Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) exert their pharmacological effects by inhibiting cyclooxygenase (COX)-1 and COX-2. Though widely prescribed for pain and inflammation, these agents have limited utility in chronic diseases due to serious mechanism-based adverse events such as gastrointestinal damage. Concomitant blockade of fatty acid amide hydrolase (FAAH) enhances the therapeutic effects of the NSAIDs while attenuating their propensity to cause gastrointestinal injury. This favorable interaction is attributed to the accumulation of protective FAAH substrates, such as the endocannabinoid anandamide, and suggests that agents simultaneously targeting COX and FAAH might provide an innovative strategy to combat pain and inflammation with reduced side effects. Here, we describe the rational design and structure-active relationship (SAR) properties of the first class of potent multi-target FAAH-COX inhibitors. A focused SAR exploration around the prototype 10r (ARN2508) led to the identification of achiral (18b) as well as racemic (29a-c and 29e) analogs. Absolute configurational assignment and pharmacological evaluation of single enantiomers of 10r are also presented. (S)-(+)-10r is the first highly potent and selective chiral inhibitor of FAAH-COX with marked in vivo activity, and represents a promising lead to discover novel analgesics and anti-inflammatory drugs.
Keywords: FAAH, COX, hybrid scaffold, multitarget inhibitors, structure-activity relationship, inflammation
Graphical Abstract
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely utilized to treat pain and inflammation, [1] but their chronic use is hindered by a variety of potentially serious adverse events that include gastrointestinal (GI) mucosal lesions, bleeding and perforations. [2-5] Conventional NSAIDs inhibit the two isoforms of cyclooxygenase (COX), COX-1 and COX-2, which catalyze the first committed steps in the biosynthetic pathway that converts arachidonic acid (AA) into inflammatory prostanoids such as prostaglandin E2 (PGE2) and thromboxane A2 (TXA2). [6] The dual role of COX-1-derived PGE2 as inflammation promoter and mucosal tissue protectant explains, at least in part, why NSAIDs cause damage to the GI tract. [7-10] Efforts to overcome this problem have led to the development of selective COX-2 inhibitors, which combine a high level of anti-inflammatory efficacy with a reduced propensity to cause injury to the GI mucosa. [6] Nevertheless, the use of COX-2 inhibitors has been linked to a distinctive set of adverse cardiovascular effects. [11, 12] Thus, the need for safe and effective drugs that can be used in the treatment of chronic inflammatory disorders remains urgent.
A promising approach to meet this need is offered by targeting with a single agent more than one component of the inflammatory cascade. [13-15] Agents designed to achieve this objective include nitric oxide (NO) donors-NSAIDs, [16, 17] COX-2 inhibitors-NO-donors, [18, 19] hydrogen sulfide (H2S) donors-NSAIDs, [20-22] as well as compounds that block distinct enzymes of the AA pathway, such as COX/lipoxygenase [23, 24] and COX-2/soluble epoxy hydrolase (sEH). [25] Another potential multi-target strategy to treat inflammation is the concomitant inhibition of COX and fatty acid amide hydrolase (FAAH) [26], [27-33] a serine hydrolase that deactivates a family of analgesic and anti-inflammatory lipid amides that are produced by host-defense cells and other cells in the body. [34, 35] These lipid mediators include the endocannabinoid anandamide (arachidonoylethanolamide) – which engages cannabinoid-1 (CB1) and CB2 receptors to suppress neutrophil migration [36] and prevent immune-cell recruitment [37, 38] – as well as the endogenous peroxisome proliferator-activate receptor-α (PPAR-α) agonists, palmitoylethanolamide (PEA) and oleoylethanolamide (OEA). [39-41] In addition to opposing pain and inflammation, these FAAH substrates are also protective of the GI mucosa. [42, 43] Indeed, studies in animal pain models have shown that co-administration of FAAH and COX inhibitors results in a synergistic potentiation of analgesia along with reduced gastric damage. [44-46]
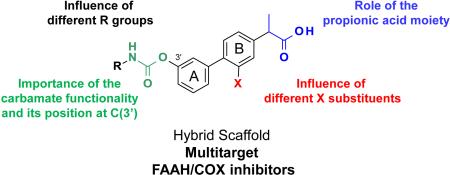
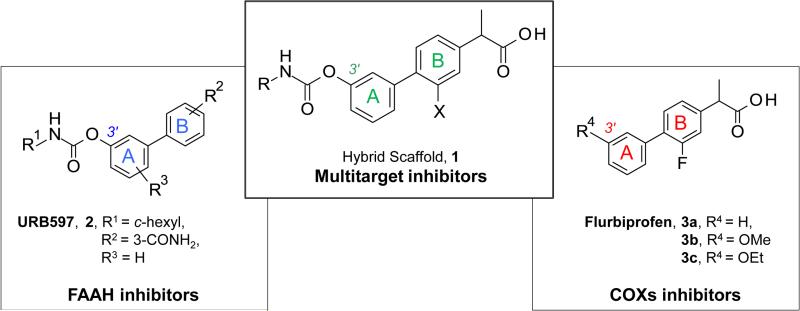
In several chronic inflammatory conditions, including inflammatory bowel disease (IBD), FAAH [47-49] and COX-2 [50] are expressed at abnormally high levels. This simultaneous up-regulation may help establish a pathological state that exacerbates inflammation by amplifying inflammatory COX-dependent signals at the expense of defensive FAAH-regulated mediators. This hypothesis predicts that drugs targeting both COX and FAAH should have substantial anti-inflammatory efficacy combined with reduced GI toxicity. In a recent study, we provided support to this hypothesis using a multi-target modulator based on the hybrid scaffold 1 (Figure 1). [51] This scaffold merges key pharmacophores of two known classes of FAAH and COX inhibitors – O-aryl carbamates [52-58] such as [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate (URB597, 2) [54, 57], and 2-aryl propionic acids [6] such as flurbiprofen, 3a [59-61] – which share a biphenyl core as a common structural motif (A and B rings, Figure 1). Moreover, structure-activity relationship (SAR) studies of these scaffolds supported the hypothesis of additional elements of structural overlapping, such as the oxygenated substituents at the 3’-position of the A phenyl ring, corresponding to the carbamate functionality of 2 [53, 54, 56] and the ether moieties of 3b or 3c, [61] respectively (Figure 1).
Figure 1.
Rational design of a ‘hybrid scaffold’ for FAAH and COX inhibition.
This SAR work led to the identification of compound 10r ((±)-2-[3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid, ARN2508) [51] as a potent in vivo active inhibitor of intracellular FAAH and COX activities, which exerts profound anti-inflammatory effects in mouse models of IBD without causing COX-dependent gastric toxicity. [51] In the present study, (a) we outline the in-depth SAR investigations that led to the discovery of compound 10r [51]; (b) we report an expansion of this SAR work, which culminated in the identification of several new and potent multitarget inhibitors (18b, 29a-c and 29e); and, finally (c) we describe the absolute configurational assignment and pharmacological properties of single enantiomers of 10r, identifying (S)-(+)-10r as the first chiral inhibitor of FAAH-COX with marked in vivo activity.
2. Results and discussion
2.1 Chemistry
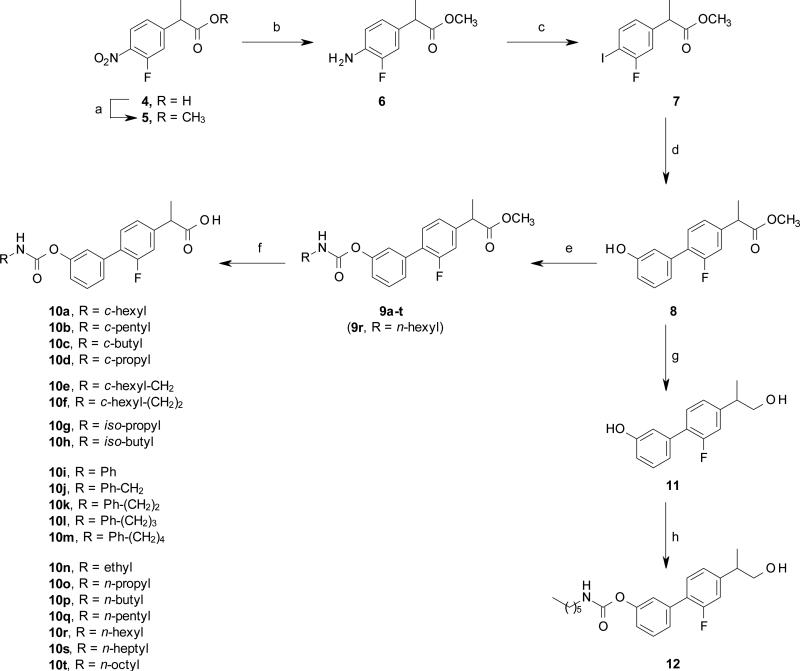
Compounds 10a-t were synthetized from the corresponding phenol 8 through a carbamoylation reaction, using commercially available isocyanates, followed by the hydrolysis of the methyl esters 9a-t, under acidic conditions (Scheme 1).
Scheme 1.
Synthesis of compounds 10a-t and 12. Reagents and conditions: (a) MeOH, conc. H2SO4, rt, 15 h, 93%; (b) HCO2NH4, 10% Pd/C, MeOH, rt, 3 h, 94%; (c) NaNO2, 3M HCl, 0 °C, 30 min, then NaI, 60 °C, 2 h, 55%; (d) (3-hydroxyphenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 84%; (e) RNCO, DMAP, MeCN, rt, 15 h, 38-99%; (f) 6M HCl, THF, rt, 2 d, 26-73%; (g) ZrCl4, NaBH4, THF, rt, 2 h, 96%; (h) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 73%.
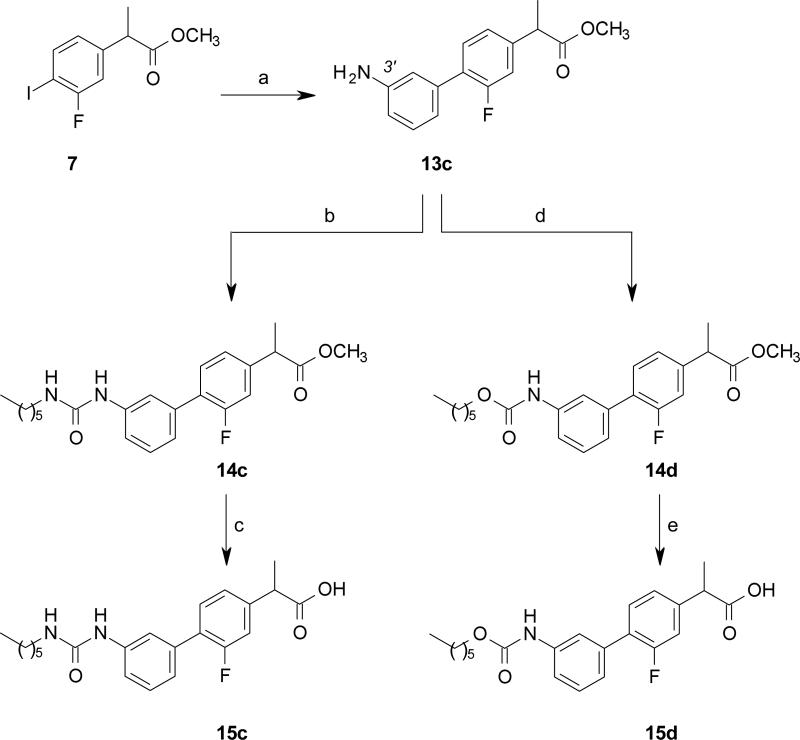
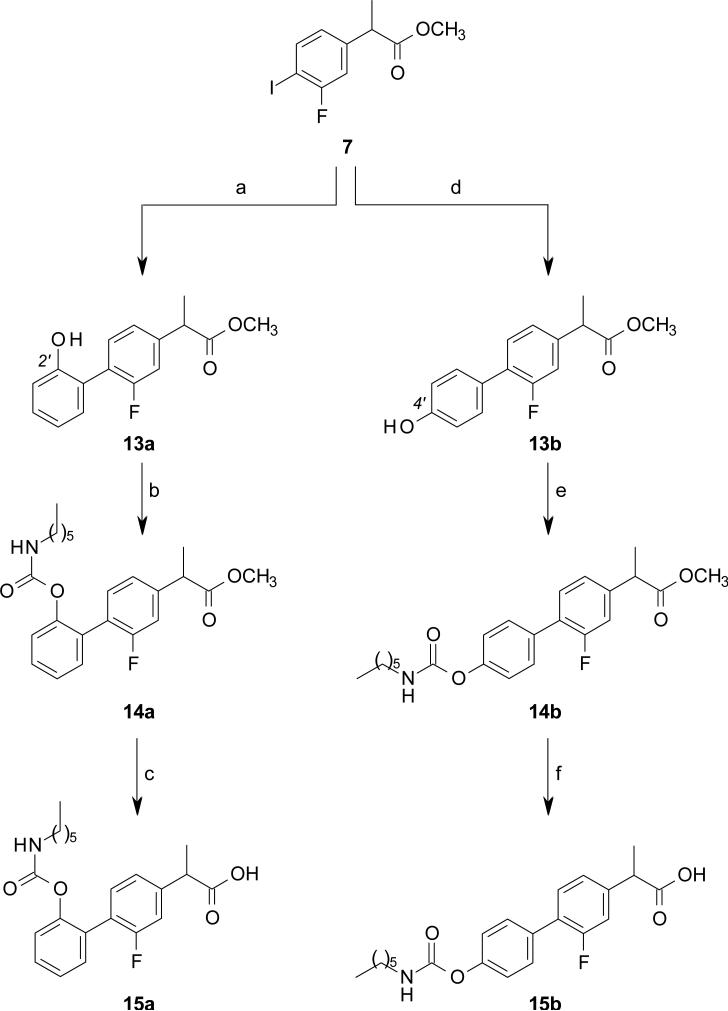
The intermediate 8 was prepared in four steps, starting from the acid 4, obtained as previously described. [62] Compound 4 was converted to the corresponding methyl ester 5, under standard acidic conditions, to afford, after catalytic hydrogenation with ammonium formate in the presence of Pd/C, the resulting aniline 6. Compound 6 was then transformed into the corresponding diazonium salt, that was reacted in situ with NaI to obtain the phenyl iodide 7 in good yield, which was converted, under ligand less Suzuki cross coupling conditions, [63] to the biphenyl derivatives 8 and 13a-c in excellent yield (Schemes 1-3).
Scheme 3.
Synthesis of compounds 15c-d. Reagents and conditions: (a) (3-aminophenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 91%; (b) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 65%; (c) 6M HCl, THF, rt, 2 d, 38%; (d) triphosgene, toluene, reflux, 15 h, then n-hexanol, rt, 15 h, 82%; (e) 6M HCl, THF, rt, 2 d, 59%.
3-Hydroxypropyl derivative 12 was synthesized by reduction of the methyl ester 8 to the alcohol 11 (Scheme 1). Although lithium aluminum hydride succeeded in reducing the ester 8, a significant des-fluorinated side product was observed and separation of the two compounds was troublesome. Therefore, a milder reducing agent, such as zirconium borohydride generated in situ, was used to afford a clean conversion of 8 to 11, [64] which was then converted to 12 under standard carbamoylation reaction conditions (Scheme 1).
Carbamates 15a-b and urea 15c were prepared from the corresponding phenols 13a-b and aniline 13c, respectively, through a carbamoylation reaction using n-hexyl-isocyanate, followed by acidic hydrolysis of the methyl esters 14a-c (Scheme 2 and 3). The reverse carbamate 15d was prepared upon activation of the aniline 13c with triphosgene, and, then, reaction with n-hexanol, followed by acidic hydrolysis of the methyl ester 14d (Scheme 3).
Scheme 2.
Synthesis of compounds 15a-b. Reagents and conditions: (a) (2-hydroxyphenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 84%; (b) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 88%; (c) 6M HCl, THF, rt, 2 d, 90%; (d) (4-hydroxyphenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 59%; (e) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 72%; (f) 6M HCl, THF, rt, 2 d, 46%.
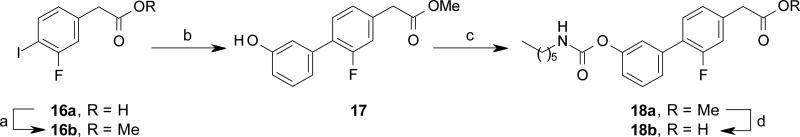
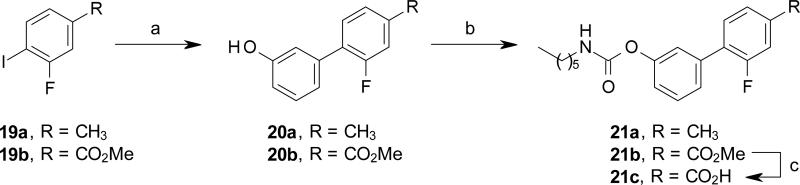
Compounds 18a and 21a-b were synthetized by reacting the phenyl iodides 16b and 19a-b, with (3-hydroxyphenyl)boronic acid under Suzuki cross coupling conditions, followed by carbamoylation reaction of phenols 17 and 20a-b under standard conditions (Scheme 4 and 5).
Scheme 4.
Synthesis of compound 18b. Reagents and conditions: (a) MeOH, conc. H2SO4, rt, 15 h, quant.; (b) (3-hydroxyphenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 71%; (c) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 64%; (d) 6M HCl, THF, rt, 2 d, 62%.
Scheme 5.
Synthesis of compound 21a and 21c. Reagents and conditions: (a) (3-hydroxyphenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 86-92%; (c) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 86%-quant.; (d) 6M HCl, THF, rt, 2 d, 34%.
Compounds 18a and 21b were then transformed into the corresponding acids 18b and 21c under standard acidic hydrolysis (Scheme 4 and 5).
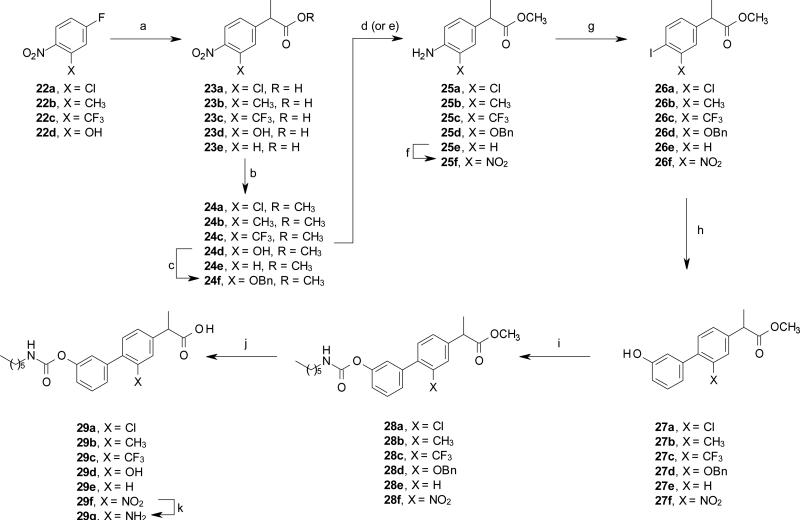
Compounds 29a-g were synthesized following the synthetic sequence described in Scheme 6. p-Nitrofluorobenzenes 22a-d were reacted with diethylmethylmalonate followed by decarboxylation to the corresponding acids 23a-d. 23a-d and the commercially available 23e were converted into methyl esters 24a-e in acidic MeOH. In addition, the phenolic intermediate 24d was directly converted into the corresponding O-Bn protected 24f, under standard reaction conditions.
Scheme 6.
Synthesis of compounds 29a-g. Reagents and conditions: (a) diethyl methylmalonate, NaOH, DMF, rt, 15 h; then AcOH, H2SO4, H2O 110 °C, 24 h, 48-87%; (b) MeOH, conc. H2SO4, rt, overnight, 81-98%; (c) BnBr, K2CO3, acetone, 60 °C, 15 h, 63%; (d) Fe, HCl, MeOH, 65 °C, 2 h, 64-94%, for 24a and 24f; (e) HCO2NH4, 10% Pd/C, MeOH, rt, 3 h, quant., for 24b-c, and 24e; (f) Ac2O, HNO3 0 °C, 2 h then H2SO4, MeOH, 65 °C, 2 h, 98%; (g) NaNO2, 3M HCl, 0 °C, 30 min, then NaI, 60 °C, 2 h, 55-72%; (h) (3-hydroxyphenyl)boronic acid, Pd(OAc)2, K2CO3, EGME/H2O, rt, 15 h, 59-84%; (i) n-hexyl-NCO, DMAP, MeCN, rt, 15 h, 89%-quant.; (j) 2M HCl, dioxane, 80 °C, 15 h, 73-95%; (k) cyclohexene, 10% Pd/C 80 °C, 2 h then 2M HCl, 55%.
Reduction of the nitro group was carried out using iron in presence of HCl for compounds 24a and 24f, and ammonium formate in the presence of Pd/C for compounds 24b-c and 24e. Compound 25f was obtained from 25e by standard nitration reaction. Diazotation/Sandmayer reaction of the anilines 25a-f gave the iodides 26a-f, which were converted to carbamates 28a-f via Suzuki and carbamoylation reactions. Compounds 28a-f were then transformed into the corresponding acids 29a-f under standard acidic hydrolysis. Finally, the aniline 29g was obtained from the nitrophenyl 29f, under standard hydrogenation conditions.
2.2. SAR exploration of the first class of potent multi-target FAAH-COX inhibitors
2.2.1 Rational drug design: merging strategy and identification of hit 10a
We started our SAR exploration with compound 10a, [51] which was designed by merging essential pharmacophores of the FAAH inhibitor, URB597, 2, and those of the NSAID, flurbiprofen, 3a (Figure 1). The inhibitory potencies of 2, 3a and 10a against rat brain FAAH, ovine testis COX-1 and human recombinant COX-2 are reported in Table 1.
Table 1.
