Abstract
Purpose
This study aimed to investigate the mid- to long-term outcomes of cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) for the treatment of 1-level or 2-level symptomatic cervical disc disease.
Methods
Medline, Embase, and the Cochrane Central Register of Controlled Trials databases were searched to identify relevant randomized controlled trials that reported mid- to long-term outcomes (at least 48 months) of CDA versus ACDF. All data were analyzed by Review Manager 5.3 software. The relative risk (RR) and 95% confidence intervals (CIs) were calculated for dichotomous variables. The weighted mean difference (WMD) and 95%CIs were calculated for continuous variables. A random effect model was used for heterogeneous data; otherwise, a fixed effect model was used.
Results
Eight prospective randomized controlled trials (RCTs) were retrieved in this meta-analysis, including 1317 and 1051 patients in CDA and ACDF groups, respectively. Patients after an ACDF had a significantly lower rate of follow-up than that after CDA. Pooled analysis showed patients in CDA group achieved significantly higher rates of overall success, Neck Disability Index (NDI) success, neurological success and significantly lower rates of implant/surgery-related serious adverse events and secondary procedure compared with that in ACDF group. The long-term functional outcomes (NDI, Visual Analog Scale (VAS) neck and arm pain scores, the Short Form 36 Health Survey physical component score (SF-36 PCS)), patient satisfaction and recommendation, and the incidence of superior adjacent segment degeneration also favored patients in CDA group with statistical difference. Regarding inferior adjacent segment degeneration, patients in CDA group had a lower rate without statistical significance.
Conclusions
This meta-analysis showed that cervical disc arthroplasty was superior over anterior discectomy and fusion for the treatment of symptomatic cervical disc disease in terms of overall success, NDI success, neurological success, implant/surgery-related serious adverse events, secondary procedure, functional outcomes, patient satisfaction and recommendation, and superior adjacent segment degeneration.
Introduction
Anterior cervical discectomy and fusion (ACDF) is considered the gold standard for the treatment of radiculopathy and myelopathy due to cervical disc disease [1–4]. Although it generally provides good outcomes [5–7], potential risks include pseudoarthrosis [3,8] and acceleration of adjacent segment degeneration [9–11]. Cervical disc arthroplasty (CDA), as a motion-preserving alternative, was introduced to address these adverse events. The biomechanical advantage of CDA has been demonstrated previously that it can maintain segmental range of motion and cervical kinematics, theoretically reducing or avoiding adjacent segment degeneration [12–16]. However, CDA has its own potential disadvantages, such as higher incidence of heterotopic ossification [17–20] and implant migration or subsidence [21–24]. Many investigators have reported RCTs comparing CDA with ACDF for the treatment of symptomatic cervical disc disease [25–41]. However, the findings of these studies are inconsistent. Some studies reported that compared to ACDF, CDA could provide better neurological outcomes and reduce the rate of adjacent segment degeneration [25–34], whereas other studies reported no difference between the two procedures [35–41]. To clarify these ambiguous findings, we performed a meta-analysis of the current literature to compare mid- to long-term efficacy and safety of CDA with ACDF for the treatment of symptomatic cervical disc disease.
Materials and Methods
Search Strategy
The study was conducted following the Preferred Reporting Items for systematic Reviews and Meta-analysis (PRISMA) guidelines [42]. Medline, Embase, the Cochrane Central Register of Controlled Trials databases were searched through January 2016 by using the following key terms: “cervical disc arthroplasty”, “fusion”, “arthrodesis”, and “randomized controlled trial” (S1 Fig). The searches were limited to studies published in English. The reference lists of selected articles and relevant reviews were also reviewed to identify studies not identified in the original search. Two investigators independently reviewed all subjects, abstracts, and the full text of articles that were potentially eligible based on abstract review. Any disagreements between the investigators were discussed and resolved by consensus.
Eligibility Criteria
We included studies that met the following conditions: (1) prospective randomized controlled trials comparing CDA with ACDF with a minimum 48 months of follow-up; (2) subjects who were older than 18 years of age and had 1-level or 2-level symptomatic cervical disc disease unresponsive to non-operative treatment for at least 6 weeks; (3) at least one desirable outcome should be reported. Articles were excluded if they had any of the following characteristics: (1) non-RCTs, retrospective studies, or case series; (2) follow-up duration was less than 48 months; (3) duplicated publications from the same investigational site.
Methodological Quality Evaluation
Two reviewers independently performed the quality of the included studies using the 12 criteria recommended by the Cochrane Back Review Group. According to the recommendation by the Cochrane Back Review Group, studies were rated as having “low risks of bias” when at least 6 of the 12 criteria were met without serious flaws. Otherwise, the studies were rated as having “high risk of bias”.
Data Extraction
Two reviewers independently extracted the following data: study country, publication year, study design, sample size, follow-up duration, patient demographics, prosthesis type, overall success, neurological success, Neck Disability Index (NDI) success, patient satisfaction and recommendation, implant/surgery-related serious adverse events (classified as WHO grade 3 or 4), secondary procedure, NDI, neck and arm pain scores, the Short Form 36 Health Survey physical component score (SF-36 PCS), and adjacent segment degeneration.
Data Analysis
The analysis was carried out using Reviewer Manager 5.3 software (Cochrane Collaboration, Oxford, UK). For dichotomous variables, the relative risk (RR) and 95% confidence intervals (CIs) were calculated. For continuous variables, the weighted mean difference (WMD) and 95% CIs were calculated. The level of significance was set as P< 0.05. Standard errors, confidence intervals, P values for difference in means, and interquartile ranges were transformed into standard deviation (SD), where necessary, according to the Cochrane Handbook for Systematic Reviews of Interventions. Statistical heterogeneity was evaluated using the chi-square test and Higgin’s I2 test. A P value of chi-square test < 0.10 or I2 > 50% indicated statistical heterogeneity, prompting a random effects modeling estimate. Otherwise, a fixed effects model was used. Subgroup analysis was performed on patients with only 1-level cervical disc disease.
