Abstract
Developing physiologically-based pharmacokinetic (PBPK) models for chemicals can be resource-intensive, as neither chemical-specific parameters nor in vivo pharmacokinetic data are easily available for model construction. Previously developed, well-parameterized, and thoroughly-vetted models can be a great resource for the construction of models pertaining to new chemicals. A PBPK knowledgebase was compiled and developed from existing PBPK-related articles and used to develop new models. From 2,039 PBPK-related articles published between 1977 and 2013, 307 unique chemicals were identified for use as the basis of our knowledgebase. Keywords related to species, gender, developmental stages, and organs were analyzed from the articles within the PBPK knowledgebase. A correlation matrix of the 307 chemicals in the PBPK knowledgebase was calculated based on pharmacokinetic-relevant molecular descriptors. Chemicals in the PBPK knowledgebase were ranked based on their correlation toward ethylbenzene and gefitinib. Next, multiple chemicals were selected to represent exact matches, close analogues, or non-analogues of the target case study chemicals. Parameters, equations, or experimental data relevant to existing models for these chemicals and their analogues were used to construct new models, and model predictions were compared to observed values. This compiled knowledgebase provides a chemical structure-based approach for identifying PBPK models relevant to other chemical entities. Using suitable correlation metrics, we demonstrated that models of chemical analogues in the PBPK knowledgebase can guide the construction of PBPK models for other chemicals.
Author Summary
Physiologically-based pharmacokinetic (PBPK) models are complex kinetic models describing the absorption, distribution, metabolism and excretion of chemicals in humans or animals in vivo. They can be utilized in many applications, such as dosimetry testing, toxicological investigations, and chemical risk assessment. De novo construction of PBPK models can be very challenging when chemical data are limited. Previously developed PBPK models from structurally similar chemicals can provide valuable insight in the construction of a new model. We compiled a PBPK knowledgebase that contains the chemical space covered by existing PBPK models. This knowledgebase indexes PBPK publications with chemical names, animal species, routes of administration, and model compartments. The knowledgebase aids new PBPK model generation by providing a structure-based approach to identify literatures related to provisional nearest-neighbor chemicals. Such approaches can complement efforts to develop de novo PBPK models that might act as supporting computational tools in modern risk assessment.
Introduction
Developing physiologically-based pharmacokinetic (PBPK) models for chemicals can be resource-intensive, as the formulation of new PBPK models is dictated by multiple factors. These factors include intended use for the model, target organism, target subpopulation (e.g., life stage, gender), endpoint of interest (which can affect which organs are modeled individually and which are lumped together), routes of exposure, dosing regimen or exposure scenarios, and availability of relevant data for model calibration or evaluation. Among these factors, collecting chemical-specific data for parameterizing and calibrating a PBPK model is often the most resource-intensive task. For example, tissue-blood partition coefficients, or data that can be used to estimate these coefficients (e.g., log KOW), can be missing. Several in silico models are available for predicting tissue-specific partition coefficients based on chemical structure or properties [1–7]. On the other hand, computational tools for predicting a chemical’s metabolic pathway and rates of metabolism have been more difficult to develop due to widely variable interspecies (e.g., rat vs. human), intraspecies/interindividual (i.e., fast vs. slow metabolizers), and intra-individual (i.e., liver vs. kidney) variation in metabolic activities [8]. Many environmental chemicals and most pharmaceuticals are metabolized in the body, and metabolites often exhibit drastically different pharmacokinetic properties and toxic effects than the parent compound or alternative metabolites of the parent compound [9]. While progress has been made toward increasing the accuracy of in silico predictions of metabolic parameters [10–12], experimental data is always preferable, but much more costly to obtain. In addition to a dearth of chemical-specific data, time course measurements of tissue concentrations, along with dose-response measurements that reflect the disposition of a chemical and its metabolites inside the body, often do not exist, further impeding model validation.
Chemical-specific parameters or in vivo pharmacokinetic data are unavailable for the vast majority of chemicals in commerce. Previously published PBPK articles are great resources to search for well-parameterized and thoroughly-vetted models that can inspire the structural design, code implementation, parameter optimization and experimental validation of models for additional chemicals. Incremental improvements, adaptations or modifications of existing models are common strategies used in the PBPK field to extrapolate chemical effects from laboratory animals to humans [13–17], to incorporate additional exposure routes or life stages [18–22], to link to pharmacodynamic endpoints [23–27], or to build new models for similar chemicals [28–33].
While adapting a model to use for a different chemical has been demonstrated previously [4,34–41], the actual process of selecting the most suitable published PBPK model for use as a starting template is not trivial. One strategy is to identify existing PBPK models that describe chemical analogues of the chemical entity of interest. This approach also works when adapting only a portion of the model (i.e., its compartmental structure) for chemicals that are similar to previously modeled chemicals [42–44]. A simple, yet efficient way to identify analogous chemicals is by conducting a similarity search in a comprehensive knowledgebase. Many online tools, such as PubChem (https://pubchem.ncbi.nlm.nih.gov/) and ChemSpider (http://www.chemspider.com/) provide similarity-searching capabilities on generic sets of chemicals, but currently no repository exists specifically for PBPK models. Thus, the objective of this study is to compile a knowledgebase that contains PBPK modeling-related literature annotated with respective chemical structures along with several easily accessible molecular descriptors for these chemicals. These molecular descriptors can then be used to build a correlation matrix for each unique chemical in the knowledgebase. The knowledgebase can be queried by inputting the structure of the chemical of interest so that existing PBPK-related literature containing that chemical’s close analogues might be found. Illustration of this approach involved two case studies. In the first, the PBPK knowledgebase and correlation matrix were applied in the development of a new PBPK model for ethylbenzene using parameter values from six chemicals. These new models were then evaluated by comparing model-simulated blood concentrations of ethylbenzene against measured literature values. In the second case study, a published model of gefitinib was used to predict blood concentrations of its close-analogues and non-analogues categorized using the PBPK knowledgebase and correlation matrix. In addition to enhancing the efficiency of analogue-based PBPK model construction for additional chemicals, the power of the PBPK knowledgebase lies in its compilation of a wealth of information related to these PBPK chemicals, such as time course tissue concentration data, dose-response data, the authors’ assumptions about the model, limitations and applications of the model, and cited material. The PBPK knowledgebase directs users to published knowledge describing a specific chemical in order to aid in the development of new PBPK models for additional chemicals of interest.
Methods
Development of the PBPK knowledgebase
A compilation of all Supplementary Tables from the current study were summarized in a separate web repository in csv format (https://sites.google.com/site/pbpkknowledgebase/supplementary-materials). An open-source web interface is currently under development to provide intuitive navigation to data of interest for users.