SAR exploration on the nature of R group: cycloalkanes, small-branched alkanes and phenyls.
| ||||
|---|---|---|---|---|
| Compound | R | FAAHa,b IC50(μM)±SD | COX-1a,b IC50 (μM)±SD | COX-2a,b IC50 (μM)±SD |
| 2, URB597 | - | 0.0017±0.001 | >100 | >100 |
| 3a, flurbiprofen | - | >100 | 0.15±0.018 | 1.06±0.53 |
| 10a | c-hexyl | 8.2±2.4 | 7.9±2.1 | >100 |
| 10b | c-pentyl | 4.8±3.2 | 4.4±2.0 | > 100 |
| 10c | c-butyl | 48.7±9.0 | 0.72±0.02 | >100 |
| 10d | c-propyl | >100 | 5.4±2.9 | 74.3±6.1 |
| 10e | c-hexyl-CH2 | 0.36±0.06 | 0.60±0.04 | >100 |
| 10f | c-hexyl-(CH2)2 | 0.018±0.007 | 0.15±0.03 | 10.8±2.2 |
| 10g | iso-propyl | >100 | 3.9±2.1 | >100 |
| 10h | iso-butyl | 4.1±2.1 | 8.2±2.1 | >100 |
| 10i | Ph | 41.2±3.4 | 0.27±0.07 | 2.7±0.3 |
| 10j | Ph-CH2 | 4.18±2.8 | 1.3±0.6 | >100 |
| 10k | Ph-(CH2)2 | 0.17±0.07 | 6.3±2.2 | >100 |
| 10l | Ph-(CH2)3 | 0.09±0.01 | 0.58±0.09 | 6.2±0.3 |
| 10m | Ph-(CH2)4 | 0.027±0.010 | 3.7±2.8 | >100 |
Values are reported as mean values of ≥3 experiments performed
IC50 values were not determined for compounds showing less than 50% inhibition at concentrations of 100 μM for FAAH and COXs.
Compound 10a inhibited FAAH and COX activities with relatively weak potencies (IC50 values, in μM: FAAH = 8.2; COX-1 = 7.9; COX-2 > 100). Nevertheless, these initial results encouraged us because 10a was one of the most potent FAAH/COX-1 inhibitors previously reported. [27, 28, 30, 32, 33, 65]
We started, therefore, an SAR exploration around 10a with the objective of identifying chemical and structural determinants that might improve potency on the three targets in a balanced manner.
2.2.2. Study of the effect of the nature of R group: cycloalkanes, small-branched alkanes and phenyls
We prepared a series of analogs bearing cycloalkyl groups with different ring size at the N-terminal of the carbamate functionality (Table 1).
We observed that, while the potency against FAAH was retained with the c-pentyl analog 10b (IC50=4.8 μM), a 10-fold loss in potency occurred with the c-butyl derivative 10c (IC50=48.7 μM) and complete loss of activity (IC50>100 μM) with the c-propyl derivative 10d. With regard to COX activity, while the c-pentyl analog 10b showed a comparable potency against COX-1 (IC50=4.4 μM), the c-butyl analog 10c was 10-fold more potent than compound 10a (IC50=0.72 μM). Conversely, the c-propyl analog 10d displayed an IC50 value similar to compounds 10a and 10b against COX-1 (=5.4 μM) and was indeed the only compound in this series that showed modest activity against COX-2 (IC50=74.3 μM).
The N-terminal region of the carbamate functionality in 10a may engage in beneficial interactions with the acyl chain-binding domain of FAAH [26], [56, 57] as well as the hydrophobic channels present in COX-1 and COX-2. [6] [61] To capture such interactions, we prepared a series of analogs bearing lipophilic aliphatic and aromatic N-terminal substituents with diverse steric properties (Table 1).
The insertion of a methylene group adjacent to the c-hexyl ring of 10a - compound 10e- led to a significant increase of potency toward FAAH (23-fold) and COX-1 (10-fold), but no COX-2 inhibition (IC50>100 μM). A further homologation, compound 10f, showed a 400-fold increase in potency toward FAAH and a 50-fold increase in potency toward COX-1, compared to 10a. Interestingly, 10f also inhibited COX-2 with an IC50 of 10.8 μM.
Next, we investigated the effects of small and branched alkyl groups, the iso-propyl 10g and the iso-butyl 10h - as truncated analogs of 10a and 10e, respectively. These modifications were detrimental for FAAH and COX inhibitory activities compared to 10a and 10e, respectively.
While the replacement of the c-hexyl ring with a phenyl group (10i) was not tolerated by FAAH, in analogy to previous reports on the class of O-aryl carbamates, [56, 57] this modification led to a gain in inhibitory activity toward COX-1 and COX-2. The insertion of a methylene group adjacent to the phenyl ring of 10i - compound 10j- caused a 10-fold increase in potency toward FAAH, compared to 10i, but had almost no impact on COX-1 activity and dramatic loss on COX-2. Homologation (10k-m) resulted in a progressive enhancement of the inhibitory potency toward FAAH, but this trend was more erratic for COX-1 and COX-2: compound 10l was most active analog with IC50 = 0.58 μM and 6.2 μM against COX-1 and COX-2, respectively.
These findings might reflect differences in the depth of lipophilic pockets of FAAH and COX enzymes. [6, 26]
2.2.3. Study of the effect of the nature of the R group: linear alkanes. Identification of 10r (ARN2508)
Since the (CH2)n homologation at the N-terminal site appeared to be critical for the modulation of the biological activities at both targets, we prepared a series of carbamates bearing linear alkyl groups (alkyl = (CH3(CH2)n) with n= 1 to 7) at N- terminal region (Table 2).
Table 2.
SAR exploration on the nature of the R group: linear alkanes
| ||||
|---|---|---|---|---|
| Compound | R | FAAHa,b IC50(μM)±SD | COX-1a,b IC50 (μM)±SD | COX-2a,b IC50 (μM)±SD |
| 10n | ethyl | >100 | 2.1±0.9 | >100 |
| 10o | n-propyl | >100 | 1.65±0.06 | >100 |
| 10p | n-butyl | 7.0±1.8 | 0.26±0.07 | >100 |
| 10q | n-pentyl | 0.57±0.15 | 0.020±0.009 | 0.16±0.02 |
| 10r, ARN2508 | n-hexyl | 0.031±0.002 | 0.012±0.002 | 0.43±0.02 |
| 10s | n-heptyl | 0.011±0.003 | 0.37±0.10 | 0.32±0.005 |
| 10t | n-octyl | 0.003±0.002 | 0.99±0.07 | 28.8±8.4 |
Values are reported as mean values of ≥3 experiments performed
IC50 values were not determined for compounds showing less than 50% inhibition at concentrations of 100 μM for FAAH and COXs.
In analogy to the reported SAR results on the class of O-aryl carbamates, [56] potency toward FAAH increased with increased length of the (CH2)n chain (n = 1-7). A different trend was observed for COX-1 and COX-2, where insertion of short (CH2)n chains (n = 1-2) led to compounds (10n-o) that were weak COX-1 inhibitors and had no activity against COX-2. On the other hand, insertion of n = 3-5 (CH2)n chains (10p-r) increased the inhibitory potencies for COX-1 and COX-2 from sub-micromolar to nano-molar IC50, whereas insertion of n = 6-7 (CH2)n chains (10s-t) was detrimental.
These results are in agreement with those above reported in the homologation of the Ph(CH2)n chain series (n = 1-4, compounds 10i-m, Table 1).
From this SAR exploration, we identified 10r (ARN2508), [51] which bears a n-hexyl chain at the N-terminal site, as a potent multi-target inhibitor of FAAH, COX-1 and COX-2 (IC50: FAAH = 31 nM; COX-1 = 12 nM; COX-2 = 430 nM) (Table 2). In addition to its high balanced potency, the highest reported thus far, [27, 28, 30, 32, 33, 65] we found that 10r displays no off-target activities on a panel of >90 biologically relevant targets, and effectively engages its intended targets after oral administration in mice. [51]
These results encouraged us to initiate a more focused SAR exploration to define the effect of additional chemical and structural modifications in various regions of 10r scaffold.
2.2.4. Focused SAR exploration around 10r (ARN2508) and identification of 18b, 29a-c, e and (S)-(+)-10r
In particular, we focused our interest on the role and position of carbamate group in the A phenyl ring (Table 3 and Table 4), as well as the role of the propionic acid functionality and the fluorine atom in the B phenyl ring (Table 5 and Table 6).
Table 3.
Effect of the position of the carbamate functionality on the A phenyl ring.
| ||||
|---|---|---|---|---|
| Compound | position | FAAHa,b IC50 (μM) ±SD | COX-1a,b IC50 (μM) ±SD | COX-2a,b IC50 (μM) ±SD |
| 15a | C(2’) | 2.2±0.6 | 0.72±0.04 | >100 |
| 15b | C(4’) | 0.068±0.012 | >100 | >100 |
Values are reported as mean values of ≥3 experiments performed
IC50 values were not determined for compounds showing less than 50% inhibition at concentrations of 100 μM for FAAH and COXs.
Table 4.
Carbamate replacement: urea and reversed carbamate derivatives
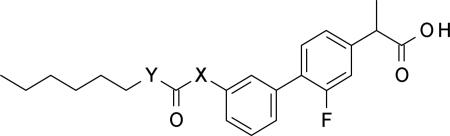
| |||||
|---|---|---|---|---|---|
| Compound | Y | X | FAAHa IC50 (μM) ±SD | COX-1a IC50 (μM) ±SD | COX-2a IC50 (μM) ±SD |
| 15c | NH | NH | 88.4±2.3 | 0.014±0.003 | 0.56±0.12 |
| 15d | O | NH | 14.9±1.6 | 0.03±0.01 | 0.17±0.01 |
Values are reported as mean values of ≥3 experiments performed.
Table 5.
SAR exploration on the R group: role of the propionic acid functionality on the B phenyl ring
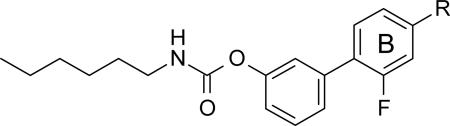
| ||||
|---|---|---|---|---|
| Compound | R | FAAHa,b IC50 (μM) ±SD | COX-1a,b IC50 (μM) ±SD | COX-2a,b IC50 (μM) ±SD |
| 9r | CH(CH3)CO2CH3 | 0.052±0.010 | >100 | >100 |
| 12 | CH(CH3)CH2OH | 0.003±0.002 | 1.1±0.3 | >100 |
| 18b | CH2CO2H | 0.063±0.010 | 2.1±0.1 | 0.24±0.04 |
| 21a | CH3 | 0.026±0.09 | >100 | >100 |
| 21c | CO2H | 0.085±0.006 | >100 | >100 |
Values are reported as mean values of ≥3 experiments performed
IC50 values were not determined for compounds showing less than 50% inhibition at concentrations of 100 μM for FAAH and COXs.
Table 6.
SAR exploration on the role of the X substituent on the B phenyl ring
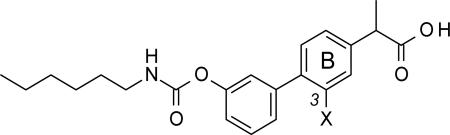
| ||||
|---|---|---|---|---|
| Compound | X | FAAHa IC50(μM)±SD | COX-1a IC50 (μM)±SD | COX-2a IC50 (μM)±SD |
| 29a | Cl | 0.023±0.008 | 0.009±0.001 | 0.73±0.21 |
| 29b | CH3 | 0.010±0.001 | 0.011±0.001 | 1.40±0.31 |
| 29c | CF3 | 0.005± 0.001 | 0.01±0.003 | 0.2±0.08 |
| 29d | OH | 0.035±0.010 | 0.65±0.07 | 13.0 ±2.1 |
| 29e | H | 0.003±0.001 | 0.054±0.011 | 0.69±0.02 |
| 29f | NO2 | 0.009±0.002 | 0.13±0.03 | 0.930±0.15 |
| 29g | NH2 | 0.049±0.023 | 0.22±0.09 | 12.1±0.6 |
Values are reported as mean values of ≥3 experiments performed.
2.2.4.1. Role and position of carbamate group in the A phenyl ring
We first investigated the effect of the position of the carbamate group in the A phenyl ring, which indeed appeared to play an important role in the inhibition of both FAAH and COX (Table 3). In agreement with the rational design of our hybrid scaffold 1 (Figure 1), the C(2’)-derivative 15a (ortho derivative) showed a 70-fold decrease in potency toward FAAH, a 60-fold decrease in potency toward COX-1, and a complete loss of activity toward COX-2, when compared to the C(3’)-isomer 10r (meta derivative) (Table 3).
On the other hand, the C(4’)-derivative 15b (para derivative) exhibited a slight loss of potency toward FAAH compared to 10r, but both COX inhibitions were completely suppressed (Table 3). These results support the hypothesis that the bent shape of the O-biphenyl moieties, which is known to better fit the FAAH enzyme surface, [53] is also important in the recognition by COX-1 and COX-2, possibly through a better superimposition to the conformations adopted by the fatty acyl chain of the natural substrate/product (the first two cis-double bonds of AA) when bound to COX-1 [66] and COX-2 [67].
Next, we replaced the carbamate moiety with alternative functional groups, such as urea (15c) [51] and reversed carbamate (15d) [51] (Table 4).
As expected from the rational design of our class of multitarget inhibitors, 15c and 15d showed a significant decrease in potency toward FAAH, whilst retaining COX-1 and COX-2 inhibitory activities compared to 10r.
These results support the hypothesis that the mechanism of action of this class of compounds is similar to the one reported for the O-aryl carbamates (acylation of FAAH Ser 241) [57] and that COX inhibition does not rely on any irreversible binding mode at the expense of the carbamate group of 10r. Reported dialysis experiments on 10r are in agreement with this mechanistic speculation. [51]
2.2.4.2. Role of the propionic acid functionality in the B phenyl ring
We then turned our attention to the role of the propionic acid in the B phenyl ring (Table 5). Replacing the propionic acid group of 10r with several substituents had only a minor impact on the potency toward FAAH, compared to the effect observed on COX activities. In fact, methyl ester 9r retained FAAH inhibitory activity, compared to 10r, but completely lost activity toward both COX-1 and COX-2. Replacement of the carboxylic acid of 10r with the corresponding primary alcohol 12 resulted in a 10-fold improvement in potency toward FAAH (IC50=3 nM), a 100-fold loss in potency toward COX-1 (IC50=1.1 μM) and in a complete loss of activity toward COX-2.
On the other hand, the removal of the α-methyl group, as in the achiral des-methylated derivative 18b, caused a 2-fold decrease of the potency toward FAAH, compared to 10r (IC50 = 63 nM and 31 nM, respectively), and a 180-fold reduction of potency toward COX-1 (IC50 = 2.1 μM and 12 nM, respectively). The activity against COX-2 was slightly improved (IC50 = 0.24 μM and 0.43 μM respectively). The methyl analog 21a [51] was active against FAAH in the same potency range of 10r (IC50 = 26 nM and 31 nM, respectively), while a completely loss of activity against COX enzymes was observed. A similar result was obtained with the carboxylic analog 21c, which also showed a 3-fold reduction in potency toward FAAH, compared to 10r (IC50 = 85 nM and 31 nM, respectively).
We conclude that FAAH tolerates substituents with different steric and electronic properties at the 4-position of the B phenyl ring, while COX-1 and COX-2 display a stringent requirement for a propionic or acetic acid groups in the same position.
2.2.4.3. Role of the fluorine atom in the B phenyl ring
To complete the SAR exploration of the B phenyl ring, we evaluated the effect of substituents with different electronic and steric properties, alternative to the fluorine atom (Table 6).
Substituting the fluorine with chlorine was tolerated: indeed, 29a was virtually equipotent against FAAH and COX-1, and marginally less potent on COX-2, compared to 10r. The same trend was observed with the methyl derivative 29b, which was slightly more potent than 10r against FAAH and equally potent on COX-1, but less active against COX-2. The CF3 derivative 29c showed a 6-fold and 2-fold increase in potency toward FAAH and COX-2, respectively, and was as potent as 10r on COX-1.
Removal of the fluorine atom (29e) resulted in a 10-fold increase in potency toward FAAH, compared to 10r, and a slight decrease in activity for COX-1 and COX-2.
Compounds 29d and 29g, which bear −OH or −NH2 groups, respectively, inhibited FAAH with potencies similar to that of 10r, whereas a clear loss in potency for both COX-1 and COX-2 was observed. On the other hand, the NO2 derivative 29f had higher potency toward FAAH but loss lower potency toward both COX-1 and COX-2. We interpret these results to suggest that the electronic and steric properties of the substituents in the 3-position of the B phenyl ring affect FAAH recognition only slightly, whereas these same substituents influence COX-1 and COX-2 more markedly, with lipophilic groups being better tolerated than polar or H-bond donator groups.
2.2.4.4. Stereochemical and pharmacological studies of 10r enantiomers
Finally, we subjected the best studied member of this class of inhibitors, the racemic compound 10r, [51] to chiral HPLC separation and tested each of its enantiomers – (−)-10r (first eluted) and (+)-10r (second eluted) – for the ability to inhibit FAAH, COX-1 and COX-2 (Table 7). FAAH showed no preference for either enantiomer, with each being more active than the racemate 10r. By contrast, in analogy to prior studies on different classes of FAAH/COX inhibitors, [30, 33] substantial differences were observed on COX-1 and COX-2. Compound (+)-10r was highly potent on both COX-1 (IC50=0.29 nM) and COX-2 (IC50=50 nM), whereas (−)-10r was weakly active on either target.
Table 7.
Evaluation of the enantiomers of 10r
| Compound | r-FAAHa IC50(μM) ±SD | COX-1a IC50 (μM) ±SD | COX-2a IC50 (μM) ±SD |
|---|---|---|---|
| (±)-10r | 0.031±0.002 | 0.012±0.002 | 0.43±0.02 |
| (−)-10rb | 0.0099±0.002 | 4.0±1.3 | 22.8±8.7 |
| (+)-10rc | 0.0094±0.0003 | 0.00029±0.00004 | 0.050±0.012 |
Values are reported as mean values of ≥3 experiments performed
(R)- configurated enantiomer of 10r (see Supporting Information for details)
(S)-configurated enantiomer of 10r (see Supporting Information for details).
We completed our exploration on the two enantiomers of 10r by assigning their absolute stereo-configurations. As reported in Supporting Information (Scheme S1), a stereochemical correlation study allowed us unambiguously to assign the absolute stereochemistry of (−)-10r and (+)-10r to the (R)- and (S)- configurations, respectively. These results are in agreement with earlier reports showing that the (S)-enantiomer is responsible for the COX-inhibiting activity of aryl-propionic acid derivatives such as flurbiprofen. [29, 31, 33, 59]
2.2.4.5. In vivo experiments on (S)-(+)-10r
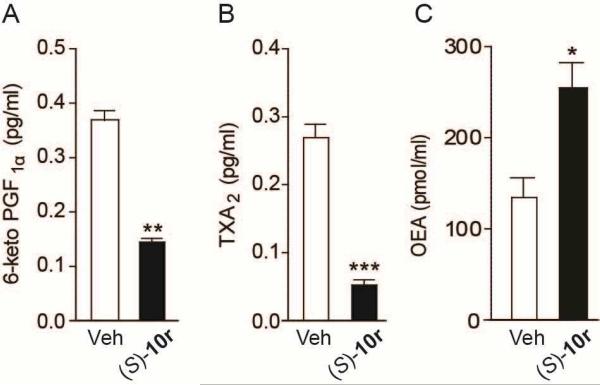
Finally, pharmacological experiments indicate that compound (S)-(+)-10r strongly engages its intended molecular targets in live mice. Intravenous administration of (S)-(+)-10r (1 mg/kg) lowered the concentrations of two COX products in circulation, prostacyclin and TXA2, as assessed surveying the stable metabolites, 6-keto-PGF1α and TXB2 (Figure 2A and 2B). Moreover, (S)-(+)-10r increased plasma levels of the FAAH substrate, OEA (Figure 2C). In addition, (S)-(+)-10r demonstrated no off-target activities on a panel of >90 biologically relevant receptors, enzymes [including N-acylethanolamine amide hydrolase (NAAA), which is the primary enzyme involved in the deactivation of PEA and OEA in innate immune cells] and ion channels (Table S1). Further pharmacological studies on the (R)- and (S)- series of this class of inhibitors will be reported in due course.
Figure 2.
Plasma levels of COX metabolites and FAAH substrate after intravenous administration of (S)-(+)-10r (1 mg/kg): 6-keto-PGF1α (A) TXA2 (B) and OEA (C). Results are expressed as mean ± s.e.m. of 6 independent determinations. *P<0.05, **P<0.01 and ***P<0.001 compared to vehicle mice, two-tailed Student's t test.
3. Conclusions
The present study outlines key SAR properties of a novel class of dual inhibitors of intracellular FAAH and COX activities, which are based on the hybrid scaffold 1. Several chemical variations of this scaffold were considered, which involved the carbamate moiety at the 3’-position of the A phenyl ring, the R groups, and the propionic acid moiety and fluorine atom in the B phenyl ring. Introduction of different alkyl and aromatic groups in the N-terminal region of the carbamate functionality improved inhibitory potency toward both FAAH and COX. A more focused exploration around the potent, selective and orally available racemic inhibitor 10r [51] led to the identification of novel potent analogs, 29a-c, and e. Because of the problems associated with the development of racemic compounds, we extended our studies and identified two additional molecules, the achiral compound 18b and the enantiomer (S)-(+)-10r, which also display high inhibitory potency for FAAH/COX-1/COX-2.
The in vivo activity of (S)-(+)-10r suggests that this agent may be used to probe the therapeutic utility of simultaneous FAAH-COX inhibition, especially in pathologies in which these enzymes are abnormally expressed.
