee of Sendai Open Hospital and the institutional review board of Tohoku University Graduate School of Medicine approved the research for this study.
Table 1. Characteristics of healthy subjects.
| All | Age | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 20–29 | 30–39 | 40–49 | 50–59 | ≥ 60 years | 50–59 vs. 20–29 | ≥ 60 years vs. 20–29 | Trend for age | ||
| Number of eyes | 189 | 36 | 29 | 34 | 63 | 27 | - | - | - |
| Number of patients | 189 | 36 | 29 | 34 | 63 | 27 | - | - | - |
| Age (years) | 45 ± 14 | 25 ± 3 | 35 ± 3 | 45 ± 3 | 54 ± 3 | 65 ± 5 | - | - | - |
| Sex (M: F) | 93: 96 | 16: 20 | 10: 19 | 18: 16 | 31: 32 | 18: 9 | 0.648a | 0.115a | 0.233a |
| IOP (mmHg) | 13.8 ± 2.5 | 15.0 ± 2.1 | 14.3 ± 2.6 | 12.3 ± 2.0 | 14.0 ± 2.8 | 12.7 ± 2.1 | 0.511 | 0.007 | 0.002 |
| SE (diopter) | -1.9 ± 2.1 | -1.9 ± 1.6 | -2.7 ± 1.8 | -2.2 ± 2.0 | -1.9 ± 2.3 | -0.9 ± 2.0 | 1.000 | 0.246 | 0.082 |
| SBP (mmHg) | 118.8 ± 15.6 | 113.6 ± 13.5 | 113.8 ± 14.9 | 115.2 ± 15.0 | 120.4 ± 13.3 | 128.0 ± 19.4 | 0.254 | 0.046 | 0.006 |
| DBP (mmHg) | 71.7 ± 12.0 | 69.1 ± 10.8 | 71.5 ± 10.5 | 68.4 ± 12.3 | 73.5 ± 12.1 | 74.2 ± 13.3 | 0.596 | 0.727 | 0.283 |
| MV (AU) | 51.8 ± 6.8 | 54.1 ± 6.3 | 52.6 ± 8.4 | 52.0 ± 7.7 | 51.5 ± 6.2 | 48.7 ± 4.2 | 0.259 | 0.003 | 0.035 |
| MT (AU) | 13.5 ± 2.0 | 13.0 ± 2.1 | 13.2 ± 2.3 | 13.6 ± 1.8 | 13.8 ± 2.2 | 13.9 ± 1.5 | 0.430 | 0.277 | 0.297 |
| MA (AU) | 29.0 ± 4.2 | 30.5 ± 4.2 | 29.0 ± 4.9 | 28.7 ± 4.6 | 28.6 ± 3.9 | 27.9 ± 3.2 | 0.124 | 0.059 | 0.140 |
DBP = diastolic blood pressure, IOP = intraocular pressure, MA = overall mean blur rate, MT = mean blur rate in tissue area, MV = mean blur rate in vessel area, SBP = systolic blood pressure, SE = spherical equivalent
Unmarked P value: Steel-Dwass test, a: Chi-square test
Physical and Ophthalmological Measurements
Systolic blood pressure and diastolic blood pressure (SBP and DBP) were measured after the patients had rested in a sitting position for 10 min. Measurements were made in the left brachial artery at the height of the heart by an automated blood pressure monitor (HEM-759E, Omron Corporation, Kyoto, Japan) before LSFG was used to measure ONH circulation. Ophthalmological examinations included fundus photography, measurement of IOP, spherical equivalent (SE), diopter (D), and optical coherence tomography (OCT) measurements of cpRNFLT (3D-OCT2000; TOPCON Corporation, Tokyo, Japan).