Creation of an abstract-based PBPK corpus
An abstract-based PBPK corpus was created to provide a comprehensive composition of PBPK-related literature using PubMed (http://www.ncbi.nlm.nih.gov/pubmed). Query parameters included: “pbpk OR (“physiologically based” AND (pharmacokinetic OR toxicokinetic))”.
Additional search filters included “Abstract/title only.” No publication date boundaries were set for the query. Search results returned articles that were available only as early as 1977. All search results were saved and exported as a text file (S1 Table).
Extraction of chemical names from the abstract corpus and formation of the PBPK knowledgebase
The PBPK abstract corpus (as a text file) was loaded into Google sites to be processed through www.chemicalize.org (developed by ChemAxon), which is a public web resource that uses chemical named-entity recognition (NER) and a chemical taxonomy mark-up utility to identify unique chemical structures from text. The entire corpus was subdivided into smaller sections (~400 abstracts per set) to accommodate the processing capability of chemicalize.org. The marked-up page source was copied into Microsoft Excel 2007, parsed, and filtered so that the only entries remaining were chemical names and PubMed manuscript ID (PMID) (a unique database-designated index for cataloging purposes). This process identified 795 abstracts containing specific chemical names; results are summarized elsewhere (S2 Table). Because many chemicals have more than one abstract associated with each chemical name, the CAS registry number and SMILES string for these chemicals were obtained from other databases (e.g., ACToR [http://actor.epa.gov/actor/faces/ACToRHome.jsp), DSSTox [http://www.epa.gov/ncct/dsstox/], and ChemSpider [http://www.chemspider.com/]). Duplicates and synonyms were removed based on the CAS registry number and SMILES strings. For quality control purposes, two authors manually curated the chemical list to ensure that the knowledgebase contains only specific chemical entities (e.g., “ethyl” was excluded) and that PBPK models exist for these chemicals (e.g., existing studies measuring kinetic data for a specific chemical that could be used to build a PBPK model). After the manual curation, 307 unique chemicals remained. Their chemical names, CAS registry numbers and SMILES strings are provided elsewhere (S3 Table). The 795 abstracts (S2 Table) and corresponding 307 unique chemicals (S3 Table) are referred to as the “PBPK knowledgebase” throughout this article.
Mining the knowledgebase for PBPK-related terms and binary-vector determination
The abstracts in the PBPK knowledgebase were analyzed in order to identify the presence or absence of PBPK-associated word-stems. The purpose of this analysis was to improve our knowledge of the type of PBPK model information that could be expected from a publication. The PBPK-associated word-stems selected for our analyses were as follows: Species included “rat, rats, mouse, mice, human, pig, cow, goat, guinea pig, hamster, marmoset, monkey, rabbit, rhesus, rodent, sheep, bird, chicken, fish, pony, swine, turkey, and whale.” Life stages included “adult, pregnant, children, lactating, fetus, infant, dam, neonate, pediatric, pup, child, fetal, neonatal, and maternal.” Gender included “female, male, man, woman, men, and women.” Compartmental organs included “cutaneous, venous, arterial, carcass, body, fin, skin, lungs, heart, adipose, fat, brain, kidney, liver, bone, placenta, testes, ovary, breast, hepatic, blood, urine, plasma, plasma, feces, fecal, renal, milk, and hair.” Mining for these terms in each chemical name-containing abstract was performed using the open-source statistical program R (R Foundation for Statistical Computing, Vienna, Austria) to create a presence (1) or absence (0) vector (summarized in S2 Table).
Calculating physicochemical descriptors for compounds in the corpus
Absorption, distribution, metabolism and elimination (ADME) of chemicals are largely governed by their physicochemical properties [2,3,45–48]. For each of the chemicals identified in the PBPK abstract corpus, eight easily obtainable 2D physicochemical molecular descriptors were calculated using the proprietary software Molecular Operating Environment (MOE) (Chemical Computing Group Inc., Montreal, QC, Canada). These descriptors include molecular weight (MW), hydrogen bond acceptor count (hba), hydrogen bond donor count (hbd), number of rotatable bonds (nRotB), polar surface area or topological polar surface area (PSA), octanol:water partition coefficient (logP), log transformation of solubility (logS) and area of van der Waals surface (vdw_area). Descriptor values are summarized in S3 Table.
These descriptors are commonly accepted by the research community as correlated with chemicals’ pharmacokinetic properties. MW, hba, PSA, logP and logS have been associated with human intestinal absorption [49–51]. MW hba, hbd, nRotB and PSA can be used to predict clearance and volume of distribution [52]. MW, logP, hba, hbd, PSA, nRotB and logS have been associated with percent binding to plasma and liver microsomal proteins [53,54]. MW, hba, hbd, PSA, logP were included in the in silico identification of cytochrome P450 isoform-specific substrates [55,56].
Because other studies have shown that increasing the number of descriptors does not necessarily increase the predictive power from descriptor to PK properties [47,57], and to limit descriptors to those that are easily accessible to the public, no additional descriptors were calculated for this study.
Normalizing descriptors and calculating correlation coefficients
In this study, the similarity between chemicals was calculated as correlation coefficients based on the eight descriptors described above. Since the scientific community lacks consensus on the weight of importance for each descriptor toward a chemical’s pharmacokinetic properties, each descriptor was considered to contribute equally to the calculation of correlation coefficients. Six of the eight descriptors, hba, hbd, nRotB, PSA, vdw_area and MW, have values 0 or above and are positively skewed to the right. Thus, a log transformation was conducted to normalize these descriptors. The remaining two descriptors, logP and logS, exist in their transformed states. All the log-transformed descriptors were converted to standard normal distribution ~N(0,1), based on Eq 1.
| (1) |
Where is the normalized value of the kth descriptor for chemical i, X_k_i is the value of the log-transformed kth descriptor for chemical i, and μ_k and σ_k are the respective mean and standard deviation values of the log-transformed kth descriptor for all chemicals. Normalized molecular descriptors for all chemicals in the PBPK knowledgebase are summarized in S4 Table.
A correlation coefficient was then calculated based on the normalized molecular descriptors, as shown in Eq 2.
| (2) |
Where Cij represent the correlation coefficient between chemicals i and j in the knowledgebase. XST_k_i and XST_k_j represent the normalized kth descriptor of chemical i and j, respectively. The pairwise correlation coefficients matrix for each chemical in the PBPK knowledgebase is summarized elsewhere (S5A Table). Each cell in the matrix represents the correlation coefficient between two chemicals (column and row names). This matrix has been further flattened into chemical-pairs, and then ordered by rank based on their correlation coefficient values. The rank-ordered correlation coefficients of chemical pairs are provided in S5B Table.