4. Experimental part
4.1. Synthesis
Solvents and reagents were obtained from commercial suppliers and were used without further purification. URB597 was prepared following a reported procedure. [54] Flurbiprofen was purchased from Sigma-Aldrich (Milan, Italy). Melting points were determined on a Büchi M-560 capillary melting point apparatus and are uncorrected. Automated column chromatography purifications were done using a Teledyne ISCO apparatus (CombiFlash® Rf) with pre-packed silica gel columns of different sizes (from 4 g until 120 g). Mixtures of increasing polarity of Cy and EtOAc or DCM and MeOH were used as eluents. Preparative TLC analyses were performed using Macherey-Nagel pre-coated 0.05 mm TLC plates (SIL G-50 UV254). 1H and 13C-NMR experiments were run on a Bruker Avance III 400 system (400.13 MHz for 1H, and 100.62 MHz for 13C), equipped with a BBI probe and Z-gradient coil. 19F-NMR experiments were run on a Bruker Avance III 600 system (546.6 MHz for 19F), equipped with a 5 mm CryoProbe QCI 1H/19F–13C/15N–D quadruple resonance and a Z-gradient coil. Spectra were acquired at 300 K, using deuterated dimethylsulfoxide (DMSO-d6) or deuterated chloroform (CDCl3) as solvents. Chemical shifts for 1H and 13C spectra were recorded in parts per million using the residual non-deuterated solvent as the internal standard (for DMSO-d6: 2.50 ppm, 1H; 39.52 ppm, 13C; for CDCl3: 7.26 ppm, 1H and 77.16 ppm, 13C). Data are reported as follows: chemical shift (ppm), multiplicity (indicated as: bs, broad signal; s, singlet; d, doublet; t, triplet; q, quartet; p, quintet, sx, sextet; m, multiplet and combinations thereof), coupling constants (J) in Hertz (Hz) and integrated intensity. UPLC/MS analyses were run on a Waters ACQUITY UPLC/MS system consisting of a SQD (Single Quadropole Detector) Mass Spectrometer equipped with an Electrospray Ionization interface and a Photodiode Array Detector. PDA range was 210-400 nm. Analyses were performed on an ACQUITY UPLC BEH C18 column (50×2.1 mmID, particle size 1.7 μm) with a VanGuard BEH C18 pre-column (5×2.1 mmID, particle size 1.7 μm). Mobile phase was either 10 mM NH4OAc in H2O at pH 5 adjusted with AcOH (A) and 10 mM NH4OAc in MeCN-H2O (95:5) at pH 5 (B). Electrospray ionization in positive and negative mode was applied. Analyses were performed with method A or B. Method A for compounds 10a-t, 15a-d, 18b, 21c and 29b-g: Gradient: 5 to 95% B over 3 min. Flow rate 0.5 mL/min. Temperature 40 °C. Method B for compounds 9r, 12, 21a and 29a: Gradient: 50 to 100% B over 3 min. Flow rate 0.5 mL/min. Temperature 40 °C. Purifications by preparative HPLC/MS were run on a Waters Autopurification system consisting of a 3100 Single Quadropole Mass Spectrometer equipped with an Electrospray Ionization interface and a 2998 Photodiode Array Detector. HPLC system included a 2747 Sample Manager, 2545 Binary Gradient Module, System Fluidic Organizer and 515 HPLC Pump. PDA range was 210-400 nm. Purifications were performed on a XBridgeTM Prep C18 OBD column (100× 19 mmID, particle size 5 μm) with a XBridgeTM Prep C18 (10× 19 mmID, particle size 5 μm) Guard Cartridge. Mobile phase was 10 mM NH4OAc in H2O at pH 5 adjusted with AcOH (A) and 10 mM NH4OAc in MeCN-H2O (95:5) at pH 5 (B). Electrospray ionization in positive and negative mode was used. Analyses by chiral HPLC were run on a Waters Alliance HPLC instrument consisting of an e2695 Separation Module and a 2998 Photodiode Array Detector. PDA range was 210-400 nm. Analyses were performed isocratic on a Daicel ChiralPak AD column (250×4.6 mmID, particle size 10 μm). Mobile phase was 0.1 % TFA Heptane/2-Propanol (75:25). Separations of 10r by preparative chiral HPLC were run on a Waters Alliance HPLC instrument consisting of a 1525 Binary HPLC Pump, Waters Fraction Collector III and a 2998 Photodiode Array Detector. UV detection was at 240 nm. Purifications were performed isocratic on a Daicel ChiralPak AD column (250 × 10mmID, particle size 10 μm). Mobile phase was 0.1 % TFA Heptane/2-Propanol (75: 25). Optical rotations were measured on a Rudolf Research Analytical Autopol II Automatic polarimeter using a sodium lamp (589 nm) as the light source; concentrations expressed in g/100 mL using CHCl3 as a solvent and a 1 dm cell. Accurate mass measurement was performed on a Synapt G2 Quadrupole-ToF Instrument (Waters, USA), equipped with an ESI ion source; compounds were diluted to 50 μM in H2O/MeCN and analyzed. Leucine Enkephalin (2 ng/mL) was used as lock mass reference compound for spectra calibration. All final compounds displayed ≥ 95% purity as determined by NMR and UPLC/MS analysis.
All the analytical data of intermediate compounds are reported in Supporting Material.
4.1.1. (±)-2-(3-fluoro-4-nitro-phenyl)propanoic acid (4)
Compound 4 was obtained as brown clear oil (4.50 g, 81 %), according to the procedure reported in the literature starting from 2,4-difluoronitrobenzene (4.77 g, 30 mmol). [62]
4.1.2. (±)-Methyl 2-(4-nitro-3-fluoro-phenyl)propanoate (5)
To a solution of 4 (4.50 g, 21.11 mmol) in MeOH (40 mL), concentrated H2SO4 (0.1 mL) was added and the resulting solution was stirred at rt overnight. After solvent evaporation, the crude oil was diluted with Et2O (15 mL) and filtered through a pad of SiO2 to afford 5 as orange-brown oil (4.45 g, 93%).
4.1.3. (±)-Methyl 2-(4-amino-3-fluoro-phenyl)propanoate (6)
To a solution of 5 (12.60 g, 55.46 mmol) in MeOH (222 mL) was added 10% Pd/C (2.35 g, 2.22 mmol) followed by addition of HCO2NH4 (20.98 g, 332.8 mmol). The solution was stirred at rt for 3 h, then, filtered through a pad of Celite and the filtrate was concentrated under reduced pressure. The residue was dissolved in EtOAc and filtered through a pad of SiO2 to afford 6 as an orange oil (10.33 g, 94%).
4.1.4. (±)-Methyl 2-(3-fluoro-4-iodo-phenyl)propanoate (7)
A solution of NaNO2 (0.70 g, 10.21 mmol) in H2O (1.5 mL) was added slowly to a solution of 6 (1.75 g, 9.76 mmol) in a 3N HCl solution (29 mL) at 0 °C. After 30 min, NaI (1.54 g, 10.25 mmol) was added at 0 °C under stirring. The resulting mixture was slowly warmed to rt in 5 min, and then heated at 60 °C for 3 h. After cooling down to rt, the mixture was extracted with Et2O and the organic phase was then washed with a 1M solution of Na2SO3 (15 mL) and dried over Na2SO4. The residue was dissolved in EtOAc (50 mL), treated with activated carbon and then filtered through a pad of Celite. The filtrate was concentrated under reduced pressure and the yellow oil was purified by column chromatography (Cy: EtOAc, 95:5) to give 7 as a pale yellow oil (1.70 g, 55%).
4.1.5. General procedure for Suzuki cross coupling reaction (Procedure A, 8, 13a-c, 17, 20a, b, 27a-f)
To a solution of the corresponding boronic acid (1.2 mmol) in EGME/H2O (3:1, 0.25 M) were added Pd(OAc)2 (0.05 mmol) and K2CO3 (1.2 mmol), followed by the addition of the corresponding phenyl iodide (1.0 mmol). The dark reaction mixture was stirred at rt for 15 h, then diluted with EtOAc (40 mL) and filtered through a pad of Celite. The resulting filtrate was washed with H2O (20 mL) and a 1M solution of Na2SO3 (20 mL). After separation, the organic phase was dried over Na2SO4 and concentrated under reduced pressure. The residues were purified by column chromatography (Cy/EtOAc).
4.1.5.1. (±)-Methyl 2-[3-fluoro-4-(3-hydroxyphenyl)phenyl]propanoate (8)
Compound 8 was prepared according to general procedure A using 7 (3.27 g, 10.61 mmol) and 3-hydroxyphenylboronic acid (1.76 g, 12.74 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 8 as a colorless oil (2.46 g, 84%).
4.1.5.2. (±)-Methyl 2-[3-fluoro-4-(2-hydroxyphenyl)phenyl]propanoate (13a)
Compound 13a was prepared according to general method A using 7 (0.31 g, 1 mmol) and 2-hydroxyphenylboronic acid (0.17 g, 1.2 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 13a as a white oil (230 mg, 84%).
4.1.5.3. (±)-Methyl 2-[3-fluoro-4-(4-hydroxyphenyl)phenyl]propanoate (13b)
Compound 13b was prepared according to general procedure A using 7 (0.31 g, 1 mmol) and 4-hydroxyphenylboronic acid (0.17 g, 1.2 mmol). The crude was purified by column chromatography (Cy/EtOAc, 95: 5) to afford 13b as a white solid (173 mg, 59%).
4.1.5.4. (±)-Methyl 2-[4-(3-aminophenyl)-3-fluoro-phenyl]propanoate (13c)
Compound 13c was prepared according to general procedure A using 7 (0.92 g, 3 mmol) and (3-aminophenyl)boronic acid monohydrate (0.56 g, 3.6 mmol). The crude was purified by column chromatography (Cy/EtOAc, 8: 2) to afford 13c as a yellow oil (750 mg, 91%).
4.1.5.5. Methyl 2-[3-fluoro-4-(3-hydroxyphenyl)phenyl]acetate (17)
Compound 17 was prepared according to general procedure A using 16b (1.00 g, 3.50 mmol) and 3-hydroxyphenylboronic acid (0.58 g, 4.20 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 19a as white solid (0.65 g, 71%).
4.1.5.6. 3-(2-fluoro-4-methyl-phenyl)phenol (20a)
Compound 20a was prepared according to general procedure A using aryl iodide 19a (236 g, 1 mmol) and 3-hydroxyphenylboronic acid (0.17 g, 1.2 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 20a as a colorless oil (187 mg, 92 %).
4.1.5.7. Methyl 3-fluoro-4-(3-hydroxyphenyl)benzoate (20b)
Compound 20b was prepared according to general procedure A using aryl iodide 19b (1 g, 3.57 mmol) and 3-hydroxyphenylboronic acid (0.59 g, 4.29 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 20b as a white solid (0.84 g, 86 %).
4.1.5.8. (±)-Methyl 2-[3-chloro-4-(3-hydroxyphenyl)phenyl]propanoate (27a)
Compound 27a was prepared according to general procedure A using 26a (2.74 g, 8.44 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to give 27a as a white solid (1.57 g, 64%).
4.1.5.9. (±)-Methyl 2-[4-(3-hydroxyphenyl)-3-methyl-phenyl]propanoate (27b)
Compound 27b was prepared according to general procedure A using 26b (1.14 g, 3.75 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to give 27b as a colorless oil (0.72 g, 71%).
4.1.5.10. (±)-Methyl 2-[4-(3-hydroxyphenyl)-3-(trifluoromethyl)phenyl]propanoate (27c)
Compound 27c was prepared according to general procedure A using 26c (1.07 g, 3 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to give 27c as a white solid (0.78 g, 80%).
4.1.5.11. (±)-Methyl 2-[3-benzyloxy-4-(3-hydroxyphenyl)phenyl]propanoate (27d)
Compound 27d was prepared according to general procedure A using 26d (1.00 g, 2.52 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to give 27d as a clear oil (0.58 g, 63%).
4.1.5.12 (±)-Methyl 2-[4-(3-hydroxyphenyl)phenyl]propanoate (27e)
Compound 27e was prepared according to general procedure A using 26e (1.45 g, 5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 8: 2) to afford 27e as a white oil (760 mg, 59%).
4.1.5.13 (±)-Methyl 2-[4-(3-hydroxyphenyl)-3-nitro-phenyl]propanoate (27f)
Compound 27f was prepared according to general procedure A using 26f (1.76 g, 5.25 mmol). The crude was purified by column chromatography (Cy/EtOAc, 8 : 2) to give 27f as a yellow solid (1.1 g, 74%).
4.1.6. General procedure for carbamoylation reaction (Procedure B, 9a-t, 12, 14a-c, 18a, 28af)
To a solution of the corresponding phenol or aniline (1 mmol) in MeCN (0.5 M) was added DMAP (0.1 mmol) and the corresponding isocyanate (3.0 mmol). The resulting solution was stirred at rt for 15 h then the solvent was concentrated under reduced pressure. The residues were purified by column chromatography (Cy/EtOAc or DCM/MeOH).
4.1.6.1. (±)-Methyl 2-[4-[3-(cyclohexylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9a)
Compound 9a was prepared according to general procedure B using 8 (274 mg, 1 mmol) and c-hexyl isocyanate (376 mg, 3 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9a as a white solid (261 mg, 65%).
4.1.6.2. (±)-Methyl 2-[4-[3-(cyclopentylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9b)
Compound 9b was prepared according to general procedure B using 8 (274 mg, 1 mmol) and c-pentyl isocianate (333 mg, 3 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9b as a white solid (235 mg, 61%).
4.1.6.3. (±)-Methyl 2-[4-[3-(cyclobutylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9c)
Compound 9c was prepared according to general procedure B using 8 (274 mg, 1 mmol) and c-butyl isocyanate (291 mg, 3 mmol). The crude colorless oil of 9c was used in the next step without further purification.
4.1.6.4. (±)-Methyl 2-[4-[3-(cyclopropylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9d)
Compound 9d prepared according to general procedure B using 8 (274 mg, 1 mmol) and c-propyl isocyanate (250 mg, 3 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9d as a white solid (59 mg, 38%).
4.1.6.5. (±)-Methyl 2-[4-[3-(cyclohexylmethylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9e)
Compound 9e was prepared according to general procedure B using 8 (137 mg, 0.50 mmol) and c-hexyl methyl isocyanate (209 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9e as a white solid (165 mg, 80%).
4.1.6.6. (±)-Methyl 2-[4-[3-(2-cyclohexylethylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9f)
Compound 9f was prepared according to general procedure B using 8 (137 mg, 0.50 mmol) and c-hexyl ethyl isocyanate (230 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9f as a white solid (179 mg, 84%).
4.1.6.7. (±)-Methyl 2-[3-fluoro-4-[3-(isopropylcarbamoyloxy)phenyl]phenyl]propanoate (9g)
Compound 9g was prepared according to general procedure B using 8 (157 mg, 0.57 mmol) and isopropyl isocyanate (145 mg, 1.71 mmol). The crude was purified by column chromatography (Cy/EtOAc, 8: 2) to afford 9g as a white solid (159 mg, 77%).
4.1.6.8. (±)-Methyl 2-[3-fluoro-4-[3-(isobutylcarbamoyloxy)phenyl]phenyl]propanoate (9h)
Compound 9h was prepared according to general procedure B using 8 (129 mg, 0.47 mmol) and isobutyl isocyanate (140 mg, 1.41 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9h as a white solid (138 mg, 78%).
4.1.6.9. (±)-Methyl 2-[3-fluoro-4-[3-(phenylcarbamoyloxy)phenyl]phenyl]propanoate (9i)
Compound 9i was prepared according to general procedure B using 8 (137 mg, 0.5 mmol) and phenyl isocyanate (179 mg, 3 mmol) to afford 9i as a colorless oil (161 mg, 82%).
4.1.6.10. (±)-Methyl 2-[4-[3-(benzylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9j)
Compound 9j was prepared according to general procedure B using 8 (137 mg, 0.5 mmol) and benzylisocyanate (199 mg, 1.5 mmol) to afford 9j as a colorless oil which was used in the next step without further purification.
4.1.6.11. (±)-Methyl 2-[3-fluoro-4-[3-(phenethylcarbamoyloxy)phenyl]phenyl]propanoate (9k)
Compound 9k was prepared according to general procedure B using 8 (137 mg, 0.5 mmol) and phenylethyl isocyanate (221 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9k as a white solid (165 mg, 71%).
4.1.6.12. (±)-Methyl 2-[3-fluoro-4-[3-(3-phenylpropylcarbamoyloxy)phenyl]phenyl]propanoate (9l)
Compound 9l was prepared according to general procedure B using 8 (137 mg, 0.5 mmol) and phenylpropyl isocyanate (241 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9l as a white solid (174 mg, 79%).
4.1.6.13. (±)-Methyl 2-[3-fluoro-4-[3-(4-phenylbutylcarbamoyloxy)phenyl]phenyl]propanoate (9m)
Compound 9m was prepared according to general procedure B using 8 (121 mg, 0.44 mmol) and phenylbutyl isocyanate (231 mg, 1.32 mmol). The crude was purified by column chromatography (Cy/EtOAc, 8: 2) to afford 9m as a white solid (171 mg, 86%).
4.1.6.14. (±)-Methyl 2-[4-[3-(ethylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9n)
Compound 9n was prepared according to general procedure B using 8 (185 mg, 0.68 mmol) and ethyl isocyanate (145 mg, 2.04 mmol). The crude was purified by column chromatography (Cy/EtOAc, 8: 2) to afford 9n as a white solid (176 mg, 75%).
4.1.6.15. (±)-Methyl 2-[3-fluoro-4-[3-(propylcarbamoyloxy)phenyl]phenyl]propanoate (9o)
Compound 9o was prepared according to general procedure B using 8 (137 mg, 0.50 mmol) and n-propyl isocyanate (128 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9o as a white solid (87 mg, 48%).
4.1.6.16. (±)-Methyl 2-[4-[3-(butylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoate (9p)
Compound 9p was prepared according to general procedure B using 8 (137 mg, 0.50 mmol) and n-butyl isocyanate (149 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9p as a white solid (135 mg, 72%).
4.1.6.17. (±)-Methyl 2-[3-fluoro-4-[3-(pentylcarbamoyloxy)phenyl]phenyl]propanoate (9q)
Compound 9q was prepared according to general procedure B using 8 (128 mg, 0.47 mmol) and n-pentyl isocyanate (159 mg, 1.41 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1), the title compound to afford 9q as a white solid (158 mg, 87%).
4.1.6.18. (±)-Methyl 2-[3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoate (9r)
Compound 9r was prepared according to general procedure B using 8 (137 mg, 0.5 mmol) and n hexyl isocyanate (191 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9r as a white solid (170 mg, 85%). Mp: 89-91 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.77 (t, J = 5.6 Hz, 1H, NH), 7.50 (t, J = 8.2 Hz, 1H, H-5), 7.47 (t, J = 7.9 Hz, 1H, h-11), 7.37 (d, J = 7.2 Hz, 1H, H-10), 7.24 (m, 3H, H-2 H-6 H-8), 7.13 (dd, J = 8.0, 1.4 Hz, 1H, H-12), 3.91 (q, J = 7.1 Hz, 1H, CH), 3.62 (s, 3H, OCH3), 3.06 (q, J = 6.7 Hz, 2H, R-H-1’), 1.48 (p, J = 6.22 Hz, 2H, R-H-2’), 1.43 (d, J = 7.2 Hz, 3H, CH3), 1.28 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.87 (t, J = 6.9 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 173.74 (COOH), 158.82 (d, J = 246.7 Hz, C-3), 154.17 (C-9), 151.21 (HNCOO), 142.75 (d, J = 7.8 Hz, C-7), 135.82 (C-1), 130.76 (d, J = 3.5 Hz, C-5), 129.42 (C-11), 125.98 (d, J = 13.0 Hz, C-4), 125.20 (C-10), 124.02 (C-6), 121.86 (C-8), 121.20 (C-12), 115.20 (d, J = 23.4 Hz, C-2), 51.95 (OCH3), 43.77 (CH), 40.45 (R-C-1’), 30.91 (R-C-4’), 29.12 (R-C-2’), 25.88 (R-C-3’), 22.02 (R-C-5’), 18.28 (CH3), 13.87 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 117.0. UPLC/MS analysis: Rt 2.00 min. MS (ES) C23H28FNO4 requires: 401, found 402 [M+H]+. HRMS C23H29NO4F [M+H]+: calculated 402.2081 measured 402.2087 Δppm 1.5.
4.1.6.19 (±)-Methyl 2-[3-fluoro-4-[3-(heptylcarbamoyloxy)phenyl]phenyl]propanoate (9s)
Compound 9s was prepared according to general procedure B using 8 (137 mg, 0.50 mmol) and n-heptyl isocyanate (212 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9s as a white solid (171 mg, 82%).
4.1.6.20. (±)-Methyl 2-[3-fluoro-4-[3-(octylcarbamoyloxy)phenyl]phenyl]propanoate (9t)
Compound 9t was prepared according to general procedure B using 8 (109 mg, 0.40 mmol) and n-octyl isocyanate (186 mg, 1.2 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 9t as a white solid (171 mg, 99%).
4.1.6.21. (±)-[3-[2-fluoro-4-(2-hydroxy-1-methyl-ethyl)phenyl]phenyl] N-hexylcarbamate (12)
Compound 12 was prepared according to general procedure B using 11 (123 mg, 0.50 mmol) and n-hexyl isocyanate (127 mg, 1 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 12 as a colorless oil (137 mg, 73%). Mp: 59-60 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.77 (t, J = 5.7 Hz, 1H, NH), 7.46 (t, J = 7.7 Hz, 1H, H-5), 7.44 (t, J = 8.5 Hz, 1H, H-11), 7.36 (m, 1H, H-10), 7.23 (m, 1H, H-8), 7.17 (m, 2H, H-2 H-6), 7.12 (ddd, J = 8.1, 2.3, 1.1 Hz, 1H, H-12), 4.69 (t, J = 5.2 Hz, 1H, OH), 3.51 (m, 2H, CH2), 3.06 (q, J = 6.8 Hz, 2H, R-H-1’), 2.87 (h, J = 6.8 Hz, 1H, CH), 1.46 (h, J = 7.1 Hz, 2H, R-H-2’), 1.29 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 1.21 (d, J = 7.0 Hz, 3H, CH3), 0.87 (t, J = 6.7 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 158.8 (d, J = 246.0 Hz, C-3), 154.1 (C-9), 151.1 (HNCOO), 147.7 (d, J = 7.3 Hz, C-7), 136.2 (C-1), 130.1 (d, J = 2.4 Hz, C-5), 129.3 (C-11), 125.1 (C-10), 124.8 (d, J = 12.7 Hz, C-4), 124.0 (C-6), 121.7 (C-8), 120.9 (C-12), 114.9 (d, J = 22.4 Hz, C-2), 66.5 (CH2), 41.4 (CH), 40.4 (R-C-1’), 30.9 (R-C-4’), 29.1 (R-C-2’), 25.8 (R-C-3’), 22.0 (R-C-5’), 17.7 (CH3), 13.8 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 118.0. UPLC/MS analysis: Rt 2.60 min. MS (ES) C22H28FNO3 requires 373, found 374 [M+H]+. HRMS C22H29NO3F [M+H]+: calculated 374.2131 measured 374.2149 Δppm 4.8.
4.1.6.22. (±)-Methyl 2-[3-fluoro-4-[2-(hexylcarbamoyloxy)phenyl]phenyl]propanoate (14a)
Compound 14a was prepared according to general procedure B using 13a (137 mg, 0.5 mmol) and n-hexyl isocyanate (191 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 14a as a white oil (178 mg, 88%).
