Abstract
The insula (IC) and cingulate are key components of the central autonomic network and central nodes of the salience network (SN), a set of spatially distinct but temporally correlated brain regions identified with resting-state (task free) functional MRI (rsMRI). To examine the SN's involvement in sympathetic outflow, we tested the hypothesis that individual differences in intrinsic connectivity of the SN correlate positively with resting postganglionic muscle sympathetic nerve activity (MSNA) burst incidence (BI) in subjects without and with obstructive sleep apnea (OSA). Overnight polysomnography, 5-min rsMRI, and fibular MSNA recording were performed in 36 subjects (mean age 57 yr; 10 women, 26 men). Independent component analysis (ICA) of the entire cohort identified the SN as including bilateral IC, pregenual anterior cingulate cortex (pgACC), midcingulate cortex (MCC), and the temporoparietal junction (TPJ). There was a positive correlation between BI and the apnea-hypopnea index (AHI) (P < 0.001), but dual-regression analysis identified no differences in SN functional connectivity between subjects with no or mild OSA (n = 17) and moderate or severe (n = 19) OSA. Correlation analysis relating BI to the strength of connectivity within the SN revealed large (i.e., spatial extent) and strong correlations for the left IC (P < 0.001), right pgACC/MCC (P < 0.006), left TPJ (P < 0.004), thalamus (P < 0.035), and cerebellum (P < 0.013). Indexes of sleep apnea were unrelated to BI and the strength of SN connectivity. There were no relationships between BI and default or sensorimotor network connectivity. This study links connectivity within the SN to MSNA, demonstrating several of its nodes to be key sympathoexcitatory regions.
Keywords: microneurography, obstructive sleep apnea, resting-state magnetic resonance imaging, salience network, sympathetic nervous system
the sympathetic and parasympathetic efferent limbs of the autonomic nervous system (ANS) regulate cardiovascular, respiratory, digestive, and endocrine homeostasis. While its peripheral elements were characterized in the late nineteenth century (Saper 2002), its central circuits have been elucidated directly only recently through electrical stimulation and tract tracing and, more recently in humans, via deep brain stimulation (Cechetto and Shoemaker 2009; Sverrisdottir et al. 2014). These efforts identified a cluster of cortical and brain stem regions through which the brain controls autonomic function (Thayer and Lane 2000). The insula (IC), anterior cingulate cortex (ACC), ventromedial prefrontal cortex (vmPFC), amygdala, and cerebellum are anatomically core, but not exclusive, components of this central autonomic network (CAN) (Cechetto and Shoemaker 2009; Gianaros and Sheu 2009; Thayer and Lane 2000). Importantly, these cortical regions have reciprocal connections with brain stem premotor autonomic neurons that ultimately project to and influence preganglionic sympathetic and parasympathetic outflow (Thayer and Lane 2000).
Our present aim was to elucidate the functional significance of such connections for sympathetic outflow in conscious humans by integrating resting-state blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) with microneurographic recordings of postganglionic muscle sympathetic nerve activity (MSNA). Over the last decade, several groups have investigated cortical cardiovascular regulation during Valsalva's maneuver (Henderson et al. 2002) and cold pressor testing (Harper et al. 2003) with fMRI or have evaluated the effect of inspiratory capacity apnea (Macefield et al. 2006), lower body negative pressure (LBNP) (Kimmerly et al. 2005), Mueller maneuvers and end-expiratory breath holds (Kimmerly et al. 2013), and task-free conditions (Fatouleh et al. 2014; James et al. 2013b) on both fMRI and MSNA. There is now a large body of evidence documenting the IC, ACC, amygdala, and cerebellum as being core cardiovascular-related CAN components.
Resting-state fMRI (rsMRI) identifies, without explicit stimuli, spatially distinct brain areas that display temporally correlated BOLD oscillations, at frequencies < 0.1 Hz (Andrews-Hanna 2012; Taylor et al. 2009). With rsMRI, several resting-state networks (RSNs) comprising anatomically distinct, but functionally connected (i.e., temporally correlated), brain regions have been identified (Biswal et al. 1995; DeLuca et al. 2006). In healthy subjects RSNs are consistent within (Shirer et al. 2015) and across individuals and stable across repeated sessions (Damoiseaux et al. 2006; Zuo et al. 2010). RSNs have also been demonstrated in anesthetized monkeys (Vincent et al. 2007), and their functional connectivity can be modified by disease states (Fox and Greicius 2010). One distinct RSN, the salience network (SN), is composed of the dorsal ACC (dACC), anterior IC (aIC), frontal opercular cortex, and temporoparietal junction (TPJ) (Kucyi et al. 2012; Seeley et al. 2007). These areas are activated by attention-demanding and cognitive tasks (Menon and Uddin 2010), autonomic challenges (Kimmerly et al. 2005, 2013; Macefield et al. 2006; Wong et al. 2007), pain (Apkarian et al. 2005; Downar et al. 2003; Kucyi et al. 2013), and homeostatic threats (Craig 2002). Because all of these stimuli elicit acute changes in sympathetic tone, the SN is of particular interest to autonomic investigators (Critchley et al. 2004, 2005; Seeley et al. 2007).
Until recently, the majority of imaging studies that have investigated task-related activations (whether they be cognitive, affective-emotional, or sensory-motor) and resting-state data either have omitted the measurement, or analysis, of autonomic variables or have mathematically eliminated variation attributable to heart rate (HR) and respiration (Iacovella and Hasson 2011). Recent task-based fMRI studies utilizing autonomic challenges clearly demonstrate cortical involvement of regions previously associated with cognitive, executive, and sensorimotor brain functions (i.e., the ACC and IC). These findings have led some authors to postulate that these brain areas are actually, or additionally, signaling brain stem regions underlying autonomic processing (Beissner et al. 2013; Craig 2002, 2003; Critchley et al. 2004; Seeley et al. 2007).
Examination of RSNs in a “spontaneous” (Fox and Raichle 2007) (i.e., unprompted or “task free”) state permits investigation of the CAN without the potential for confounding by autonomic responses specific to cognitive, emotional, or sensorimotor task-induced activations. Thus far, combined rsMRI and ANS investigations are limited to two studies that linked intraindividual indirect estimates of efferent sympathetic and parasympathetic outflow (i.e., skin conductance and HR variability) (Chang et al. 2013; Fan et al. 2012) with variations in resting-state connectivity and one seed-based analysis, situated on the ventromedial hypothalamus, that was not specifically correlated with a physiological measure (i.e., MSNA) (James et al. 2013b). Here we use independent component analysis (ICA) to identify interindividual differences in SN connectivity and direct recordings to quantify postganglionic MNSA.
ICA is a blind source separation technique that is used to decompose a multivariate signal into source signals that are independent of each other. In the context of neuroimaging ICA is employed conventionally to remove motion and scanner artifacts, identify task-induced activations without explicit modeling, and consistently identify RSNs (Beckmann et al. 2005; McKeown et al. 1998; Zuo et al. 2010). The utility of multisubject (or group) ICA has been demonstrated in diverse clinical populations such as depression (Greicius et al. 2007), Alzheimer's disease or dementia (Greicius et al. 2004; Rombouts et al. 2009; Seeley et al. 2009), Huntington's disease (Wolf et al. 2008), schizophrenia (Jafri et al. 2008), epilepsy (Zhang et al. 2009), and amyotrophic lateral sclerosis (Mohammadi et al. 2009). In sum, group ICA is a powerful, well-validated, and commonly used tool to identify and examine RSNs at the group or population level (Filippini et al. 2009; Zuo et al. 2010).
Modulated by baroreceptor reflex, nonbaroreceptor reflex, and cortical input (Floras 2009), sympathetic nerve firing to skeletal muscle vasculature is entrained to the cardiac cycle and thus for evaluation of its influence by temporal- and HR-independent mechanisms is expressed as burst incidence (BI; bursts/100 cardiac cycles). Unlike the arterial baroreceptor reflex modulation of HR, which is impaired in heart failure, hypertension, and healthy aging, the baroreflex modulation of MSNA by blood pressure is preserved under these conditions (Floras 2009; Seravalle et al. 2015).
The purpose of this study was to utilize ICA to specifically extract the SN in middle-aged adults to correlate individual differences in the strength of its intrinsic connectivity with directly recorded sympathetic outflow. We hypothesized that MSNA BI would correlate positively with the strength of intrinsic connectivity within the SN. Our secondary objective was to determine whether obstructive sleep apnea (OSA) affected the strength of intrinsic connectivity or its relationship with MSNA BI. As internal controls, between-group comparisons and correlations with BI and the connectivity within the default mode and somatosensory networks also were performed.