Results
Literature Search
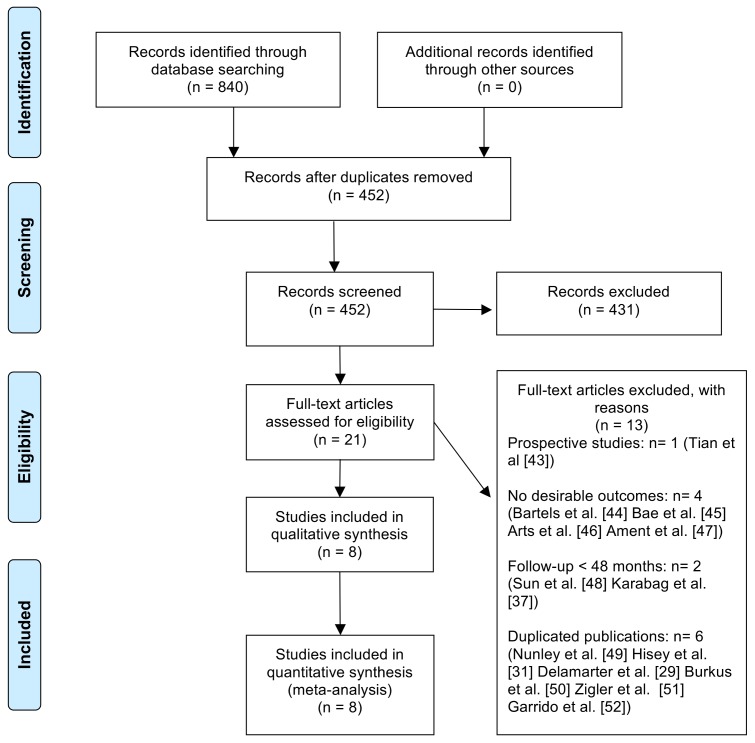
The details of the literature search and selection are discussed in Fig 1. A total of 840 articles were identified through three electronic database searches. After removal of duplicate and irrelevant articles by title and abstract review, 21 potential articles were retrieved for further full-text evaluation [29, 31, 37, 43–60]. Among them, 13 articles were excluded for not meeting the eligibility criteria [29,31,37,43–52]. Finally, 8 RCTs involving 2368 patients were included in the meta-analysis [53–60]. The basic characteristics of the included studies are shown in Table 1.
Fig 1. Flow chart showing search strategy.
Table 1. Characteristics of all included studies.
| Study | Year | Country | Design | Levels | Enrolled patients | Followed patients | Mean age (years) | Male (%) | Prosthesis | Mean follow-up (months) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDA | ACDF | CDA | ACDF | CDA | ACDF | CDA | ACDF | |||||||
| Burkus et al | 2014 | USA | PRCT, FDA, 31 centers | 1 | 276 | 265 | 212 | 183 | 43.3 | 43.9 | 46.4 | 46 | Prestige | 84 |
| Coric et al | 2013 | USA | PRCT, FDA, 1 center | 1 | 41 | 33 | 36 | 27 | 49.5 | 49.3 | 39 | 43.8 | Bryan or Kineflex/C | 72 |
| Davis et al | 2015 | USA | PRCT, FDA, 24 centers | 2 | 225 | 105 | 202 | 89 | 45.3 | 46.2 | 50.2 | 42.9 | Mobi-C | 48 |
| Hisey et al | 2015 | USA | PRCT, FDA, 23 centers | 1 | 164 | 81 | 128 | 55 | 43.3 | 44 | 47.6 | 44.4 | Mobi-C | 48 |
| Phillips et al | 2015 | USA | PRCT, FDA, 24 centers | 1 | 211 | 184 | 163 | 130 | 45.3 | 43.7 | 51.8 | 51.9 | PCM | 60 |
| Sasso et al | 2011 | USA | PRCT, FDA, 30 centers | 1 | 242 | 221 | 181 | 138 | 44.4 | 44.7 | 45.5 | 51.1 | Bryan | 48 |
| Zhang et al | 2014 | China | PRCT, 11 centers | 1 | 55 | 56 | 55 | 56 | 44.8 | 46.7 | 45.5 | 46.4 | Mobi-C | 48 |
| Janssen et al | 2015 | USA | PRCT, FDA, 13 centers | 1 | 103 | 106 | 79 | 73 | 42.1 | 43.5 | 44.7 | 46.2 | ProDisc-C | 84 |
PRCT: prospective randomized controlled trial, FDA: food and drug administration, CDA: cervical disc arthroplasty, ACDF: anterior cervical discectomy and fusion
Methodological Quality Assessment
The methodological quality of all included studies is presented in Table 2. All eight studies were rated as “low risk of bias” according to the Cochrane Back Review Group criteria. One study [59] failed to clearly report adequate randomization and only one study provided the information of allocation concealment [59]. Blinding of patients, surgeons, and assessors were not considered achieved because of the nature of the studies and evident difference of implant design. Missing information such as the absence of intention-to-treatment analysis and follow-up loss were presented in seven studies [53–58,60].
Table 2. Risk of bias assessment of all included studies.
| Burkus et al. | Coric et al. | Davis et al. | Hisey et al. | Phillips et al. | Sasso et al. | Zhang et al. | Janssen et al. | |
|---|---|---|---|---|---|---|---|---|
| Adequate randomization | + | + | + | + | + | + | Unclear | + |
| Allocation concealment | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | + |
| Blinding of patients | - | - | - | - | - | - | - | - |
| Blinding of care provider | - | - | - | - | - | - | - | - |
| Blinding of outcome assessor | - | - | - | - | - | - | - | - |
| Acceptable drop-out rate | Unclear | + | + | - | + | - | + | - |
| ITT analysis | - | - | - | - | - | - | + | - |
| Free of selective reporting | + | + | + | + | + | + | + | + |
| Similar baseline | + | + | + | + | + | + | + | + |
| Avoided or similar co-interventions | + | + | + | + | + | + | + | + |
| Acceptable compliance | + | + | + | + | + | + | + | + |
| Similar timing | + | + | + | + | + | + | + | + |
| Total score | 6 | 7 | 7 | 6 | 7 | 6 | 7 | 7 |
Overall Success, NDI Success, and Neurological Success
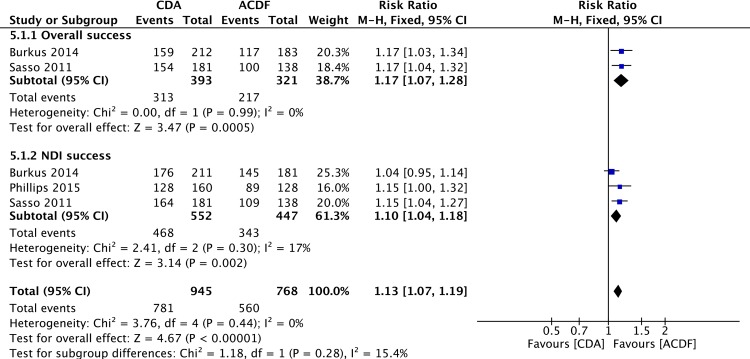
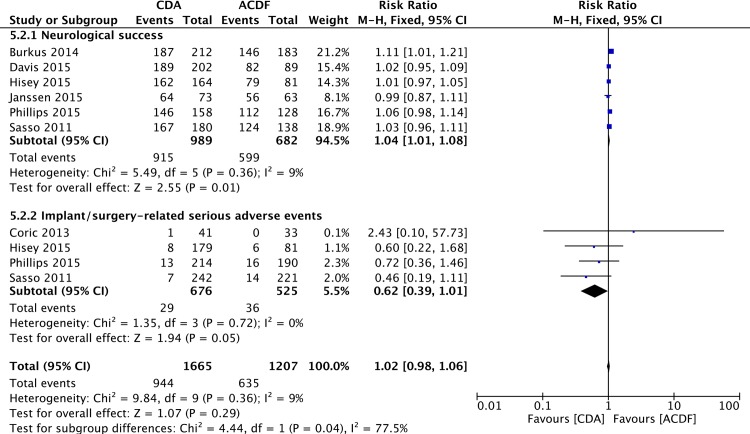
Overall success was considered achieved if a patient met all of the following items: NDI success, neurological success, absences of implant/surgery-related serious adverse events and secondary procedure. Two studies [53, 58] reported the overall success data. Pooled analysis showed that patients in CDA group had a higher rate of overall success compared with that in ACDF group (RR = 1.17; 95%CI: 1.07, 1.28; P = 0.0005; I2 = 0%; Fig 2). NDI success was defined as postoperative NDI score improvement of at least a 15-point increase from preoperative score. Three studies [53, 57, 58] reported the NDI success data. Pooled analysis revealed a higher rate of NDI success in CDA group (RR = 1.10; 95%CI: 1.04, 1.18; P = 0.002; I2 = 17%; Fig 2). Neurological success was determined as postoperative maintenance or improvement in each of the individual neurological evaluations (muscle strength, sensory deficit, and reflex functions) compared with the preoperative status. Six studies [53,55–58,60] reported the neurological success data. Pooled analysis found a higher rate of neurological success in CDA group (RR = 1.04; 95%CI: 1.01, 1.08; P = 0.01; I2 = 9%; Fig 3).