Case study with ethylbenzene
To demonstrate the utility of the PBPK knowledgebase in finding analogous chemicals with existing PBPK models that could act as a starting template to build a new model, ethylbenzene was used as a case study. Six chemicals with varying structural similarities towards ethylbenzene were selected from the PBPK knowledgebase, and the equations/parameters from their existing models were used for the construction of an ethylbenzene PBPK model. The simulation results from these newly constructed models were compared to the experimental data on ethylbenzene [29].
Correlation coefficient-based selection of entries in the PBPK knowledgebase
First, eight molecular descriptors for ethylbenzene were calculated in MOE and normalized as described above for the chemicals contained within the PBPK knowledgebase. Second, the correlation coefficients of ethylbenzene with all chemicals in the PBPK knowledgebase were calculated and rank ordered (S6 Table). Because the experimental data [29] for ethylbenzene were extracted from a rat inhalation study, only the PBPK knowledgebase entries that had “rats” and “inhalation” in the title and/or abstract were considered for our case study. Six chemicals were selected from three categories, including: (1) exact matches (ethylbenzene), which have a correlation coefficient of 1; (2) close-analogues (xylene, toluene and benzene), which have high-ranked correlation coefficients among chemicals in the PBPK knowledgebase; and (3) non-analogues (dichloromethane and methyl iodide), which have low-ranked correlation coefficients among chemicals in the PBPK knowledgebase. The chemical names, CAS number, correlation coefficients (toward ethylbenzene), rank of correlation coefficient (among a total of 307 chemicals in the PBPK knowledgebase) and PBPK literature references of the six selected chemicals are summarized below (Table 1).
Table 1. Selected analogues* of ethylbenzene.
| Name | CAS No. | Correlation Coefficient | Rank* | PBPK ref. |
|---|---|---|---|---|
| Ethylbenzene | 100-41-4 | 1 | [58] | |
| Xylene | 1330-20-7 | 0.999979 | 1 | [58] |
| Toluene | 108-88-3 | 0.999957 | 3 | [58] |
| Benzene | 71-43-2 | 0.999848 | 7 | [58] |
| Dichloromethane | 75-09-2 | 0.988299 | 54 | [59] |
| Methyl iodide | 21410-51-5 | 0.910114 | 283 | [60] |
*Chemical analogues were selected based on their similar published experimental designs (animal species = rats, route of administration = inhalation, time span = short term) relative to that of ethylbenzene-associated literature.
*The ranking of correlation coefficient among a total of 307 unique chemicals in the PBPK knowledgebase.
Using newly developed models to simulate ethylbenzene blood concentrations
PBPK models for the selected entries (ethylbenzene, xylene, toluene, benzene, dichloromethane, and methyl iodide) were extracted from the literature in Table 1 [58–60]. The models were coded in MATLAB R2014b (version 8.4; The MathWorks, Natick, MA) and modified to simulate the time course of ethylbenzene blood concentrations in rats weighing 250 g after inhalation exposure to 100 ppm ethylbenzene for 4 hours. For simplicity and illustrative purposes, uncertainties in model parameters, model predictions and experimental observations were not considered: All parameters in the newly built models were set as fixed, and all data points extracted from the literature were fixed at their mean values.
The simulation results were then compared to experimental data for ethylbenzene obtained from the literature [29]. Only blood concentrations were compared for illustrative purposes. Organ-specific or tissue-specific concentration data were not measured in many studies, especially human studies, due to economic and ethical reasons. Therefore, organ-specific data were not used in the current studies.
For each of the six models, goodness-of-fit between predicted blood concentrations and measured values was calculated through the calculation of Chi Square statistics (χ2), using Eq 3 as follows.
| (3) |
Where Oi is the model-predicted concentration and ei is the experimentally observed concentration at the ith time point. p—Values for the χ2 statistics were obtained through MS Excel function “CHIDIST”. Calculated χ2 statistics and p-values for each model versus experimental data are stored in S7 Table.
Comparing model parameters
Parameters from the existing models (Table 1) for ethylbenzene, xylene, toluene, benzene, dichloromethane, and methyl iodide were extracted from published studies associated with abstracts contained within the PBPK knowledgebase [58–60]. These parameters can be grouped into two general types: physiology-specific and chemical-specific. Rat physiology-specific parameters (e.g., cardiac output, alveolar ventilation rate, tissue volumes, blood flows to tissues) from these models were generally consistent [58–60], while chemical-specific parameters varied widely among models (Table 2).
Table 2. Partition coefficients and metabolic parameters from existing PBPK models for ethylbenzene and selected analogues.
| Parameters | Ethyl Benzene | Xylene | Toluene | Benzene | Dichloromethane | Methyl iodide |
|---|---|---|---|---|---|---|
| Blood:air | 42.7 | 46 | 18 | 15 | 19.2 | 39 |
| Fat:air | 1556 | 1859 | 1021 | 500 | 120 | 89 |
| Slowly perfused tissue:air | 26 | 41.9 | 27.7 | 15 | 7.9 | 7.5 |
| Rapidly perfused tissue:air | 60.3 | 90.9 | 83.6 | 17 | 14.2 | 9 |
| Liver:air | 83.8 | 90.9 | 83.6 | 17 | 14.2 | 24 |
| Maximum rate of metabolism (mg/h/kg) | 6.39 | 6.49 | 3.44 | 2.11 | 4.3 | 831 |
| Michaelis-Menten constant (mg/L) | 1.04 | 0.45 | 0.13 | 0.01 | 0.4 | 3.6 |
Case study with gefitinib
Models for the anti-cancer drug gefitinib [61] were coded and executed in Matlab in order to predict blood concentrations for seven other chemicals of varying similarity to gefitinib. Predicted blood concentrations for these seven structural analogues were then compared against measured values [26,61–67]. All entries in the PBPK knowledgebase were first ranked based on their similarity toward gefitinib, as described above (S8 Table). Close- and non-analogues of gefitinib were selected from the top and bottom of the ranking list. Because some of the top or bottom ranked chemicals do not have published experimental data, only entries that are associated with experimental data were kept as examples. Four close-analogues (itraconazole, cocaine, diclofenac and 3,3'-diindolylmethane) and 3 non-analogues (perchlorate, phosphorothioate oligonucleotide, and melamine) of gefitinib were selected for experimental data extraction (Table 3). The existing gefitinib model [61] was used to simulate the blood concentrations for these selected example chemicals. Dose and body weight (BW) were obtained from references for each chemical [26,61–67]. The volume of distribution (V1, V2) for each new chemical was linearly scaled by body weight. For example, V1_itraconazole = (BW_itraconazole /BW_ gefitinib)* V1_ gefitinib. Other parameters were not altered from the original gefitinib model (Table 4). Predicted blood concentrations were then compared to published experimental concentrations for each chemical. Calculation of Chi Square statistics (χ2) as an indication of goodness-of-fit was performed as described above.