MATERIALS AND METHODS
Subjects
Recognizing that subjects with OSA would be more likely than not to exhibit high MSNA BI (Floras 2015), and seeking to ensure a range of interindividual variability in resting BI to compare with intrinsic SN connectivity, we initiated these experiments with the intent of studying a similar proportion of male and female subjects with either a low or a high pretest likelihood of moderate to severe OSA [apnea-hypopnea index (AHI) > 15 events/h of sleep]. To do so, we assembled, primarily by advertisement, two groups (otherwise healthy middle-aged subjects who considered themselves free of OSA or subjects with a history of snoring, witnessed apnea during sleep, or high-risk anthropometric characteristics) who were agreeable to participating in a research protocol involving overnight polysomnography followed by a daytime physiological recording session and an MRI session. We anticipated, in light of current epidemiologic estimates, that the prevalence of unrecognized asymptomatic OSA in such a nonsleepy cohort would be high (Kasai et al. 2012). This study population therefore is distinctively different from that of most previous published reports, in which OSA patients were identified only after a clinical syndrome prompted physician referral for polysomnography and in which control subjects were assumed to be free of OSA based upon self-reports.
Before participating in this University Health Network Research Ethics Board-approved protocol, all subjects provided written informed consent. During enrollment subjects completed routine medical and MRI-readiness questionnaires to determine their eligibility and to ensure safety within a strong magnetic field. No one with a history or the presence of heart failure, myocardial infarction, kidney disease, Raynaud's disease, autonomic neuropathy, excessive daytime sleepiness, predominantly central sleep apnea, refractory hypertension, neurological impairment, chronic back pain, current smoking, current use of continuous positive airway pressure therapy, claustrophobia, imbedded magnetic objects, or current pregnancy was studied. To maintain clinical stability, and assuming that if any had central actions these would affect concurrently and proportionately the SN and MSNA, prescribed medications were continued in 10 participants (see Table 2).
Table 2.
Prescribed medications
| Medication | n |
|---|---|
| Angiotensin-converting enzyme inhibitor | 6 |
| Angiotensin II antagonist | 1 |
| β-Blocker | 4 |
| Ca2+ channel blocker | 1 |
| Diuretic | 5 |
| Anticoagulant | 2 |
| Statin | 7 |
| Thyroid hormone | 2 |
| Tapazole | 1 |
| Allopurinol | 1 |
| Nonsteroidal anti-inflammatory | 5 |
| Proton pump inhibitor | 3 |
| Testosterone | 1 |
Data Acquisition
After overnight polysomnography, MRI and physiological recording sessions were performed in random order on different days separated by ∼1 wk. All MRI scans and physiological recordings were acquired at the same time of day, 2–3 h after a similar light meal. Subjects also were instructed to abstain from nicotine, alcohol, and caffeine for at least 12 h before data acquisition. Before each test session subjects were instructed to void to minimize the established effects of a distended bladder on sympathetic activity (Fagius and Karhuvaara 1989).
Polysomnography
To quantify their subjective daytime sleepiness, the Epworth Sleepiness Scale questionnaire (Johns 1994) was administered to all participants before sleep by a trained technician unaware of the purpose of the present study. Subjects then underwent overnight polysomnography using standard techniques and scoring criteria for sleep stages and arousals from sleep (Rechtschaffen and Kales 1968; Sleep Disorders Atlas Task Force 1992). Thoracoabdominal movements and tidal volume were measured by respiratory inductance plethysmography (American Academy of Sleep Medicine Task Force 1999). Airflow was measured by nasal pressure cannulas (American Academy of Sleep Medicine Task Force 1999) and arterial oxyhemoglobin saturation (SaO2) by oximetry. Apneas and hypopneas were scored according to the American Academy of Sleep Medicine criteria (American Academy of Sleep Medicine Task Force 1999; Iber et al. 2007), and OSA severity was graded by a continuum with an AHI < 5 events/h of sleep classified as no sleep apnea; AHI 5–15 as mild OSA, AHI 15–30 as moderate OSA, and AHI > 30 as severe OSA (American Academy of Sleep Medicine Task Force 1999; Iber et al. 2007).
Physiological Data Acquisition
All study data were acquired in a quiet, temperature-controlled room with participants lying supine, with eyes closed but awake, and breathing spontaneously. Lead II of an electro-cardiogram was utilized to calculate HR. Arterial blood pressure was recorded continuously with noninvasive automated photoelectric plethysmography (Portapres Model-2, Finapres Medical Systems, Amsterdam, The Netherlands). Approximately every minute throughout the experimental session these values were referenced to noninvasive oscillometric determinations of brachial artery blood pressure acquired from the opposite arm with a standard 23- to 33-cm adult cuff (Dinamap Pro 100, Critikon, Tampa, FL). Breathing was recorded with a pneumobelt connected to a pressure transducer. Multiunit recordings of postganglionic MSNA were acquired with a unipolar tungsten microelectrode inserted percutaneously into a fascicle of the right common fibular nerve (Hagbarth and Vallbo 1968; Notarius et al. 2015). MSNA, blood pressure, and HR were acquired over a 10-min rest period.
MR Data Acquisition
MRI data were obtained while subjects lay supine within a 3-T GE (HDxt 16.0) MRI system fitted with an eight-channel phased-array head coil. A three-dimensional (3D) high-resolution anatomical scan of the whole brain (120 slices, 24 × 24-cm FOV, 256 × 256 matrix, 1.5 × 0.859 × 0.859 voxels) was acquired with a T1-weighted 3D spoiled gradient echo sequence (flip angle = 45°, TE = 5 ms, TR = 25 ms). Resting-state functional MRI data were acquired with a T2*-weighted echo planar imaging sequence (32 axial slices, FOV = 20 × 20 cm, 64 × 64 matrix, 3.75 × 3.75 × 4.4-mm voxels, TE = 40 ms, TR = 2,000 ms). The scan was 5 min long, for a total of 150 frames. During resting-state imaging subjects were instructed to keep their eyes closed, but stay awake, and think of nothing in particular (Damoiseaux et al. 2006; Greicius et al. 2004; Taylor et al. 2009).
Physiological Data Analysis
All physiological signals underwent analog-to-digital conversion to a standard PC desktop for storage and analysis with the LabVIEW software platform (National Instruments, Austin, TX). Spontaneous postganglionic sympathetic nerve bursts were accepted for analysis if they 1) were synchronized with HR; 2) were not elicited by skin stroking or arousing stimuli; 3) increased in response to voluntary apnea; and 4) exhibited a signal-to-noise ratio of ≥3:1 (Notarius et al. 2015). To control for between-subject variation in HR, MSNA was expressed as a cardiac frequency-independent measure of the intensity of sympathetic discharge, bursts per 100 heartbeats or BI.
Imaging Data Analysis
All data were analyzed with FSL (FMRIB's Software Library v.5.0.1, http://www.fmrib.ox.ac.uk/fsl) (Smith et al. 2004). A spatial group ICA was performed to identify the SN with the Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC v.3.10) toolbox implemented through FSL (Beckmann and Smith 2004). Before the group ICA was run, preprocessing of each subject's raw resting-state data was performed as follows: removal of the first three volumes to allow for T1 equilibration effects, high-pass filtering (0.01-Hz cutoff), motion correction with FMRIB's Linear Imaging Registration Tool (FLIRT) (Jenkinson et al. 2002), brain extraction with FSL's brain extraction tool (BET) (Smith 2002), spatial smoothing with a 5-mm Gaussian kernel, linear registration between each subject's T2*-weighted and T1-weighted images (6 degrees of freedom), and registration between the subject's T1-weighted space and standard MNI152 2-mm3 stereotaxic space (12 degrees of freedom). Group ICA involves three steps: 1) image concatenation, 2) dual regression, and 3) statistical inference.
Image concatenation.
Each subject's time course was variance-normalized and temporally concatenated across subjects to form a 2D Space × Concatenated Time data matrix (Beckmann and Smith 2004; Filippini et al. 2009). ICA was then applied to this matrix to identify large-scale patterns of functional connectivity common to all subjects (Beckmann et al. 2005; Filippini et al. 2009). In accordance with previous practice, to identify the SN we limited our analysis to 25 spatially independent components (Kucyi et al. 2012; Uddin et al. 2011).
Dual regression.
