Abstract
Recent experimental findings suggest that there may be rich physiological information embedded within the power spectrum of neurophysiological recordings, which, in addition to power in specific oscillatory frequencies, can be extracted with the appropriate model. This article reviews previous empirical and modeling results, as well as the canonical power law model that is often used to describe the power spectrum. In addition, a modified power law model with parameters estimating synaptic and spiking contributions is proposed.
Keywords: power spectrum, power law, electrophysiology, local field potential
extracellular electrophysiological recordings, including local field potential (LFP), electrocorticogram (ECoG), and electroencephalogram (EEG), have been invaluable in investigating the relationship between neurophysiology and behavior. Like all time series data, they can be perfectly characterized by their frequency domain representation through the Fourier transform, i.e., their power and phase spectra. Neuroscientists have leveraged this identity for decades to examine the relationship between neural activity and cognition, behavior, and disease, with an interest in specific oscillatory bands such as theta (4–8 Hz) and alpha (8–12 Hz). These oscillations are often quantified as changes in signal power in specific narrow frequency bands and are assumed to be caused solely by periodic neural activity. Recently, however, evidence has been accumulating regarding the significance of the power spectrum as a unified statistical entity (Voytek and Knight 2015). In this view, oscillations are defined as a special subset of the information contained in the power spectrum, often identified as perturbations (or “bumps”) that rise above the background activity (Lopes da Silva 2013). Equally important, though, is the background activity itself, where recent empirical evidence (Manning et al. 2009; Miller et al. 2007; Podvalny et al. 2015) and computational models (Baranauskas et al. 2012; Freeman and Zhai 2009; Miller et al. 2009; Milstein et al. 2009) have demonstrated that its characterization does not only serve as a convenient description of the equivalent time series but also is closely linked with behavioral variables.
Decomposing the Power Spectrum
I begin from the fact that the electrophysiological power spectral density (PSD) can be said to follow an inverse power law relationship of the form P(f) ∼ Afχ, with χ typically ranging from −4 to −1.5, in addition to oscillatory peaks on top of the 1/f background (see Fig. 2C in the Podvalny et al. 2015, the target of this Neuro Forum, as well as Buzsáki and Draguhn 2004; Buzsáki et al. 2012). This is mathematically equivalent to a negative linear relationship in the log-log domain [log P(f) ∼ log A + χlog f]. I thus refer to χ as the slope and log A as the offset. In 2009, Manning et al. observed that the firing rate of a local neuronal population correlates with an increased offset in the spectrum of the concurrently recorded LFP in human neurological patients performing a virtual navigation task, while the slope remained qualitatively unchanged. Miller and colleagues (2007, 2009) subsequently supported this observation through computational modeling of the LFP, while also having observed activation-related shifts and a stable slope in power spectra of human ECoG, after accounting for a bend, or knee, at ∼70 Hz. Recently, Podvalny et al. (2015) reported slope changes in the 10- to 100-Hz range in human ECoG PSD during a visuomotor attention task, corresponding to a “rotation” of the spectrum with slopes between −3 and −1. Similar observations were made in both rabbit and human ECoG data previously, where Freeman and Zhai (2009) attributed the overall rotation of the spectrum to changes in rise times of the impulse response of a recurrent excitatory network.
Although both an increase in overall slope and an increase in offset of the PSD have been linked, broadly speaking, to neural activation, it is unclear whether the two effects are interdependent and whether they arise from the same neurological mechanism. Nonetheless, the power law model is able to accurately capture changes in both. A separate phenomenon is observed, however, where more broadband high gamma (>80 Hz), a nonoscillatory section of the LFP, can increase in power through an increase in slope only in that particular frequency range, independent of the slope of the lower frequencies (see Fig. 2A in Podavlny et al. 2015, as well as Freeman and Zhai 2009). In other words, it appears that rotation of the high-frequency spectrum can occur independently of the overall PSD, at an axis frequency in the gamma range, producing an upward deflection about a “knee” frequency. Furthermore, the data reported by Podvalny et al. shows the axis of overall rotation (named “intersection frequency”) to be around 30 Hz and that the shifting of this rotational axis to lower frequencies is correlated with increased gamma power. These findings imply that the change in spectrum slope may be more than simply a change in the parameter χ, as would be the case if there existed a constant axis of rotation at 1 Hz. This pair of observations appears to be inconsistent with the current power law model, because high-frequency deflections cannot be captured through changing the slope and offset due to the constraint that the slope remains constant over all frequencies.
Population Spiking Contribution in the Power Law PSD
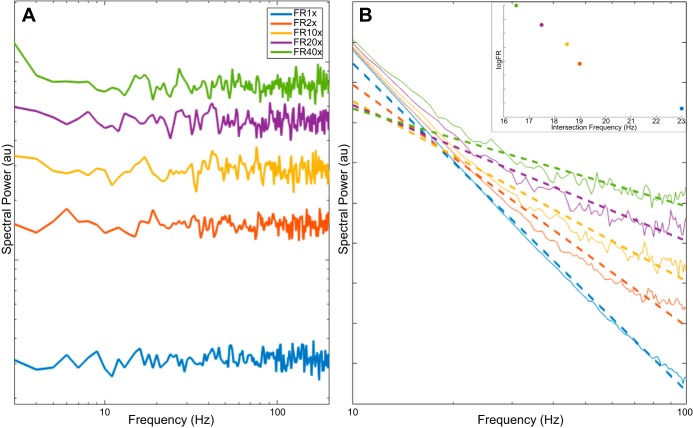
To account for this independent rotation, I propose an updated model of the LFP power spectrum, with the addition of a constant B such that P(f) ∼ Afχ + B, with B representing the addition of a signal with a flat spectrum. Furthermore, I propose that this flat-spectrum signal corresponds to the detection of decorrelated (Poisson-like) firing in a large neuronal population, each with a small Dirac delta-like spike waveform at the resolution detectable in ECoG, the summation of which results in a noisy broadband signal that adds linearly to the LFP and can be detected by meso- and macroscale measurements. Using a population of Poisson spiking neurons, I simulate their spontaneous spike train and sum across the population for the aggregate activity. The PSD of the aggregate spike train has a flat spectrum, resembling that of white noise, and experiences a constant gain in all frequencies in response to an increased firing rate (Fig. 1A).