4.1.6.23. (±)-Methyl 2-[3-fluoro-4-[4-(hexylcarbamoyloxy)phenyl]phenyl]propanoate (14b)
Compound 14b was prepared according to general procedure B using 13b (137 mg, 0.5 mmol) and n-hexyl isocyanate (191 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 14b as a white solid (146 mg, 72%).
4.1.6.24. (±)-Methyl 2-[3-fluoro-4-[3-(hexylcarbamoylamino)phenyl]phenyl]propanoate (14c)
Compound 14c was prepared according to general procedure B using 13c (153 mg, 0.56 mmol) and n-hexyl isocyanate (214 mg, 1.7 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 14c as a white solid (146 mg, 65%).
4.1.6.25. Methyl 2-[3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]acetate (18a)
Compound 18a was prepared according to general procedure B using 17 (130 mg, 0.50 mmol) and n-hexyl isocyanate (191 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 18a as a white solid (123 mg, 64%).
4.1.6.26. [3-(2-fluoro-4-methyl-phenyl)phenyl] N-hexylcarbamate (21a)
Compound 21a was prepared according to general procedure B using 20a (101 mg, 0.50 mmol) and n-hexyl isocyanate (191 mg, 1.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 20a as a white solid (142 mg, 86%). Mp: 56-57 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.76 (t, J = 5.7 Hz, 1H, NH), 7.45 (t, J = 7.9 Hz, 1H, H-5), 7.41 (t, J = 8.0, 1H, H-11), 7.35 (d, J = 6.8 Hz, 1H, H-10), 7.22 (m, 1H, H-8), 7.12 (m, 3H, H-2 H-6 H-11 ), 3.06 (q, J = 6.8 Hz, 2H, R-H-1’), 2.36 (s, 3H, CH3), 1.47 (p, J = 6.9 Hz, 2H, R-H-2’), 1.28 (m, 6H, H-3’ H-4’ H-5’), 0.87 (t, J = 6.8 Hz, 3H, H-6’). 13C NMR (101 MHz, DMSO-d6) δ 158.8 (d, J = 247.0 Hz, C-3), 154.2 (C-9), 151.1 (HNCOO), 140.0 (d, J = 8.3 Hz, C-7), 136.2 (C-1), 130.2 (d, J = 3.6 Hz, C-5), 129.3 (C-11), 125.5 (C-10), 125.1 (C-6), 124.3 (d, J = 12.9 Hz, C-4), 121.7 (C-8), 120.9 (C-12), 116.4 (d, J = 22.3 Hz, C-2), 40.4 (R-C-1’), 30.9 (R-C-4’), 29.1 (R-C-2’), 25.8 (R-C-3’), 22.0 (RC-5’), 20.4 (CH3), 13.8 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 118.0. UPLC/MS analysis: Rt 2.17 min. MS (ES) C20H24FNO2 requires 329, found 330 [M+H]+. HRMS C20H25NO2F [M+H]+: calculated 330.1869 measured 330.189 Δppm 6.4.
4.1.6.27. Methyl 3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]benzoate (21b)
Compound 21b was prepared according to general procedure B using 20b (0.84 g, 3.41 mmol) and n-hexyl isocyanate (1.30 g, 10.23 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 21b as a white solid (1.27 g, quant.).
4.1.6.28 (±)-Methyl 2-[3-chloro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoate (28a)
Compound 28a was prepared according to general procedure B using 27a (1.57 g, 5.40 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to give 28a as a colorless oil (2.12 g, 94%).
4.1.6.29 (±)-Methyl 2-[4-[3-(hexylcarbamoyloxy)phenyl]-3-methyl-phenyl]propanoate (28b)
Compound 28b was prepared according to general procedure B using 27b (0.72 g, 2.66 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to give 28b a white solid (0.94 g, 89%).
4.1.6.30 (±)-Methyl 2-[4-[3-(hexylcarbamoyloxy)phenyl]-3-(trifluoromethyl)phenyl]propanoate (28c)
Compound 28c was prepared according to general procedure B using 27c (0.78 g, 2.41 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to give 28c as a white solid (1.02 g, 94%).
4.1.6.31 (±)-Methyl 2-[3-benzyloxy-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoate (28d)
Compound 28d was prepared according to general procedure B using 27d (1.00 g, 2.52 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to obtain 28d as a clear oil (0.72 g,quant.).
4.1.6.32 (±)-Methyl 2-[4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoate (28e)
Compound 28e was prepared according to general procedure B using 27e (128 mg, 0.5 mmol). The crude was purified by column chromatography (Cy/EtOAc, 9: 1) to afford 28e as a white solid (123 mg, 64%).
4.1.6.33 (±)-Methyl 2-[4-[3-(hexylcarbamoyloxy)phenyl]-3-nitro-phenyl]propanoate (28f)
Compound 28f was prepared according to general procedure B using 27f (0.85 g, 2.82 mmol). The crude was purified by column chromatography (Cy/EtOAc 9 : 1) to give 28f as a yellow oil (1.17 g, 97%).
4.1.7. General procedure for methyl ester hydrolysis (Procedure C, 10a-t, 15a-d, 18b, 21c, 29a-f)
To a solution of the corresponding methyl ester (1.0 mmol) in THF (0.1 M) was added 6M HCl (5 mL) and the mixture was stirred at rt until the disappearance of the starting material was noted by UPLC-MS analysis. H2O (10 mL) was added and the suspension was extracted with EtOAc (20 mL). After evaporation, the organic phase was dried over Na2SO4 and concentrated under reduced pressure. The residues were purified by crystallization (Et2O/Cy, Et2O/ pentane, TBME), preparative TLC (Cy/EtOAc) or preparative HPLC.
4.1.7.1. (±)-2-[4-[3-(cyclohexylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10a)
Compound 10a was prepared according to general procedure C using 9a (261 mg, 0.65 mmol). The crude was purified by preparative HPLC to afford 10a as a white solid (65 mg, 26%). Mp: 152-153 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.45 (s, 1H, COOH), 7.73 (d, J =7.8, 1H, NH), 7.50 (t, J =8.3, 1H, H-5), 7.47 (t, J =7.9, 1H, H-11), 7.37 (d, J = 7.7, 1H, H-10), 7.24 (m, 3H, H-2 H-6 H-8), 7.14 (d, J =8.0, 1H, H-12), 3.78 (q, J =7.1, 1H, CH), 3.33 (m, 1H, R-H-1’), 1.84 (m, 2H, R-H-2’), 1.71 (m, 2H, R-H-3’), 1.56 (m, 1H, R-H-4’), 1.41 (d, J =7.1, 3H, CH3), 1.23 (m, 5H, R-H-2’ R-H-3’, R-H-4’). 13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.7 (d, J = 246.1 Hz, C-3), 153.3 (C-9), 151.2 (HNCOO), 143.4 (d, J = 8.0 Hz, C-7), 135.9 (C-1), 130.6 (C-5), 129.4 (C-11), 125.7 (d, J = 13.0 Hz, C-4), 125.1 (C-10), 124.0 (C-6), 121.8 (C-8), 121.1 (C-12), 115.1 (d, J = 23.2 Hz, C-2), 49.7 (R-C-1’), 44.0 (CH), 32.4 (R-C-2’), 25.1 (R-C-4’), 24.5 (R-C-3’), 18.2 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.41 min. MS (ES) C22H24FNO4 requires 385, found 386 [M+H]+. HRMS C22H25NO4F [M+H]+: calculated 386.1768 measured 386.1781 Δppm 3.4.
4.1.7.2. (±)-2-[4-[3-(cyclopentylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10b)
Compound 10b was prepared according to general procedure C using 9b (235 mg, 0.61 mmol). The crude was purified by crystallization from TBME to afford 10b as a white solid (95 mg, 42%). Mp: 151-152°C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (s, 1H, COOH), 7.81 (d, J =7.2, 1H, NH), 7.50 (t, J =8.3, 1H, H-5), 7.47 (t, J =7.9, 1H, H-11), 7.37 (d, J =7.5, 1H, H-10), 7.23 (m, 3H, H-2 H-6 H-8), 7.14 (dd, J =7.9, 2.3, 1H, H-12), 3.85 (h, J =6.6, 1H, R-H-1’), 3.78 (q, J =7.1, 1H, CH), 1.83 (m, 2H, R-H-2’), 1.67 (m, 2H, R-H-3’), 1.50 (m, 4H, R-H-2’ R-H-3’), 1.41 (d, J =7.1, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.7 (d, J = 247.4 Hz, C-3), 153.6 (C-9), 151.1 (HNCOO), 143.4 (d, J = 7.8 Hz, C-7), 135.9 (C-1), 130.6 (C-5), 129.4 (C-11), 125.7 (d, J = 13.0 Hz, C-4), 125.1 (C-10), 124.0 (C-6), 121.8 (C-8), 121.2 (C-12), 115.1 (d, J = 23.2 Hz, C-2), 52.3(R-C-1’), 44.0 (CH), 32.1 (R-C-2’), 23.2 (R-C-3’), 18.2 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.41 min. MS (ES) C21H22FNO4 requires 371, found 372 [M+H]+. HRMS C21H23NO4F [M+H]+: calculated 372.1611 measured 372.1603 Δppm −2.1.
4.1.7.3. (±)-2-[4-[3-(cyclobutylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10c)
Compound 10c was prepared according to general procedure C using 9c. The crude was purified by preparative HPLC to afford 10c as a white solid (66 mg, 37% over 2 steps). Mp: 140-141°C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (s, 1H, COOH), 8.10 (d, J =7.9, 1H, NH), 7.49 (t, J =8.3, 1H, H-5), 7.46 (t, J =7.9, 1H, H-11), 7.38 (d, J =7.0, 1H, H-10), 7.23 (m, 3H, H-2 H-6 H-8), 7.13 (m, 1H, H-12), 4.02 (h, J =8.2, 1H, R-H-1’), 3.78 (q, J =7.1, 1H, CH), 2.18 (m, 2H, R-H-2’), 1.98 (m, 2H, R-H-2’), 1.61 (m, 2H, R-H-3’), 1.41 (d, J =7.1, 3H). 13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.7 (d, J = 246.2 Hz, C-3), 152.9 (C-9), 151.0 (HNCOO), 143.43 (d, J = 7.7 Hz, C-7), 135.9 (C-1), 130.6 (d, J = 2.9 Hz, C-5), 129.4 (C-11), 125.7 (d, J = 12.7 Hz, C-4), 125.2 (C-10), 124.0 (C-6), 121.9 (C-8), 121.2 (C-12), 115.1 (d, J = 23.3 Hz, C-2), 45.7 (R-C-1’), 44.0 (CH), 30.1 (R-C-2’), 18.2 (CH3), 14.3 (R-C-3’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.17 min. MS (ES) C20H20FNO4 requires 357, found 358 [M+H]+. HRMS C20H21NO4F [M+H]+: calculated 358.1455 measured 358.1452 Δppm −0.8.
4.1.7.4. (±)-2-[4-[3-(cyclopropylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10d)
Compound 10d was prepared according to general procedure C using 9d (59 mg, 0.17 mmol). The crude was purified by preparative HPLC to afford 10d as a white solid (35 mg, 60%). Mp: 117-188 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H, COOH), 7.97 (d, J =2.3, 1H, NH), 7.50 (t, J =8.4, 1H, H-5), 7.47 (t, J =7.9, 1H, H-11), 7.38 (d, J =7.5, 1H, H-10), 7.23 (m, 3H, H-2 H-6 H-8), 7.14 (d, J = 7.9, 1H, H-12), 3.78 (q, J = 7.1, 1H, CH), 2.57 (m, 1H, R-H-1’), 1.41 (d, J = 7.1, 3H, CH3), 0.64 (m, 2H, R-H-2’), 0.50 (m, 2H, R-H-2’). 13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.7 (d, J = 246.5 Hz, C-3), 154.8 (C-9), 151.0 (HNCOO), 143.4 (d, J = 7.9 Hz, C-7), 135.9 (C-1), 130.6 (d, J = 2.2 Hz, C-5), 129.4 (C-11), 125.7 (d, J = 13.1 Hz, C-4), 125.2 (C-10), 124.0 (C-6), 121.8 (C-8), 121.2 (C-12), 115.1 (d, J = 23.0 Hz, C-2), 44.0 (CH), 23.0 (R-C-1’), 18.2 (CH3), 5.7 (R-C-2’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 1.96 min. MS (ES) C19H18FNO4 requires 343, found 344 [M+H]+. HRMS C19H19NO4F [M+H]+: calculated 344.1298 measured 344.1298 Δppm 0.
4.1.7.5. (±)-2-[4-[3-(cyclohexylmethylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10e)
Compound 10e was prepared according to general procedure C using 9e (157 mg, 0.38 mmol). The crude was purified by crystallization from pentane/Et2O to afford 10e as a white solid (93 mg, 61%). Mp: 142-143 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.46 (s, 1H, COOH), 7.79 (t, J = 5.9 Hz, 1H, NH), 7.50 (t, J = 8.1, 1H, H-5), 7.47 (t, J = 7.9, 1H, H-11), 7.37 (d, J = 7.6 Hz, 1H, H-10), 7.24 (m, 3H, H-2 H-6 H.8), 7.13 (dd, J = 7.6, 1.8 Hz, 1H, H-12), 3.78 (q, J = 7.1 Hz, 1H, CH), 2.92 (t, J = 6.3 Hz, 2H, R-H-1’), 1.67 (m, 5H, R-H-3’ R-H-4’ R-H-5’), 1.46 (m, 1H, R-H-2’), 1.41 (d, J = 7.1 Hz, 3H, CH3), 1.18 (m, 3H, R-H-4’ R-H-5’), 0.90 (m, 2H, R-H-3’).13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.4 Hz, C-3), 154.8 (C-9), 151.7 (HNCOO), 143.9 (d, J = 7.6 Hz, C-7), 136.4 (C-1), 131.1 (d, J = 3.7 Hz, C-5), 129.9 (C-11), 126.2 (d, J = 13.0 Hz, C-4), 125.6 (C-10), 124.5 (d, J = 2.9 Hz, C-6), 122.3 (d, J = 3.0 Hz, C-8), 121.6 (C-12), 115.6 (d, J = 23.3 Hz, C-2), 47.2 (R-C-1’), 44.5 (CH), 38.1 (R-C-2’), 30.7 (R-C-3’), 26.5 (R-C-5’), 25.8 (R-C-4’), 18.7 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.57 min. MS (ES) C23H26FNO4 requires 399, found 400 [M+H]+. HRMS C23H27NO4F [M+H]+: calculated 400.1924 measured 400.193 Δppm 1.5.
4.1.7.6. (±)-2-[4-[3-(2-cyclohexylethylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10f)
Compound 10f was prepared according to general procedure C using 9f (149 mg, 0.35 mmol). The crude was purified by crystallization from pentane/Et2O to afford 10f as a white solid (98 mg, 68%). Mp: 118-119 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.07 (s, 1H, COOH), 7.74 (t, J = 5.6 Hz, 1H, NH), 7.48 (m, 2H, H-5), 7.37 (d, J = 7.0 Hz, 1H, H-11), 7.23 (m, 3H, H-2 H-6 H-8), 7.13 (dd, J = 7.7, 1.8 Hz, 1H, H-12), 3.78 (q, J = 7.1 Hz, 1H, CH), 3.09 (q, J = 6.7 Hz, 2H, R-H-1’), 1.66 (m, 5H, R-H-5’ R-H-4’ R-H-6’), 1.41 (d, J = 7.1 Hz, 3H, CH3), 1.34 (m, 3H, R-H-2’ R-H-3’), 1.19 (m, 3H, R-H-5’ R-H-6’), 0.88 (m, 2H, R-H-4’). 13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.7 (d, J = 246.5 Hz, C-3), 154.1 (C-9), 151.2 (HNCOO), 143.4 (d, J = 7.8 Hz, C-7)), 135.9 (C-1), 130.6 (d, J = 3.5 Hz, C-5), 129.4 (C-11), 125.7 (d, J = 13.1 Hz, C-4), 125.1 (C-10), 124.0 (d, J = 3.1 Hz, C-6), 121.8 (C-8), 121.1 (C-12), 115.1 (d, J = 23.1 Hz, C-2), 44.0 (CH), 38.2 (R-C-1’), 36.6 (R-C-2’), 34.4 (R-C-4’), 26.0 (R-C-6’), 25.7 (R-C-5’), 18.2 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.69 min. MS (ES) C24H28FNO4 requires 413, found 414 [M+H]+. HRMS C24H29NO4F [M+H]+: calculated 414.2081 measured 414.2096 Δppm 3.6.
4.1.7.7. (±)-2-[3-fluoro-4-[3-(isopropylcarbamoyloxy)phenyl]phenyl]propanoic acid (10g)
Compound 10g was prepared according to general procedure C using 9g (159 mg, 0.44 mmol). The crude was purified by crystallization from pentane/Et2O to afford 10g as a white solid (65 mg, 43%). Mp: 131-132 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.43 (s, 1H, COOH), 7.73 (d, J = 7.6 Hz, 1H, NH), 7.51 (t, J = 8.1 Hz, 1H, H-5), 7.48 (t, J = 7.9 Hz, 1H, H-11), 7.39 (d, J = 7.5 Hz, 1H, H-10), 7.25 (m, 3H, H-2 H-6 H-8), 7.15 (d, J = 7.9 Hz, 1H, H-12), 3.79 (q, J = 7.1 Hz, 1H, CH), 3.67 (m, J = 6.9 Hz, 1H, R-H-1’), 1.42 (d, J = 7.1 Hz, 3H, CH3), 1.15 (d, J = 6.5 Hz, 6H, R-H-2’). 13C NMR (101 MHz, DMSO-d6) δ 175.3 (COOH), 159.2 (d, J = 246.5 Hz, C-3), 153.7 (C-9), 151.6 (HNCOO), 143.9 (d, J = 8.0 Hz, C-7), 136.4 (C-1), 131.1 (d, J = 3.7 Hz, C-5), 129.8 (C-11), 126.2 (d, J = 13.1 Hz, C-4), 125.6 (C-10), 124.5 (d, J = 3.2 Hz, C-6), 122.3 (C-8), 121.7 (C-12), 115.6 (d, J = 23.1 Hz, C-2), 44.5 (CH), 43.1 (R-C-1’), 22.8 (R-C-2’), 18.7 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.09 min. MS (ES) C19H20FNO4 requires 345, found 346 [M+H]+. HRMS C19H21NO4F [M+H]+: calculated 346.1455 measured 346.1458 Δppm 0.9.
4.1.7.8. (±)-2-[3-fluoro-4-[3-(isobutylcarbamoyloxy)phenyl]phenyl]propanoic acid (10h)
Compound 10h was prepared according to general procedure C using 9h (138 mg, 0.38 mmol). The crude was purified by crystallization from pentane/Et2O to afford 10h as a white solid (57 mg, 42%). Mp: 128-130 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.45 (s, 1H, COOH), 7.84 (t, J = 5.9 Hz, 1H, NH), 7.51 (t, J = 8.1 Hz, 1H, H-5), 7.48 (t, J = 7.9 Hz, 1H, H-11), 7.39 (d, J = 7.7 Hz, 1H, H-10), 7.25 (m, 3H, H-2 H-6 H-8), 7.15 (dd, J = 8.0, 1.5 Hz, 1H, H-12), 3.79 (q, J = 7.1 Hz, 1H, CH), 2.91 (t, J = 6.4 Hz, 2H, R-H-1’), 1.76 (hept, J = 6.7 Hz, 1H, R-H-2’), 1.42 (d, J = 7.1 Hz, 3H, CH3), 0.90 (d, J = 6.7 Hz, 6H, R-H-3’). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.3 Hz, C-3), 154.9 (C-9), 151.7 (HNCOO), 143.9 (d, J = 7.7 Hz, C-7), 136.4 (C-1), 131.1 (d, J = 3.5 Hz, C-5), 129.9 (C-11), 126.2 (d, J = 13.1 Hz, C-4), 125.6 (C-10), 124.5 (d, J = 3.1 Hz, C-6), 122.3 (d, J = 2.9 Hz, C-8), 121.6 (C-12), 115.6 (d, J = 23.1 Hz, C-2), 48.5 (R-C-1’), 44.5 (CH), 28.7 (R-C-2’), 20.4 (R-C-3’), 18.7 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.24 min. MS (ES) C20H22FNO4 requires 359, found 360 [M+H]+. HRMS C20H23NO4F [M+H]+: calculated 360.1611 measured 360.1631 Δppm 5.6.
4.1.7.9. (±)-2-[3-fluoro-4-[3-(phenylcarbamoyloxy)phenyl]phenyl]propanoic acid (10i)
Compound 10i was prepared according to general procedure C using 9i (87 mg). The crude was purified by preparative HPLC to afford 10i as a white solid (31 mg, 37%). Mp: 145-146 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H, COOH), 10.26 (s, 1H, NH), 7.53 (m, 4H, H-5 H-11 R- Ph-3’), 7.45 (d, J = 6.8 Hz, 1H, H-12), 7.40 (s, 1H, H-8), 7.33 (t, J = 7.9 Hz, 2H, R-Ph-2’), 7.26 (m, 3H, H-2 H-6 H-10), 7.05 (t, J = 7.4 Hz, 1H, R- Ph-4’), 3.78 (q, J = 7.1 Hz, 1H, CH), 1.41 (d, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.8 (d, J = 246.5 Hz, C-3), 151.5 (C-9), 150.5 (HNCOO), 143.5 (d, J = 8.0 Hz, C-7), 138.5 (R-Ph-1’), 136.1 (C-1), 130.6 (d, J = 2.9 Hz, C-5), 129.6 (C-11), 128.8 (R-Ph-3’), 125.7 (C-10), 125.6 (d, J = 13.1 Hz, C-4), 124.0 (C-6), 122.9 (R-Ph-4’), 122.0 (C-8), 121.3 (C-12), 118.4 (R-Ph-2’), 115.2 (d, J = 23.3 Hz, C-2), 44.1, 18.2. 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.05 min. MS (ES) C22H18FNO4 requires 379, found 380 [M+H]+. HRMS C22H19NO4F [M+H]+: calculated 380.1298 measured 380.1296 Δppm −0.5.