The set of 25 independent spatial maps (components) from the group-average analysis was then used to generate subject-specific versions of the spatial maps, and to generate their associated time series, using dual regression (Filippini et al. 2009). First, for each subject, the unthresholded group-average set of spatial maps was regressed (as spatial regressors in a multiple regression) into that subject's 4D space-time data set. This resulted in a set of subject-specific time series, one per group-level spatial map [i.e., 25 in total (1 for each group-level component) for each subject]. Next, those time series were regressed (as temporal regressors, again in a multiple regression) into the same 4D data set, resulting in a set of subject-specific spatial maps, one per group-level spatial map. Using the 25 group ICA components we identified the SN as the component that included the anterior IC (aIC), perigenual ACC (pgACC), midcingulate (MCC), and TPJ (Kucyi et al. 2012; Weissman-Fogel et al. 2010).
Statistical inference.
Using FSL's nonparametric permutation-testing tool (randomize), we then tested whether the subject-specific spatial maps, corresponding to the group ICA SN component 1) were different between HC and OSA groups and 2) correlated with BI. FSL's stringent Threshold Free Cluster Enhancement (TFCE) statistical analysis was utilized to identify brain regions with a corrected P < 0.05. Significant regions are those where the degree of connectivity with the SN (z score) is associated with individual differences in BI.
To ensure the specificity of our findings we also interrogated two additional independent components: the default mode network (DMN) and the sensorimotor network. The DMN was chosen because, in the context of autonomic regulation, most of its regions are thought to be associated with parasympathetic functions (Beissner et al. 2013) and the sensorimotor network was not expected to be significantly engaged in sympathetic nervous system regulation. The DMN was identified as the component comprising the posterior cingulate (PCC)/precuneus (PCu), medial prefrontal, and lateral parietal cortices (Weissman-Fogel et al. 2010); the sensorimotor network consisted of the sensorimotor cortices, secondary somatosensory cortices, and the supplementary motor area (Wu et al. 2008). While standard motion correction techniques were applied prior to group ICA analysis combined with the knowledge that ICA inherently identifies and separates signals associated with movement and scanner artifacts (Tohka et al. 2008), to further control for subject head motion the dual-regression analyses were rerun with the inclusion of mean framewise displacement (Power et al. 2015) for each subject (extracted from the MCFLIRT preprocessing file) included as a covariate within the model. A correlation analysis was also performed between BI and mean framewise displacement to ensure that significant connectivity within the SN could not be attributed to collinearity between these measures. Finally, to further delineate the mechanism(s) responsible for the connectivity relationship with the SN, dual-regression analysis was performed with measures specific to the sleep state obtained during overnight polysomnography [mean SaO2, minimum SaO2 (MinSaO2), number of arousals, and AHI], patient perceptions of daytime sleepiness (Epworth Sleepiness Score, ESS), and body mass index (BMI). All images were familywise error corrected to z > 2.3 and a cluster-based threshold of P < 0.05 and were displayed with FSLview and Caret (http://brainvis.wustl.edu/wiki/index.php/Caret:About).
RESULTS
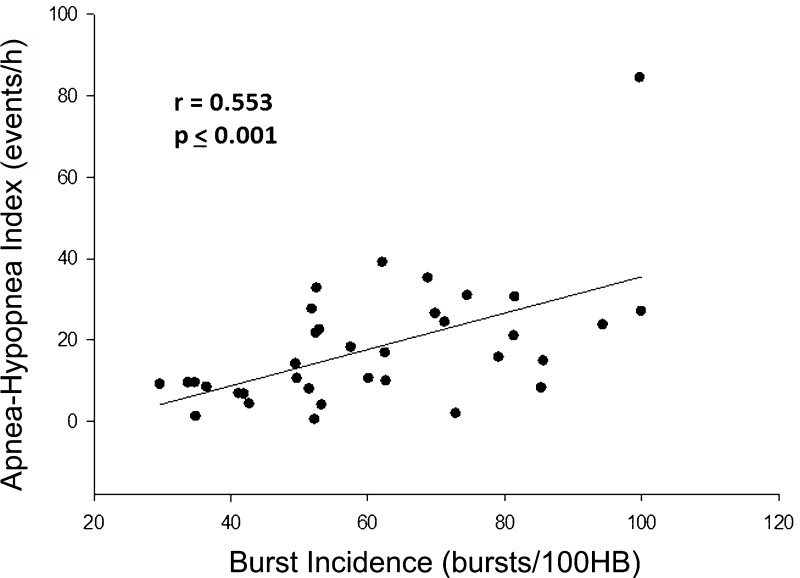
All but one subject completed the full protocol (one 38-yr-old male, considered clinically not to have OSA, did not undergo polysomnography). Participant characteristics and hemodynamic and microneurographic values appear in Table 1. Resting sympathetic BI ranged from 29 to 100 bursts/100 heartbeats, confirming that our recruitment strategy fulfilled its objective of creating a high degree of interindividual variability. Moreover, there was, in this cohort, a significant positive relationship between AHI and MSNA BI (Fig. 1).
Table 1.
Participant characteristics
| Characteristic | All Subjects | No or Mild Apnea | Moderate or Severe Apnea | P Value |
|---|---|---|---|---|
| No. of subjects (female) | 36 (10) | 17 (6) | 19 (4) | |
| Age, yr | 57 ± 4 | 57 ± 4 | 58 ± 4 | 0.592 |
| AHI, events/h | 18 ± 3 | 6 ± 2 | 28 ± 2 | < 0.001 |
| BI, bursts/100 heartbeats | 60 ± 4 | 49 ± 4 | 71 ± 4 | < 0.001 |
| BMI, kg/m2 | 29 ± 1 | 27 ± 1 | 30 ± 1 | 0.311 |
| HR, bpm | 60 ± 2 | 59 ± 3 | 61 ± 2 | 0.440 |
| SBP, mmHg | 120 ± 3 | 118 ± 3 | 122 ± 2 | 0.166 |
| DBP, mmHg | 69 ± 2 | 67 ± 7 | 70 ± 9 | 0.833 |
| ESS | 5 ± 1 | 3 ± 3 | 6 ± 2 | 0.385 |
| Mean SaO2, % | 95 ± 2 | 94 ± 2 | 95 ± 2 | 0.929 |
| MinSaO2, % | 88 ± 4 | 87 ± 2 | 82 ± 2 | 0.103 |
All values are means ± SE. P values relate to between-group differences.
AHI, apnea-hypopnea index; BI, burst incidence; BMI, body mass index; HR, heart rate; bpm, beats per minute; SBP, systolic blood pressure; DBP, diastolic blood pressure; ESS, Epworth Sleepiness Score; SaO2, arterial oxyhemoglobin saturation; MinSaO2, minimum SaO2.
Fig. 1.
Correlation analysis between burst incidence (BI) and apnea-hypopnea index (AHI) across all subjects. A significant positive correlation is demonstrated. HB, heartbeat.
With the exception of one subject with OSA, in whom the ESS on the night before polysomnography was recorded as 13, none had self-reported daytime sleepiness. Of those participants who reported no symptoms or signs of OSA when recruited, and who considered themselves free of any significant medical condition, only 5 had an AHI < 5 events/h, 12 were found to have 5–10 events/h, and, importantly, 5 had moderate OSA (AHI > 15). Comparison of the mean subgroup characteristics of subjects classified after polysomnography either as having no or mild OSA or as having moderate or severe OSA is also presented in Table 1. In the no or mild OSA subgroup, comprising 17 subjects [6 women, 11 men; 57 ± 4 yr (mean ± SE)], the AHI ranged between 1 and 10 events/h. The AHI ranged between 15 and 84 events/h in the other 19 subjects (4 women, 15 men; 58 ± 4 yr). As anticipated, those with OSA had higher average BI (71 ± 4 vs. 49 ± 4 bursts/100 heartbeats; P < 0.001). No subject with no or mild OSA was prescribed medications (Table 2).