Laser Speckle Flowgraphy Measurements
Before LSFG measurement, the pupils of each subject were dilated with 0.5% tropicamide and 0.5% phenylephrine hydrochloride. The details of the underlying principles of LSFG (Softcare, Fukutsu, Japan) have been described in previous reports.[15,16] Briefly, the LSFG device consists of a fundus camera equipped with a diode laser (wavelength 830 nm) and a camera. MBR (measured in arbitrary units; AU), the relative speed of blood flow, is derived from the pattern of speckle contrast produced by the interference of a laser scattered by blood cells moving in the ocular fundus.[17] MBR images are acquired continuously at the rate of 30 frames per second for a 4-second time period and transferred to a computer file. Integrated analysis software then synchronizes the captured MBR images in each cardiac cycle, and averages the MBR in each heartbeat to produce a heartbeat map of the ONH. The LSFG software automatically divides this map into the large vessel and capillary areas, and blood flow parameters are assessed separately for the vessel area (referred to as MV, “mean value vessel area”), the tissue area (referred to as MT, “mean value tissue area”) and the total area of the ONH (MA, “mean value all areas”). The statistical analysis was based on values for ONH circulation that represented the average of three measurements made with LSFG.
Statistical Analysis
The Steel-Dwass test and chi-square test were used to determine the significance of differences between groups and trends related to age. Spearman’s rank correlation test was used to evaluate single correlations between age and each measurement variable (i.e., SE, IOP, SBP, DBP, MV, MT and MA). A multiple linear regression analysis was performed to determine independent variables affecting MT. The statistical analyses were performed with JMP software (Pro version 10.0.2, SAS Institute Japan Inc., Tokyo, Japan). The significance level was set at P < 0.05.
Results
One hundred and eighty-nine healthy subjects were divided into 5 age groups: 20–29, 30–39, 40–49, 50–59, and ≥ 60 YO; these groups included 36, 29, 34, 63 and 27 subjects, respectively. Each group had a statistically similar sex distribution (P = 0.233, Table 1). Overall, there was a trend towards decreased IOP and MV and increased SBP with age (P = 0.002, P = 0.035 and P = 0.006, respectively, Table 1). However, there were no age trends for SE, DBP, MT, or MA (P = 0.082, P = 0.283, P = 0.297, and P = 0.140, respectively, Table 1). MV was lower in the ≥ 60 YO group than in the 20-29-YO group (P = 0.007, Table 1), but there were no differences in MT or MA (P = 0.227 and P = 0.059, Table 1). IOP was also lower in the ≥ 60 YO group than in the 20-29-YO group (P = 0.007, Table 1). SBP was higher in the ≥ 60 YO group than the 20-29-YO group (P = 0.046, Table 1). Furthermore, there were age trends for IOP, MV and SBP (P = 0.002, P = 0.006 and P = 0.035, respectively, Table 1). MV was weakly correlated with age (r = -0.24, P < 0.001, Fig 1 right) and MT was negatively correlated with IOP (r = -0.29, P = 0.001, Fig 2 right). There were no correlations between MV, MT or MA and SE, SBP or DBP. MA and MV were higher in the female subjects than in the male subjects (P < 0.01, Fig 3 left and P < 0.05, Fig 1 left, respectively), while there was no such trend for MT (Fig 2 left). A series of multiple regression analyses of independent contributing factors revealed that sex, IOP and age contributed to MV (P < 0.001, P = 0.003 and P = 0.024, respectively, Table 2), IOP contributed to MT (P = 0.003, Table 3) and sex and IOP contributed to MA (P < 0.001 and P = 0.002, respectively, Table 4). SBP made no contribution to MA, MV, or MT (P = 0.979, P = 0.234, and P = 0.080, respectively, Tables 2–4).
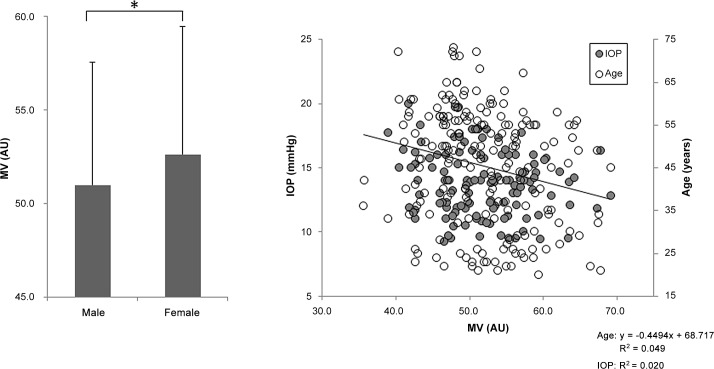
Fig 1. Relationship between vessel-area mean blur rate (MV) and clinical findings.