4.1.7.10. (±)-2-[4-[3-(benzylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10j)
Compound 10j was prepared according to general procedure C using 9j. The crude was purified by preparative HPLC to afford 10j as a white solid (69 mg, 35% over 2 steps). Mp: 120-122 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (s, 1H, COOH), 8.36 (t, J =6.1, 1H, NH), 7.49 (m, 2H H-5 H-11), 7.36 (m, 5H, H-10 R-Ph-2’ R- Ph-3’), 7.27 (m, 2H, H-8 R-Ph-4’), 7.23 (m, 2H, H-2 H-6), 7.17 (dd, J =7.8, 1.7, 1H, H-12), 4.29 (d, J =6.1, 2H, R-H-1’), 3.78 (q, J =7.1, 1H, CH), 1.41 (d, J =7.1, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.7 (d, J = 246.3 Hz, C-3), 154.5 (C-9), 151.1 (HNCOO), 143.4 (d, J = 7.6 Hz, C-7), 139.1 (R-Ph-1), 135.9 (C-1), 130.6 (d, J = 2.8 Hz, C-5), 129.4 (C-11), 128.3 (R-Ph-3), 127.1 (R-Ph-2), 126.9 (R-Ph-4), 125.7 (d, J = 12.9 Hz, C-4), 125.3 (C-10), 124.0 (C-6), 121.8 (C-8), 121.2 (C-12), 115.1 (d, J = 23.2 Hz, C-2), 44.1 (CH), 44.0 (R-C-1’), 18.2 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.27 min. MS (ES) C23H20FNO4 requires 393, found 394 [M+H]+. HRMS C23H21NO4F [M+H]+: calculated 394.1455 measured 394.1462 Δppm 1.8.
4.1.7.11. (±)-2-[3-fluoro-4-[3-(phenethylcarbamoyloxy)phenyl]phenyl]propanoic acid (10k)
Compound 10k was prepared according to general procedure C using 9k. The crude was purified by crystallization from Et2O/ pentane to afford 10k as a white solid (58 mg, 41%). Mp: 104°C. 1H NMR (400 MHz, DMSO-d6) δ 12.45 (s, 1H, COOH), 7.88 (t, J = 5.6 Hz, 1H, NH), 7.51 (t, J = 8.0 Hz, 1H, H-5), 7.48 (t, J = 7.9 1H, H-11), 7.37 (m, 1H, R-Ph-4), 7.31 (m, 2H, R-Ph-3), 7.23 (m, 6H, H-10 H-2 H-6 H-8 R- Ph-2), 7.11 (dd, J = 7.7, 1.7 Hz, 1H, H-12), 3.78 (q, J = 7.1 Hz, 1H, CH), 3.31 (q, J = 7.7 Hz, 2H, R-H-1’), 2.80 (t, J = 7.4 Hz, 2H, R-H-2’), 1.41 (d, J = 7.1 Hz, 3H, CH3).13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 245.8 Hz, C-3), 154.6 (C-9), 151.1 (HNCOO), 143.9 (d, J = 7.7 Hz, C-7), 139.5 (R-Ph-1), 136.4 (C-1), 131.1 (d, J = 3.4 Hz, C-5), 129.9 (C-11), 129.1 (R-Ph-2), 128.8 (R-Ph-3), 126.6 (R-Ph-4), 126.2 (d, J = 13.0 Hz, C-4), 125.7 (C-10), 124.5 (d, J = 2.8 Hz, C-6), 122.3 (d, J = 2.6 Hz, C-8), 121.6 (C-12), 115.6 (d, J = 23.1 Hz, C-2), 44.5 (CH), 42.5 (R-C-1’), 35.6 (R-C-2’), 18.7 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.37 min. MS (ES) C24H22FNO4 requires 407, found 408 [M+H]+. HRMS C24H23NO4F [M+H]+: calculated 408.1611 measured 408.1626 Δppm 3.7.
4.1.7.12. (±)-2-[3-fluoro-4-[3-(3-phenylpropylcarbamoyloxy)phenyl]phenyl]propanoic acid (10l)
Compound 10l was prepared according to general procedure C using 9l (174 mg, 0.40 mmol). The crude was purified by preparative TLC (Cy/EtOAc, 5: 5) to afford 10l as a white solid (44 mg, 26%). Mp: 82-83 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (s, 1H, COOH), 7.87 (t, J = 5.53 Hz, 1H, NH), 7.51 (t, J = 8.04 Hz, 1H, H-5), 7.48 (t, J = 8.20 Hz, 1H, H-11), 7.38 (d, J = 7.2 Hz, 1H, R- Ph-4), 7.22 (m, 9H, H-2 H-6 H-8 H-10 H-12 R- Ph-2 R- Ph-3), 3.78 (q, J = 6.5 Hz, 1H, CH), 3.09 (q, J = 6.3 Hz, 2H, R-H-1’), 2.63 (t, J = 7.3 Hz, 2H, R-H-2’), 1.78 (p, J = 6.7 Hz, 2H, R-H-3’), 1.41 (d, J = 6.9 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.1 Hz, C-3), 154.7 (C-9), 151.6 (HNCOO), 143.9 (d, J = 8.1 Hz, C-7), 142.0 (R-Ph-1), 136.4 (C-1), 131.1 (d, J = 3.3 Hz, C-5), 129.9 (C-11), 128.8 (R-Ph-3), 128.7 (RPh-2), 126.2 (R-Ph-4), 125.7 (d, J = 3.5 Hz, C-4), 124.6 (C-10) 124.5 (C-6), 122.3 (C-8), 121.70 (C-12), 115.6 (d, J = 23.5 Hz, C-2), 44.5 (CH), 40.5 (R-C-1’), 32.8 (R-C-3’), 31.4 (R-C-2’), 18.7 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.46 min. MS (ES) C25H24FNO4 requires 421, found 422 [M+H]+ . HRMS C25H25NO4F [M+H]+: calculated 422.1768 measured 422.1776 Δppm 1.9.
4.1.7.13. (±)-2-[3-fluoro-4-[3-(4-phenylbutylcarbamoyloxy)phenyl]phenyl]propanoic acid (10m)
Compound 10m was prepared according to general procedure C using 9m (171 mg, 0.38 mmol). The crude was purified by preparative TLC (Cy/EtOAc, 5: 5) to afford 10m as a white solid (70 mg, 43%). Mp: 101-102°C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (s, 1H, COOH), 7.80 (t, J = 5.7 Hz, 1H, NH), 7.47 (m, 2H, H-5 H-8), 7.37 (d, J = 7.3 Hz, 1H, R- Ph-4), 7.19 (m, 9H, H-2 H-6 H-8 H-10 H-12 R- Ph-2 R- Ph-3), 3.78 (q, J = 7.1 Hz, 1H, CH), 3.10 (q, J = 6.6 Hz, 2H, R-H-1’), 2.60 (t, J = 7.5 Hz, 2H, R-H-4’), 1.60 (q, J = 7.9 Hz, 2H, R-H-3’), 1.50 (q, J = 7.2 Hz, 2H, R-H-2’), 1.41 (d, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.3 Hz, C-3), 154.7 (C-9), 151.6 (HNCOO), 143.9 (d, J = 7.5 Hz, C-7), 142.5 (R-Ph-1), 136.4 (C-1), 131.0 (d, J = 3.6 Hz, C-5), 129.9 (C-11), 128.7 (R-Ph-3), 128.6 (RPh-2), 126.1 (R-Ph-4), 125.7 (d, J = 2.4 Hz, C-4), 124.6 (C-10) 124.5 (C-6), 122.3 (d, J = 3.1 Hz, C-8), 121.6 (C-12), 115.6 (d, J = 23.2 Hz, C-2), 44.5 (CH), 40.7 (R-C-1’), 35.2 (R-C-4’), 29.3 (R-C-2’), 28.7 (R-C-3’), 18.7 (CH3). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.59 min. MS (ES) C26H26FNO4 requires 435, found 436 [M+H]+. HRMS C26H27NO4F [M+H]+: calculated 436.1924 measured 436.1936 Δppm 2.8.
4.1.7.14. (±)-2-[4-[3-(ethylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10n)
Compound 10n was prepared according to general procedure C using 8 (104 mg, 0.30 mmol). The crude was purified by crystallization from Et2O/ pentane to afford 10n as a white solid (37 mg, 37%). Mp: 93-94 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H, COOH), 7.79 (t, J = 5.5 Hz, 1H, NH), 7.51 (t, J = 8.3 Hz, 1H, H-5), 7.48 (t, J = 7.9 Hz, 1H, H-11), 7.39 (d, J = 7.5 Hz, 1H, H-10), 7.25 (m, 3H, H-2 H-6 H-8), 7.15 (d, J = 7.9 Hz, 1H, H-12), 3.79 (q, J = 7.1 Hz, 1H, CH), 3.12 (p, J = 7.1 Hz, 2H, R-H-1’), 1.42 (d, J = 7.1 Hz, 3H, CH3), 1.10 (t, J = 7.2 Hz, 3H, R-H-2’). 13C NMR (101 MHz, DMSO-d6) δ 175.3 (COOH), 159.2 (d, J = 245.9 Hz, C-3), 154.5 (C-9), 151.6 (HNCOO), 143.9 (d, J = 7.6 Hz, C-7), 136.4 (C-1), 131.1 (d, J = 3.6 Hz, C-5), 129.9 (C-11), 126.2 (d, J = 13.0 Hz, C-4), 125.7 (C-10), 124.5 (d, J = 3.0 Hz, C-6), 122.3 (d, J = 3.1 Hz, C-8), 121.7 (C-C-12), 115.6 (d, J = 23.4 Hz, C-2), 44.5 (CH), 35.7 (R-C-1’), 18.7 (CH3), 15.3 (R-C-2’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 1.95 min. MS (ES) C18H18FNO4 requires 331, found 332 [M+H]+. HRMS C18H19NO4F [M+H]+: calculated 332.1298 measured 332.1304 Δppm 1.8.
4.1.7.15. (±)-2-[3-fluoro-4-[3-(propylcarbamoyloxy)phenyl]phenyl]propanoic acid (10o)
Compound 10o was prepared according to general procedure C using 8 (87 mg, 0.24 mmol). The crude was purified by preparative TLC (Cy/EtOAc, 5: 5) to afford 10o as a white solid (57 mg, 68%). Mp: 113-114 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (s, 1H), 7.79 (t, J = 5.7 Hz, 1H), 7.50 (t, J = 8.3 Hz, 1H), 7.47 (t, J = 7.9 Hz, 1H), 7.37 (d, J = 6.9 Hz, 1H), 7.24 (ddt, J = 9.9, 3.9, 1.7 Hz, 3H), 7.14 (ddd, J = 8.2, 2.4, 1.1 Hz, 1H), 3.78 (q, J = 7.1Hz, 1H), 3.03 (td, J = 7.1, 5.9 Hz, 2H), 1.49 (h, J = 7.3 Hz, 2H), 1.41 (d, J = 7.2 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H).13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.7 (d, J = 246.5 Hz, C-3), 154.2 (C-9), 151.2 (HNCOO), 143.4 (d, J = 7.8 Hz, C-7), 135.9 (C-1), 130.6 (C-5), 129.4 (C-11), 125.7 (d, J = 12.9 Hz, C-4), 125.1 (C-10), 124.0 (C-6), 121.8 (C-8), 121.1 (C-12), 115.1 (d, J = 23.2 Hz, C-2), 44.0 (CH), 42.2 (R-C-1’), 22.4 (R-C-2’), 18.2 (CH3), 11.2 (R-C-3’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.10 min. MS (ES) C19H20FNO4 requires 345, found 346 [M+H]+. HRMS C19H21NO4F [M+H]+: calculated 346.1455 measured 346.1459 Δppm 1.2.
4.1.7.16. (±)-2-[4-[3-(butylcarbamoyloxy)phenyl]-3-fluoro-phenyl]propanoic acid (10p)
Compound 10p was prepared according to general procedure C using 9p (135 mg, 0.36 mmol). The crude was purified by crystallization from Cy/Et2O to afford 10p as a white solid (75 mg, 58%). Mp: 110-111 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H, COOH), 7.79 (t, J = 5.7 Hz, 1H, NH), 7.50 (t, J = 8.3 Hz, 1H, H-5), 7.47 (t, J = 7.9 Hz, 1H, H-11), 7.37 (d, J = 7.0 Hz, 1H, H-10), 7.23 (m, 3H, H-2 H-6 H-8), 7.13 (ddd, J = 8.1, 2.4, 1.0 Hz, 1H, H-12), 3.78 (q, J = 7.1 Hz, 1H, CH), 3.06 (q, J = 6.8 Hz, 2H, R-H-1’), 1.46 (p, J = 6.9 Hz, 2H, R-H-2’), 1.40 (d, J = 7.2 Hz, 3H, CH3), 1.32 (h, J = 7.1 Hz, 2H, R-H-3’), 0.89 (t, J = 7.3 Hz, 3H, R-H-4’). 13C NMR (101 MHz, DMSO-d6) δ 174.8 (COOH), 158.8 (d, J = 246.0 Hz, C-3), 154.2 (C-9), 151.2 (HNCOO), 143.4 (d, J = 8.0 Hz, C-7), 135.9 (C-1), 130.6 (d, J = 2.9 Hz, C-5), 129.4 (C-11), 125.7 (d, J = 12.8 Hz, C-4), 125.2 (C-10), 124.0 (C-6), 121.8 (C-8), 121.2 (C-12), 115.2 (d, J = 23.3 Hz, C-2), 44.1 (CH), 40.1 (R-C-1’), 31.3 (R-C-2’), 19.4 (R-C-3’), 18.2 (CH3), 13.6 (R-C-4’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.25 min. MS (ES) C20H22FNO4 requires 359, found 360 [M+H]+. HRMS C20H23NO4F [M+H]+ : calculated 360.1611 measured 360.1615 Δppm 1.1.
4.1.7.17. (±)-2-[3-fluoro-4-[3-(pentylcarbamoyloxy)phenyl]phenyl]propanoic acid (10q)
Compound 10q was prepared according to general procedure C using 9q (149 mg, 0.39 mmol). The crude was purified by crystallization from pentane/Et2O to afford 10q as a white solid (85 mg, 59%). Mp: 105-106 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.40 (s, 1H, COOH), 7.77 (t, J = 5.7 Hz, 1H, NH), 7.51 (t, J = 7.9 Hz, 1H, H-5), 7.48 (t, J = 7.7 Hz, 1H, H-11), 7.37 (d, J = 8.6 Hz, 1H, H-10), 7.23 (m, 3H, H-2 H-6 H-8), 7.14 (dd, J = 8.1, 2.2 Hz, 1H, H-12), 3.78 (q, J = 7.1 Hz, 1H, CH), 3.06 (q, J = 6.8 Hz, 2H, R-H-1’), 1.48 (p, J = 7.2 Hz, 2H, R-H-2’), 1.41 (d, J = 7.2 Hz, 3H, CH3), 1.29 (m, 4H, R-H-3’ R-H-4’), 0.88 (t, J = 6.9 Hz, 3H, R-H-5’). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.4 Hz, C-3), 154.6 (C-9), 151.7 (HNCOO), 143.9 (d, J = 7.6 Hz, C-7), 136.4 (C-1), 131.1 (d, J = 3.6 Hz, C-5), 129.9 (C-11), 126.2 (d, J = 13.2 Hz, C-4), 125.6 (C-10), 124.5 (d, J = 3.3 Hz, C-6), 122.3 (C-8), 121.6 (C-12), 115.6 (d, J = 23.4 Hz, C-2), 44.5 (CH), 40.9 (R-C-1’), 29.3 (R-C-2’), 28.9 (R-C-4’), 22.2 (R-C-3’), 18.7 (CH3), 14.38 (R-C-5’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.42 min. MS (ES) C21H24FNO4 requires 373, found 374 [M+H]+. HRMS C21H25NO4F [M+H]+: calculated 374.1768 measured 374.1778 Δppm 2.7.
4.1.7.18. (±)-2-[3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid (10r)
Compound 10r was prepared according to general procedure C using 9r (142 mg, 0.35 mmol). The crude was purified by crystallization from Et2O/ pentane to afford 10r as a white solid (41 mg, 30%). Mp: 102-103 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.29 (s, 1H, COOH), 7.79 (t, J = 5.7 Hz, 1H, NH), 7.51 (t, J = 8.1 Hz, 1H, H-5), 7.48 (t, J = 7.9 Hz, 1H, H-11), 7.39 (d, J = 7.7 Hz, 1H, H-10), 7.25 (m, 3H, H-2 H-6 H-8), 7.15 (dd, J = 8.7, 1.5 Hz, 1H, H-12), 3.79 (q, J = 7.1 Hz, 1H, CH), 3.07 (q, J = 6.8 Hz, 2H, R-H-1’), 1.47 (p, J = 6.3 Hz, 2H, R-H-2’), 1.42 (d, J = 7.1 Hz, 3H, CH3), 1.30 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.88 (t, J = 7.0 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.4 Hz, C-3), 154.6 (C-9), 151.7 (HNCOO), 143.9 (d, J = 8.0 Hz, C-7), 136.4 (C-1), 131.0 (d, J = 3.5 Hz, C-5), 129.9 (C-11), 126.2 (d, J = 13.1 Hz, C-4), 125.6 (C-10), 124.5 (d, J = 3.0 Hz, C-6), 122.3 (d, J = 2.6 Hz, C-8), 121.6 (C-12), 115.6 (d, J = 23.2 Hz, C-2), 44.5 (CH), 40.9 (R-C-1’), 31.4 (R-C-4’), 29.6 (R-C-2’), 26.3 (R-C-3’), 22.5 (R-C-5’), 18.7 (CH3), 14.3 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.60 min. MS (ES) C22H26FNO4 requires 387, found 388 [M+H]+. HRMS C22H27NO4F [M+H]+: calculated 388.1924 measured 388.1927 Δppm 0.8.
4.1.7.19. (±)-2-[3-fluoro-4-[3-(heptylcarbamoyloxy)phenyl]phenyl]propanoic acid (10s)
Compound 10s was prepared according to general procedure C using 9s (160 mg, 0.38 mmol). The crude was purified by crystallization from pentane/Et2O to afford 10s as a white solid (85 mg, 55%). Mp: 104-105 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (s, 1H, COOH), 7.77 (t, J = 5.7 Hz, 1H, NH), 7.50 (t, J = 8.0 Hz, H-5), 7.47 (t, J = 7.7 Hz, H-11), 7.37 (d, J = 7.7 Hz, 1H, H-10), 7.24 (m, 3H, H-2 H-6 H-8), 7.13 (dd, J = 8.0, 2.2 Hz, 1H, H-12), 3.78 (q, J = 7.1 Hz, 1H, CH), 3.06 (q, J = 6.8 Hz, 2H, R-H-1’), 1.47 (p, J = 7.0 Hz, 2H, R-H-2’), 1.41 (d, J = 7.1 Hz, 3H, CH3), 1.27 (m, 8H, R-H-3’ R-H-4’ R-H-5’ R-H-6’), 0.86 (t, J = 6.8 Hz, 3H, R-H-7’). 13C NMR (101 MHz, DMSO-d6) δ 174.7 (COOH), 158.7 (d, J = 246.5 Hz, C-3), 154.1 (C-9), 151.2 (HNCOO), 143.4 (d, J = 7.6 Hz, C-7), 135.9 (C-1), 130.6 (d, J = 3.6 Hz, C-5), 129.4 (C-11), 125.7 (d, J = 13.1 Hz, C-4), 125.2 (C-10), 124.0 (d, J = 3.2 Hz, C-6), 121.8 (C-8), 121.1 (C-12), 115.1 (d, J = 23.3 Hz, C-2), 44.0 (CH), 40.4 (R-C-1’), 31.2 (R-C-4’), 29.1 (R-C-2’), 28.3 (R-C-5’), 26.1 (R-C-3’), 22.0 (R-C-6’), 18.2 (CH3), 13.9 (R-C-7’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.70 min. MS (ES) C23H28FNO4 requires 401, found 402 [M+H]+ . HRMS C23H29NO4F [M+H]+: calculated 402.2081 measured 402.2096 Δppm 3.7.
4.1.7.20. (±)-2-[3-fluoro-4-[3-(octylcarbamoyloxy)phenyl]phenyl]propanoic acid (10t)
Compound 10t was prepared according to general procedure C using 9t (141 mg, 0.33 mmol). The crude was purified by crystallization from pentane/Et2O to afford 10t as a white solid (41 mg, 30%). Mp: 103-104 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.49 (s, 1H, COOH), 7.78 (t, J = 5.6 Hz, 1H, NH), 7.50 (t, J = 8.1 Hz, 1H, H-5), 7. 48 (t, J = 7.8 Hz, 1H, H-11), 7.39 (d, J = 7.3 Hz, 1H, H-10), 7.25 (m, 3H, H-2 H-6 H-8), 7.15 (d, J = 7.9 Hz, 1H, H-12), 3.79 (q, J = 7.1 Hz, 1H, Ch), 3.07 (q, J = 6.7 Hz, 2H, R-H-1’), 1.48 (p, J = 6.6, 2H, R-H-2’), 1.42 (d, J = 7.1 Hz, 3H, CH3), 1.28 (d, J = 6.2 Hz, 10H, R-H-3’ R-H-4’ R-H-5’ R-H-6’ R-H-7’), 0.87 (t, J = 6.6 Hz, 3H, R-H-8’). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.4 Hz, C-3), 154.6 (C-9), 151.7 (HNCOO), 143.9 (d, J = 8.0 Hz, C-7), 136.4 (C-1), 131.0 (d, J = 3.6 Hz, C-5), 129.9 (C-11), 126.23 (d, J = 13.1 Hz, C-4), 125.6 (C-10), 124.5 (d, J = 3.1 Hz, C-6), 122.3 (C-8), 121.6 (C-12), 115.6 (d, J = 23.3 Hz, C-2), 44.5 (CH), 40.9 (R-C-1’), 31.7 (R-C-6’), 29.6 (R-C-2’), 29.1 (R-C-4’), 29.1 (R-C-5’), 26.7 (R-C-3’), 22.5 (R-C-7’), 18.7 (CH3), 14.4 (R-C-8’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.86 min. MS (ES) C24H30FNO4 requires 415, found 416 [M+H]+. HRMS C24H31NO4F [M+H]+: calculated 416.2237 measured 416.2249 Δppm 2.9.