Table 3. Chemical names, CAS numbers, rank of correlation coefficient toward gefitinib, and related references of selected analogues of gefitinib.
| Name | CAS No. | Rank | Species | Route of Administration | PBPK ref. |
|---|---|---|---|---|---|
| gefitinib | 184475-35-2 | 1 | mice | oral | [61] |
| itraconazole | 84625-61-6 | 2 | human | oral | [62] |
| cocaine | 50-36-2 | 5 | human | intravenous | [63] |
| diclofenac | 15307-86-5 | 7 | human | oral | [64] |
| 3,3'-diindolylmethane | 1968-05-4* | 8 | mice | oral | [65] |
| perchlorate | 14797-73-0 | 301 | rat | intravenous | [26] |
| phosphorothioate oligonucleotide | 10101-88-9 | 302 | rat | intravenous | [66] |
| melamine | 108-78-1 | 304 | pig | intravenous | [67] |
Table 4. Model parameters for selected gefitinib’s close-analogues and non-analogues.
| Name | Dose (mg/kg) | BW (g) | Bioavailability | Absorption rate (1/h) | Elimination rate (1/h) | High- to low- permeability tissue rate (1/h) | Low- to high- permeability tissue rate (1/h) | High- permeability tissue volume (V1) (ml) | Low permeability tissue volume (V2) (ml) |
|---|---|---|---|---|---|---|---|---|---|
| gefitinib | 55 | 22.5 | 0.45 | 0.88 | 0.54 | 1.65 | 0.55 | 75 | 0.3 |
| itraconazole | 2.9 | 70000 | 0.45 | 0.88 | 0.54 | 1.65 | 0.55 | 233330 | 933 |
| cocaine | 0.3 | 70000 | 0.45 | 0.88 | 0.54 | 1.65 | 0.55 | 233330 | 933 |
| diclofenac | 0.7 | 70000 | 0.45 | 0.88 | 0.54 | 1.65 | 0.55 | 233330 | 933 |
| 3,3'-diindolylmethane | 250 | 25 | 0.45 | 0.88 | 0.54 | 1.65 | 0.55 | 83 | 0.33 |
| perchlorate | 3.3 | 330 | 0.45 | 0.88 | 0.54 | 1.65 | 0.55 | 1100 | 4.4 |
| phosphorothioate oligonucleotide | 10 | 250 | 0.45 | 0.88 | 0.54 | 1.65 | 0.55 | 833 | 3.3 |
| melamine | 6.13 | 100000 | 0.45 | 0.88 | 0.54 | 1.65 | 0.55 | 333333 | 1333 |
Results
Trends in PBPK-related literature
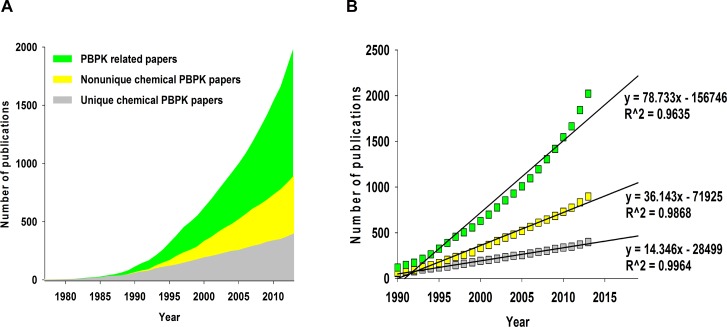
The 2,039 PBPK-related articles were assigned to one of three categories (Fig 1A): publications on unique chemicals that appeared for the first time (likely to be a newly-developed PBPK model); publications on chemicals that appeared in previous publications (likely to be an application or refinement of a previously-developed PBPK model); and reports on general PBPK concepts, methods, commentaries, perspectives, or reviews.
Fig 1. Trends of PBPK literatures.
(A) The 2,039 PBPK-related articles are placed into one of three categories: (1) unique chemical PBPK papers (grey), pioneering articles in which specific chemical names have appeared for the first time; (2) non-unique chemical PBPK papers (yellow), articles in which chemical names have appeared in previous publications; or (3) PBPK related papers (green), articles that are not associated with specific chemical names. (B) Linear regression of the number of articles in three categories over time.
Regression analysis was performed for the three categories (Fig 1B). Linear relationships between the number of publications and the year of publication were calculated to help identifying the growth rates. The growth rates for publications are 14/year, 36/year, and 78/year for the first, second, and third categories, respectively. These trends reflect the difficulty in developing a new PBPK model due to the great quantity of experimental data required. While our search suggests ongoing development and expansion of PBPK-related modeling methodologies, the low output of new PBPK models limits the utility of PBPK modeling for examination of health risks resulting from chemical exposures.
Species, life stage, gender, and organ coverage of the PBPK knowledgebase
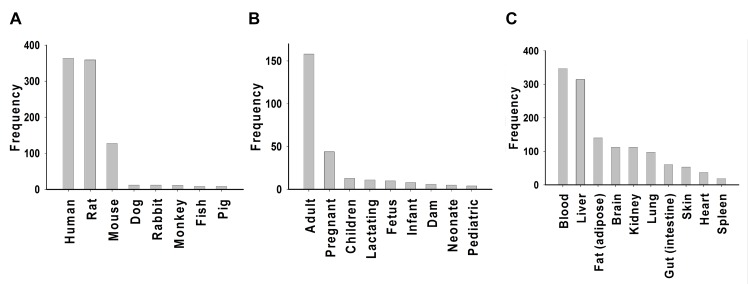
When comparing the two gender keywords, “male” appeared much more frequently than “female” (66% vs. 34%). “Human” and “rat” were the most frequently mentioned species, and these key words appeared at about three times the frequency of “mouse” (Fig 2A). “Dog,” “rabbit,” “monkey,” “fish,” and “pig” comprised the 4th to 8th most common animal species mentioned in the abstracts, but with noticeably lower frequency than the top 3 species, which accounted for >94% of the total (Fig 2A).
Fig 2. Keywords extraction from PBPK literatures.
The abstracts in the PBPK knowledgebase were analyzed to identify PBPK-associated word-stems: (A) Frequency of the top 10 species; (B) Frequency of the top 10 life stages; (C) Frequency of the top 10 compartments.
The most frequently mentioned life stage was “adult,” appearing three times more frequently than the second-most frequent term “pregnant” (Fig 2B). For the top 9 life stage terms mentioned in PBPK-related literature, the key words “pregnant,” “dam,” and “lactating” refer to the reproductive cycle of the female parent; the key words “fetus,” “neonate,” “infant,”“pediatric,” and “children” refer to growth and developmental stages of offspring (Fig 2B).