MV was higher in the female subjects than in the male subjects (P < 0.05, left). MV was weakly correlated with age (r = -0.24, P < 0.001, right).
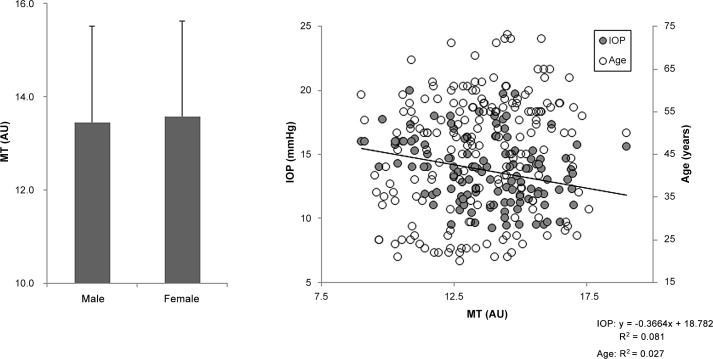
Fig 2. Relationship between tissue-area mean blur rate (MT) and clinical findings.
There was no significant difference in MT between the female and male subjects (left). MT was weakly correlated with IOP (r = -0.29, P = 0.001, right).
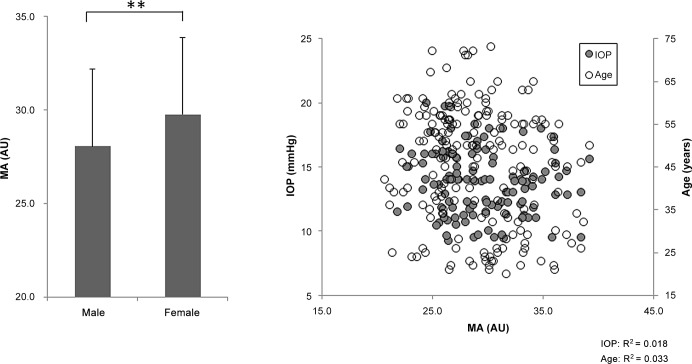
Fig 3. Relationship between overall mean blur rate (MA) and clinical findings.
MA was higher in the female subjects than in the male subjects (P < 0.01, left). MA was not correlated with age or IOP (right).
Table 2. Multiple regression analysis for factors independently contributing to MV.
| Variable | |||
|---|---|---|---|
| Dependent | Independent | β | P value |
| MV | Age | -0.221 | 0.024 |
| Sex (F) | 0.339 | <0.001 | |
| IOP | -0.286 | 0.003 | |
| SE | -0.026 | 0.771 | |
| SBP | 0.156 | 0.234 | |
| DBP | -0.051 | 0.691 | |
β = standard partial regression coefficient, DBP = diastolic blood pressure, F = female, IOP = intraocular pressure, MV = mean blur rate in vessel area, SBP = systolic blood pressure, SE = spherical equivalent
Table 3. Multiple regression analysis for factors independently contributing to MT.
| Variable | |||
|---|---|---|---|
| Dependent | Independent | β | P value |
| MT | Age | 0.150 | 0.136 |
| Sex (F) | 0.147 | 0.124 | |
| IOP | -0.294 | 0.003 | |
| SE | 0.066 | 0.466 | |
| SBP | -0.238 | 0.080 | |
| DBP | 0.155 | 0.244 | |
β = standard partial regression coefficient, DBP = diastolic blood pressure, F = female, IOP = intraocular pressure, MT = mean blur rate in tissue area, SBP = systolic blood pressure, SE = spherical equivalent
Table 4. Multiple regression analysis for factors independently contributing to MA.