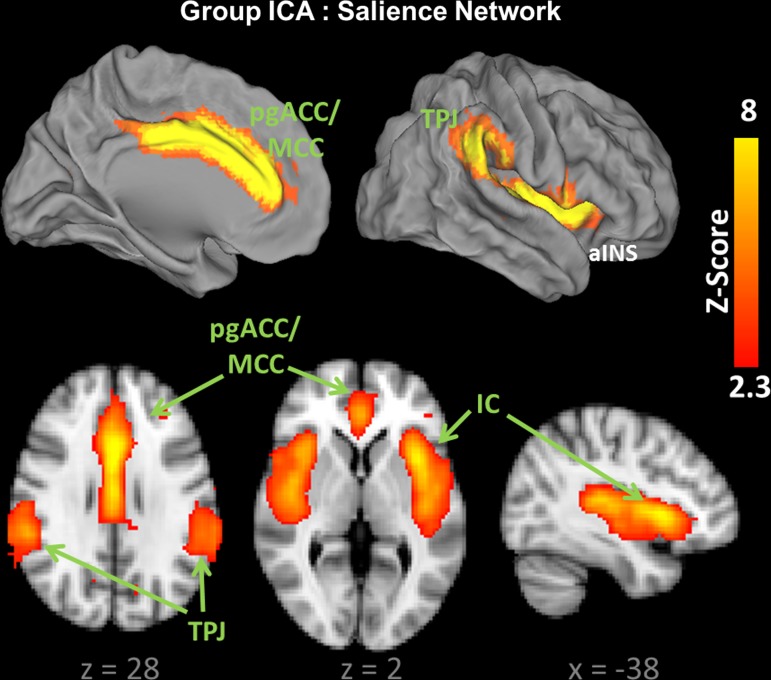
The component representing the SN was easily identified from the group ICA maps. Consistent with prior reports, the SN in this cohort of subjects included bilateral IC, pgACC and MCC, and TPJ regions (Fig. 2) (Kucyi et al. 2012; Seeley et al. 2007; Weissman-Fogel et al. 2010). Examination of MELODIC's power-spectral analysis for the SN component demonstrated low-frequency oscillations within the 0.01–0.02 Hz range, satisfying criteria for a low-frequency, resting-state, network.
Fig. 2.
The salience network (SN) identified by group independent component analysis (ICA) (n = 36). L, left; R, right; pgACC, perigenual anterior cingulate cortex; MCC, midcingulate cortex; IC, insula; TPJ, temporoparietal junction. Coordinates (x,y,z) refer to millimeters in MNI space.
Importantly, dual-regression between-group comparison between HC and OSA subjects did not reveal significant differences in the strength of connectivity of the SN with any brain regions (TFCE P > 0.05); the mean ESS in the moderate to severe OSA group was only 6 (≥11 indicates excessive daytime sleepiness); there were no significant correlations between the strength of connectivity within the SN and the ESS; there were no significant differences with respect to mean values between the two subgroups with respect to any other patient characteristic related to the severity of OSA or confounding factors with the potential also to influence MSNA (ESS, mean SaO2, MinSaO2, BMI, HR, systolic blood pressure, diastolic blood pressure, P > 0.05 for all; Table 1); and the strength of connectivity within the SN was not related to BMI or three indexes of the severity of obstructive apnea during sleep: mean SaO2, MinSaO2, and the total number of arousals (P > 0.05). Dual regression between AHI and the strength of connectivity within the SN revealed only a small cluster (3 voxels) of positively correlated activity within the right aIC [P = 0.037; x = 13, y = 35, z = 17 (peak MNI coordinates)]. No inverse correlations were detected. On the basis of these several concordant findings, we proceeded to interrogate the relationship between BI and SN connectivity in the entire study population.
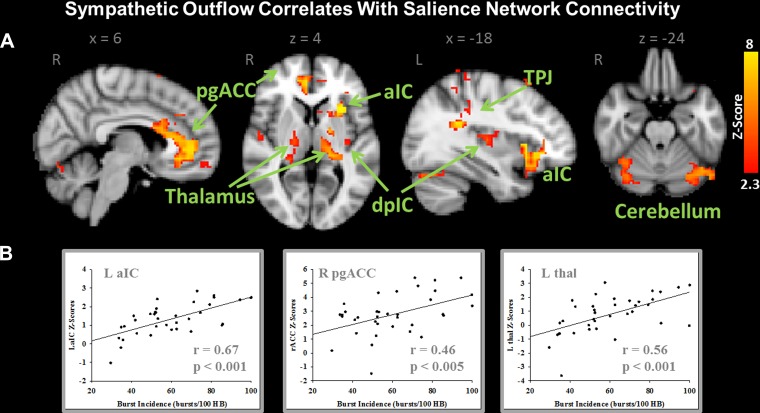
Analysis involving the entire cohort identified a strong positive correlation with connectivity of several brain regions within the SN. In total, 43 distinct cortical clusters were found to be significantly correlated with BI (see Table 3 for a full list of regions) after FSL's stringent TFCE statistical analysis. Of particular note are the extent (i.e., number of voxels) and strength (z score) of the correlations between BI and resting SN connectivity within left aIC, right pregenual/anterior MCC, bilateral posterior IC, TPJ, thalamus, and cerebellum (lobule V/VI and crus I) (see Fig. 3A). For visualization purposes the TFCE threshold was increased from P = 0.05 to P = 0.025. Subsequently, the maps were binarized and the z-score values were extracted from the regions of interest that encompassed the left aIC, right pgACC, and left thalamus. These z-score values are plotted against BI measures for each subject in Fig. 3B. Clearly, as BI increases in this population so too does the strength of connectivity of these areas within the SN. No regions within the SN were found to be negatively correlated with BI.
Table 3.
Anatomical location, number of voxels, and peak MNI coordinates for regions exhibiting positive correlations between sympathetic outflow and functional connectivity within the salience network
| MNI Coordinates |
|||||
|---|---|---|---|---|---|
| Anatomical Location (Brodmann area) | # Vox | x | y | z | P Value (corr) |
| L anterior insula/caudate | 314 | −38 | 18 | −4 | 0.001 |
| L cerebellum (crus I) | 46 | −42 | −66 | −24 | 0.018 |
| L cerebellum (lobule V) | 2 | −14 | −50 | −16 | 0.041 |
| L frontal pole (BA 9) | 1 | −30 | 50 | 32 | 0.043 |
| L frontal pole/medial frontal gyrus (BA 9) | 14 | −26 | 38 | 32 | 0.037 |
| L inferior parietal lobule (BA 40; TPJ) | 121 | −34 | −46 | 24 | 0.004 |
| L medial frontal gyrus (BA 6) | 6 | −34 | 22 | 60 | 0.027 |
| L postcentral gyrus (BA 3/4) | 73 | −38 | −26 | 72 | 0.015 |
| L posterior insula | 19 | −34 | −18 | 8 | 0.03 |
| L precentral gyrus/medial frontal gyrus (BA 6) | 1 | −38 | −2 | 48 | 0.05 |
| L precentral gyrus/medial frontal gyrus (BA 4) | 2 | −30 | −6 | 52 | 0.047 |
| L precuneus/cuneus (BA 18) | 15 | −2 | −74 | 28 | 0.019 |
| L superior frontal gyrus (BA 6) | 16 | −10 | 10 | 72 | 0.026 |
| L superior parietal lobule | 4 | −14 | −54 | 64 | 0.043 |
| L superior parietal lobule (BA 5/7) | 6 | −30 | −46 | 72 | 0.031 |
| L superior temporal gyrus (BA 42) | 5 | −58 | −10 | 8 | 0.025 |
| L supramarginal gyrus/angular gyrus | 1 | −50 | −50 | 20 | 0.05 |
| R anterior insula | 1 | 42 | 18 | −4 | 0.05 |
| R cerebellum (crus I) | 132 | 42 | −54 | −28 | 0.013 |
| R cerebellum (lobule V/VI) | 10 | 18 | −50 | −16 | 0.021 |
| R cerebellum (lobule VI) | 5 | 38 | −38 | −32 | 0.036 |
| R frontal pole (BA 11) | 18 | 18 | 58 | −16 | 0.026 |
| R frontal pole/medial frontal cortex (BA 11) | 5 | 6 | 58 | −12 | 0.045 |
| R lateral occipital cortex (BA 19) | 1 | 34 | −74 | 40 | 0.042 |
| R lateral parietal cortex (BA 7) | 1 | 30 | −66 | 60 | 0.051 |
| R medial frontal gryus (BA 6) | 48 | 42 | 10 | 60 | 0.027 |
| R medial frontal gyrus (BA 9) | 3 | 30 | 14 | 44 | 0.045 |
| R medial temporal gyrus | 10 | 34 | −54 | 0 | 0.023 |
| R postcentral gyrus (BA 1/2) | 3 | 42 | −34 | 68 | 0.037 |
| R precentral gyrus (BA 4) | 2 | 38 | −10 | 56 | 0.044 |
| R precuneus | 24 | 26 | −58 | 20 | 0.032 |
| R precuneus (BA 18/31) | 9 | 16 | −66 | 28 | 0.032 |
| R precuneus/superior parietal lobule (BA 7) | 100 | 18 | −62 | 50 | 0.023 |
| R precuneus (BA 7) | 6 | 2 | −34 | 52 | 0.044 |
| R precuneus (BA 7) | 1 | 22 | −42 | 56 | 0.05 |
| R pregenual anterior cingulate cortex (BA 32) | 189 | 6 | 46 | 0 | 0.006 |
| R superior frontal gyrus (BA 8) | 17 | 2 | 26 | 60 | 0.029 |
| R superior frontal gyrus (BA 8) | 2 | 10 | 34 | 60 | 0.049 |
| R superior temporal gyrus (BA 22)/posterior insula | 105 | 50 | −10 | −5 | 0.014 |
| R supramarginal gyrus | 2 | 38 | −46 | 16 | 0.045 |
| R supramarginal gyrus/angular gyrus (BA 40) | 1 | 46 | −46 | 44 | 0.047 |
| R supramarginal gyrus/postcentral gyrus (BA 3) | 4 | 62 | −22 | 40 | 0.047 |
| R thalamus (peak on VPL) | 33 | 18 | −14 | 4 | 0.035 |
Values are anatomical location (including Brodmann area), no. of 4-mm3 voxels (# Vox), and peak MNI coordinates for regions exhibiting positive correlations between sympathetic outflow and functional connectivity within the salience network.