“Blood” and “liver” were the two most frequent organs incorporated into PBPK models, with appearance frequencies twice as high as the 3rd most frequent organ, “fat (adipose)” (Fig 2C). “Brain,” “kidney,” “lungs,” “gut (intestine),” “skin,” “heart,” and “spleen” comprised the 4th to 10th most frequent organs mentioned in these publications.
Calculation of physicochemical molecular descriptors and correlation coefficients
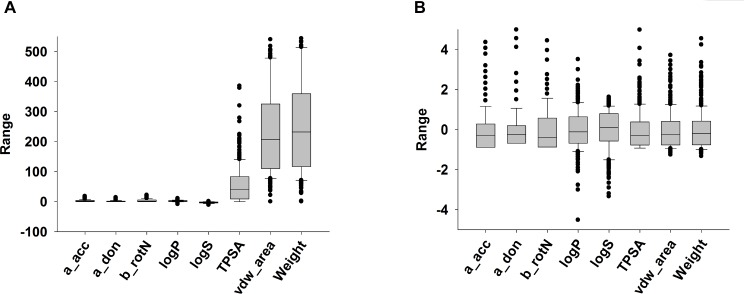
The means and standard deviations of hba, hbd, nRotB, logP and logS were much smaller than those of PSA, vdw_area and MW (Fig 3A). After normalization of the physicochemical molecular descriptors for these chemicals using Eq 1 above to reduce bias in calculation of correlation coefficients, the mean and standard deviation of each descriptor was set as 0 and 1, respectively (Fig 3B).
Fig 3. Physicochemical molecular descriptors.
Summary of the values of eight physicochemical molecular descriptors, calculated using the Molecular Operating Environment (MOE), for 307 chemicals in the PBPK knowledgebase. The eight descriptors are molecular weight (MW), hydrogen bond acceptor count (hba), hydrogen bond donor count (hbd), number of rotatable bonds (nRotB), polar surface area or topological polar surface area (PSA), octanol:water partition coefficient (LogP), log transformation of solubility (logS), and area of van der Waal surface (vdw_area). (A) The original calculated descriptor values; (B) The normalized descriptor values using Eq 1 from the Methods section.
Each cell in the correlation matrix contains the correlation coefficient of one chemical toward another chemical in the knowledgebase. The top five correlation coefficients were equal to 1, because those five chemical pairs contained identical molecular descriptors. These chemicals were either chiral isomers or isotopically-labeled compounds. The remaining chemical-pair combinations exhibited a maximum correlation coefficient of 0.999990409 (between 1,2,4-trimethylbenzene and 1,2,3,5-tetramethylbenzene) and a minimum correlation coefficient of 0.589788342 (between ethylene and methyl mercury).
Case study with ethylbenzene
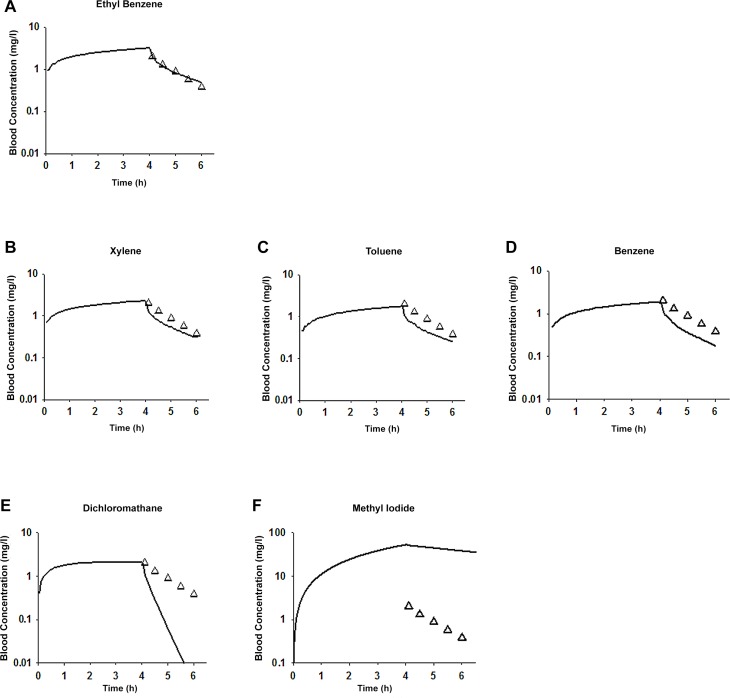
Simulated blood concentrations from the PBPK model based on an “exact match” (ethylbenzene) [58] aligned extremely well with the experimental data [29] (Fig 4A). Simulated blood concentrations from PBPK models based on “close-analogues” (xylene, toluene and benzene) [58] deviated slightly from the ethylbenzene data (Fig 4B and 4C and 4D). In contrast, simulated blood concentrations from the models based on “non-analogues” (dichloromethane and methyl iodide) [59,60] exhibited significant deviations from the experimental data on ethylbenzene (Fig 4E and 4F).
Fig 4. Case study with ethylbenzene.
Comparing blood concentrations of ethylbenzene (triangle symbols) from rats exposed to 100 ppm ethylbenzene for four hours [29] and simulated blood concentrations of ethylbenzene (solid lines) based on the (A) ethylbenzene PBPK model [58]; (B) xylene PBPK model [58]; (C) toluene PBPK model [58]; (D) benzene PBPK model [58]; (E) dichloromethane PBPK model [59]; and (F) methyl iodide PBPK model [60].
The PBPK model based on an “exact match” (ehtylbenzene) resulted in the highest χ2 goodness-of-fit p-value of 0.9991; PBPK models based on “close-analogues” had p-values of 0.8603, 0.5789 and 0.1479 for xylene, toluene, and benzene, respectively; PBPK models based on “non-analogues” (dichloromethane and methyl iodide) resulted in much lower p-values of <6 × 10−215.