| Variable | |||
|---|---|---|---|
| Dependent | Independent | β | P value |
| MA | Age | -0.189 | 0.050 |
| Sex (F) | 0.377 | <001 | |
| IOP | -0.302 | 0.002 | |
| SE | -0.038 | 0.664 | |
| SBP | 0.003 | 0.979 | |
| DBP | 0.094 | 0.463 | |
β = standard partial regression coefficient, DBP = diastolic blood pressure, F = female, IOP = intraocular pressure, MA = overall mean blur rate, SBP = systolic blood pressure, SE = spherical equivalent
Discussion
We analyzed variations in MBR in a population of normal individuals in order to determine the potential of MBR as a normative value for inter-individual and inter-group comparisons. We found that SBP increased with age but that IOP and MV decreased with age. Furthermore, MV differed significantly between the 20-29-YO subjects and the ≥ 60-YO subjects, although there were no differences in MA or MT. Additionally, while MA and MV tended to be higher in the female subjects than the male subjects, this was not true for MT. Finally, a series of multiple regression analyses of independent contributing factors revealed that sex and IOP contributed to MA, that sex, IOP and age contributed to MV, but that IOP was the only contributor to MT. SBP did not contribute to either MA, MV, or MT.
Our cross-sectional study supports previous research showing that IOP tends to decrease with age while SBP increases.[18,19] However, while previous reports have described a negative correlation between MA and age,[20,21] we found that this relationship was not significant in the current study (P = 0.05). Several other reports that used laser Doppler flowmetry to investigate blood flow and volume in the ONH of healthy subjects also found that it had a negative correlation with age.[22,23] Our study also supports earlier data showing that MT values are stable regardless of the age of the subject.[20] Interestingly, the multivariate analysis revealed a number of differences in factors to contributing to MV and MT, i.e., age, sex and IOP vs. IOP only, respectively. Though the exact reason is unclear, one possibility is related to measurement area: while the area measured by MV contains large vessels, the area measured by MT lacks these vessels and contains only capillaries. MT is thus less affected than MV by changes related to age-dependent atherosclerosis, and blood flow measured with MT is accordingly more stable with age.
Our observation that MA and MV were higher in female subjects than males was interesting, and is likely due to the lower rate of atherosclerosis in women than in men.[24,25] This would most directly affect MV, and then affect MA in turn, which was also confirmed recently.[14] Furthermore, previous reports have shown that cerebral blood flow is significantly higher in women than in men among normal adult individuals[26] and that the maintenance of cerebral blood flow speed during posture change and cerebral autoregulation is better in female subjects.[27] The existence of ocular blood flow autoregulation in response to posture change has also been confirmed in a study that used LSFG,[28] and although the mechanism underlying sex-based differences in ocular circulation and autoregulation remains unclear, we speculate that female hormones, such as estradiol, an estrogen, may affect ocular circulation, especially in the vessel area of the ONH.[29]
This study confirmed that MT was more stable with age and sex than MA or MV. This is an important new finding, as it shows that MT can serve as a normative value for inter-individual and inter-group comparisons. Currently, LSFG measurements are mainly used to monitor changes in ONH or choroid circulation over time at a single site in the same eye.[30] This limitation arises from the bias introduced to the laser speckle signal by fundus pigmentation.[31] Previous efforts to overcome this bias have relied on analyzing pulse waveform parameters of MBR, such as blowout time and falling rate. These waveform parameters were found to be comparable between individuals and to correlate with age.[20,32,33] Additionally, to answer the basic question of whether MBR itself is also an inter-individually comparable parameter, we recently completed a study in which we induced ONH ischemia in pigmented and albino rabbits and then compared measurements taken with MBR and CBF. Our report showed that MT was closely correlated with hydrogen gas clearance-measured CBF in albino and pigmented rabbits with or without chronic ischemia-induced ONH atrophy.[34] This implied that individual pigmentation has little effect on MT, and that MT should thus also be suitable for inter-individual comparisons. Furthermore, since the ONH is pigment-free in all human individuals, regardless of ethnicity, inter-individual comparisons of MT should be valid in all patients. Finally, we showed that relative flow volume, an index of blood flow derived from MBR, is an accurate and reliable index of blood flow speed and volume in the human retina.[35] Now, building on these previous reports, the findings reported here suggest that measurements of MT are stable independent of age or sex in the normal population, that MV decreases with the normal aging process and that MV differs between the sexes. The clear implication of all these recent findings, in particular that MT is stable, while MV is age- and sex-dependent, is that LSFG is a valuable tool to evaluate ocular blood flow and MBR values, and should enable valuable future comparisons of ONH circulation between individuals and groups, in addition to its current role in comparing circulation at a single site over time in an individual eye.