Fig 2. Forest plot for overall success and NDI success.
Fig 3. Forest plot for neurological success and implant/surgery-related serious adverse events.
Implant/Surgery-Related Serious Adverse Events
Serious adverse events were defined as grade 3 or 4 adverse events based on the WHO criteria. Four studies [54,56–58] reported the data of implant/surgical procedure-related serious adverse events. Overall, there was a significant difference in favor of the CDA group (RR = 0.62; 95%CI: 0.39, 1.01; P = 0.05; I2 = 0%; Fig 3).
Secondary Procedure
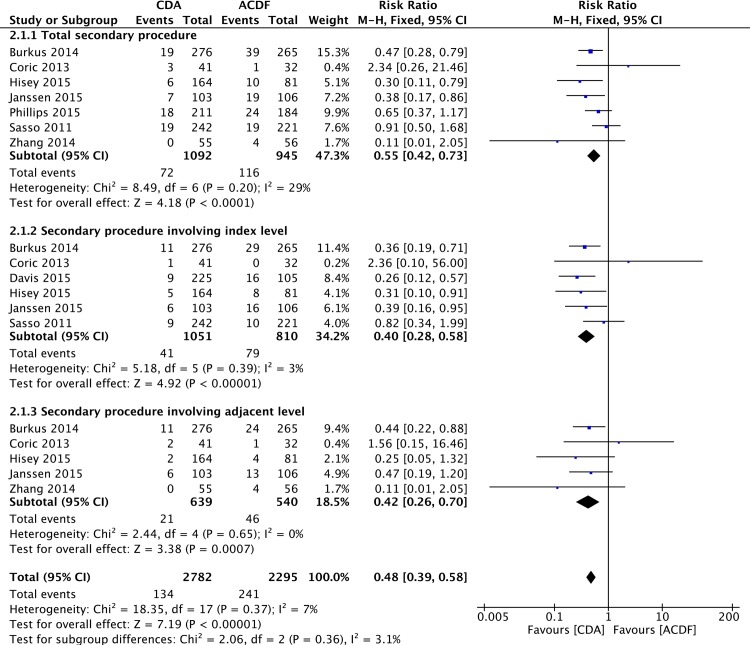
Secondary procedure was defined as any reoperation, revision, supplemental fixation, or implant removal. Total secondary procedure data were available in seven studies [53,54,56–60] and pooled analysis showed a lower rate in CDA group (RR = 0.55; 95%CI: 0.42, 0.73; P< 0.0001; I2 = 29%; Fig 4). Six studies [53–56,58,60] reported the data of secondary procedure involving the index level. Pooled analysis revealed a lower rate in CDA group (RR = 0.40; 95%CI: 0.28, 0.58; P< 0.00001; I2 = 3%; Fig 4). The data of secondary procedure involving the adjacent level were available in five studies [53,54,56,59,60]. Overall, the percentage of patients undergoing secondary procedure involving the adjacent level was lower in CDA group (RR = 0.42; 95%CI: 0.26, 0.70; P = 0.0007; I2 = 0%; Fig 4).
Fig 4. Forest plot for secondary procedure.