Case study with gefitinib
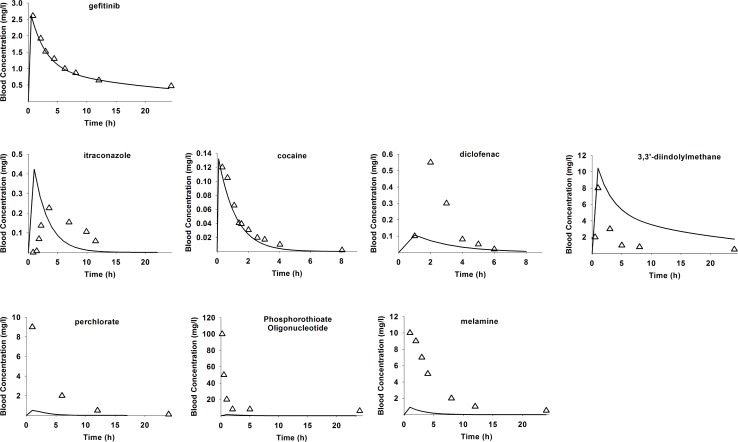
Fig 5 shows published experimental observed blood concentrations for each example chemicals compared to their predicted values from the each of the accommodated gefitinib models (dose, BW, V1,V2 adjusted). Chi Square statistics (χ2) were calculated and stored in S9 Table. The gefitinib model fit the best with its own experimental data, with χ2 test p-values equal to 0.999. The predictive ability of the gefitinib model for the structural analogues cocaine and 3,3'-diindolylmethane were high, with χ2 goodness-of-fit p-values of 0.994 and 0.898, respectively. The χ2 test p-values for the other two structural analogues itraconazole and diclofenac were not as high, but still better than those of non-analogues, with χ2 test p-values of 2.81 × 10−21 and 5.71 × 10−14, respectively. The χ2 test p-values for non-analogues were all zero.
Fig 5. Case study with gefitinib.
Comparing simulated (solid lines) and experimentally observed (triangle symbols) blood concentrations for compounds. PBPK models were extracted from the gefitinib study [61], and executed to predict pharmacokinetics of gefitinib’s close-analogues (itraconazole, cocaine, diclofenac, 3,3'-diindolylmethane) and non-analoguse (perchlorate, phosphorothioate oligonucleotide, melamine, carbamateon). The experimental observations were extracted from PBPK literature listed in Table 3.
Discussion
Researchers have used molecular modeling approaches (e.g., quantitative structure-activity relationships) to predict parameters, such as volume of distribution and clearance rate, to fill data gaps when building new PBPK models for chemicals lacking these data [52,68–70]. This approach can be labor intensive and requires background knowledge in computational chemistry and statistics. Utilizing pre-existing models with well-calibrated parameters to help new model construction is a more efficient approach and has been widely implemented [28–33]. However, reviewing or sorting through publications for relevant information often would be an overwhelming task for investigators. The PBPK knowledgebase presented in this current work serves as an effective means for finding analogues whose publications contain necessary model information and/or data that can aid the construction or validation of new PBPK models for new chemicals.
One-compartment (whole body) and two-compartment (blood and the remainder of the body) models are simplest classical pharmacokinetic models. In these models, the organ-structure and mathematical equations remain the same for different chemicals, so extracting parameters from pre-existing models would be reasonable approach [61]. When a classical PK model extends beyond two compartments, grouping and integration of organs into hypothetical compartments often occurs [71]. For example, the liver might be integrated into one compartment (e.g., compartment 3 of a five-compartment model) for one chemical but integrated into an entirely different compartment (e.g., compartment 4) for another chemical. This difference in integration could lead to changes in not only chemical-specific parameters, but also in physiological parameters (e.g. compartmental volume, protein content, metabolic capacity) for compartment 3 and 4, respectively. Therefore, extraction from pre-existing models would not be an appropriate parameterization approach for high-dimension classical PK models.
Compartments in PBPK models correspond to real biological organs (tissues), so the physiological parameters of compartments (e.g., blood flow, organ volume, and protein content) are highly conserved among PBPK models [72]. Chemical-specific parameters for each organ, such as tissue:blood partition coefficients and fraction of protein binding, are related to organ structure and the compound’s physicochemical properties. These characteristics enable researchers to use parameters from an analogue’s pre-existing model for a new PBPK model of new chemicals. However, mathematical equations in PBPK models are determined on a case-by-case basis, with variation in the number of compartments, type of compartments (flow-limited or diffusion-limited), chemical-specific elimination routes, active transport of the parent chemical and/or metabolites, and other factors [73]. Therefore, borrowing parameters from other PBPK models would be a case-by-case practice and require extensive browsing and reading of relevant publications. The PBPK knowledgebase not only ranked the publications based on structural similarity (S5A Table), but also summarized much essential information, such as chemical names, organs, genders and species (S2 Table). It will help expedite selection and reading through relevant publications for locating appropriate parameter values.
The current estimate for the number of chemicals in commerce in the United States is nearly 100,000, with 500 to 1000 new chemicals being produced each year [74]. The current rate of PBPK model development (~14 chemicals/year; Fig 1B) will likely never catch up with rate of new chemical production. Just covering the 1,800 chemicals found in consumer products would take more than 100 years. The new strategy presented in this current work is designed to facilitate the generation of provisional pharmacokinetic models as chemical inventories continue to expand. The PBPK knowledgebase can be used to gauge the chemical space of existing PBPK models, as well as developing a methodology to search for existing PBPK models for structural analogues of chemicals of interest. It is up to the discretion of future investigators whether to use our proposed approach based on eight molecular descriptors to select an analogue or to use a different similarity testing method based on the intended purpose of the new model.
Identifying structural analogues is an ongoing area of research. Commonly accepted numerical measurements of chemical similarity are distance coefficients based on chemical descriptors, such as binary (0 or 1) values, indicating the absence or presence of some particular feature, topological indices, physicochemical properties (sometimes estimated using in silico approaches), or on/off indications for molecular fingerprints [75]. A large number of similarity calculations have been defined and used in the literature, including Euclidean [75,76], Hamming [75,76], Minkowsky [76,77], Correlation [76,78], Tanimoto [75,76], Molecular Access System (MACCS) [79,80], and Artificial Neural Network (ANN) [81,82]. Currently, there is no consensus regarding the best practices in selecting molecular descriptors and similarity calculation methods. Pearson’s correlation coefficient has previously been used in similarity calculations [76,78]. We chose this metric because it equally-weighted the descriptors and also can easily be calculated through a simple R programming language script. The eight molecular descriptors (Fig 3) presented here were selected based on their relationship with pharmacokinetic properties [47,49,52,57,83] and their ease of accessibility to the general public.
Route of administration, as well as molecular properties of chemicals, can influence chemical behavior entering into a biological system. Route of administration determines the bioavailability, peak blood/tissue concentrations (Cmax), time of peak concentrations (tmax), biological half-life (t1/2), and other pharmacokinetic characteristics [84,85]. When intravenously injected, ethylbenzene is 100% bioavailable and reaches tmax at time 0. When exposed through oral administration, the bioavailability of ethylbenzene is influenced by metabolic degradation in gut tissue, by gut lumen bacteria, and by liver hepatocytes [86–88]. If exposed through inhalation, ethylbenzene’s bioavailability and rate of absorption is determined by the air:blood partition coefficients and gas exchange rate of the lung [89,90]. The physiological parameters of the model change with a given animal species. Although extrapolation between species is a common practice in PBPK modeling [91], the original model is more appropriate when using the same species as that used to derive the experimental data. Therefore, in our ethylbenzene case study which used model predictions comparable to measured time course data obtained from a rat inhalation study [29], “inhalation” and “rats” were used as filters for the PBPK knowledgebase before chemical selection.