TPJ, temporoparietal junction; VPL, ventral posterolateral nucleus.
Fig. 3.
A: dual-regression analysis demonstrating positive correlations between resting efferent sympathetic BI and the strength of connectivity within the SN. B: z-score values were extracted from L anterior IC (aIC), R anterior cingulate cortex (ACC), and L thalamus (thal) in all subjects and plotted against their BI to demonstrate specific regional correlations. dpIC, dorsal posterior IC. Images are thresholded with a Threshold Free Cluster Enhancement-corrected P < 0.025. Coordinates (x,y,z) refer to millimeters in MNI space.
Supporting the specificity of our findings, connectivity within the DMN and the sensory network did not correlate with BI (P > 0.05). In addition, the inclusion of mean framewise displacement (head motion) in the dual-regression analysis with BI measures did not significantly alter the connectivity maps. Collinearity was not observed between BI and mean framewise displacement (R = 0.260, P = 0.125).
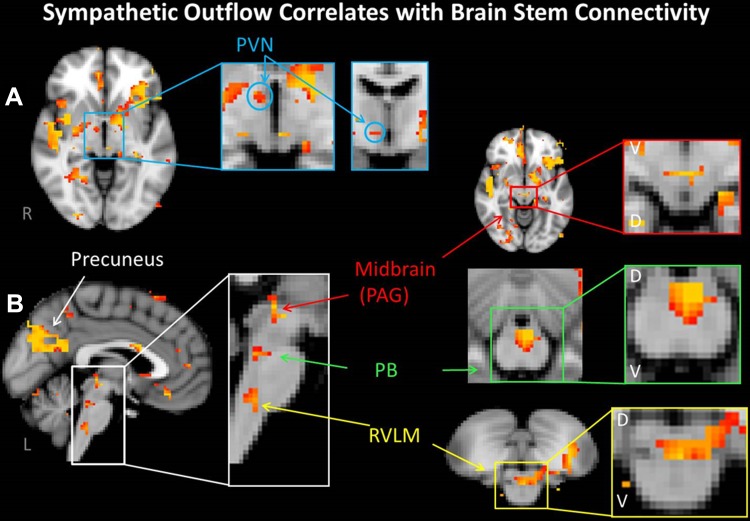
To satisfy standards of the neuroimaging community at large we initially employed the statistically rigorous TFCE methodology with a threshold set to a corrected P ≤ 0.05 (i.e., all aforementioned data). On review of the relevant literature specifically examining cortical and brain stem cardiovascular regulation, it became apparent that current analytical conventions include the application of lower uncorrected P values (i.e., P < 0.001 uncorrected) or fixed effects (as opposed to random effects) analysis (James et al. 2013a, 2013b; Kimmerly et al. 2013; Macefield and Henderson 2010). One reason for this approach, particularly relevant to the brain stem, is that increased physiological “noise,” derived from major arteries and pulsatile cerebrospinal fluid-filled spaces adjacent to and within this structure, reduces severely the signal-to-noise ratio and increases the risk of false-negative determination (Brooks et al. 2013). Following these investigators' lead, we reinterrogated our images at a lower threshold (uncorrected P = 0.001). Shifting to this lower threshold uncovered correlations between efferent sympathetic discharge and several brain stem regions known to be involved in autonomic cardiovascular regulation that were not identified by the initial, more rigorous, analysis: the rostral ventral lateral medulla (RVLM) (Macefield and Henderson 2010), the parabrachial nucleus (PB) in the rostral pons (Dampney 1994), the midbrain periaqueductal gray (PAG) (Verberne and Owens 1998), and the paraventricular nucleus of the hypothalamus (PVN) (Dampney 2011) (Fig. 4). Also, in addition to those cortical centers identified in Table 3, the PCu emerged as a prominent connected region.
Fig. 4.
Images are thresholded at a reduced uncorrected P = 0.001. A: in the SN, connectivity within the paraventricular nucleus of the hypothalamus (PVN) was correlated with resting BI. B: likewise, SN connectivity within the midbrain [periaqueductal gray (PAG)], pons [parabrachial nucleus (PB)], and medulla [rostral ventral lateral medulla (RVLM)] was also positively correlated with resting BI. Note also connectivity with the precuneus. D, dorsal; V, ventral.
DISCUSSION
The concept that efferent sympathetic discharge might relate to intrinsic, low-frequency (<0.1 Hz), resting-state cortical activity has thus far received little attention. The present study demonstrates a positive relationship during a state of wakeful rest, with spontaneous breathing, between directly measured postganglionic muscle sympathetic outflow and individual differences in the strength of resting-state functional connectivity within the SN. Moreover, there was in this cohort a significant positive relationship between the AHI and MSNA BI (Fig. 1). This itself is a novel observation.
The SN, first identified as a distinct RSN by Seeley et al. (2007), was posited to identify, from internal or extrapersonal stimuli, the most homeostatically relevant information. The IC and the ACC are two of its key nodes (Menon and Uddin 2010; Seeley et al. 2007). Over the last decade task-based fMRI has ascribed to these two cortical sites several functions, but principally cognitive processes such as attentional demand and executive control. By contrast, their contribution (particularly that of the cingulate) to autonomic regulation has received less attention. While these two regions are anatomically distinct, coactivation over a wide variety of behavioral tasks is consistently reported (Craig 2009; Menon and Uddin 2010), suggesting an interrelated role for these cortical areas. Identification of the SN with resting-state functional connectivity analysis (Seeley et al. 2007) provides support for interactions between these brain areas. The amalgamation of current evidence suggests that the IC and ACC are responsive to emotional, cognitive, or homeostatic stimuli or events, whether in the internal or external environment, i.e., subjective salience in general (Craig 2009).
The IC has been implicated in gustatory, visceral sensory/motor, cardiovascular, ocular, vestibular, memory, auditory, and language processes (Augustine 1996) and in response to acute noxious stimuli (Apkarian et al. 2005). The posterior IC, termed the primary interoceptive cortex (Craig 2004), connects reciprocally with the nucleus of the solitary tract (NTS) (Verberne 2011), the main medullary gateway to the central nervous system for afferent input from cardiovascular, respiratory, gastrointestinal, and other peripheral receptors (Andresen and Paton 2011). A parallel pathway from the NTS via the PB to the IC underscores its importance in integrating afferent autonomic input (Verberne 2011). The major path by which the posterior IC influences cardiovascular responses is via the lateral hypothalamus, which connects with the RVLM, the brain stem origin of most premotor sympathetic outflow (Sun and Guyenet 1986). Anatomical pathways from the aIC to the RVLM (Gabbott et al. 2005; M'hamed et al. 1993) and lateral hypothalamus (Yasui et al. 1991) provide additional routes by which this region could modulate medullary autonomic function. Depending on its exact location, electrical stimulation within the IC elicits either tachycardia and pressor responses or bradycardia and depressor responses (Oppenheimer et al. 1991; Oppenheimer and Cechetto 1990). In humans, both types of responses could be elicited from either hemisphere, but bradycardia and depressor responses were evoked primarily by left IC stimulation, whereas increases in HR and blood pressure occurred more often with right-sided stimulation (Oppenheimer et al. 1992). Thus the IC integrates ascending interoceptive and visceromotor afferent information. This enables the determination and assessment of salience, ultimately guiding behavior through direct and indirect connections with multiple cortical and brain stem regions (including medullary autonomic regions that modulate autonomic homeostatic outflow) (Uddin 2015).