Functional Outcomes
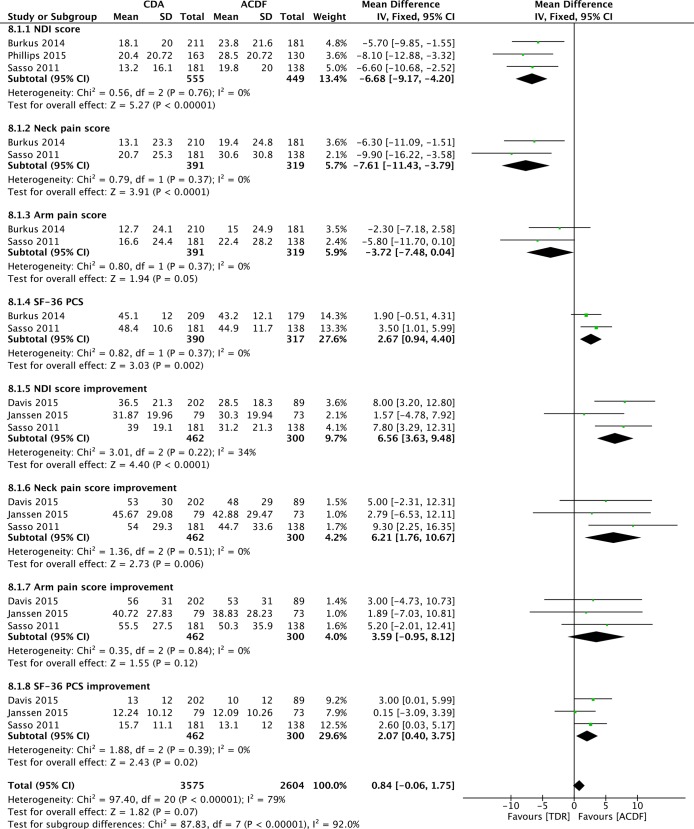
Three studies [53,57,58] reported the NDI score data. Pooled analysis indicated patients in CDA group had a better NDI score on last follow-up (WMD = -6.68; 95%CI: -9.17, -4.20; P< 0.00001; I2 = 0%; Fig 5). Neck and arm pain and SF-36 PCS were available in two studies [53,58]. Overall, patients in CDA group had a better neck pain score (WMD = -7.61; 95%CI: -11.43, -3.79; P< 0.0001; I2 = 0%; Fig 5), better arm pain score (WMD = -3.72; 95%CI: -7.48, 0.04; P = 0.05; I2 = 0%; Fig 5), and better SF-36 PCS (WMD = 2.67; 95%CI: 0.94, 4.40; P = 0.002; I2 = 0%; Fig 5). Three studies [55,58,60] reported the mean improvement from baseline through last follow-up in NDI score, neck and arm pain scores, and SF-36 PCS. Pooled estimate showed CDA group had greater improvement in NDI score (WMD = 6.56; 95%CI: 3.63, 9.48; P< 0.0001; I2 = 34%; Fig 5), neck pain score (WMD = 6.21; 95%CI: 1.76, 10.67; P = 0.006; I2 = 0%; Fig 5), and SF-36 PCS (WMD = 2.07; 95%CI: 0.40, 3.75; P = 0.02; I2 = 0%; Fig 5). In addition, CDA group had a greater improvement in arm pain score without statistical significance (WMD = 3.59; 95%CI: -0.95, 8.12; P = 0.12; I2 = 0%; Fig 5).
Fig 5. Forest plot for functional outcomes.
Patient Satisfaction and Recommendation
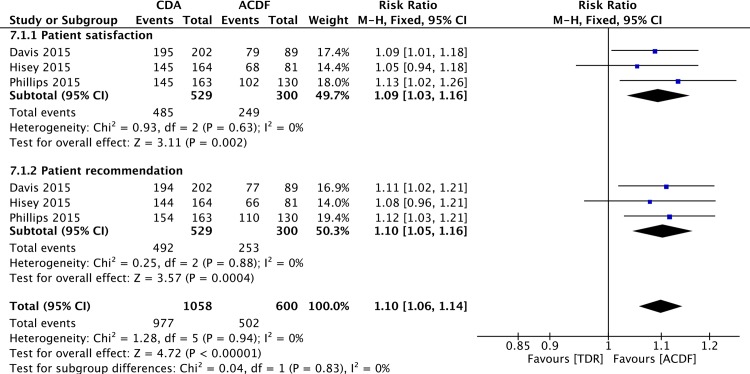
Three studies [55–57] reported the data about patient satisfaction and recommendation. The pooled results indicated that the percentage of patients satisfied with their treatment was higher in the CDA group (RR = 1.09, 95%CI: 1.03,1.16; P = 0.002; I2 = 0%; Fig 6). Pooled analysis showed a higher percentage of patients would recommend their treatment to a friend in CDA group (RR = 1.10; 95%CI: 1.05, 1.16; P = 0.0004; I2 = 0%; Fig 6).
Fig 6. Forest plot for patient satisfaction and recommendation.
Radiological Adjacent Segment Degeneration
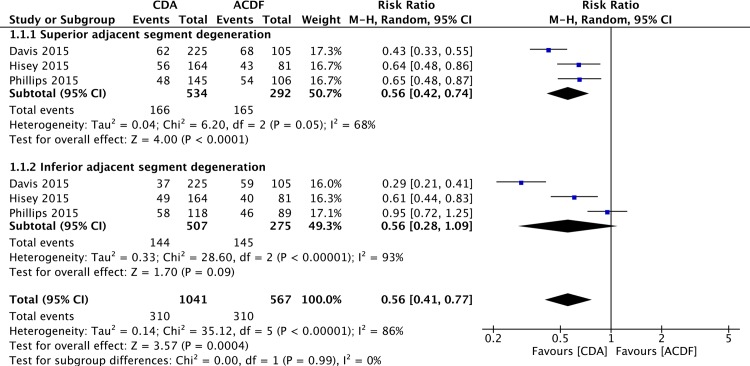
Two studies [55,56] employed Kellgren-Lawrence scale [61] to assess adjacent segment degeneration. Pooled analysis of these two studies showed lower rates of adjacent segment degeneration in CDA group (total: 0.01; superior: P = 0.002; inferior: P = 0.02). One study [57] reported the incidence of adjacent segment degeneration determined by Walraevens’s grading system [62]. Their results showed there was significant difference between CDA and ACDF groups regarding superior adjacent segment degeneration (P = 0.004), but not inferior adjacent segment degeneration (P = 0.72) [57]. Pooled analysis of these three studies [55–57] revealed a lower rate of superior adjacent segment degeneration in CDA group (RR = 0.56; 95%CI: 0.42, 0.74; P< 0.0001; I2 = 68%; Fig 7). Regarding inferior adjacent segment degeneration, patients in CDA group had a lower rate without statistical significance (RR = 0.56; 95%CI: 0.28, 1.09; P = 0.09; I2 = 93%; Fig 7).
Fig 7. Forest plot for radiological adjacent segment degeneration.
Follow-Up Rate
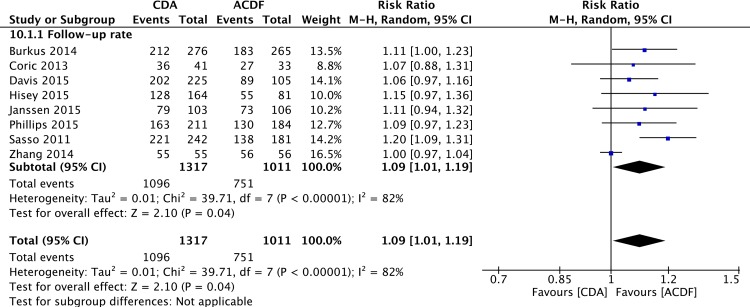
Pooled analysis of all studies found a significantly lower follow-up rate in ACDF group (RR = 1.09; 95%CI: 1.01, 1.19; P = 0.04; I2 = 82%; Fig 8). Only one study [59] did not have follow-up loss at last follow-up. Pooled analysis of seven studies with follow-up loss also found a significantly lower rate of follow-up rate in ACDF group (RR = 1.12; 95%CI: 1.07, 1.17; P< 0.00001; I2 = 0%).