The three categories of “exact match,” “close-analogue,” and “non-analogue” in our case study represent the major scenarios that can aid researchers in model development using the PBPK knowledgebase. For a given chemical, if an “exact match” entry is found, the existing model should have the best predictive capability. Although the same information may be retrievable through a PubMed search for the chemical, our PBPK knowledgebase can be used to search existing models that might have been built based on alternatives for a chemical (e.g., synonyms, chiral isomers or isotopically-labeled compounds). For example, ChemSpider lists 21 synonyms for ethylbenzene. The PBPK knowledgebase provides a more efficient and more precise solution, especially for those without a background in chemistry: any synonyms, chiral isomers or isotopically-labeled compounds would have a calculated correlation coefficient of 1 (S5B Table).
Besides being able to search for an “exact match,” the power of the PBPK knowledgebase lies in its ability to detect “close-analogues” of a chemical simply by searching for the highest correlation-ranked entries. Searching for “close-analogues” without the knowledgebase could potentially be achieved by a two-step process of (1) searching public chemical databases for structural analogues; and then (2) searching PubMed for existing PBPK models for each analogue. This two-step process, however, is unnecessarily time-consuming. Although many publications exist that contain PBPK-related models and information, this number pales in comparison to the hundreds of thousands of chemical structures found in public chemistry databases (e.g., ChemSpider, PubChem) [92]. It is more efficient to start the search of analogues found within the PBPK knowledgebase, which contains 307 entries rather than with the entire universe of chemicals. For example, in a structural-similarity search using ethylbenzene on ChemSpider, more than 10,000 results were retrieved with a Tanimoto score >99%. Xylene and toluene, which ranked 1st and 3rd in the PBPK knowledgebase, were not included in the top 100 of this list of chemical analogues for ethylbenzene identified in ChemSpider. Studies have shown that not all structural properties are associated with chemicals’ PK properties [2,3,45–48]. Using all available molecular descriptors, such as the structural analogue algorithm in the public chemical database, may result in a less accurate estimation of the desired PK property analogue.
The two “non-analogues” of ethylbenzene were selected from lower correlation-ranked entries in our ethylbenzene case study to confirm that the parameter values for non-analogues differ most from parameter values for “exact matches” (Table 2), and that simulations from PBPK models of non-analogues deviate most from experimental data (Fig 4E and 4F). Since experimental data for the target chemical, ethylbenzene, was available in our case study, it was straightforward for us to categorize the knowledgebase entries as “close-analogues” or “non-analogues” by comparing model predictions with data. For a chemical of interest lacking experimental data, there is no clear way to select a threshold for similarity rankings. A proposed rule of thumb is to select three to five chemicals from the first ten correlation-ranked entities, and then the “best” published model is picked from this shortlist. We caution that this recommendation is subjective, and the choice of the best model should be rooted in the quantity and applicability of the data that is available from published research to calibrate the model. For example, a model that was calibrated using time course data in multiple tissues in animals and evaluated against human data would be considered a better model than one that was calibrated using only urinary metabolite data and was not evaluated against any human data.
Our second case study with gefitinib further demonstrates the utility and versatility of the PBPK knowledgebase. A pre-existing PBPK model of gefitinib was accommodated to predict the experimental observations of other chemicals, selected from the top and bottom of similarity-ranked PBPK knowledgebase entries. The simulated drug kinetics in Fig 5 and calculated χ2 test p-values in S9 Table, demonstrated that the gefitinib model gave better predictions for the closer structure analogues (cocaine, 3,3'-diindolylmethane) than to non-analogues (perchlorate, phosphorothioate oligonucleotide, melamine). These results supported the theoretical assumption for using close-analogue’s existing parameters for a new chemical’s model construction [4,34]. The PBPK knowledgebase not only contains the chemical names, animal species, route of administrations, and tissue compartments (all of which were extracted and used for indexing and searching in the current manuscript), but also can facilitate the discovery of corresponding experimental data that can be easily extracted from publications. Such extracted data was used to test the appropriateness of gefitinib’s model in our second case study. These data can also serve additional needs and interests of knowledgebase users.
Commercial software packages such as SimCyp, Gastroplus or PKSim are used extensively in the pharmaceutical industry, as well as in academia, to support rapid PBPK model development [93–96]. We wish to emphasize several fundamental differences between these commercial software packages and our PBPK knowledgebase. Firstly, values of chemical-specific parameters in the knowledgebase are either measured or optimized against experimental data; while in SimCyp, Gastroplus and PKSim, chemical-specific parameters are often QSAR-based predictions. Secondly, our knowledgebase provides more information than merely model code and parameter values. Through its abstract corpus compilation, the knowledgebase also refers users to relevant articles containing time course tissue concentration data, dose-response data, the authors’ assumptions, limitations and applications of the model, and cited resources, in regards to chemicals of interest. Thirdly, our knowledgebase is free to the public and acts as a central location for abstract information relevant to chemicals of interest. Users accessing this information can contact the authors of the publications for additional information pertaining to model code or related data, while commercial software packages require licensing fees. Finally, the model structures in the published literature, which can be easily located from abstract information provided in the knowledgebase, were constructed based on the authors’ expert judgement and modeling philosophy (e.g., top-down vs. bottom-up); in SimCyp, Gastroplus, and PKsim, the model structure is primarily generic. Although a modifiable, generic model structure is easy to use, more experienced users may prefer to construct their own models based on data availability and purposes of the study.
The knowledgebase herein provides abstracts for previously published PBPK articles, beginning from 1977 onwards. With extracted information from these published articles, the knowledgebase can aid users in identifying the most relevant publications. Use of this extracted information is highly dependent on the scientific questions and problems of interest and is applicable mostly on a case-by-case basis. The two case studies presented here represent two specific circumstances addressing different scientific queries. Future users should select a strategy that meets their individual needs, based on data availability and study purpose, when using the knowledgebase.