4.1.7.21. (±)-2-[3-fluoro-4-[2-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid (15a)
Compound 15a was prepared according to general procedure C using 14a (200 mg, 0.5 mmol). The crude was purified by preparative TLC (Cy/EtOAc, 5: 5) to afford 15a as a white oil (175 mg, 90%). Mp: 61-63 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.47 (s, 1H, COOH), 7.55 (t, J = 5.7 Hz, 1H, NH), 7.42 (td, J = 7.6, 1.9 Hz, 1H, H-10), 7.36 (dd, J = 7.7, 1.9 Hz, 1H, H-12), 7.28 (m, 2H, H-5 H-11), 7.17 (m, 3H, H-2 H-6 H-9), 3.76 (q, J = 7.1 Hz, 1H, CH), 2.92 (q, J = 6.6 Hz, 2H, R-H-1’), 1.40 (d, J = 7.1 Hz, 3H, CH3), 1.32 (p, J = 6.6 Hz,2H, R-H-2’), 1.25 (m, 6H, R H-3’ R-H-4’ R-H-5’), 0.86 (t, J = 6.8 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 174.8 (COOH), 158.9 (d, J = 246.2 Hz, C-3), 154.0 (C-8), 148.5 (HNCOO), 143.2 (d, J = 7.5 Hz, C-7), 131.3 (C-5), 131.0 (C-12), 128.9 (C-10), 128.1 (C-1), 125.0 (C-11), 123.4 (C-9), 123.3 (C-6), 123.1 (C-4), 114.5 (d, J = 22.9 Hz, C-2), 44.1 (CH), 40.2 (R-C-1’), 30.8 (R-C-4’), 28.9 (R-C-2’), 25.6 (R-C-3’), 22.0 (R-C-5’), 18.3 (CH3), 13.8 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.47 min. MS (ES) C22H26FNO4 requires 387, found 388 [M+H]+. HRMS C22H27NO4F [M+H]+: calculated 388.1924 measured 388.1945 Δppm 5.4.
4.1.7.22. (±)-2-[3-fluoro-4-[4-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid (15b)
Compound 15b was prepared according to general procedure C using 14b (146 mg, 036 mmol). The crude was purified by crystallization from Et2O/Cy to afford 15b as a white solid (65 mg, 46%). Mp: 136-137 °C. 1H NMR (400 MHz, DMSO-d6) δ12.48 (s, 1H, COOH), 7.78 (t, J =5.6, 1H, NH), 7.53 (d, J =7.5, 2H, H-8 H-12), 7.48 (t, J =8.3, 1H, H-5), 7.23 (m, 2H, H-2 H-6), 7.19 (d, J =8.6, 2H, H-9 H-11), 3.77 (q, J =7.1, 1H, CH), 3.06 (q, J =6.7, 2H, R-H-1’), 1.47 (p, J =6.7, 2H, R-H-2’), 1.41 (d, J =7.4, 3H, CH3), 1.29 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.88 (t, J =6.7, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ174.8 (COOH), 158.8 (d, J = 245.9 Hz, C-3), 154.1 (C-10), 150.7 (HNCOO), 143.0 (d, J = 8.0 Hz, C-7), 131.4 (C-1), 130.5 (C-5), 129.5 (C-8 C-12), 125.9 (d, J = 13.2 Hz, C-4), 124.0 (C-6), 121.8 (C-9 C-11), 115.1 (d, J = 23.3 Hz, C-2), 44.0 (CH), 40.4 (R-C-1’), 30.9 (R-C-4’), 29.1 (R-C-2’), 25.8 (R-C-3’), 22.0 (R-C-5’), 18.2 (CH3), 13.8 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.59 min. MS (ES) C22H26FNO4 requires 387, found 388 [M+H]+. HRMS C22H27NO4F [M+H]+: calculated 388.1924 measured 388.194 Δppm 4.1.
4.1.7.23. (±)-2-[3-fluoro-4-[3-(hexylcarbamoylamino)phenyl]phenyl]propanoic acid (15c)
Compound 15c was prepared according to general procedure C using 14c (117 mg, 0.29 mmol). The crude was purified by preparative TLC (DCM/MeOH, 9: 5) to afford 15c as a white solid (43 mg, 38%). Mp: 84-85 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.46 (s, 1H, COOH), 8.49 (s, 1H, NH’), 7.59 (m, 1H, H-8), 7.43 (t, J = 8.1 Hz, 1H, H-5), 7.39 (d, J = 8.8 Hz, 1H, H-10), 7.30 (t, J = 7.9 Hz, 1H, H-11), 7.22 (d, J = 2.5 Hz, 1H, H-6), 7.20 (m, 1H, H-2), 7.04 (d, J = 7.1 Hz, 1H, H-12), 6.14 (t, J = 5.6 Hz, 1H, NH), 3.76 (q, J = 7.1 Hz, 1H, CH), 3.07 (q, J = 6.6 Hz, 2H, R-H-1’), 1.45 (m, 2H, R-H-2’), 1.40 (d, J = 7.1 Hz, 3H, CH3), 1.29 (m, 6H, R-H-3’ R-H-4’ R-H-5), 0.87 (t, J = 6.7 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 175.3 (COOH), 159.2 (d, J = 245.8 Hz, C-3), 155.6 (HNCONH’), 143.47 (d, J = 8.0 Hz, C-7), 141.3 (C-9), 135.7 (C-1), 130.9 (d, J = 3.8 Hz, C-5), 129.3 (C-11), 127.3 (d, J = 13.2 Hz, C-4), 124.3 (C-10), 121.7 (C-12), 118.2 (C-6), 117.4 (C-8), 115.5 (d, J = 23.1 Hz), 44.5 (CH), 39.5 (R-C-1’), 31.4 (R-C-4’), 30.1 (R-C-2’), 26.5 (R-C-2’), 22.5 (R-C-3’), 18.7 (CH3), 14.4 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 117.2. UPLC/MS analysis: Rt 2.32 min. MS (ES) C22H27FN2O3 requires 386, found 387 [M+H]+. HRMS C22H28N2O3F [M+H]+: calculated 387.2084 measured 387.2091 Δppm 1.8.
4.1.7.24. (±)-2-[3-fluoro-4-[3-(hexoxycarbonylamino)phenyl]phenyl]propanoic acid (15d)
Compound 15d was prepared according to general procedure C using 14d (143 mg, 0.36 mmol). The crude was purified by preparative TLC (Cy/EtOAc, 5: 5) to afford 15d as a white solid (82 mg, 59%). Mp: 63-64 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.46 (s, 1H, COOH), 9.70 (s, 1H, NH), 7.66 (s, 1H, H-8), 7.49 (d, J = 8.3 Hz, 1H, H-10), 7.42 (t, J = 8.3 Hz, 1H, H-5), 7.36 (t, J = 7.9 Hz, 1H, H-11), 7.23 (m, 1H, H-6), 7.21 (m, 1H, H-2), 7.14 (d, J = 6.8 Hz, 1H, H-12), 4.08 (t, J = 6.6 Hz, 2H, R-H-1’), 3.77 (q, J = 7.1 Hz, 1H, CH), 1.62 (p, J = 6.7 Hz, 2H, R-H-2’), 1.40 (d, J = 7.1 Hz, 3H, CH3), 1.30 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.87 (t, J = 6.9 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 175.3 (COOH), 159.2 (d, J = 246.1 Hz, C-3), 154.1 (OCONH), 143.6 (d, J = 8.0 Hz, C-7), 139.9 (C-9), 135.8 (C-1), 130.9 (d, J = 3.7 Hz, C-5), 129.44 (C-11), 127.0 (d, J = 13.2 Hz, C-4), 124.4 (d, J = 3.1 Hz, C-6), 123.1 (C-12), 118.9 (C-8), 118.0 (C-10), 115.6 (d, J = 23.4 Hz, C-2), 64.7 (R-C-1’), 44.5 (CH), 31.3 (R-C-2’), 28.9 (R-C-4’), 25.5 (R-C-2’), 22.5 (R-C-3’), 18.7 (CH3), 14.3 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 117.2. UPLC/MS analysis: Rt 2.64 min. MS (ES) C22H26FNO4 requires 387, found 388 [M+H]+. HRMS C22H27NO4F [M+H]+: calculated 388.1924 measured 388.1934 Δppm 2.6.
4.1.7.25. 2-[3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]acetic acid (18b)
Compound 18b was prepared according to general procedure C using 18a (123 mg, 0.32 mmol). The crude was purified by crystallization from pentane/Et2O to afford 18b as a white solid (73 mg, 62%). Mp: 90-92 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.44 (s, 1H, COOH), 7.77 (t, J = 5.7 Hz, 1H, NH), 7.48 (t, J = 8.1 Hz, 1H, H-5), 7.47 (t, J = 8.0 Hz, 1H, H-11), 7.37 (dq, J = 7.8, 1.5 Hz, 1H, H-10), 7.23 (m, 2H, H-2 H-8), 7.20 (dd, J = 7.9, 1.7 Hz, 1H, H-6), 7.13 (ddd, J = 8.1, 2.4, 1.0 Hz, 1H, H-12), 3.66 (s, 2H, CH2), 3.06 (td, J = 7.1, 5.8 Hz, 2H, R-H-1’), 1.47 (p, J = 7.1 Hz, 2H, R-H-2’), 1.29 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.87 (t, J = 7.0 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 172.1 (COOH), 158.6 (d, J = 245.9 Hz, C-3), 154.2 (C-9), 151.2 (HNCOO), 137.2 (d, J = 8.4 Hz, C-7), 136.0 (C-1), 130.3 (d, J = 3.5 Hz, C-5), 129.4 (C-11), 126.0 (d, J = 3.1 Hz, C-6), 125.5 (d, J = 13.2 Hz, C-4), 125.2 (C-10), 121.8 (C-8), 121.1 (C-12), 117.1 (d, J = 23.2 Hz, C-2), 40.4 (CH), 39.8 (R-C-1’), 30.9 (R-C-4’), 29.1 (R-C-2’), 25.9 (R-C-3’), 22.0 (R-C-5’), 13.8 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 118.0. UPLC/MS analysis: Rt 2.43 min. MS (ES) C20H21FNO2 requires 373, found 374 [M+H]+. HRMS C21H25NO4F [M+H]+: calculated 374.1768 measured 374.1763 Δppm -1.3.
4.1.7.26. 3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]benzoic acid (21c)
Compound 21c was prepared according to general procedure C using 21b (1.27 g, 3.40 mmol). Mp: 180-181 °C. The crude was purified by column chromatography (DCM/MeOH, 99: 1) to afford 21b as a white solid (0.41 g, 34%). 1H NMR (400 MHz, DMSO-d6) δ 13.32 (s, 1H, COOH), 7.85 (dd, J = 8.0, 1.4 Hz, 1H, H-6), 7.80 (t, J = 5.7 Hz, 1H, NH), 7.76 (dd, J = 11.3, 1.2 Hz, 1H, H-2), 7.68 (t, J = 7.9 Hz, 1H, H-5), 7.51 (t, J = 7.9 Hz, 1H, H-11), 7.44 (dq, J = 7.7, 1.5 Hz, 1H, H-12), 7.32 (q, J = 1.7 Hz, 1H, H-8), 7.19 (ddd, J = 8.0, 2.4, 1.1 Hz, 1H, H-10), 3.06 (td, J = 7.1, 6.0 Hz, 2H, R-H-1’), 1.46 (p, J = 6.8 Hz, 2H, R-H-2’), 1.28 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.87 (t, J = 6.6 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 166.0 (COOH), 158.7 (d, J = 247.8 Hz, C-3), 154.2 (C-9), 151.3 (HNCOO), 135.2 (C-1), 132.3 (d, J = 6.6 Hz, C-7), 131.6 (d, J = 13.4 Hz, C-4), 131.1 (C-5), 129.6 (C-11), 125.7 (C-6), 125.4 (C-12), 122.1 (C-8), 122.0 (C-10), 116.7 (d, J = 24.3 Hz, C-2), 40.5 (R-C-1’), 30.9 (R-C-4’), 29.1 (R-C-2’), 25.9 (R-C-3’), 22.0 (R-C-5’), 13.9 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 116.4. UPLC/MS analysis: Rt 2.28 min. MS (ES) C20H22FNO4 requires 359, found 360 [M+H]+. HRMS C20H23NO4F [M+H]+: calculated 360.1611 measured 360.1617 Δppm 1.7.
4.1.7.27. (±)-2-[3-chloro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid (29a)
Compound 29a was prepared according to general procedure C using 28a (2.12 g, 5.07 mmol). The crude was purified by column chromatography (DCM/MeOH, 98: 2) to give 29a as a white solid (1.95 g, 98%). Mp: 48-59 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.49 (s, 1H, COOH), 7.76 (t, J = 5.6 Hz, 1H, NH), 7.48 (d, J = 1.4 Hz, 1H, H-2), 7.45 (t, J = 8.3 Hz, 1H, H-11), 7.39 (d, J = 7.9 Hz, 1H, H-5), 7.34 (dd, J = 8.0 Hz 1.4 Hz, 1H, H-6), 7.26 (d, J = 7.7 Hz, 1H, H-10), 7.14 (m, 2H, H-8 H-12), 3.78 (q, J = 7.1 Hz, 1H, CH), 3.05 (q, J = 6.7 Hz, 2H, R-H-1’), 1.46 (p, J = 7.3 Hz, 2H, R-H-2’), 1.41 (d, J = 7.1 Hz, 3H, CH3), 1.29 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.87 (t, J = 6.7 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 174.8 (COOH), 154.1 (C-9), 150.8 (NHCOO), 142.7 (C-7), 139.4 (C-1), 137.3 (C-3), 131.4 (C-5), 131.0 (C-4), 129.0 (C-11), 128.9 (C-2), 126.7 (C-6), 125.7 (C-10), 122.3 (C-12), 121.0 (C-8), 43.9 (CH), 40.4 (R-C-1’), 30.9 (RC-4’), 29.1 (R-C-2’), 25.9 (R-C-3’), 22.0 (R-C-5’), 18.3 (CH3), 13.9 (R-C-6’). UPLC/MS analysis: Rt 1.24 min. MS (ES) C22H26ClNO4 requires 403, found 404, 406 [M+H]+, 402, 404 [M-H]−. HRMS C22H27NO4Cl [M+H]+: calculated 404.1629; measured 404.1644 Δppm 3.7.
4.1.7.28. (±)-2-[4-[3-(hexylcarbamoyloxy)phenyl]-3-methyl-phenyl]propanoic acid (29b)
Compound 29b was prepared according to general procedure C using 28b (0.94 g, 2.36 mmol).The crude was purified by column chromatography (DCM/MeOH, 98: 2) to give 29b as a white solid (0.76 g, 84%). Mp: 89-90 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.31 (s, 1H, COOH), 7.75 (t, J = 5.7 Hz, 1H, NH), 7.43 (t, J = 7.9 Hz, 1H, H-11), 7.22 (s, 1H, H-2), 7.17 (m, 3H, H-5 H-6 H-10), 7.09 (ddd, J = 8.1, 2.4, 1.0 Hz, 1H, H-12), 7.05 (m, 1H, H-8), 3.69 (q, J = 7.1 Hz, 1H, CH), 3.06 (q, J = 6.8 Hz, 2H, R-H-1’), 2.24 (s, 3H, Ph-CH3), 1.47 (p, J = 7.3 Hz, 2H, R-H-2’), 1.40 (d, J = 7.1 Hz, 3H, CH3), 1.29 (m, 6H, H-3’ H-4’ H-5’), 0.88 (t, J = 6.8 Hz, 3H, H-6’). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 154.2 (C-9), 150.9 (HNCOO), 142.0 (C-7), 140.4 (C-1), 138.9 (C-3), 134.7 (C-4), 129.5 (C-5), 129.4 (C-2), 129.0 (C-11), 125.4 (C-6), 125.0 (C-10), 122.1 (C-8), 120.1 (C-12), 44.3 (CH), 40.4 (R-C-1’), 30.9 (R-C-4’), 29.1 (R-C-2’), 25.9 (R-C-3’), 22.0 (R-C-5’), 20.1 (Ph-CH3), 18.4 (CH3), 13.9 (R-C-6’). UPLC/MS analysis: Rt 2.70 min. MS (ES) C23H29NO4 requires 383, found 384 [M+H]+ , 382 [M-H]+. HRMS C23H30NO4 [M+H]+: calculated 384.2175; measured 384.2177 Δppm 0.5.
4.1.7.29. (±)-2-[4-[3-(hexylcarbamoyloxy)phenyl]-3-(trifluoromethyl)phenyl]propanoic acid (29c)
Compound 29c was prepared according to general procedure C using 28c (1.02 g, 2.26 mmol). The crude was purified by column chromatography (DCM/MeOH, 98: 2) to give 29c as a white solid (0.8 g, 81%). Mp: 95-97°C [dec]. 1H NMR (400 MHz, DMSO-d6) δ 12.55 (s, 1H, COOH), 7.75 (t, J = 5.7 Hz, 1H, NH), 7.73 (d, J = 1.7 Hz, 1H, H-2), 7.64 (dd, J = 8.0, 1.6 Hz, 1H, H-6), 7.43 (t, J = 7.9 Hz, 1H, H-11), 7.39 (d, J = 7.9 Hz, 1H, H-5), 7.15 (m, 2H, H-10 H-12), 7.02 (s, 1H, H-8), 3.91 (q, J = 7.1 Hz, 1H, CH), 3.05 (q, J = 6.9 Hz, 2H, R-H-1’), 1.47 (p, J = 6.9 Hz, 2H, R-H-2’), 1.44 (d, J = 7.3 Hz, 1H, CH3 ), 1.27 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.85 (t, J = 6.6 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 174.8 (COOH), 154.0 (C-9), 150.5 (HNCOO), 141.4 (C-7), 140.0 (C-4), 138.2 (C-1), 132.3 (C-5), 131.3 (C-6), 128.8 (C-11), 126.7 (q, J = 29.1 Hz, C-3), 125.2 (C-10), 125.1 (C-2), 124.0 (q, J = 274.2 Hz, CF3), 121.8 (C-8), 121.1 (C-12), 44.0 (CH), 40.4 (R-C-1’), 30.9 (R-C-4’), 29.1 (R-C-2’), 25.9 (R-C-3’), 22.0 (R-C-5’), 18.3 (CH3), 13.8 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 54.3. UPLC/MS analysis: Rt 2.63 min. MS (ES) C24H28F3NO3 requires 437, found 438 [M+H]+, 436 [M-H]−. HRMS C23H27NO4F3 [M+H]+: calculated 438.1892; measured 438.189 Δppm −0.5.
4.1.7.30. (±)-2-[4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid (29e)
Compound 29e was prepared according to general procedure C using 28e (123 mg, 0.32 mmol). The crude was purified by crystallization from Et2O/ pentane to afford 29e as a white solid (73 mg, 62%). Mp: 125-127 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.34 (s, 1H, COOH), 7.76 (t, J = 5.7 Hz, 1H, NH), 7.62 (d, J = 8.2 Hz, 2H, H-3 H-5), 7.42 (d, J = 8.2 Hz, 1H, H-12), 7.44 (t, J = 7.7 Hz, 1H, H-11), 7.38 (d, J = 8.2 Hz, 2H, H-2 H-6), 7.34 (s, 1H, H-8), 7.08 (d, J = 7.7 Hz, 1H, H-10), 3.73 (q, J = 7.1 Hz, 1H, CH), 3.06 (q, J = 6.8 Hz, 2H, R-H-1’), 1.47 (p, J = 6.9 Hz, 2H, R-H-2’), 1.39 (d, J = 7.1 Hz, 3H, CH3), 1.29 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.88 (t, J = 6.7 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 175.7 (COOH), 154.7 (C-9), 152.1 (HNCOO), 141.6 (C-7), 141.2 (C-4), 138.3 (C-1), 130.1 (C-11), 128.5 (C-2 C-6), 127.2 (C-3 C-5), 123.5 (C-12), 121.1 (C-10), 120.2 (C-8), 44.7 (CH), 40.9 (R-C-1’), 31.4 (R-C-4’), 29.6 (R-C-2’), 26.3 (R-C-3’), 22.5 (R-C-5’), 18.9 (CH3), 14.3 (R-C-6’). UPLC/MS analysis: Rt 2.30 min. MS (ES) C22H27NO4 requires 369, found 370 [M+H]+. HRMS C22H28NO4 [M+H]+: calculated 370.2018; measured 370.2027 Δppm 2.4.
4.1.7.31. (±)-2-[4-[3-(hexylcarbamoyloxy)phenyl]-3-nitro-phenyl]propanoic acid (29f)
Compound 29f was prepared according to general procedure C using 28f (0.26 g, 0.51 mmol). The crude was purified by column chromatography (DCM/MeOH, 97: 3) to give 29f as a cream colored solid (870 mg, 77%). Mp: 93-94 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.66 (s, 1H, COOH), 7.91 (d, J = 1.7 Hz, 1H, H-2), 7.79 (t, J = 5.7 Hz, 1H, NH), 7.69 (dd, J = 8.0, 1.7 Hz, 1H, H-6), 7.53 (d, J = 8.0 Hz, 1H, H-5), 7.44 (t, J = 7.9 Hz, 1H, H-11), 7.16 (m, 2H, H-10 H-12), 7.07 (t, J = 1.9 Hz, 1H, H-8), 3.92 (q, J = 7.1 Hz, 1H, CH), 3.05 (q, J = 6.8 Hz, 2H, R-H-1’), 1.46 (p, J = 7.1 Hz, 2H, R-H-2’), 1.45 (d, J = 7.2 Hz, 3H, CH3), 1.28 (m, 6H, R-H-3’ R-H-4’ RH-5’), 0.86 (t, J = 7.1 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 174.6 (COOH), 154.0 (C-9), 151.2 (HNCOO), 148.6 (C-3), 142.6 (C-7), 137.8 (C-1), 132.7 (C-4), 132.1 (C-6), 131.9 (C-5), 129.6 (C-11), 124.3 (C-12), 123.2 (C-2), 121.6 (C-10), 120.9 (C-8), 43.9 (CH), 40.5 (R-C-1’), 30.9 (R-C-4’), 29.1 (R-C-2’), 25.9 (R-C-3’), 22.0 (R-5’), 18.2 (CH3), 13.9 (R-C-6’). UPLC/MS analysis: Rt 2.44 min. MS (ES) C22H26N2O6 requires 414, found 415 [M+H]+, 369 [M-H – CO2]−. HRMS C22H27N2O6 [M+H]+: calculated 415.1869; measured 415.188 Δppm 2.6.
4.1.8. (±)-3-[2-fluoro-4-(2-hydroxy-1-methyl-ethyl)phenyl]phenol (11)
To a solution of ZrCl4 (291 mg, 1.25 mmol) in THF (5 mL), NaBH4 (189 mg, 5 mmol) was added at rt. Upon mixing the reagents, gas evolution is immediately observed and a cream colored suspension was obtained. A solution of 8 (274 mg, 1 mmol) in THF (1 mL) was added and the mixture was stirred at rt for 2 h. The reaction was carefully quenched by the addition of 2M HCl (5 mL) and then extracted with EtOAc. The solvent was removed under reduced pressure and the residue was purified by column chromatography (Cy/EtOAc, 7: 3) to afford 11 as a white solid (235 mg , 96%).