Fig 8. Forest plot for follow-up rate.
Subgroup Analysis
Subgroup analyses were performed on patients with only 1-level cervical disc disease. The results are shown in Table 3.
Table 3. Subgroup analysis of patients with 1-level cervical disc disease.
| Outcomes | No. Studies | No. Patients | Statistical method | Effect estimate | P | X2 | I2 (%) |
|---|---|---|---|---|---|---|---|
| Overall success | 2 | 714 | Risk Ratio (M-H, Fixed, 95% CI) | 1.17 (1.07, 1.28) | 0.0005 | 0 | 0% |
| NDI success | 3 | 999 | Risk Ratio (M-H, Fixed, 95% CI) | 1.10 (1.04, 1.18) | 0.002 | 2.41 | 17% |
| Neurological success | 5 | 1380 | Risk Ratio (M-H, Fixed, 95% CI) | 1.05 (1.01, 1.09) | 0.01 | 5.7 | 30% |
| Implant/surgery-related serious adverse events | 4 | 1201 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 (0.39, 1.01) | 0.05 | 1.35 | 0% |
| Total secondary procedures | 7 | 2037 | Risk Ratio (M-H, Fixed, 95% CI) | 0.55 (0.42, 0.73) | < 0.0001 | 8.49 | 29% |
| Secondary procedures involving index level | 5 | 1531 | Risk Ratio (M-H, Fixed, 95% CI) | 0.45 (0.30, 0.68) | 0.0001 | 3.8 | 0% |
| Secondary procedures involving adjacent levels | 5 | 1179 | Risk Ratio (M-H, Fixed, 95% CI) | 0.42 (0.26, 0.70) | 0.0007 | 2.44 | 0% |
| NDI score | 3 | 1004 | Mean Difference (IV, Fixed, 95%CI) | —6.68 (-9.17, -4.20) | < 0.00001 | 0.56 | 0% |
| Neck pain score | 2 | 710 | Mean Difference (IV, Fixed, 95%CI) | —7.61 (-11.43, -3.79) | < 0.0001 | 0.79 | 0% |
| Arm pain score | 2 | 710 | Mean Difference (IV, Fixed, 95%CI) | —3.72 (-7.48, 0.04) | 0.05 | 0.8 | 0% |
| SF-36 PCS | 2 | 707 | Mean Difference (IV, Fixed, 95%CI) | 2.67 (0.94, 4.40) | 0.002 | 0.82 | 0% |
| NDI score improvement | 2 | 471 | Mean Difference (IV, Random, 95%CI) | 5.10 (-0.95, 11.15) | 0.1 | 2.46 | 59% |
| Neck pain score improvement | 2 | 471 | Mean Difference (IV, Fixed, 95%CI) | 6.93 (1.31, 12.55) | 0.02 | 1.19 | 16% |
| Arm pain score improvement | 2 | 471 | Mean Difference (IV, Fixed, 95%CI) | 3.89 (-1.71, 9.50) | 0.17 | 0.32 | 0% |
| SF-36 PCS improvement | 2 | 471 | Mean Difference (IV, Fixed, 95%CI) | 1.65 (-0.36, 3.67) | 0.11 | 1.35 | 26% |
| Patient satisfaction | 2 | 538 | Risk Ratio (M-H, Fixed, 95% CI) | 1.10 (1.02, 1.18) | 0.02 | 0.91 | 0% |
| Patient recommendation | 2 | 538 | Risk Ratio (M-H, Fixed, 95% CI) | 1.10 (1.03, 1.18) | 0.006 | 0.24 | 0% |
| Superior ASD | 2 | 496 | Risk Ratio (M-H, Fixed, 95% CI) | 0.65 (0.52, 0.80) | < 0.0001 | 0 | 0% |
| Inferior ASD | 2 | 452 | Risk Ratio (M-H, Random, 95% CI) | 0.76 (0.49, 1.19) | 0.24 | 4.44 | 77% |
X2, chi-squared heterogeneity statistics; I2, index of heterogeneity; M-H, Mantel-Haenszel; CI, confidence interval.
Discussion
Although artificial cervical discs have been utilized in spinal surgery for several years, ACDF remains the gold standard for the treatment of symptomatic cervical disc disease. It is partly attributable to the uncertainty of the long-term outcomes of cervical disc arthroplasty compared with the well-perceived long-term success of ACDF. To our knowledge, there are many meta-analysis studies available in the literature comparing the efficacy and safety of CDA with ACDF for the treatment of symptomatic cervical disc disease. However, most of them included the studies with short-term follow-up, which made it impossible to conclude the long-term comparativeness. Therefore, we performed a meta-analysis of eight RCTs with at least 48 months follow-up to determine whether CDA was superior over ACDF.
This meta-analysis found that patients in CDA group had a significantly higher overall success rate compared with that in ACDF group. Pooled analysis of NDI success and neurological success data also revealed to be in favor of CDA. Moreover, we extracted NDI, VAS, and SF-36 scores at last follow-up to evaluate functional outcomes. Pooled estimates of these data showed superiority in CDA except for arm pain score improvement data, which showed no significant difference. These findings suggested that CDA seemed to be more effective than ACDF for the treatment of cervical spondylosis.
Secondary procedure is an important clinical event with substantial clinical and financial burdens for the patient as well as additional cost for the payor. In this meta-analysis, we found that CDA was superior to ACDF regarding the rate of total secondary procedures. Pooled results of the data of secondary procedure involving index level or adjacent level also revealed superiority in CDA group. These results were consistent with Wu et al.’ findings [63]. However, they only included four randomized controlled trials with only 921 patients in total.
We observed that most of the adverse events reported in the included studies were medical problems unrelated to the index surgery or the cervical spine. Therefore, we chose implant/surgery-related serious adverse events for the assessment of safety. Pooled results showed a lower rate in CDA patients, suggesting CDA seemed to be surgically safer than ACDF for the treatment of symptomatic cervical disc disease. In addition, three studies reported the data of patient satisfaction and patient recommendation. Regarding these self-assessed data, this meta-analysis revealed better results reported in CDA patients, supporting the superior efficacy in CDA over ACDF.