Fig. 1.
Effect of adding Poisson population spiking to a power law local field potential (LFP). A: power spectral density (PSD) of a summed Poisson spike train (10,000 neurons) is flat and increases as a function of firing rate (FR). B: robust linear fit (dashed lines) is applied to the sum of power law PSD and flat-spectrum signals from A and demonstrates flattening of slope as a result of increased FR, although the power law parameter χ remains unchanged. Colors correspond to firing rates in A. Inset: intersection frequency between fitted PSD and f−2 decreases as FR (and broadband gamma power) increases.
With this, several key findings raised by Podvalny et al. (2015) can be explained within a simplified mathematical framework. First, it is observed that the addition of a flat-spectrum signal dominates the low-power (high frequency) portions of the power law PSD, flattening the slope at those frequencies while having no effect in the lower frequencies, thus introducing an upward deflection (Fig. 1B). Second, when a robust linear fit is applied in the range of 10–100 Hz, a rotational effect is seen on the slope of the line of best fit, mainly due to the increase in the higher frequencies, although it is not a true rotation of the whole spectrum, as would be obtained by only increasing χ. Last, as the magnitude of the flat-spectrum signal increases as a result of increased firing rate, the axis of rotation (knee) varies in response to the magnitude of the upward deflection. Specifically, an increase of flat-spectrum signal power will shift the axis of rotation to a lower frequency (Fig. 1B, inset), consistent with experimental observations by Podvalny et al. (see Fig. 3F in Podvalny et al. 2015). Code for simulation can be found at http://voyteklab.com/code-data/.
Discussion
Electrophysiological recordings are invaluable in illuminating the neural correlates of behavior and cognition, although simply working with the signals in time domain is not always informative. The Fourier power spectrum, or frequency representation, can often shed more insight on the phenomenon of interested, as exemplified by the target article, a recent study from Podvalny et al. (2015). The target article showed several behaviorally relevant changes in the power spectrum slope that are not captured by the inverse power law model [P(f) ∼ Afχ]. Here, I demonstrate that the addition of a flat-spectrum signal to the current power law model can explain a range of experimental observations, particularly the independent increase in high-frequency slope, as well as the apparent changes in slope with a shifting axis of rotation.
Under the proposed model, the LFP has two explicitly separate contributors: the first is synaptic events, which canonically have been the leading explanation for the origin of the LFP and depend largely on the activity of the presynaptic population. These events give rise to the general 1/f background. The flat-spectrum signal, I propose, arises from the aggregate spiking activity of the local population, which has been previously cited as a probable contributor to the LFP (Buzsaki et al. 2012; Schomburg et al. 2012). In reality, the two signals are likely interrelated, because changes in presynaptic activity, which are possibly reflected in the broadband shift seen in Manning et al. (2009) and Miller et al. (2007), will certainly cause changes in local population activity. As such, the proposed model will undoubtedly benefit from further empirical work that would delineate the two contributors. From a broader perspective, the new model of the PSD moves from a strictly descriptive point of view to a semimechanistic one, with parameters that correspond to physiological variables, thus enabling further theories and testable hypotheses in behavioral and cognitive neuroscience.
GRANTS
This research was supported by the University of California, San Diego Katzin Prize and Frontiers of Innovation Scholars Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
R.G. drafted manuscript.
ACKNOWLEDGMENTS
I thank the Voytek Lab Ignite team for the insightful discussion that inspired this manuscript, as well as Bradley Voytek for extensive help and feedback in preparing it.
REFERENCES
- Baranauskas G, Maggiolini E, Vato A, Angotzi G, Bonfanti A, Zambra G, Spinelli A, Fadiga L. Origins of 1/f2 scaling in the power spectrum of intracortical local field potential. J Neurophysiol 107: 984–994, 2012. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science 304: 1926–1929, 2004. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13: 407–420, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ, Zhai J. Simulated power spectral density (PSD) of background electrocorticogram (ECoG). Cogn Neurodyn 3: 97–103, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva F. EEG and MEG: relevance to neuroscience. Neuron 80: 1112–1128, 2013. [DOI] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci 29: 13613–13620, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. Spectral changes in cortical surface potentials during motor movement. J Neurosci 27: 2424–2432, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput Biol 5: e1000609, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein J, Mormann F, Fried I, Koch C. Neuronal shot noise and Brownian 1/f2 behavior in the local field potential. PLoS One 4: e4338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podvalny E. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J Neurophysiol 114: 505–519, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg E, Anastassiou CA, Buzsáki G, Koch C. The spiking component of oscillatory extracellular potentials in the rat hippocampus. J Neurosci 32: 11798–11811, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Knight RT. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol Psychiatry 77: 1089–1097, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]