It is known from early electrical stimulation studies conducted in animals (Burns and Wyss 1985; Kaada 1951; Kaada et al. 1949; Ward 1948) and humans (Pool and Ransohoff 1949) that the cingulate also has a role in autonomic regulation. Homeostatically relevant interconnections between the cingulate and autonomic nuclei located within the brain stem and hypothalamus as well as cortical regions (i.e., medial temporal and IC) have also been demonstrated (Barbas et al. 2003; Carmichael and Price 1995a, 1995b, 1996; Ongur et al. 1998; Vogt et al. 1987; Vogt and Pandya 1987). It is therefore surprising that more recent neuroimaging studies have implicated the cingulate in predominantly cognitive processes (Critchley 2004). The ACC (pregenual and subgenual) is often linked with nociceptive (Apkarian et al. 2005) and emotional (Vogt 2005) processes, while the MCC has principally been linked with nociception, attention, error detection, anticipation, decision making, and motor functions (Bush et al. 2000, 2002; Davis et al. 1997, 2000; Rushworth et al. 2007; Rushworth and Behrens 2008; Vogt 2005). That such cognitive, emotional, and sensorimotor (including nociceptive) tasks also directly evoke autonomic responses is often ignored when interpreting these observations. Interestingly, the recent meta-analysis by Beissner et al. (2013) demonstrated that the pgACC/MCC is the cortical region most consistently activated across 43 autonomic fMRI studies.
More recently, a handful of groups have utilized task-based fMRI to elucidate the cortical regions specifically associated with cardiovascular autonomic regulation. These studies have reported activation of the IC and ACC (often coactivation) with sympathetic modulation of HR variability, cardiac rhythm (Critchley et al. 2003), blood pressure changes during the Stroop task (Gianaros et al. 2005), the cold pressor and Valsalva's maneuver (Harper et al. 2000), MSNA responses to inspiratory capacity apnea (Macefield et al. 2006), (nonvolitional) baroreceptor afferent unloading (Kimmerly et al. 2005, 2007a, 2007b), and end-expiratory breath holds and the Mueller maneuver (Kimmerly et al. 2013). James et al. (2013b) recently mastered the technical challenge of performing concurrent MSNA and fMRI, demonstrating that BOLD signal intensity within several cortical regions, including the left midinsula, covaried with the intensity of resting MSNA bursts. Finally, utilizing direct electrical brain stimulation of the anterior MCC in two patients with refractory epilepsy, Parvizi et al. (2013) reported increased HR, “hot flashes,” and a sensation of “shakiness” in the upper chest and neck. Using rsMRI, these authors mapped their regions of cortical stimulation to the SN (Parvizi et al. 2013).
In the present experiment, we utilized a task-free state to avoid the potential confounds associated with task-related activations. We demonstrated that connectivity within bilateral aIC, posterior IC, and pgACC/MCC correlated significantly with directly recorded efferent sympathetic outflow. The largest clusters linked to sympathoexcitation were in the left aIC and right pgACC. Overall, these data support the notion that the IC and cingulate are not only responding in a task-specific manner but are instead, or additionally, premotor autonomic nuclei that respond to salient stimuli with adaptive changes in sympathetic tone (Beissner et al. 2013; Critchley 2004; Critchley et al. 2004; Seeley et al. 2007).
Resting-state activity in several other SN regions correlated significantly with sympathetic BI. One, the TPJ (a region encompassing the supramarginal and angular gyrus), often coactivated with the cingulate and IC during response to a range of sensory stimuli (Downar et al. 2002), is considered part of a network responsible for general salience monitoring (Kucyi et al. 2012). The cerebellum also exhibited increased connectivity. In experimental preparations, cerebellar stimulation results in sympathoexcitation, cardiovascular, and respiratory changes (Dampney 1994). Although its involvement in autonomic control is less well characterized in humans, cerebellar fMRI responses are evident in healthy subjects during inspiratory capacity apneas (Macefield et al. 2006) and in patients with OSA during an inspiratory loading challenge (Macey et al. 2006). Recently, rsMRI was used to demonstrate that cerebellar lobule VI and adjacent crus I regions are part of the SN (Habas et al. 2009). Our findings agree, demonstrating that BI significantly correlates with cerebellar connectivity within lobule V/VI and crus I.
After our initial analyses, we adopted the current, more liberal, conventions reported in the literature examining cortical autonomic cardiovascular regulation and reduced the threshold from a corrected P ≤ 0.05 to an uncorrected P = 0.001. Interestingly, this process uncovered several brain stem regions implicated in cardiovascular regulation, namely, RVLM, PB, PAG, and the PVN (Dampney 1994; Verberne and Owens 1998). There is extensive literature supporting the involvement of each of these regions in autonomic regulation. The RVLM, which in humans is displaced dorsally by the large olivary nuclei (Allen et al. 1988; Macefield and Henderson 2010), is well established as the primary medullary output nucleus from which sympathetic vasoconstrictor control of arterial blood pressure arises (Dampney 1994). The PB, located in the rostral pons, provides an integrative role as it possesses reciprocal connections with forebrain structures (including IC, hypothalamus, and thalamus) and with the brain stem NTS and RVLM (Card and Sved 2011; Dampney 1994; Verberne and Owens 1998). The PAG is also highly interconnected with cortical anterior cingulate cortex, prefrontal cortex, hypothalamus, and brain stem autonomic regions (Cersosimo and Benarroch 2013). Finally, the PVN regulates autonomic outflow via direct connections with sympathetic and parasympathetic preganglionic neurons and indirectly through connections with PAG, PB, and RVLM (Dampney 2011). Interestingly, the PVN is implicated in the sympathetic overactivity demonstrated in animal models of heart failure (Li and Patel 2003). These additional observations therefore are concordant with the experimental literature, add to our current understanding, in humans, of connectivity between brain regions involved in tonic sympathetic cardiovascular regulation under task-free conditions, and provide a model for future clinical research concerning hypertension or heart failure.
Obstructive Sleep Apnea
To secure a broad range of MSNA BIs within an essentially asymptomatic cohort, we sought to recruit otherwise healthy middle-aged subjects who either considered themselves free of OSA or had a high pretest probability of having as yet undiagnosed moderate to severe OSA based upon witnessed snoring or apnea, anthropometric characteristics, or other factors. In contrast to most previous investigations of such cohorts, all subjects first underwent formal polysomnography. Consistent with contemporary sleep laboratory experience, this procedure uncovered in many of these asymptomatic self-identified healthy control subjects mild, moderate, or even severe OSA.
As per conventional practice in the OSA literature, we then subdivided participants on the basis of the polysomnographic findings into subgroups comprising those with no or mild OSA and those with moderate and severe OSA (AHI ≥ 15). No between-group differences in resting-state connectivity were identified by comparative analysis. Importantly, with the exception of AHI, no other indexes of OSA severity differed between the two groups (Table 1), permitting us to focus on relationships between SN functional connectivity and BI across all subjects as a continuum. Importantly, the connectivity demonstrated within the bilateral aIC, posterior IC, and pgACC/MCC, which correlated significantly with directly recorded efferent sympathetic outflow, could not be attributed to OSA. There were either no or trivial (i.e., 3 voxels in right aIC correlated with AHI) relationships between measures specific to the severity of OSA (i.e., mean SaO2, MinSaO2, number of arousals, AHI, ESS, and BMI) and intrinsic connectivity within the SN. Thus, rather than being a maladaptive response to longstanding OSA, the increased connectivity with MSNA BI identified for these regions provides evidence for their engagement in amplifying (independently of any coexisting sleep disorder) efferent sympathetic outflow.
This study has several important characteristics that merit emphasis. First, because of the high prevalence of asymptomatic and undiagnosed OSA in the general population, all subjects, including self-reported healthy control subjects, underwent overnight polysomnography. Importantly, and in contrast to much prior literature in which subjects were recruited for research purposes only after OSA was identified by referral for diagnostic polysomnography on clinical grounds, our OSA subjects were not sleepy (Table 1). In 5 of our self-reported healthy subjects, polysomnography identified previously unrecognized moderate to severe OSA, and 12 self-reported healthy control subjects were diagnosed with mild OSA (AHI > 5 but < 15 events/h). The common practice in previous investigations has been to label volunteers as healthy control subjects if they report no nighttime snoring or daytime sleepiness. The present study reveals that assumption to be incorrect and calls into question the conclusions of prior studies in middle-aged subjects reliant on self-reported normal sleep for dichotomization into OSA and non-OSA cohorts. Second, our population comprised primarily middle-aged and older participants, and hence our findings are more relevant to the general population at cardiovascular risk. Previous microneurographic studies have often focused on young healthy control subjects, since they are in general easier to recruit and lack potentially confounding comorbidities. Third, the groups did not differ significantly with respect to mean values for any other measure related to the severity of OSA, such as the BMI, ESS, mean SaO2, or MinSaO2, that might be associated with difference in gray matter volume that may be present in OSA (Macey et al. 2002). Fourth, ∼30% of our subjects were women. Finally, our principal findings were found to be independent of the presence and severity of other commonly described indexes of OSA severity and unrelated to resting-state connectivity within default mode and sensory networks.