The earliest version of LSFG was introduced in the 1990s as a non-invasive method of imaging tissue circulation in the choroid and ONH.[16] Originally, LSFG imaging represented the difference between average speckle intensity in a series of scans and speckle intensity in each scan. This ratio, a quantitative index of blood speed, was defined as normalized blur (NB)[16], and was displayed as a colorized, two-dimensional map.[16] Later versions of LSFG replaced NB with square blur rate (SBR; the square of NB), and the current version uses MBR (SBR multiplied by two). Other improvements in modern LSFG systems include a much improved spatial resolution, due to a larger, 750 × 360-pixel, charge-coupled-device-based image sensor (in contrast to the 100 × 100-pixel, specialized sensor in NB-based LSFG)[17,36,37,38]and the use of eye-tracking.[39] Together, these changes have considerably broadened the usefulness of LSFG. Originally, LSFG was mainly used to monitor changes in blood circulation over time at a single site in the same eye, while interindividual comparisons relied on the analysis of pulse waveform parameters.[20,32] By contrast, the latest versions of LSFG, with their large measurement area and reduced measurement errors, should allow us to directly compare MBR, particularly MT, interindividually.[34]
Our study was somewhat limited by its nature as a cross-sectional retrospective case series enrolling only healthy Japanese subjects undergoing regular medical check-ups. Thus, we could not ensure that the subjects fasted or abstained from alcohol before the examination, and patients with a current smoking habit, which can affect blood circulation, might have been included in this study. However, the subjects were carefully selected in order to exclude those with a history of ocular disease, blood flow-affecting systemic disease requiring medical treatment, hypertension, hyperlipidemia, and diabetes. Therefore, since a number of existing papers, including a total of approximately 150 eyes, have provided normative values for age-dependent ocular changes measured with optical coherence tomography,[40,41] we consider that our results, which were obtained with a multiple regression analysis of 189 subjects, lend sufficient support to our conclusion: MV is closely related to age, sex and IOP, MT is stable independent of age or sex and is only related to IOP, and both are independent of SBP. Additionally, although MBR has previously been reported to decrease in glaucoma and ischemic ocular diseases,[7,9,42] this is the first report of a normative stable value for MT (13.5 ± 2.0 AU) over a wide age range. Nevertheless, although our findings promise to be useful in the clinical diagnostic and decision-making process, it may be advisable to use data gathered with our technique as supplementary information, similar to the "normative" IOP range of 10–21 mmHg.
In conclusion, our findings show that LSFG measurements of MBR are affected by IOP, regardless of which ONH region is measured, in a normal population. MV was affected by age and sex, and MT was stable independent of age or sex. Therefore, it should be possible to use LSFG measurements of MT to compare individuals or groups, and to make comparisons with nominal MT values. Thus, MT may be a quantitative, clinically useful way of identifying circulatory disturbances in ocular diseases. Further investigation is needed to determine the relationship between MBR and other clinical parameters, in order to establish MBR as a surrogate marker for the non-invasive, objective evaluation of ocular circulatory diseases or vascular aging.
Acknowledgments
Informed consent for treatment and participation in the research for this retrospective study was obtained from all patients. The institutional review board of Tohoku University Graduate School of Medicine approved this study.