Adjacent segment degeneration has been considered as one major concern for patients undergoing ACDF for degenerative disc disease [3–7]. Compared to cervical fusion, disc arthroplasty provides theoretical biomechanical advantage of motion preservation and stress reduction at adjacent levels [12–16]. However, it remains unclear whether CDA can decrease the incidence of adjacent segment degeneration compared to ACDF [33, 36, 40, 49, 64]. In this meta-analysis, no studies reported the rate of symptomatic adjacent segment disease while three studies reported the rate of radiological adjacent segment degeneration where pooled outcomes demonstrated a significantly lower rate of superior adjacent segment degeneration and an insignificantly lower rate of inferior adjacent segment degeneration in CDA patients. These findings suggested that CDA seemed to have positive effect on the process of adjacent segment degeneration. We noticed the statistical heterogeneity was high for these outcomes. This level of heterogeneity might be due to the difference of radiological criteria determined for adjacent segment degeneration and number of surgical levels. Of note, radiological adjacent segment degeneration is known to not directly correlate with symptomology [6,65]. Therefore, prospective RCTs with long-term follow-up reporting symptomatic adjacent segment disease as an outcome are warranted to clarify this question.
Several potential limitations should be acknowledged in our meta-analysis. First, only eight RCTs with follow-up between 4 to 7 years were included in this meta-analysis. Further studies with larger sample sizes and longer follow-up are warranted. Second, there were some methodological weaknesses in the included studies, such as unclear methods of allocation concealment and inadequate blinding procedures. Moreover, missing information such as the absence of ITT analysis and follow-up loss was presented in almost every study. All these methodological drawbacks would weaken the credibility of pooled outcomes. Third, patients undergoing an ACDF had a tendency to have poorer follow-up rate than those in CDA group (P = 0.04), which might lead to biased results. The reasons for this bias are not clear and probably multifactorial because of the lack of blinding of patients in all studies. Fourth, almost all the studies utilized a non-inferiority study design, which is typically less stringent in demonstrating efficacy than standard clinical trials. Despite these limitations, we still believe that this meta-analysis supports the superiority of CDA over ACDF on efficacy and safety for the treatment of symptomatic cervical disc disease in mid- to long-term follow-up.
Supporting Information
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funded by China Scholarship Council No.201306370115 (YH) http://www.csc.edu.cn.
References
- 1.Buchowski JM, Anderson PA, Sekhon L, Riew KD. Cervical disc arthroplasty compared with arthrodesis for the treatment of myelopathy. Surgical technique. J Bone Joint Surg Am. 2009; 91 Suppl 2:223–32. 10.2106/JBJS.I.00564 [DOI] [PubMed] [Google Scholar]
- 2.Rao RD, Currier BL, Albert TJ, Bono CM, Marawar SV, Poelstra KA, et al. Degenerative cervical spondylosis: clinical syndromes, pathogenesis, and management. J Bone Joint Surg Am. 2007; 89(6): 1360–78. [DOI] [PubMed] [Google Scholar]
- 3.Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993; 75(9): 1298–307. [DOI] [PubMed] [Google Scholar]
- 4.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999; 81(4): 519–28. [DOI] [PubMed] [Google Scholar]
- 5.Majd ME, Vadhva M, Holt RT. Anterior cervical reconstruction using titanium cages with anterior plating. Spine (Phila Pa 1976). 1999; 24(15): 1604–10. [DOI] [PubMed] [Google Scholar]
- 6.Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11- year radiologic and clinical follow-up study. Spine (Phila Pa 1976). 2005; 30(19): 2138–44. [DOI] [PubMed] [Google Scholar]
- 7.Goffin J, Geusens E, Vantomme N, Quintens E, Waerzeggers Y, Depreitere B, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004; 17(2): 79–85. [DOI] [PubMed] [Google Scholar]
- 8.Shriver MF, Lewis DJ, Kshettry VR, Rosenbaum BP, Benzel EC, Mroz TE. Pseudoarthrosis rates in anterior cervical discectomy and fusion: a meta-analysis. Spine J. 2015; 15(9): 2016–27. 10.1016/j.spinee.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 9.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004; 4(6 Suppl): 190S–194S. [DOI] [PubMed] [Google Scholar]
- 10.Wigfield C, Gill S, Nelson R, Langdon I, Metcalf N, Robertson J. Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg. 2002; 96(1 Suppl): 17–21. [DOI] [PubMed] [Google Scholar]
- 11.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999; 81(4): 519–28. [DOI] [PubMed] [Google Scholar]
- 12.DiAngelo DJ, Roberston JT, Metcalf NH, McVay BJ, Davis RC. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech. 2003; 16(4): 314–23. [DOI] [PubMed] [Google Scholar]
- 13.Dmitriev AE, Cunningham BW, Hu N, Sell G, Vigna F, McAfee PC. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine (Phila Pa 1976). 2005; 30(10): 1165–72. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi AA, Kode S, DeVries NA, Grosland NM, Smucker JD, Fredericks DC. Biomechanical Analysis of Cervical Disc Replacement and Fusion Using Single Level, Two level and Hybrid Constructs. Spine (Phila Pa 1976). 2015. 40(10): 1578–1585. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Kim JS, Lee JH, Chung ER, Shim CS, Lee SH. Comparison of cervical kinematics between patients with cervical artificial disc replacement and anterior cervical discectomy and fusion for cervical disc herniation. Spine J. 2014; 14(7): 1199–204. 10.1016/j.spinee.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 16.Anakwenze OA, Auerbach JD, Milby AH, Lonner BS, Balderston RA. Sagittal cervical alignment after cervical disc arthroplasty and anterior cervical discectomy and fusion: results of a prospective, randomized, controlled trial. Spine (Phila Pa 1976). 2009; 34(19): 2001–7. 10.1097/BRS.0b013e3181b03fe6 [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Wang X, Bai W, Shen X, Yuan W. Prevalence of heterotopic ossification after cervical total disc arthroplasty: a meta-analysis. Eur Spine J. 2012; 21(4): 674–80. 10.1007/s00586-011-2094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung C, Casey AT, Goffin J, Kehr P, Liebig K, Lind B, et al. Clinical significance of heterotopic ossification in cervical disc replacement: a prospective multicenter clinical trial. Neurosurgery. 2005; 57(4): 759–63. [DOI] [PubMed] [Google Scholar]