4.1.9. (±)-Methyl 2-[3-fluoro-4-[3-(hexoxycarbonylamino)phenyl]phenyl]propanoate (14d)
To a suspension of 13c (114 mg, 0.44 mmol) in toluene (10 mL), triphosgene was added (392 mg, 1.32 mmol) and the resulting mixture was refluxed for 15 h. n-hexanol (224 mg, 2.20 mmol) was added and stirring was continued at rt for further 15 h. The solvent was removed under reduced pressure and the residue was purified by column chromatography (Cy/EtOAc, 9:1) to afford 14d as a white solid (145 mg, 82%).
4.1.10. Methyl 2-(3-fluoro-4-iodo-phenyl)acetate (16b)
To a solution of 16a (1g, 3.57 mmol) in MeOH (54 mL), concentrated H2SO4 (0.1 mL) was added and the resulting solution was stirred at rt overnight. After solvent evaporation, the crude oil was diluted with Et2O (15 mL) and filtered through a pad of SiO2 to afford 16b as a yellow liquid (1.03 g, quant.).
4.1.11. (±)-2-(3-chloro-4-nitro-phenyl)propanoic acid (23a)
Step 1: To a solution of 22a (4.70 g, 27.0 mmol) and diethyl methylmalonate (4.13 mL, 25.0 mmol) in DMF (31 mL), NaOH (1.11 g, 28 mmol) was added. The mixture was stirred at rt for 15 h. The dark red solution was poured into ice, acidified with concentrated HCl (4 mL) and extracted with TBME. The organic solvent was washed with brine, dried over Na2SO4 and evaporated under reduced pressure to give orange oil (8.24 g) which was used for the next step without any further purification. Step 2: H2O (25 mL), AcOH (38 mL) and H2SO4 (13 mL) were added to the orange oil (8.24 g, 25 mmol) and the reaction mixture was refluxed for 24 h. AcOH was removed under reduced pressure, and the mixture was extracted with DCM. The organic layer was then extracted with a saturated aqueous Na2CO3 solution and the aqueous layer was acidified with 1N HCl, and extracted with DCM. The organic layer was dried over Na2SO4 and evaporated to give 23a as an orange oil (5.00 g, 87%).
4.1.12. (±)-Methyl 2-(3-chloro-4-nitro-phenyl)propanoate (24a)
23a (5.00 g, 21.78 mmol) was dissolved in MeOH (27 mL), H2SO4 (58 mL, 1.09 mmol) was added and the mixture was stirred for 15 h. The solvent was removed under reduced pressure and the residue taken up in TBME, activated carbon was added and then the mixture passed through an allumina pad. The solvent was removed under reduced pressure to give 24a as a yellow oil (4.57 g, 86%).
4.1.13. (±)-Methyl 2-(4-amino-3-chloro-phenyl)propanoate (25a)
Iron powder (4.19 g, 75 mmol) was added to a solution of 24a (4.57 g, 18.76 mmol) in MeOH/HCl (7:1, 40 mL). The mixture was refluxed for 2 h, then filtered through a pad of Celite. The solvent was removed under reduced pressure and taken up in H2O, the thick slurry was basified with K2CO3, EtOAc was added, filtered through a pad of Celite and the two phases separated. The organic layer was dried over Na2SO4 and evaporated to give 25a as an orange oil (2.57 g, 64%).
4.1.14 (±)-Methyl 2-(3-chloro-4-iodo-phenyl)propanoate (26a)
A solution of NaNO2 (0.91 g, 13.2 mmol) in H2O (2 mL) was added slowly to a solution of 25a (2.57 g, 12.03 mmol) in a mixture of 2N HCl (54 mL) and dioxane (24 mL) at 0 °C. After stirring for 15 min at 0 °C, NaI (1.98 g, 13.23 mmol) was added, and then the mixture was stirred for 15 h, after which Na2SO3 was added. The solution was extracted with TBME, dried over Na2SO4, passed through an alumina pad and evaporated. The residue was purified by column chromatography (Cy: EtOAc, 95: 5) to obtain 26a as a colorless oil (2.74 g, 70%).
4.1.15. (±)-2-(3-methyl-4-nitro-phenyl)propanoic acid (23b)
Step 1: To a solution of 22b (3.26 mL, 26.75 mmol) and diethyl methylmalonate (4.59 mL, 25 mmol) in DMF (31 mL), NaOH (1.11 g, 27.75 mmol) was added. The mixture was stirred at rt for 15 h. The dark red solution was poured into ice, acidified with concentrated HCl (10 mL) and extracted with Et2O. The organic solvent was washed with brine, dried over Na2SO4 and evaporated under reduced pressure to give yellow oil (7.73 g) which was used for the next step without any further purification. Step 2: H2O (25 mL), AcOH (41 mL) and H2SO4 (12 mL) were added to the oil (7.73 g, 25 mmol) and the mixture was refluxed for 24 h. AcOH was removed under reduced pressure and the product was extracted with DCM and washed with brine. The organic layer was treated with aqueous K2CO3, and the separated aqueous layer was acidified with concentrated HCl, extracted with DCM, washed with brine and dried over Na2SO4. After removal of the solvent 23b was obtained as brown clear oil (2.50 g, 48%) which was used for the next step without any further purification.
4.1.16. (±)-Methyl 2-(3-methyl-4-nitro-phenyl)propanoate (24b)
To a solution of 23b (2.5 g, 11.95 mmol) in MeOH (120 mL), H2SO4 (0.22 mL, 1.2 mmol) was added and the mixture was stirred at rt for 15 h. The solvent was removed under reduced pressure and the residue was dissolved in Et2O and passed through a pad of alumina. The solvent was evaporated to obtain 24b as a yellow oil (2.17 g, 81%).
4.1.17. (±)-Methyl 2-(4-amino-3-methyl-phenyl)propanoate (25b)
To a mixture of 24b (2.17 g, 9.72 mmol) and Pd/C (0.52 g, 0.49 mmol), HCO2NH4 (3.68 g, 58.33 mmol) was added and stirred for 1 h. The catalyst was filtered through a pad of Celite and the solvent evaporated. The residue was taken up in EtOAc, passed through a pad os SiO2 and evaporated to 25b as a yellow oil (1.85 g, 98%).
4.1.18. (±)-Methyl 2-(4-iodo-3-methyl-phenyl)propanoate (26b)
A solution of NaNO2 (0.71 g, 10.24 mmol) in H2O (2 mL) was added slowly to a solution of 25b (1.85 g, 9.57 mmol) in 2N HCl (43 mL) at 0 °C. After stirring for 30 min a solution of NaI (2.15 g, 14.36 mmol) was added dropwise and the mixture was allowed to reach rt and stirred for 2 h, then warmed to 60 °C for other 2 h. Na2SO3 was added and the product was extracted with Et2O, dried over Na2SO4 and evaporated. The residue was purified by column chromatography (Cy/EtOAc, 95: 5) to give 26b as clear oil (1.14 g, 39%).
4.1.18. (±)-2-[4-nitro-3-(trifluoromethyl)phenyl]propanoic acid (23c)
Step 1: To a solution of 22c (3.74 mL, 26.75 mmol) and diethyl methylmalonate (4.13 mL, 25 mmol) in DMF (30 mL), NaOH (1.11 g, 27.75 mmol) was added. The mixture was stirred at rt for 15 h. The mixture was stirred at rt for 15 h. The dark red solution was poured into ice, acidified with concentrated HCl (4 mL) and extracted with TBME. The organic solvent was washed with brine, dried over Na2SO4 and evaporated under reduced pressure to give orange oil (9.1 g) which was used for the next step without any further purification. Step 2: H2O (25 mL), AcOH (37 mL) and H2SO4 (12 mL) were added to the orange oil and the mixture was refluxed for 24 h. AcOH was removed under reduced pressure, and the mixture was extracted with DCM. The organic layer was then extracted with a saturated aqueous Na2CO3 solution and the aqueous layer was acidified with 1N HCl, and extracted with DCM. The organic layer was dried over Na2SO4 and evaporated to give 23c as an orange oil (5.59 g, 85%).
4.1.19. (±)-Methyl 2-[4-nitro-3-(trifluoromethyl)phenyl]propanoate (24c)
23c (5.59 g, 21.24 mmol) was dissolved in MeOH (28 mL), H2SO4 (58 mL, 1.06 mmol) was added and the mixture was stirred for 19 h. The solvent was removed under reduced pressure and the residue taken up in TBME, activated carbon was added and then the mixture passed through an allumina pad. The solvent was removed under reduced pressure to give 24c as an orange oil (5.70 g, 97%).
4.1.20. (±)-Methyl 2-[4-amino-3-(trifluoromethyl)phenyl]propanoate (25c)
To a mixture of 24c (5.59 g, 20.17 mmol) and Pd/C (1.07 g, 1.01 mmol) in MeOH (100 mL) HCO2NH4 (7.63 g, 121.00 mmol) was added and stirred at rt for 1 h. The catalyst was filtered through a pad of Celite and the solvent evaporated. The residue was taken up in EtOAc, passed through a pad os SiO2 and evaporated to 25c as a dark red oil (4.94 g, quant.).
4.1.21. (±)-Methyl 2-[4-iodo-3-(trifluoromethyl)phenyl]propanoate (26c)
A solution of NaNO2 (1.48 g, 21.38 mmol) in H2O (4 mL) was added slowly to a solution of 25c (4.94 g, 19.98 mmol) in 2N HCl (90 mL) at 0 °C. After stirring for 30 min a solution of NaI (4.49 g, 29.97 mmol) was added dropwise and the mixture was allowed to reach rt and stirred for 2 h, then warmed to 60 °C for other 2 h. Na2SO3 was added and the product was extracted with TBME, dried over Na2SO4 and evaporated. The residue was purified by column chromatography (Cy: EtOAc, 95: 5) to give 26c as clear oil (5.51 g, 77%).
4.1.22. (±)-2-[4-[3-(hexylcarbamoyloxy)phenyl]-3-hydroxy-phenyl]propanoic acid (29d)
To a solution of 28d (0.72 g, 1.49 mmol) in EtOH (29 mL), Pd/C (78 mg, 74 mmol) and cyclohexene (9 mL, 88 mmol) were added and the mixture was stirred at 80 °C for 2 h. The catalyst was filtered through a pad of Celite and the solvent was removed under reduced pressure. The residue oil was taken up in dioxane (15 mL), 2M HCl (15 mL) was added and the solution was stirred at 80 °C for 15 h. The solvent was removed under reduced pressure and the residue was purified by column chromatography (DCM/MeOH, 98: 2) to obtain 29d as a white solid (414 mg, 73%). Mp: 61-62 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.33 (s, 1H, COOH), 9.63 (s, 1H, OH), 7.74 (t, J = 5.7 Hz, 1H, NH), 7.38 (m, 2H, H-11 H-12), 7.25 (m, 1H, H-8), 7.22 (d, J = 7.9 Hz, 1H, H-5), 7.02 (m, 1H, H-10), 6.90 (d, J = 1.5 Hz, 1H, H-11), 6.81 (dd, J = 7.9, 1.5 Hz, 1H, H-2), 3.61 (q, J = 7.0 Hz, 1H, CH), 3.06 (q, J = 6.8 Hz, 2H, R-H-1’), 1.47 (p, J = 7.5 Hz, 2H, R-H-2’), 1.36 (d, J = 7.1 Hz, 3H, CH3), 1.28 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.88 (t, J = 6.8 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 154.3 (C-9), 154.2 (C-3), 150.8 (HNCOO), 141.9 (C-7), 139.4 (C-1), 130.20 (C-5), 128.6 (C-11), 125.4 (C-12), 125.3 (C-4), 122.0 (C-8), 119.8 (C-10), 118.7 (C-6), 114.9 (C-2), 44.4 (CH), 40.4 (R-C-1’), 30.9 (R-C-4’), 29.2 (R-C-2’), 25.9 (R-C-3’), 22.0 (R-C-5’), 18.4 (CH3), 13.9 (R-C-6’). UPLC/MS analysis: Rt 2.33 min. MS (ES) C22H27NO5 requires 385, found 386 [M+H]+, 384 [M-H]−. HRMS C22H28NO5 [M+H]+: calculated 386.1967; measured 386.1975 Δppm 2.1.
4.1.23. (±)-Methyl 2-(4-nitrophenyl)propanoate (24e)
To a solution of 23e (1.95 g, 10 mmol) in MeOH (20 mL), concentrated H2SO4 (0.1 mL) was added and the resulting solution was stirred overnight at rt. After solvent evaporation, the crude oil was diluted with Et2O (15 mL) and filtered through a pad of SiO2 to afford 24e as yellow oil (2.10 g, quant.)
4.1.24. (±)-Methyl 2-(4-aminophenyl)propanoate (25e)
To a solution of 24e (1.05 g, 5 mmol) in MeOH (20 mL) was added 10% Pd/C (0.37 g, 0.35 mmol) followed by the addition of HCO2NH4 (1.9 g, 30 mmol). The solution was stirred at rt for 1 h. The solution was filtered through a pad of Celite and the filtrate was concentrated under reduced pressure. The residue was dissolved in EtOAc and filtered through a pad of SiO2to afford 25e as an off-white solid (0.89 g, quant.).
4.1.25. (±)-Methyl 2-(4-iodophenyl)propanoate (26e)
A solution of NaNO2 (0.69g, 10 mmol) in H2O (1.5 mL) was added slowly to a solution of 25e (1.75g, 9.76 mmol) in 28 mL of 3N HCl at 0 °C. After stirring for 1 h at 0 °C, NaI (1.50 g, 10 mmol) was added. The resultant mixture was slowly warmed to rt for 5 min, and heated at 60 °C for 2 h. After cooling down to rt, the mixture was extracted with Et2O and the organic phase was then washed with a 1M solution of Na2SO3 (20 mL), dried over Na2SO4. After evaporation, the residue was dissolved in EtOAc (40 mL) and treated with activated carbon and filtered through a pad of Celite. The solvent was removed under reduced pressure and the orange oil was purified by column chromatography (Cy/EtOAc, 95:5) to give 26e as a clear oil (2.05 g, 72%).
4.1.26. (±)-Methyl 2-(4-amino-3-nitro-phenyl)propanoate (25f)
Step 1: A solution of 25e (3.58 g, 20 mmol) in Ac2O (100 mL) was heated at 130 °C for 1 h. The solution was poured into H2O, stirred for 3 h then evaporated. The residual solid was taken up in H2O and filtered under vaccum to obtain a yellow solid. This solid was dissolved in MeOH (100 mL) and 37% HCl (5 mL) was added. The solution was stirred for 2 h and the organic solvent was removed under reduced pressure. H2O was added and the precipitate was filtered under vacuum and washed with H2O to obtain methyl 2-(4-acetamidophenyl)propanoate as a cream colored solid (2.21 g, 50%). Step 2: (±)-Methyl 2-(4-acetamidophenyl)propanoate (2.21 g, 10 mmol) in Ac2O (10 mL) was cooled to 0 °C. HNO3 (1 mL, 14 mmol) was added and the mixture was stirred for 2 h. The yellow solution was poured in ice while stirring was continued. The aqueous layer was extracted with DCM, the organic layer was evaporated and the residue was taken up in DCM and washed with saturated aqueous NaHCO3 solution, dried over Na2SO4 and evaporated to give (±)-methyl 2-(4-acetamido-3-nitro-phenyl)propanoate as a dark orange oil (2.60 g, 98%). Step 3: To a solution of (±)-methyl 2-(4-acetamido-3-nitro-phenyl)propanoate (2.60 g, 9.77 mmol) in MeOH (98 mL) H2SO4 (10 mL, 183 mmol) was added and the mixture was stirred at reflux for 2 h. MeOH was evaporated under reduced pressure and the solution was carefully poured into a aqueous solution of Na2CO3 (2M, 120 mL), then extracted with EtOAc, dried over Na2SO4 and evaporated to give 25f as a dark orange oil (2.20 g, quant.) which was used in the next step without further purification.
4.1.27. (±)-Methyl 2-(4-iodo-3-nitro-phenyl)propanoate (26f)
A solution of NaNO2 (0.74 g, 10.79 mmol) in H2O (2 mL) was added slowly to a solution of 25f (2.20 g, 9.81 mmol) in a mixture of 2N HCl (44 mL) and dioxane (20 mL) at 0 °C. After stirring for 5 min at 0 °C, NaI (1.47 g, 9.81 mmol) was added and the reaction mixture was stirred for 30 min, after which Na2SO3 was added. The solution was extracted with TBME, dried over Na2SO4 and evaporated. The residue which was purified by column chromatography (Cy: EtOAc, 95 : 5) to obtain 26f as a yellow oil (1.00 g, 30%).
4.1.28. (±)-2-[3-amino-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid hydrochloride(29g)
To a solution of 29g (0.87 g, 2.10 mmol) in MeOH (21 mL), Pd/C (223 mg, 0.21 mmol), cyclohexene (5.32 mL, 52.48 mmol) were added and the solution was stirred at 80 °C for 2 h. The mixture was filtered through a pad of Celite and the solvent removed under reduced pressure to give a residue which was purified by column chromatography (DCM/MeOH, 96: 4) to obtain a glassy oil, which was dissolved in dioxane (10 mL) and concentrated HCl (1 mL) was added. The solvent was removed under reduced pressure and the residue oil was suspended in DCM and Et2O. The solid was filtered under vacuum to obtain 29g as an off-white solid (488 mg, 55 %). Mp: 180 °C [dec]. 1H NMR (400 MHz, DMSO-d6) δ 12.51 (s, 1H, COOH), 7.79 (t, J = 5.7 Hz, 1H, NH), 7.48 (t, J = 7.9 Hz, 1H, H-11), 7.35 (d, J = 7.8 Hz, 1H, H-12), 7.29 (d, J = 7.6 Hz, 2H, H-2 H-5), 7.22 (m, 2H, H-6 H-8), 7.16 (dd, J = 8.0, 1.9 Hz, 1H, H-10), 3.73 (q, J = 7.0 Hz, 1H, CH), 3.05 (q, J = 6.8 Hz, 2H, R-H-1’), 1.45 (p, J = 7.3 Hz, 2H, R-H-2’), 1.39 (d, J = 7.1 Hz, 3H, CH3), 1.29 (m, 6H, R-H-3’ R-H-.4’ R-H-5’), 0.87 (t, J = 6.7 Hz, 3H, R-H-6’), NH +3 not visible. 13C NMR (101 MHz, DMSO-d6) δ 174.9 (COOH), 154.2 (C-9), 151.4 (HNCOO), 145.1 (C-3), 142.1 (C-7), 138.1 (C-1), 131.3 (C-2), 129.7 (C-11), 125.5 (C-12), 124.9 (C-6), 122.1 (C-8), 121.2 (C-10), 120.8 (C-5), 44.2 (CH), 40.5 (R-C-1’), 31.0 (R-C-4’), 29.2 (R-C-2’), 25.9 (R-C-3’), 22.1 (R-C-5’), 18.3 (CH3), 13.9 (R-C-6’). UPLC/MS analysis: Rt 2.46 min. MS (ES) C22H28N2O4 requires 384, found 385 [M+H]+, 383 [M-H]−. HRMS C22H29N2O4 [M+H]+: calculated 385.2127; measured 385.2161 Δppm 8.8.
4.1.29. Chiral HPLC separation of 10r
10r (500 mg, 1.29 mmol) was subjected to chiral HPLC separation to afford the two enantiomers:
4.1.29.1. (−)-2-[3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid ((−)-10r)
First eluted enantiomer (15.2 min), 112 mg (45%). Mp: 99-100 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.29 (s, 1H, COOH), 7.79 (t, J = 5.7 Hz, 1H, NH), 7.51 (t, J = 8.1 Hz, 1H, H-5), 7.48 (t, J = 7.9 Hz, 1H, H-11), 7.39 (d, J = 7.7 Hz, 1H, H-10), 7.25 (m, 3H, H-2 H-6 H-8), 7.15 (dd, J = 8.7, 1.5 Hz, 1H, H-12), 3.79 (q, J = 7.1 Hz, 1H, CH), 3.07 (q, J = 6.8 Hz, 2H, R-H-1’), 1.47 (p, J = 6.3 Hz, 2H, R-H-2’), 1.42 (d, J = 7.1 Hz, 3H, CH3), 1.30 (m, 6H, R-H-3’ R-H-4’ R-H-5’), 0.88 (t, J = 7.0 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.4 Hz, C-3), 154.6 (C-9), 151.7 (HNCOO), 143.9 (d, J = 8.0 Hz, C-7), 136.4 (C-1), 131.0 (d, J = 3.5 Hz, C-5), 129.9 (C-11), 126.2 (d, J = 13.1 Hz, C-4), 125.6 (C-10), 124.5 (d, J = 3.0 Hz, C-6), 122.3 (d, J = 2.6 Hz, C-8), 121.6 (C-12), 115.6 (d, J = 23.2 Hz, C-2), 44.5 (CH), 40.9 (R-C-1’), 31.4 (R-C-4’), 29.6 (R-C-2’), 26.3 (R-C-3’), 22.5 (R-C-5’), 18.7 (CH3), 14.3 (R-C-6’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.58 min. MS (ES) C22H26FNO4 requires 387, found 388 [M+H]+. [α]20D -29° (c 1.0, CHCl3). >99.5% ee. HRMS C22H27NO4F [M+H]+: calculated 388.1924; measured 388.1919 Δppm -1.3.