Limitations
MSNA and rsMRI data were acquired on separate experimental days, but a considerable body of evidence confirms the reproducibility of resting MSNA (Floras and Hara 1993) and MSNA responses to a variety of challenges (i.e., head-up tilt, lower body negative pressure, and cold pressor) when repeated after short time intervals (Kimmerly et al. 2004; Schobel et al. 1995). To minimize variation, subject preparation was identical prior to MRI and MSNA. To avoid changes that might arise from drug withdrawal, the 10 treated subjects continued their prescribed medications on the study days. An optimistic assumption is that if any of these drugs altered activity within the CAN, they would affect concurrently and proportionately the SN and MSNA; a more realistic possibility is that few might have such action, or have reflex or brain stem actions that then would obscure or attenuate any relationship between the strength of SN connectivity and BI, rendering the present finding of a significant relationship all the more compelling. Importantly, however, only a minority of the medications prescribed [principally ACE inhibitors and angiotensin II antagonists, which can be sympathoinhibitory, but not β-adrenoceptor antagonists (Azevedo et al. 2001)] influence MSNA when administered chronically. A potential concern, namely, that our principal observation during quiet rest, wakefulness, and spontaneous breathing might simply be a consequence of nighttime events, is obviated by analysis of measures specific to the sleep state indicating no substantive relationship between any OSA-specific variables and connectivity within the SN. Fatouleh et al. have recently concluded, from their functional and structural experiments, “that asphyxic damage due to repeated episodes of nocturnal apnoea is not the main cause of the sympthoexcitation” of OSA (Fatouleh et al. 2014). Finally, because of technical limitations we were unable to determine in this protocol connectivity patterns within the medulla, pons, or midbrain.
In conclusion, this is the first study to utilize MSNA to link autonomic outflow with spontaneous cortical activity within the SN. In a cohort of subjects with a wide range of interindividual variability in resting sympathetic outflow we have demonstrated a strong positive correlation between BI and resting intrinsic connectivity within the SN. We did not observe a relationship between BI and the DMN and sensorimotor network, supporting specificity between BI and SN connectivity. Finally, we have demonstrated that this relationship is not related to measures specific to the severity of OSA and the sleep state. These data support a sympathoexcitatory role for the SN in middle-aged humans.
GRANTS
This work was supported by a Grant-in-Aid from the Heart and Stroke Foundation of Ontario (HSFO, Grant NA 6407). K. S. Taylor and P. J. Millar are recipients of a Canadian Institutes of Health Research Postdoctoral Fellowship. P. J. Millar also held a Heart and Stroke Foundation of Canada Post-Doctoral Fellowship. H. Murai was supported by a Bluma Appel International Fellowship of the Mount Sinai Hospital Department of Medicine Research Fund. D. S. Kimmerly was supported by a Canadian Institutes of Health Research Postdoctoral Fellowship. T. D. Bradley holds the Clifford Nordal Chair in Sleep Apnea and Rehabilitation Research. J. S. Floras holds the Canada Research Chair in Integrative Cardiovascular Biology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.S.T., D.S.K., and J.S.F. conception and design of research; K.S.T., P.J.M., H.M., D.S.K., B.L.M., and J.S.F. performed experiments; K.S.T., A.K., T.D.B., and J.S.F. analyzed data; K.S.T. and J.S.F. interpreted results of experiments; K.S.T. and A.K. prepared figures; K.S.T. drafted manuscript; K.S.T., A.K., P.J.M., D.S.K., and J.S.F. edited and revised manuscript; K.S.T., A.K., P.J.M., H.M., D.S.K., B.L.M., T.D.B., and J.S.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Andrew McReynolds, Massieh Moayedi, and Eugen Hlasny for help with data collection.
REFERENCES
- Allen AM, Chai SY, Clevers J, McKinley MJ, Paxinos G, Mendelsohn FA. Localization and characterization of angiotensin II receptor binding and angiotensin converting enzyme in the human medulla oblongata. J Comp Neurol 269: 249–264, 1988. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep 22: 667–689, 1999. [PubMed] [Google Scholar]
- Andresen MC, Paton JF. The nucleus of the solitary tract: processing information from viscerosensory afferents. In: Central Regulation of Autonomic Functions (2nd ed.), edited by Llewellyn-Smith IJ, Verberne AJ. New York: Oxford Univ. Press, 2011, p. 23–46. [Google Scholar]
- Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist 18: 251–270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484, 2005. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229–244, 1996. [DOI] [PubMed] [Google Scholar]
- Azevedo ER, Kubo T, Mak S, Al-Hesayen A, Schofield A, Allan R, Kelly S, Newton GE, Floras JS, Parker JD. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation 104: 2194–2199, 2001. [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci 4: 25, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23, 137–152, 2004. [DOI] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 33: 10503–10511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541, 1995. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Faull OK, Pattinson KT, Jenkinson M. Physiological noise in brainstem fMRI. Front Hum Neurosci 7: 623, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SM, Wyss JM. The involvement of the anterior cingulate cortex in blood pressure control. Brain Res 340: 71–77, 1985. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222, 2000. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci USA 99: 523–528, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Sved AF. Central autonomic pathways. In: Central Regulation of Autonomic Functions (2nd ed.), edited by Llewellyn-Smith IJ, Verberne AJ. New York: Oxford Univ. Press, 2011, p. 3–22. [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641, 1995a. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 363: 642–664, 1995b. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 371: 179–207, 1996. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Shoemaker JK. Functional neuroanatomy of autonomic regulation. Neuroimage 47: 795–803, 2009. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Benarroch EE. Central control of autonomic function and involvement in neurodegenerative disorders. Handb Clin Neurol 117: 45–57, 2013. [DOI] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68: 93–104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666, 2002. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13: 500–505, 2003. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends Cogn Sci 8: 239–241, 2004. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70, 2009. [DOI] [PubMed] [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 101: 6333–6334, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126: 2139–2152, 2003. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. Neuroimage 27: 885–895, 2005. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195, 2004. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. [DOI] [PubMed] [Google Scholar]
- Dampney RA. The hypothalamus and autonomic regulation: an overview. In: Central Regulation of Autonomic Functions (2nd ed.), edited by Llewellyn-Smith IJ, Verberne AJ. New York: Oxford Univ. Press, 2011, p. 47–61. [Google Scholar]
- Davis KD, Hutchison WD, Lozano AM, Tasker RR, Dostrovsky JO. Human anterior cingulate cortex neurons modulated by attention-demanding tasks. J Neurophysiol 83: 3575–3577, 2000. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol 77: 3370–3380, 1997. [DOI] [PubMed] [Google Scholar]
- DeLuca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 29: 1359–1367, 2006. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87: 615–620, 2002. [DOI] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, Davis KD. Neural correlates of the prolonged salience of painful stimulation. Neuroimage 20: 1540–1551, 2003. [DOI] [PubMed] [Google Scholar]
- Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension 14: 511–517, 1989. [DOI] [PubMed] [Google Scholar]
- Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo YJ, Hof PR. Spontaneous brain activity relates to autonomic arousal. J Neurosci 32: 11176–11186, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouleh RH, Hammam E, Lundblad LC, Macey PM, McKenzie DK, Henderson LA, Macefield VG. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin 6: 275–283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA 106: 7209–7214, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385, 2009. [DOI] [PubMed] [Google Scholar]
- Floras JS. Hypertension and sleep apnea. Can J Cardiol 31: 889–897, 2015. [DOI] [PubMed] [Google Scholar]
- Floras JS, Hara K. Sympathoneural and haemodynamic characteristics of young subjects with mild essential hypertension. J Hypertens 11: 647–655, 1993. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci 4: 19, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711, 2007. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492: 145–177, 2005. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology 42: 627–635, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 47: 922–936, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637–4642, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29: 8586–8594, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand 74: 96–108, 1968. [DOI] [PubMed] [Google Scholar]