- 19.Zechmeister I, Winkler R, Mad P. Artificial total disc replacement versus fusion for the cervical spine: a systematic review. Eur Spine J. 2011; 20(2): 177–84. 10.1007/s00586-010-1583-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganbat D, Kim K, Jin YJ, Kim YH. Heterotopic ossification in cervical total disk replacement: a finite element analysis. Proc Inst Mech Eng H. 2014; 228(2): 200–5. 10.1177/0954411914522024 [DOI] [PubMed] [Google Scholar]
- 21.Pickett GE, Sekhon LH, Sears WR, Duggal N. Complications with cervical arthroplasty. J Neurosurg Spine. 2006; 4(2): 98–105. [DOI] [PubMed] [Google Scholar]
- 22.Wagner SC, Kang DG, Helgeson MD. Implant migration after Bryan cervical disc arthroplasty. Spine J. 2014; 14(10): 2513–4. 10.1016/j.spinee.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 23.Tsermoulas G, Bhattathiri PS. Anterior migration of prosthesis following cervical arthroplasty. Br J Neurosurg. 2013; 27(1): 132–3. 10.3109/02688697.2012.703354 [DOI] [PubMed] [Google Scholar]
- 24.Hacker FM, Babcock RM, Hacker RJ. Very late complications of cervical arthroplasty: results of 2 controlled randomized prospective studies from a single investigator site. Spine (Phila Pa 1976). 2013; 38(26): 2223–6. 10.1097/BRS.0000000000000060 [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Nie L, Li M, Huo Y, Pan X. Superiority of the Bryan(®) disc prosthesis for cervical myelopathy: a randomized study with 3-year follow-up. Clin Orthop Relat Res. 2011; 469(12): 3408–14. 10.1007/s11999-011-2039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine. 2007; 6(3): 198–209. [DOI] [PubMed] [Google Scholar]
- 27.Coric D, Nunley PD, Guyer RD, Musante D, Carmody CN, Gordon CR, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex/C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine. 2011; 15(4): 348–58. 10.3171/2011.5.SPINE10769 [DOI] [PubMed] [Google Scholar]
- 28.Park DK, Lin EL, Phillips FM. Index and adjacent level kinematics after cervical disc replacement and anterior fusion: in vivo quantitative radiographic analysis. Spine (Phila Pa 1976). 2011; 36(9): 721–30. 10.1097/BRS.0b013e3181df10fc [DOI] [PubMed] [Google Scholar]
- 29.Delamarter RB, Zigler J. Five-year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine (Phila Pa 1976). 2013; 38(9): 711–7. 10.1097/BRS.0b013e3182797592 [DOI] [PubMed] [Google Scholar]
- 30.Phillips FM, Lee JY, Geisler FH, Cappuccino A, Chaput CD, DeVine JG, et al. A prospective, randomized, controlled clinical investigation comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. 2-year results from the US FDA IDE clinical trial. Spine (Phila Pa 1976). 2013; 38(15): E907–18. 10.1097/BRS.0b013e318296232f [DOI] [PubMed] [Google Scholar]
- 31.Hisey MS, Bae HW, Davis R, Gaede S, Hoffman G, Kim K, et al. Multi-center, prospective, randomized, controlled investigational device exemption clinical trial comparing Mobi-C Cervical Artificial Disc to anterior discectomy and fusion in the treatment of symptomatic degenerative disc disease in the cervical spine. Int J Spine Surg. 2014; 8 10.14444/1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis RJ, Kim KD, Hisey MS, Hoffman GA, Bae HW, Gaede SE, et al. Cervical total disc replacement with the Mobi-C cervical artificial disc compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled multicenter clinical trial: clinical article. J Neurosurg Spine. 2013; 19(5): 532–45. 10.3171/2013.6.SPINE12527 [DOI] [PubMed] [Google Scholar]
- 33.Luo J, Gong M, Huang S, Yu T, Zou X. Incidence of adjacent segment degeneration in cervical disc arthroplasty versus anterior cervical decompression and fusion meta-analysis of prospective studies. Arch Orthop Trauma Surg. 2015; 135(2): 155–60. 10.1007/s00402-014-2125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, Liu M, Li T, Huang F, Tang T, Xiang Z. A meta-analysis comparing the results of cervical disc arthroplasty with anterior cervical discectomy and fusion (ACDF) for the treatment of symptomatic cervical disc disease. J Bone Joint Surg Am. 2013; 95(6): 555–61. 10.2106/JBJS.K.00599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nabhan A, Steudel WI, Nabhan A, Pape D, Ishak B. Segmental kinematics and adjacent level degeneration following disc replacement versus fusion: RCT with three years of follow-up. J Long Term Eff Med Implants. 2007; 17(3): 229–36. [DOI] [PubMed] [Google Scholar]
- 36.Maldonado CV, Paz RD, Martin CB. Adjacent-level degeneration after cervical disc arthroplasty versus fusion. Eur Spine J. 2011; 20 Suppl 3:403–7. 10.1007/s00586-011-1916-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karabag H, Cakmak E, Celik B, Iplikcioglu AC, Soran AF. Arthroplasty versus fusion for single-level cervical disc disease. J Pak Med Assoc. 2014; 64(12): 1348–51. [PubMed] [Google Scholar]
- 38.Jawahar A, Cavanaugh DA, Kerr EJ 3rd, Birdsong EM, Nunley PD. Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: results of 93 patients in three prospective randomized clinical trials. Spine J. 2010; 10(12): 1043–8. 10.1016/j.spinee.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 39.Nabhan A, Ishak B, Steudel WI, Ramadhan S, Steimer O. Assessment of adjacent-segment mobility after cervical disc replacement versus fusion: RCT with 1 year’s results. Eur Spine J. 2011; 20(6): 934–41. 10.1007/s00586-010-1588-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma K, Gandhi SD, Maltenfort M, Albert TJ, Hilibrand AS, Vaccaro AR, et al. Rate of adjacent segment disease in cervical disc arthroplasty versus single-level fusion: meta-analysis of prospective studies. Spine (Phila Pa 1976). 2013; 38(26): 2253–7. 10.1097/BRS.0000000000000052 [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Li H, Zhang T, He X, Xu S. The incidence of adjacent segment degeneration after cervical disc arthroplasty (CDA): a meta analysis of randomized controlled trials. PLos One. 2012; 7(4): e35032 10.1371/journal.pone.0035032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Loannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analysis of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009; 6(7): e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian W, Yan K, Han X, Yu J, Jin P, Han X. Comparison of the Clinical and Radiographic Results between Cervical Artificial Disc Replacement and Anterior Cervical Fusion: A Six-year Prospective Non-randomized Comparative Study. J Spinal Disord Tech. 2014. 10.1097/BSD.0000000000000206 [DOI] [Google Scholar]