4.1.29.2. (+)-2-[3-fluoro-4-[3-(hexylcarbamoyloxy)phenyl]phenyl]propanoic acid ((+)-10r)
Second eluted enantiomer (25.7 min), 172 mg (45%). Mp: 101-102 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.29 (s, 1H, COOH), 7.79 (t, J = 5.7 Hz, 1H, NH), 7.51 (t, J = 8.1 Hz, 1H, H-5), 7.48 (t, J = 7.9 Hz, 1H, H-11), 7.39 (d, J = 7.7 Hz, 1H, H-10), 7.25 (m, 3H, H-2 H-6 H-8), 7.15 (dd, J = 8.7, 1.5 Hz, 1H, H-12), 3.79 (q, J = 7.1 Hz, 1H, CH), 3.07 (q, J = 6.8 Hz, 2H, R-H-1’), 1.47 (p, J = 6.3 Hz, 2H, R-H-2’), 1.42 (d, J = 7.1 Hz, 3H, CH3), 1.30 (m, 6H, R-H-3’ R-H-4’ RH-5’), 0.88 (t, J = 7.0 Hz, 3H, R-H-6’). 13C NMR (101 MHz, DMSO-d6) δ 175.2 (COOH), 159.2 (d, J = 246.4 Hz, C-3), 154.6 (C-9), 151.7 (HNCOO), 143.9 (d, J = 8.0 Hz, C-7), 136.4 (C-1), 131.0 (d, J = 3.5 Hz, C-5), 129.9 (C-11), 126.2 (d, J = 13.1 Hz, C-4), 125.6 (C-10), 124.5 (d, J = 3.0 Hz, C-6), 122.3 (d, J = 2.6 Hz, C-8), 121.6 (C-12), 115.6 (d, J = 23.2 Hz, C-2), 44.5 (CH), 40.9 (R-C-1’), 31.4 (R-C-4’), 29.6 (R-C-2’), 26.3 (R-C-3’), 22.5 (R-C-5’), 18.7 (CH3), 14.3 (RC-6’). 19F NMR (564 MHz, DMSO-d6): δ 117.3. UPLC/MS analysis: Rt 2.59 min. MS (ES) C22H26FNO4 requires 387, found 388 [M+H]+. [α]20D +29° (c 1.0, CHCl3). >99.5% ee. HRMS C22H27NO4F [M+H]+: calculated 388.1924; measured 388.1927 Δppm 0.8.
4.2. Enzyme assays
Quantitative 1HNMR analyses of DMSO-d6 stock solutions of tested compounds are performed using PULCON method (PUlse Length based CONcentration determination, Bruker software, topspin 3.0). [68, 69]
4.2.1. In vitro rat FAAH radiometric assay
Rat FAAH was prepared from male Sprague Dawley rat brains, homogenized in a potter in 20 mM of Tris HCl pH 7.4, 0.32 M sucrose. The radiometric assay used to measure FAAH activity was performed in eppendorf tubes: 50 μg of total rat brain homogenate were pre-incubated in 445.5 μL of assay buffer (50mM Tris-HCl pH 7.4, 0.05% Fatty acid-free bovine serum albumin (BSA)) with 4.5 μL of inhibitor (at appropriate concentration in DMSO) or DMSO alone (to measure FAAH total activity) for 10 min at 37 °C. The blank (no activity control) was prepared using 445.5 μL of assay buffer and 4.5 μL of DMSO without the 50 μg of total rat brain homogenate.
After 10 min of pre-incubation with test compounds, the reaction was started by adding of 50 μL of substrate and incubating for 30 min at 37 °C. The substrate was prepared in assay buffer in order to achieve the final concentration of 1μM arachidonoyl ethanolamide (Cayman Chemical N. 90050) and 0.6 nM anandamide [ethanolamine-1-3H] (American Radiolabeled Chemicals Inc., ART. 0626, conc. 1 mCi/mL, S.A. 60 Ci/mmol). The reaction was stopped by adding cold 1:1 CHCl3/MeOH. After 10 min of centrifugation (845xg at 4 °C) 600 μL of aqueous phase were transferred into scintillation vials previously filled with 3 mL of scintillation fluid (Ultima GoldTM, Perkin Elmer Inc., Cat. 6013329). Radioactivity was measured by liquid scintillation counting (MicroBeta2 LumiJET Perkin Elmer Inc.).
4.2.3. In vitro COX assay
COX activity was measured using a commercial kit (COX Inhibitor Screening Assay Kit - Cayman Chemical N. 560131) which includes both ovine COX-1 and human recombinant COX-2 enzymes. Inhibitors were pre-incubated with either ovine COX-1 or human COX-2 in order to screen isozyme-specific inhibition. Differently than described in the kit protocol, the reaction was carried out in the presence of 5 M arachidonic acid while for the blank sample (no activity) the two enzymes were inactivated for 40 min at 100°C. It was then measured the amount of PGF2α produced by reduction with SnCl2 of COX- derived PGH2, via enzyme immunoassay (EIA) using a PG-specific antibody and competing with a PG-acetylcholinesterase conjugate.
Absorbance was measured at 412 nm with a Tecan Infinite M200 plate reader and data were processed according to manufacturer's instructions.
The median inhibitory concentrations (IC50) were determined by non-linear regression analysis of the Log [concentration]/response curves generated with mean replicate values using a four parameter Hill equation curve fitting with GraphPad Prism 5 (GraphPad Software Inc., CAUSA). IC50 values are means of ≥3 experiments performed in duplicate.
4.2.4. Ex vivo lipid analyses
All procedures performed were in accordance with the Ethical Guidelines of the International Association for the Study of Pain, Italian regulations on the protection of animals used for experimental and other scientific purposes (D.M. 116192), and European Economic Community regulations (O.J. of E.C. L 358/1 12/18/1986). Great care was taken to minimize suffering of the animals and to reduce the number of animals used. Mice were housed in groups of 5 in ventilated cages containing autoclaved cellulose paper as nesting material with free access to food and water. They were maintained under a 12 h light/dark cycle (lights on at 08:00 a.m.), at controlled temperature (21 ± 1 °C) and relative humidity (55 ± 10%). The animals were randomly divided in groups of 6. Behavioral testing was performed between 9:00 a.m. and 5:00 p.m. Scientists running the experiments were not aware of the treatment protocol at the time of the test (blind procedure). Mice were decapitated under anesthesia 1h after intravenous injection of (S)-(+)-10r) (1 mg/kg). Blood (0.3 mL) was collected through a left cardioventricular puncture with heparinized syringes and centrifuged at 2000 × g for 30 min to obtain plasma. OEA was extracted from plasma and measured by LC/MS as described. Briefly, 300 μL of plasma were centrifuged with cold acetone (1 mL) containing [2H4]-OEA (Cayman Chemical). Lipids were extracted with CHCl3 (2 vol), the organic phases were washed with water (1 vol), collected, dried under nitrogen and reconstituted in MeOH (0.2 mL). LC/MS analyses were conducted on a Xevo TQ LC-MS/MS system (Waters) equipped with a BEH C18 column (Waters, Milford MA), using a linear gradient of MeCN in water. Quantification was performed monitoring the MRM transitions. Analyte peak areas were compared with a standard calibration curve (1 nM to 10 μM). Tissue levels of TXA2 and 6-keto-PGF1α were determined using ELISA kits (ABcam, Cambridge, UK), following manufacturer's instructions.
Supplementary Material
Highlights.
Rational design - SAR exploration of the first class of potent multitarget inhibitors of FAAH and COX enzymes;
Focused SAR studies around 10r (ARN2508) identified novel leads 18b and 29a-c, e;
Stereochemical and pharmacological studies of 10r enantiomers are reported;
Animal studies indicate that (S)-(+)-10r strongly inhibits FAAH and COX activities in vivo.
Acknowledgement
The authors thank Dr Angelo Reggiani for helpful discussions, Ms Silvia Venzano and Mr Luca Goldoni for technical support and the National Institute on Drug Abuse (grant DA012413 to D.P.) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare the following competing financial interest: Daniele Piomelli, Rita Scarpelli, Marco Migliore, Damien Habrant are inventors on the patent application WO2014023643, filed by the University of California and Fondazione Istituto Italiano di Tecnologia, which protects novel compounds disclosed in this paper.
Appendix A. Supplementary material: Supplementary data related to this article including 1H, 13C NMR data and UPLC/MS analysis of all the intermediates for the preparation of tested compounds 9r, 10a-t, 12, 15a-d, 18b, 21a, c, 29a-g; experimental details for the absolute configurational assignment of compounds (−)-10r and (+)-10r (Scheme S1); in vitro pharmacology data of (S)-(+)-10r on a panel of >90 biologically relevant targets (Table S1); copies of 1H, 13C 19F NMR data and UPLC/ MS analysis of the tested compounds 9r, 10a-t, 12, 15a-d, 18b, 21a, c, 29a-g can be found at htpp:// XXXX.
REFERENCES
- 1.Steinmeyer J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. 2000;2:379–385. doi: 10.1186/ar116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fries JF. NSAID gastropathy: the second most deadly rheumatic disease? Epidemiology and risk appraisal. J Rheumatol Suppl. 1991;28:6–10. [PubMed] [Google Scholar]
- 3.Tamblyn R, Berkson L, Dauphinee WD, Gayton D, Grad R, Huang A, Isaac L, McLeod P, Snell L. Unnecessary prescribing of NSAIDs and the management of NSAID-related gastropathy in medical practice. Ann Intern Med. 1997;127:429–438. doi: 10.7326/0003-4819-127-6-199709150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology. 1997;112:109–117. doi: 10.1016/s0016-5085(97)70225-7. [DOI] [PubMed] [Google Scholar]
- 5.Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: new insights into an old problem. J Gastroenterol. 1997;32:127–133. doi: 10.1007/BF01213310. [DOI] [PubMed] [Google Scholar]
- 6.Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxygenase inhibition. J Med Chem. 2007;50:1425–1441. doi: 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]
- 7.Wallace JL. NSAID gastroenteropathy: past, present and future. Can J Gastroenterol. 1996;10:451–459. doi: 10.1155/1996/850710. [DOI] [PubMed] [Google Scholar]
- 8.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself? Physiol Rev. 2008;88:1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JL, Vong L. NSAID-induced gastrointestinal damage and the design of GI-sparing NSAIDs. Curr Opin Investig Drugs. 2008;9:1151–1156. [PubMed] [Google Scholar]
- 10.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell JA, Warner TD. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov. 2006;5:75–86. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Ding EL, Song Y. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: meta-analysis of randomized trials. JAMA. 2006;296:1619–1632. doi: 10.1001/jama.296.13.jrv60015. [DOI] [PubMed] [Google Scholar]
- 13.Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piscitelli F, Di Marzo V. “Redundancy” of endocannabinoid inactivation: new challenges and opportunities for pain control. ACS Chem Neurosci. 2012;3:356–363. doi: 10.1021/cn300015x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maione S, Costa B, Di Marzo V. Endocannabinoids: a unique opportunity to develop multitarget analgesics. Pain. 2013;154(Suppl 1):S87–93. doi: 10.1016/j.pain.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Wallace JL, Ignarro LJ, Fiorucci S. Potential cardioprotective actions of no-releasing aspirin. Nat Rev Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- 17.Lazzarato L, Donnola M, Rolando B, Marini E, Cena C, Coruzzi G, Guaita E, Morini G, Fruttero R, Gasco A, Biondi S, Ongini E. Searching for new NO-donor aspirin-like molecules: a new class of nitrooxy-acyl derivatives of salicylic acid. J Med Chem. 2008;51:1894–1903. doi: 10.1021/jm701104f. [DOI] [PubMed] [Google Scholar]
- 18.Ranatunge RR, Augustyniak M, Bandarage UK, Earl RA, Ellis JL, Garvey DS, Janero DR, Letts LG, Martino AM, Murty MG, Richardson SK, Schroeder JD, Shumway MJ, Tam SW, Trocha AM, Young DV. Synthesis and selective cyclooxygenase-2 inhibitory activity of a series of novel, nitric oxide donor-containing pyrazoles. J Med Chem. 2004;47:2180–2193. doi: 10.1021/jm030276s. [DOI] [PubMed] [Google Scholar]
- 19.Chegaev K, Lazzarato L, Tosco P, Cena C, Marini E, Rolando B, Carrupt PA, Fruttero R, Gasco A. NO-donor COX-2 inhibitors. New nitrooxy-substituted 1,5-diarylimidazoles endowed with COX-2 inhibitory and vasodilator properties. J Med Chem. 2007;50:1449–1457. doi: 10.1021/jm0607247. [DOI] [PubMed] [Google Scholar]
- 20.Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci. 2007;28:501–505. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 22.Wallace JL, Caliendo G, Santagada V, Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346) Br J Pharmacol. 2010;159:1236–1246. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 2003;38:645–659. doi: 10.1016/s0223-5234(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 24.Rao PN, Chen QH, Knaus EE. Synthesis and structure-activity relationship studies of 1,3-diarylprop-2-yn-1-ones: dual inhibitors of cyclooxygenases and lipoxygenases. J Med Chem. 2006;49:1668–1683. doi: 10.1021/jm0510474. [DOI] [PubMed] [Google Scholar]
- 25.Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, Wecksler AT, Hammock BD. Synthesis and structure-activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem. 2011;54:3037–3050. doi: 10.1021/jm2001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seierstad M, Breitenbucher JG. Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. J Med Chem. 2008;51:7327–7343. doi: 10.1021/jm800311k. [DOI] [PubMed] [Google Scholar]
- 27.Cocco MT, Congiu C, Onnis V, Morelli M, Cauli O. Synthesis of ibuprofen heterocyclic amides and investigation of their analgesic and toxicological properties. Eur J Med Chem. 2003;38:513–518. doi: 10.1016/s0223-5234(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 28.Holt S, Paylor B, Boldrup L, Alajakku K, Vandevoorde S, Sundstrom A, Cocco MT, Onnis V, Fowler CJ. Inhibition of fatty acid amide hydrolase, a key endocannabinoid metabolizing enzyme, by analogues of ibuprofen and indomethacin. Eur J Pharmacol. 2007;565:26–36. doi: 10.1016/j.ejphar.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 29.Duggan KC, Hermanson DJ, Musee J, Prusakiewicz JJ, Scheib JL, Carter BD, Banerjee S, Oates JA, Marnett LJ. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat Chem Biol. 2011;7:803–809. doi: 10.1038/nchembio.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Favia AD, Habrant D, Scarpelli R, Migliore M, Albani C, Bertozzi SM, Dionisi M, Tarozzo G, Piomelli D, Cavalli A, De Vivo M. Identification and Characterization of Carprofen as a Multitarget Fatty Acid Amide Hydrolase/Cyclooxygenase Inhibitor. Journal of Medicinal Chemistry. 2012;55:8807–8826. doi: 10.1021/jm3011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Windsor MA, Hermanson DJ, Kingsley PJ, Xu S, Crews BC, Ho W, Keenan CM, Banerjee S, Sharkey KA, Marnett LJ. Substrate-Selective Inhibition of Cyclooxygenase-2: Development and Evaluation of Achiral Profen Probes. ACS Med Chem Lett. 2012;3:759–763. doi: 10.1021/ml3001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fowler CJ, Bjorklund E, Lichtman AH, Naidu PS, Congiu C, Onnis V. Inhibitory properties of ibuprofen and its amide analogues towards the hydrolysis and cyclooxygenation of the endocannabinoid anandamide. J Enzyme Inhib Med Chem. 2013;28:172–182. doi: 10.3109/14756366.2011.643304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cipriano M, Bjorklund E, Wilson AA, Congiu C, Onnis V, Fowler CJ. Inhibition of fatty acid amide hydrolase and cyclooxygenase by the N-(3-methylpyridin-2-yl)amide derivatives of flurbiprofen and naproxen. Eur J Pharmacol. 2013;720:383–390. doi: 10.1016/j.ejphar.2013.09.065. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, Ferri GL, Sibaev A, Storr M, Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cencioni MT, Chiurchiu V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T- lymphocytes mainly via CB2 receptors. PLoS One. 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiurchiu V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G, Centonze D, Battistini L, Maccarrone M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol. 2013;73:626–636. doi: 10.1002/ana.23875. [DOI] [PubMed] [Google Scholar]
- 39.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 40.LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- 41.Suardiaz M, Estivill-Torrus G, Goicoechea C, Bilbao A, Rodriguez de Fonseca F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain. 2007;133:99–110. doi: 10.1016/j.pain.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Wilson DE. Role of prostaglandins in gastroduodenal mucosal protection. J Clin Gastroenterol. 1991;13(Suppl 1):S65–71. doi: 10.1097/00004836-199112001-00011. [DOI] [PubMed] [Google Scholar]
- 43.Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 44.Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J Pharmacol Exp Ther. 2009;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasso O, Bertorelli R, Bandiera T, Scarpelli R, Colombano G, Armirotti A, Moreno-Sanz G, Reggiani A, Piomelli D. Peripheral FAAH inhibition causes profound antinociception and protects against indomethacin-induced gastric lesions. Pharmacol Res. 2012;65:553–563. doi: 10.1016/j.phrs.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grim TW, Ghosh S, Hsu KL, Cravatt BF, Kinsey SG, Lichtman AH. Combined inhibition of FAAH and COX produces enhanced anti-allodynic effects in mouse neuropathic and inflammatory pain models. Pharmacol Biochem Behav. 2014;124:405–411. doi: 10.1016/j.pbb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2008;10:R43. doi: 10.1186/ar2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Sabatino A, Battista N, Biancheri P, Rapino C, Rovedatti L, Astarita G, Vanoli A, Dainese E, Guerci M, Piomelli D, Pender SL, MacDonald TT, Maccarrone M, Corazza GR. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol. 2011;4:574–583. doi: 10.1038/mi.2011.18. [DOI] [PubMed] [Google Scholar]
- 49.Suarez J, Romero-Zerbo Y, Marquez L, Rivera P, Iglesias M, Bermudez-Silva FJ, Andreu M, Rodriguez de Fonseca F. Ulcerative colitis impairs the acylethanolamide-based anti- inflammatory system reversal by 5-aminosalicylic acid and glucocorticoids. PLoS One. 2012;7:e37729. doi: 10.1371/journal.pone.0037729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 51.Sasso O, Migliore M, Habrant D, Armirotti A, Albani C, Summa M, Moreno-Sanz G, Scarpelli R, Piomelli D. Multitarget fatty acid amide hydrolase/cyclooxygenase blockade suppresses intestinal inflammation and protects against nonsteroidal anti-inflammatory drug- dependent gastrointestinal damage. FASEB J. 2015;29:2616–2627. doi: 10.1096/fj.15-270637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 53.Tarzia G, Duranti A, Tontini A, Piersanti G, Mor M, Rivara S, Plazzi PV, Park C, Kathuria S, Piomelli D. Design, synthesis, and structure-activity relationships of alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase inhibitors. J Med Chem. 2003;46:2352–2360. doi: 10.1021/jm021119g. [DOI] [PubMed] [Google Scholar]
- 54.Mor M, Rivara S, Lodola A, Plazzi PV, Tarzia G, Duranti A, Tontini A, Piersanti G, Kathuria S, Piomelli D. Cyclohexylcarbamic acid 3′- or 4′-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure-activity relationships, and molecular modeling studies. J Med Chem. 2004;47:4998–5008. doi: 10.1021/jm031140x. [DOI] [PubMed] [Google Scholar]
- 55.Tarzia G, Duranti A, Gatti G, Piersanti G, Tontini A, Rivara S, Lodola A, Plazzi PV, Mor M, Kathuria S, Piomelli D. Synthesis and structure-activity relationships of FAAH inhibitors: cyclohexylcarbamic acid biphenyl esters with chemical modulation at the proximal phenyl ring. ChemMedChem. 2006;1:130–139. doi: 10.1002/cmdc.200500017. [DOI] [PubMed] [Google Scholar]
- 56.Mor M, Lodola A, Rivara S, Vacondio F, Duranti A, Tontini A, Sanchini S, Piersanti G, Clapper JR, King AR, Tarzia G, Piomelli D. Synthesis and quantitative structure- activity relationship of fatty acid amide hydrolase inhibitors: modulation at the N-portion of biphenyl-3-yl alkylcarbamates. J Med Chem. 2008;51:3487–3498. doi: 10.1021/jm701631z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mileni M, Kamtekar S, Wood DC, Benson TE, Cravatt BF, Stevens RC. Crystal structure of fatty acid amide hydrolase bound to the carbamate inhibitor URB597: discovery of a deacylating water molecule and insight into enzyme inactivation. J Mol Biol. 2010;400:743–754. doi: 10.1016/j.jmb.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 60.Selinsky BS, Gupta K, Sharkey CT, Loll PJ. Structural analysis of NSAID binding by prostaglandin H2 synthase: time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry. 2001;40:5172–5180. doi: 10.1021/bi010045s. [DOI] [PubMed] [Google Scholar]
- 61.Bayly CI, Black WC, Leger S, Ouimet N, Ouellet M, Percival MD. Structure-based design of COX-2 selectivity into flurbiprofen. Bioorg Med Chem Lett. 1999;9:307–312. doi: 10.1016/s0960-894x(98)00717-3. [DOI] [PubMed] [Google Scholar]
- 62.Lu G, Franzén R, Yu XJ, Xu YJ. Synthesis of Flurbiprofen via Suzuki Reaction Catalyzed by Palladium Charcoal in Water. Chinese Chemical Letters. 2006;17:461–464. [Google Scholar]
- 63.Del Zotto A, Amoroso F, Baratta W, Rigo P. Very Fast Suzuki-Miyaura Reaction Catalyzed by Pd(OAc)2 under Aerobic Conditions at Room Temperature in EGME/H2O. European Journal of Organic Chemistry. 2009;2009:110–116. [Google Scholar]
- 64.Narasimhan S, Balakumar R. Zirconium Borohydride - a Versatile Reducing Agent for the Reduction of Electrophilic and Nucleophilic Substrates. Synthetic Communications. 2000;30:4387–4395. [Google Scholar]
- 65.Bartolini WC, Brian M, Chen Barbara, Chien Yueh-Tyng, Currie Mark G., Milne Todd G., Pearson JJZ, Philip James, Talley Craig. Cyclooxygenase 2 (COX-2) and fatty acid amide hydrolase (FAAH) inhibitors for therapeutic use. :WO2005002525. [Google Scholar]
- 66.Malkowski MG, Ginell SL, Smith WL, Garavito RM. The productive conformation of arachidonic acid bound to prostaglandin synthase. Science. 2000;289:1933–1937. doi: 10.1126/science.289.5486.1933. [DOI] [PubMed] [Google Scholar]
- 67.Kiefer JR, Pawlitz JL, Moreland KT, Stegeman RA, Hood WF, Gierse JK, Stevens AM, Goodwin DC, Rowlinson SW, Marnett LJ, Stallings WC, Kurumbail RG. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature. 2000;405:97–101. doi: 10.1038/35011103. [DOI] [PubMed] [Google Scholar]
- 68.Wider G, Dreier L. Measuring protein concentrations by NMR spectroscopy. J Am Chem Soc. 2006;128:2571–2576. doi: 10.1021/ja055336t. [DOI] [PubMed] [Google Scholar]
- 69.Burton IW, Quilliam MA, Walter JA. Quantitative 1H NMR with external standards: use in preparation of calibration solutions for algal toxins and other natural products. Anal Chem. 2005;77:3123–3131. doi: 10.1021/ac048385h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.