The principal investigator, Dr. Naoko Aizawa, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
No authors have any potential conflicts of interest to disclose.
The authors would like to express our gratitude to Shinji Onodera (NTT East Japan Tohoku Hospital), Shinobu Saito, Rie Taima, Nobuko Tsuda and Megumi Nagano (Sendai Open Hospital) for their support of this study.
This paper was supported in part by a JST grant from JSPS KAKENHI Grants-in-Aid for Scientific Research (B) (T.N. 26293372), for Scientific Research (C) (H.K. 26462629), and for Exploratory Research (T.N. 26670751). The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Availability
Data is restricted from public sharing because patient data exists within the underlying data. Please contact n.aizawa@oph.med.tohoku.ac.jp for data requests.
Funding Statement
Support was provided by a JST grant from JSPS KAKENHI Grants-in-Aid for Scientific Research (B) (TN 26293372), Scientific Research (C) (HK 26462629), and Exploratory Research (TN 26670751). The funder had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Xu L, Wang Y, Li Y, Cui T, Li J, Jonas JB (2006) Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology 113: 1134 e1131-1111. [DOI] [PubMed] [Google Scholar]
- 2.Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y; Tajimi study group. (2006) Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology 113: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 3.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82: 844–851. [PMC free article] [PubMed] [Google Scholar]
- 4.Quigley HA (1996) Number of people with glaucoma worldwide. Br J Ophthalmol 80: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi H, Sugiyama T, Tokushige H, Maeno T, Nakazawa T, Ikeda T, et al. (2013) Comparison of CCD-equipped laser speckle flowgraphy with hydrogen gas clearance method in the measurement of optic nerve head microcirculation in rabbits. Exp Eye Res 108: 10–15. 10.1016/j.exer.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Cull GA, Piper C, Burgoyne CF, Fortune B (2012) Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and microsphere method. Invest Ophthalmol Vis Sci 53: 8303–8309. 10.1167/iovs.12-10911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aizawa N, Kunikata H, Shiga Y, Yokoyama Y, Omodaka K, Nakzawa T (2014) Correlation between structure/function and optic disc microcirculation in myopic glaucoma, measured with laser speckle flowgraphy. BMC Ophthalmol 14: 113 10.1186/1471-2415-14-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsuda S, Yokoyama Y, Chiba N, Aizawa N, Shiga Y, Yasuda M, et al. (2013) Effect of topical tafluprost on optic nerve head blood flow in patients with myopic disc type. J Glaucoma 22: 398–403. 10.1097/IJG.0b013e318237c8b3 [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama Y, Aizawa N, Chiba N, Omodaka K, Nakamura M, Otomo T, et al. (2011) Significant correlations between optic nerve head microcirculation and visual field defects and nerve fiber layer loss in glaucoma patients with myopic glaucomatous disk. Clin Ophthalmol 5: 1721–1727. 10.2147/OPTH.S23204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba N, Omodaka K, Yokoyama Y, Aizawa N, Tsuda S, Yasuda M, et al. (2011) Association between optic nerve blood flow and objective examinations in glaucoma patients with generalized enlargement disc type. Clin Ophthalmol 5: 1549–1556. 10.2147/OPTH.S22097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizawa N, Yokoyama Y, Chiba N, Omodaka K, Yasuda M, Otomo T, et al. (2011) Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin Ophthalmol 5: 1171–1176. 10.2147/OPTH.S22093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama T, Mashima Y, Yoshioka Y, Oku H, Ikeda T (2009) Effect of unoprostone on topographic and blood flow changes in the ischemic optic nerve head of rabbits. Arch Ophthalmol 127: 454–459. 10.1001/archophthalmol.2008.606 [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama T, Kojima S, Ishida O, Ikeda T (2009) Changes in optic nerve head blood flow induced by the combined therapy of latanoprost and beta blockers. Acta Ophthalmol 87: 797–800. 10.1111/j.1755-3768.2008.01460.x [DOI] [PubMed] [Google Scholar]