- 44.Bartels RH, Donk R, van der Wilt GJ, Grotenhuis JA, Venderink D. Design of the PROCON trial: a prospective, randomized multi-center study comparing cervical anterior discectomy without fusion, with fusion or with arthroplasty. BMC Musculoskelet Disord. 2006; 7:85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae HW, Kim KD, Nunley PD, Jackson RJ, Hisey MS, Davis RJ, et al. Comparison of Clinical Outcomes of 1- and 2-Level Total Disc Replacement: Four-Year Results From a Prospective, Randomized, Controlled, Multicenter IDE Clinical Trial. Spine (Phila Pa 1976). 2015; 40(11): 759–66. 10.1097/BRS.0000000000000887 [DOI] [PubMed] [Google Scholar]
- 46.Arts MP, Brand R, van den Akker E, Koes BW, Peul WC. The NEtherlands Cervical Kinematics (NECK) trial. Cost-effectiveness of anterior cervical discectomy with or without interbody fusion and arthroplasty in the treatment of cervical disc herniation; a double-blind randomized multicenter study. BMC Musculoskelet Disord. 2010; 11:122 10.1186/1471-2474-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ament JD, Yang Z, Chen Y, Green RS, Kim KD. A Novel Quality-of-Life Utility Index in Patients With Multilevel Cervical Degenerative Disc Disease: Comparison of Anterior Cervical Discectomy and Fusion With Total Disc Replacement. Spine (Phila Pa 1976). 2015; 40(14): 1072–8. 10.1097/BRS.0000000000000898 [DOI] [PubMed] [Google Scholar]
- 48.Peng-Fei S, Yu-Hua J. Cervical disc prosthesis replacement and interbody fusion: a comparative study. Int Orthop. 2008; 32(1):103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunley PD, Jawahar A, Cavanaugh DA, Gordon CR, Kerr EJ 3rd, Utter PA. Symptomatic adjacent segment disease after cervical total disc replacement: re-examining the clinical and radiological evidence with established criteria. Spine J. 2013; 13(1): 5–12. 10.1016/j.spineee.2012.11.032 [DOI] [PubMed] [Google Scholar]
- 50.Burkus JK, Haid RW, Traynelis VC, Mummaneni PV. Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: results from a prospective randomized controlled trial. J Neurosurg Spine. 2010; 13(3): 308–18. 10.3171/2010.3.SPINE09513 [DOI] [PubMed] [Google Scholar]
- 51.Zigler JE, Delamarter R, Murrey D, Spivak J, Janssen M. ProDisc-C and anterior cervical discectomy and fusion as surgical treatment for single-level cervical symptomatic degenerative disc disease: five-year results of a Food and Drug Administration study. Spine (Phila Pa 1976). 2013; 38(3): 203–9. 10.1097/BRS.0b013e318278eb38 [DOI] [PubMed] [Google Scholar]
- 52.Garrido BJ, Taha TA, Sasso RC. Clinical outcomes of Bryan cervical disc arthroplasty a prospective, randomized, controlled, single site trial with 48-month follow-up. J Spinal Disord Tech. 2010; 23(6): 367–71. 10.1097/BSD.0b013e3181bb8568 [DOI] [PubMed] [Google Scholar]
- 53.Burkus JK, Traynelis VC, Haid RW Jr, Mummaneni PV. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article. J Neurosurg Spine. 2014; 21(4): 516–28. 10.3171/2014.6.SPINE13996 [DOI] [PubMed] [Google Scholar]
- 54.Coric D, Kim PK, Clemente JD, Boltes MO, Nussbaum M, James S. Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: results in 74 patients from a single site. J Neurosurg Spine. 2013; 18(1): 36–42. 10.3171/2012.9.SPINE12555 [DOI] [PubMed] [Google Scholar]
- 55.Davis RJ, Nunley PD, Kim KD, Hisey MS, Jackson RJ, Bae HW, et al. Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: a prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine. 2015; 22(1): 15–25. 10.3171/2014.7.SPINE13953 [DOI] [PubMed] [Google Scholar]
- 56.Hisey MS, Bae HW, Davis RJ, Gaede S, Hoffman G, Kim KD, et al. Prospective, Randomized Comparison of Cervical Total Disk Replacement Versus Anterior Cervical Fusion: Results at 48 Months Follow-up. J Spinal Disord Tech. 2015; 28(4): E237–43. 10.1097/BSD.0000000000000185 [DOI] [PubMed] [Google Scholar]
- 57.Phillips FM, Geisler FH, Gilder KM, Reah C, Howell KM, McAfee PC. Long-term Outcomes of the US FDA IDE Prospective, Randomized Controlled Clinical Trial Comparing PCM Cervical Disc Arthroplasty With Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976). 2015; 40(10): 674–83. 10.1097/BRS.0000000000000869 [DOI] [PubMed] [Google Scholar]
- 58.Sasso RC, Anderson PA, Riew KD, Heller JG. Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am. 2011; 93(18): 1684–92. 10.2106/JBJS.J.00476 [DOI] [PubMed] [Google Scholar]
- 59.Zhang HX, Shao YD, Chen Y, Hou Y, Cheng L, Si M, et al. A prospective, randomised, controlled multicentre study comparing cervical disc replacement with anterior cervical decompression and fusion. Int Orthop. 2014; 38(12): 2533–41. 10.1007/s00264-014-2497-5 [DOI] [PubMed] [Google Scholar]
- 60.Janssen ME, Zigler JE, Spivak JM, Delamarter RB, Darden BV 2nd, Kopjar B. ProDisc-C Total Disc Replacement Versus Anterior Cervical Discectomy and Fusion for Single-Level Symptomatic Cervical Disc Disease: Seven-Year Follow-up of the Prospective Randomized U.S. Food and Drug Administration Investigational Device Exemption Study. J Bone Joint Surg Am. 2015; 97(21): 1738–47. 10.2106/JBJS.N.01186 [DOI] [PubMed] [Google Scholar]
- 61.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957; 16(4): 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walraevens J, Demaerel P, Suetens P, Van Calenbergh F, van Loon J, Vander Sloten J, et al. Longitudinal prospective long-term radiographic follow-up after treatment of single-level cervical disk disease with the Bryan Cervical Disc. Neurosurgery. 2010; 67(3): 679–87; discussion 687. 10.1227/01.NEU.0000377039.89725.F3 [DOI] [PubMed] [Google Scholar]
- 63.Wu AM, Xu H, Mullinix KP, Jin HM, Huang ZY, Lv QB, et al. Minimum 4-year outcomes of cervical total disc arthroplasty versus fusion: a meta-analysis based on prospective randomized controlled trials. Medicine (Baltimore). 2015; 94(15): e665 10.1097/MD.0000000000000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren C, Song Y, Xue Y, Yang X. Mid- to long-term outcomes after cervical disc arthroplasty compared with anterior discectomy and fusion: a systematic review and meta-analysis of randomized controlled trials. Eur Spine J. 2014; 23(5): 1115–23. 10.1007/s00586-014-3220-3 [DOI] [PubMed] [Google Scholar]
- 65.Shriver MF, Lubelski D, Sharma AM, Steinmetz MP, Benzel EC, Mroz TE. Adjacent segment degeneration and disease following cervical arthroplasty: a systematic review and meta-analysis. Spine J. pii: S1529-9430(15)01544-2. 10.1016/j.spinee.2015.10.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.