- Harper RM, Bandler R, Spriggs D, Alger JR. Lateralized and widespread brain activation during transient blood pressure elevation revealed by magnetic resonance imaging. J Comp Neurol 417: 195–204, 2000. [DOI] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Henderson LA, Woo MA, Macey KE, Frysinger RC, Alger JR, Nguyen KP, Yan-Go FL. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol (1985) 94: 1583–1595, 2003. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Macey PM, Macey KE, Frysinger RC, Woo MA, Harper RK, Alger JR, Yan-Go FL, Harper RM. Brain responses associated with the Valsalva maneuver revealed by functional magnetic resonance imaging. J Neurophysiol 88: 3477–3486, 2002. [DOI] [PubMed] [Google Scholar]
- Iacovella V, Hasson U. The relationship between BOLD signal and autonomic nervous system functions: implications for processing of “physiological noise.” Magn Reson Imaging 29: 1338–1345, 2011. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage 39: 1666–1681, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C, Henderson L, Macefield VG. Real-time imaging of brain areas involved in the generation of spontaneous skin sympathetic nerve activity at rest. Neuroimage 74: 188–194, 2013a. [DOI] [PubMed] [Google Scholar]
- James C, Macefield VG, Henderson LA. Real-time imaging of cortical and subcortical control of muscle sympathetic nerve activity in awake human subjects. Neuroimage 70: 59–65, 2013b. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002. [DOI] [PubMed] [Google Scholar]
- Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep 17: 703–710, 1994. [DOI] [PubMed] [Google Scholar]
- Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiol Scand Suppl 24: 1–262, 1951. [PubMed] [Google Scholar]
- Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus: a preliminary report. J Neurophysiol 12: 347–356, 1949. [DOI] [PubMed] [Google Scholar]
- Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 126: 1495–1510, 2012. [DOI] [PubMed] [Google Scholar]
- Kimmerly DS, Morris BL, Floras JS. Apnea-induced cortical BOLD-fMRI and peripheral sympathoneural firing response patterns of awake healthy humans. PLoS One 8: e82525, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly DS, O'Leary DD, Menon RS, Gati JS, Shoemaker JK. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569: 331–345, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly DS, O'Leary DD, Shoemaker JK. Test-retest repeatability of muscle sympathetic nerve activity: influence of data analysis and head-up tilt. Auton Neurosci 114: 61–71, 2004. [DOI] [PubMed] [Google Scholar]
- Kimmerly DS, Wong S, Menon R, Shoemaker JK. Forebrain neural patterns associated with sex differences in autonomic and cardiovascular function during baroreceptor unloading. Am J Physiol Regul Integr Comp Physiol 292: R715–R722, 2007a. [DOI] [PubMed] [Google Scholar]
- Kimmerly DS, Wong SW, Salzer D, Menon R, Shoemaker JK. Forebrain regions associated with postexercise differences in autonomic and cardiovascular function during baroreceptor unloading. Am J Physiol Heart Circ Physiol 293: H299–H306, 2007b. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol 108: 3382–3392, 2012. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA 110: 18692–18697, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177: 17–26, 2003. [DOI] [PubMed] [Google Scholar]
- M'hamed SB, Sequeira H, Poulain P, Bennis M, Roy JC. Sensorimotor cortex projections to the ventrolateral and the dorsomedial medulla oblongata in the rat. Neurosci Lett 164: 195–198, 1993. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Henderson LA. Neural sites involved in the sustained increase in muscle sympathetic nerve activity induced by inspiratory capacity apnea: a fMRI study. J Appl Physiol 100: 266–273, 2006. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Henderson LA. Real-time imaging of the medullary circuitry involved in the generation of spontaneous muscle sympathetic nerve activity in awake subjects. Hum Brain Mapp 31: 539–549, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey KE, Macey PM, Woo MA, Henderson LA, Frysinger RC, Harper RK, Alger JR, Yan-Go F, Harper RM. Inspiratory loading elicits aberrant fMRI signal changes in obstructive sleep apnea. Respir Physiol Neurobiol 151: 44–60, 2006. [DOI] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 166: 1382–1387. 2002. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 6: 160–188, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi B, Kollewe K, Samii A, Krampfl K, Dengler R, Munte TF. Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp Neurol 217: 147–153, 2009. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P, Floras JS. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol 593: 715–722, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol 401: 480–505, 1998. [PubMed] [Google Scholar]
- Oppenheimer SM, Cechetto DF. Cardiac chronotropic organization of the rat insular cortex. Brain Res 533: 66–72, 1990. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732, 1992. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Wilson JX, Guiraudon C, Cechetto DF. Insular cortex stimulation produces lethal cardiac arrhythmias: a mechanism of sudden death? Brain Res 550: 115–121, 1991. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Rangarajan V, Shirer WR, Desai N, Greicius MD. The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron 80: 1359–1367, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool JL, Ransohoff J. Autonomic effects on stimulating rostral portion of cingulate gyri in man. J Neurophysiol 12: 385–392, 1949. [DOI] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105C: 536–551, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute, 1968. [Google Scholar]
- Rombouts SA, Damoiseaux JS, Goekoop R, Barkhof F, Scheltens P, Smith SM, Beckmann CF. Model-free group analysis shows altered BOLD FMRI networks in dementia. Hum Brain Mapp 30: 256–266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci 11: 389–397, 2008. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol 17: 220–227, 2007. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25: 433–469, 2002. [DOI] [PubMed] [Google Scholar]
- Schobel HP, Oren RM, Mark AL, Ferguson DW. Influence of resting sympathetic activity on reflex sympathetic responses in normal man. Clin Auton Res 5: 71–80, 1995. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 62: 42–52, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seravalle G, Lonati L, Buzzi S, Cairo M, Quarti TF, Dell'Oro R, Facchetti R, Mancia G, Grassi G. Sympathetic nerve traffic and baroreflex function in optimal, normal, and high-normal blood pressure states. J Hypertens 33: 1411–1417, 2015. [DOI] [PubMed] [Google Scholar]
- Shirer WR, Jiang H, Price CM, Ng B, Greicius MD. Optimization of rs-fMRI pre-processing for enhanced signal-noise separation, test-retest reliability, and group discrimination. Neuroimage 117: 67–79, 2015. [DOI] [PubMed] [Google Scholar]
- Sleep Disorders Atlas Task Force. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15: 173–184, 1992. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, Suppl 1: S208–S219, 2004. [DOI] [PubMed] [Google Scholar]
- Sun MK, Guyenet PG. Hypothalamic glutamatergic input to medullary sympathoexcitatory neurons in rats. Am J Physiol Regul Integr Comp Physiol 251: R798–R810, 1986. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir YB, Green AL, Aziz TZ, Bahuri NF, Hyam J, Basnayake SD, Paterson DJ. Differentiated baroreflex modulation of sympathetic nerve activity during deep brain stimulation in humans. Hypertension 63: 1000–1010, 2014. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30: 2731–2745, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 61: 201–216, 2000. [DOI] [PubMed] [Google Scholar]
- Tohka J, Foerde K, Aron AR, Tom SM, Toga AW, Poldrack RA. Automatic independent component labeling for artifact removal in fMRI. Neuroimage 39: 1227–1245, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16: 55–61, 2015. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci 31: 18578–18589, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne AJ. Modulation of autonomic function by the cerebral cortex. In: Central Regulation of Autonomic Functions (2nd ed), edited by Llewellyn-Smith IJ, Verberne AJ. New York: Oxford Univ. Press, 2011, p. 202–219. [Google Scholar]
- Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol 54: 149–168, 1998. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86, 2007. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6: 533–544, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol 262: 271–289, 1987. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey. I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262: 256–270, 1987. [DOI] [PubMed] [Google Scholar]
- Ward AA., Jr The cingular gyrus, area 24. J Neurophysiol 11: 13–23, 1948. [DOI] [PubMed] [Google Scholar]
- Weissman-Fogel I, Moayedi M, Taylor KS, Pope G, Davis KD. Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum Brain Mapp 31: 1713–1726, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Schonfeldt-Lecuona C, Ecker D, Landwehrmeyer B. Aberrant connectivity of lateral prefrontal networks in presymptomatic Huntington's disease. Exp Neurol 213: 137–144, 2008. [DOI] [PubMed] [Google Scholar]
- Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage 35: 698–708, 2007. [DOI] [PubMed] [Google Scholar]
- Wu CW, Gu H, Lu H, Stein EA, Chen JH, Yang Y. Frequency specificity of functional connectivity in brain networks. Neuroimage 42: 1047–1055, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303: 355–374, 1991. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Yang Z, Liao W, Chen Z, Shi J, Liu Y. Impaired attention network in temporal lobe epilepsy: a resting fMRI study. Neurosci Lett 458: 97–101, 2009. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage 49: 2163–2177, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]