- 14.Yanagida K, Iwase T, Yamamoto K, Ra E, Kaneko H, Murotani K, et al. (2015) Sex-Related Differences in Ocular Blood Flow of Healthy Subjects Using Laser Speckle Flowgraphy. Invest Ophthalmol Vis Sci 56: 4880–4890. 10.1167/iovs.15-16567 [DOI] [PubMed] [Google Scholar]
- 15.Isono H, Kishi S, Kimura Y, Hagiwara N, Konishi N, Fujii H (2003) Observation of choroidal circulation using index of erythrocytic velocity. Arch Ophthalmol 121: 225–231. [DOI] [PubMed] [Google Scholar]
- 16.Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H (1995) Non-contact, two-dimensional measurement of tissue circulation in choroid and optic nerve head using laser speckle phenomenon. Exp Eye Res 60: 373–383. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe G, Fujii H, Kishi S (2008) Imaging of choroidal hemodynamics in eyes with polypoidal choroidal vasculopathy using laser speckle phenomenon. Jpn J Ophthalmol 52: 175–181. 10.1007/s10384-007-0521-7 [DOI] [PubMed] [Google Scholar]
- 18.Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T (2011) Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl Health Stat Report: 1–22, 24. [PubMed] [Google Scholar]
- 19.Nomura H, Shimokata H, Ando F, Miyake Y, Kuzuya F (1999) Age-related changes in intraocular pressure in a large Japanese population: a cross-sectional and longitudinal study. Ophthalmology 106: 2016–2022. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda S, Kunikata H, Shimura M, Aizawa N, Omodaka K, Shiga Y, et al. (2014) Pulse-Waveform Analysis of Normal Population using Laser Speckle Flowgraphy. Curr Eye Res 39: 1207–1215. 10.3109/02713683.2014.905608 [DOI] [PubMed] [Google Scholar]
- 21.Tamura A, Kogure A, Watanabe G, Kishi S, Hori S (2013) Association between age and chorioretinal hemodynamics in normal volunteers examined with laser speckle flowgraphy. Nihon Ganka Gakkai Zasshi 117: 110–116. [PubMed] [Google Scholar]
- 22.Boehm AG, Koeller AU, Pillunat LE (2005) The effect of age on optic nerve head blood flow. Invest Ophthalmol Vis Sci 46: 1291–1295. [DOI] [PubMed] [Google Scholar]
- 23.Groh MJ, Michelson G, Langhans MJ, Harazny J (1996) Influence of age on retinal and optic nerve head blood circulation. Ophthalmology 103: 529–534. [DOI] [PubMed] [Google Scholar]
- 24.Flora GC, Baker AB, Loewenson RB, Klassen AC (1968) A comparative study of cerebral atherosclerosis in males and females. Circulation 38: 859–869. [DOI] [PubMed] [Google Scholar]
- 25.Joakimsen O, Bonaa KH, Stensland-Bugge E, Jacobsen BK (1999) Age and sex differences in the distribution and ultrasound morphology of carotid atherosclerosis: the Tromso Study. Arterioscler Thromb Vasc Biol 19: 3007–3013. [DOI] [PubMed] [Google Scholar]
- 26.Swank RL, Roth JG, Woody DC Jr (1983) Cerebral blood flow and red cell delivery in normal subjects and in multiple sclerosis. Neurol Res 5: 37–59. [DOI] [PubMed] [Google Scholar]
- 27.Deegan BM, Sorond FA, Galica A, Lipsitz LA, O'Laighin G, Serrador JM (2011) Elderly women regulate brain blood flow better than men do. Stroke 42: 1988–1993. 10.1161/STROKEAHA.110.605618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiga Y, Shimura M, Asano T, Tsuda S, Yokoyama Y, Aizawa N, et al. (2013) The influence of posture change on ocular blood flow in normal subjects, measured by laser speckle flowgraphy. Curr Eye Res 38: 691–698. 10.3109/02713683.2012.758292 [DOI] [PubMed] [Google Scholar]
- 29.Toker E, Yenice O, Akpinar I, Aribal E, Kazokoglu H (2003) The influence of sex hormones on ocular blood flow in women. Acta Ophthalmologica Scandinavica 81: 617–624. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S (2010) Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol 88: 723–729. 10.1111/j.1755-3768.2009.01586.x [DOI] [PubMed] [Google Scholar]
- 31.Nagahara M, Tamaki Y, Tomidokoro A, Araie M (2011) In Vivo Measurement of Blood Velocity in Human Major Retinal Vessels Using the Laser Speckle Method. Investigative Ophthalmology & Visual Science 52: 87–92. [DOI] [PubMed] [Google Scholar]
- 32.Shiba T, Takahashi M, Hori Y, Maeno T (2012) Pulse-wave analysis of optic nerve head circulation is significantly correlated with brachial-ankle pulse-wave velocity, carotid intima-media thickness, and age. Graefes Arch Clin Exp Ophthalmol 250: 1275–1281. 10.1007/s00417-012-1952-5 [DOI] [PubMed] [Google Scholar]
- 33.Shiba T, Takahashi M, Hori Y, Maeno T, Shirai K (2012) Optic nerve head circulation determined by pulse wave analysis is significantly correlated with cardio ankle vascular index, left ventricular diastolic function, and age. J Atheroscler Thromb 19: 999–1005. [DOI] [PubMed] [Google Scholar]
- 34.Aizawa N, Nitta F, Kunikata H, Sugiyama T, Ikeda T, Araie M, et al. (2014) Laser speckle and hydrogen gas clearance measurements of optic nerve circulation in albino and pigmented rabbits with or without optic disc atrophy. Invest Ophthalmol Vis Sci 55: 7991–7996. 10.1167/iovs.14-15373 [DOI] [PubMed] [Google Scholar]
- 35.Shiga Y, Asano T, Kunikata H, Nitta F, Sato H, Nakazawa T, et al. (2014) Relative flow volume, a novel blood flow index in the human retina derived from laser speckle flowgraphy. Invest Ophthalmol Vis Sci 55: 3899–3904. 10.1167/iovs.14-14116 [DOI] [PubMed] [Google Scholar]
- 36.Tamaki Y, Araie M, Kawamoto E, Eguchi S, Fujii H (1994) Noncontact, two-dimensional measurement of retinal microcirculation using laser speckle phenomenon. Investigative Ophthalmology & Visual Science 35: 3825–3834. [PubMed] [Google Scholar]
- 37.Sugiyama T, Utsumi T, Azuma I, Fujii H (1996) Measurement of optic nerve head circulation: comparison of laser speckle and hydrogen clearance methods. Jpn J Ophthalmol 40: 339–343. [PubMed] [Google Scholar]
- 38.Konishi N, Tokimoto Y, Kohra K, Fujii H (2002) New laser speckle flowgraphy system using CCD camera. Optical Review 9: 163–169. [Google Scholar]
- 39.Sugiyama T (2014) Basic Technology and Clinical Applications of the Updated Model of Laser Speckle Flowgraphy to Ocular Diseases. Photonics 1: 220–234. [Google Scholar]
- 40.Demirkaya N, van Dijk HW, van Schuppen SM, Abramoff MD, Garvin MK, Sonka M, et al. (2013) Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 54: 4934–4940. 10.1167/iovs.13-11913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes LA, Huisingh C, Johnstone J, Fazio M, Smith B, Clark M, et al. (2014) Variation of laminar depth in normal eyes with age and race. Invest Ophthalmol Vis Sci 55: 8123–8133. 10.1167/iovs.14-15251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maekubo T, Chuman H, Nao IN (2013) Laser speckle flowgraphy for differentiating between nonarteritic ischemic optic neuropathy and anterior optic neuritis. Jpn J Ophthalmol 57: 385–390. 10.1007/s10384-013-0246-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is restricted from public sharing because patient data exists within the underlying data. Please contact n.aizawa@oph.med.tohoku.ac.jp for data requests.