In summary, a PBPK knowledgebase was compiled that contains a thorough documentation of the chemical space of PBPK models. This knowledgebase provides scientists with a structure-based approach to identify provisional nearest-neighbor chemicals that are described by existing PBPK models and whose existing models might be used as a template to construct new models for chemicals of interest. These comprehensive dataset initiatives can be coupled with in vitro or in vivo chemical and biological data curated and accessible from other sources (e.g., STITCH 4.0, CSS Dashboard, Comparative Toxicogenomics Database), or with more recent methods such as in silico multi-target profiling in DockScreen [97], in order to pave the way for more rapid PBPK model development. Such approaches can complement efforts to rapidly develop PBPK/PD models that are designed to act as supporting computational tools in modern risk assessment.
Supporting Information
(TXT)
(CSV)
(CSV)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors would like to thank Drs. Hisham El-Masri, Kent Thomas, Lisa Baxter, and Roy Fortmann for their suggestions and comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JL, JAL and MBP were funded by the Oak Ridge Institute for Science and Education’s Research Participation Program at the US Environmental Protection Agency. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Poulin P, Krishnan K (1996) Molecular Structure-Based Prediction of the Partition Coefficients of Organic Chemicals for Physiological Pharmacokinetic Models. Toxicology Mechanisms and Methods 6: 117–137. [Google Scholar]
- 2.Poulin P, Theil FP (2000) A priori prediction of tissue:plasma partition coefficients of drugs to facilitate the use of physiologically-based pharmacokinetic models in drug discovery. J Pharm Sci 89: 16–35. [DOI] [PubMed] [Google Scholar]
- 3.Schmitt W (2008) General approach for the calculation of tissue to plasma partition coefficients. Toxicol In Vitro 22: 457–467. [DOI] [PubMed] [Google Scholar]
- 4.Peyret T, Poulin P, Krishnan K (2010) A unified algorithm for predicting partition coefficients for PBPK modeling of drugs and environmental chemicals. Toxicol Appl Pharmacol 249: 197–207. 10.1016/j.taap.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 5.Poulin P, Dambach DM, Hartley DH, Ford K, Theil FP, et al. (2013) An algorithm for evaluating potential tissue drug distribution in toxicology studies from readily available pharmacokinetic parameters. J Pharm Sci 102: 3816–3829. 10.1002/jps.23670 [DOI] [PubMed] [Google Scholar]
- 6.Endo S, Brown TN, Goss KU (2013) General model for estimating partition coefficients to organisms and their tissues using the biological compositions and polyparameter linear free energy relationships. Environ Sci Technol 47: 6630–6639. 10.1021/es401772m [DOI] [PubMed] [Google Scholar]
- 7.Ruark CD, Hack CE, Robinson PJ, Mahle DA, Gearhart JM (2014) Predicting passive and active tissue:plasma partition coefficients: interindividual and interspecies variability. J Pharm Sci 103: 2189–2198. 10.1002/jps.24011 [DOI] [PubMed] [Google Scholar]
- 8.Shlomi T, Cabili MN, Herrgard MJ, Palsson BO, Ruppin E (2008) Network-based prediction of human tissue-specific metabolism. Nat Biotech 26: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 9.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, et al. (2008) Prodrugs: design and clinical applications. Nat Rev Drug Discov 7: 255–270. 10.1038/nrd2468 [DOI] [PubMed] [Google Scholar]
- 10.Greene N, Judson PN, Langowski JJ, Marchant CA (1999) Knowledge-based expert systems for toxicity and metabolism prediction: DEREK, StAR and METEOR. SAR QSAR Environ Res 10: 299–314. [DOI] [PubMed] [Google Scholar]
- 11.Kirchmair J, Williamson MJ, Tyzack JD, Tan L, Bond PJ, et al. (2012) Computational prediction of metabolism: sites, products, SAR, P450 enzyme dynamics, and mechanisms. J Chem Inf Model 52: 617–648. 10.1021/ci200542m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyzack JD, Mussa HY, Williamson MJ, Kirchmair J, Glen RC (2014) Cytochrome P450 site of metabolism prediction from 2D topological fingerprints using GPU accelerated probabilistic classifiers. J Cheminform 6: 29 10.1186/1758-2946-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frederick CB, Bush ML, Lomax LG, Black KA, Finch L, et al. (1998) Application of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry extrapolation of acidic vapors in the upper airways. Toxicol Appl Pharmacol 152: 211–231. [DOI] [PubMed] [Google Scholar]
- 14.Bogdanffy MS, Plowchalk DR, Sarangapani R, Starr TB, Andersen ME (2001) Mode-of-action-based dosimeters for interspecies extrapolation of vinyl acetate inhalation risk. Inhal Toxicol 13: 377–396. [DOI] [PubMed] [Google Scholar]
- 15.Timchalk C, Nolan RJ, Mendrala AL, Dittenber DA, Brzak KA, et al. (2002) A Physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol Sci 66: 34–53. [DOI] [PubMed] [Google Scholar]
- 16.Clewell RA, Merrill EA, Yu KO, Mahle DA, Sterner TR, et al. (2003) Predicting fetal perchlorate dose and inhibition of iodide kinetics during gestation: a physiologically-based pharmacokinetic analysis of perchlorate and iodide kinetics in the rat. Toxicol Sci 73: 235–255. [DOI] [PubMed] [Google Scholar]
- 17.Fisher JW, Twaddle NC, Vanlandingham M, Doerge DR (2011) Pharmacokinetic modeling: prediction and evaluation of route dependent dosimetry of bisphenol A in monkeys with extrapolation to humans. Toxicol Appl Pharmacol 257: 122–136. 10.1016/j.taap.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 18.Sarangapani R, Teeguarden J, Andersen ME, Reitz RH, Plotzke KP (2003) Route-specific differences in distribution characteristics of octamethylcyclotetrasiloxane in rats: analysis using PBPK models. Toxicol Sci 71: 41–52. [DOI] [PubMed] [Google Scholar]
- 19.Tornero-Velez R, Mirfazaelian A, Kim KB, Anand SS, Kim HJ, et al. (2010) Evaluation of deltamethrin kinetics and dosimetry in the maturing rat using a PBPK model. Toxicol Appl Pharmacol 244: 208–217. 10.1016/j.taap.2009.12.034 [DOI] [PubMed] [Google Scholar]
- 20.Price PS, Schnelle KD, Cleveland CB, Bartels MJ, Hinderliter PM, et al. (2011) Application of a source-to-outcome model for the assessment of health impacts from dietary exposures to insecticide residues. Regul Toxicol Pharmacol 61: 23–31. 10.1016/j.yrtph.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Tornero-Velez R, Davis J, Scollon EJ, Starr JM, Setzer RW, et al. (2012) A pharmacokinetic model of cis- and trans-permethrin disposition in rats and humans with aggregate exposure application. Toxicol Sci 130: 33–47. 10.1093/toxsci/kfs236 [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Doerge DR, Fisher JW (2013) Prediction and evaluation of route dependent dosimetry of BPA in rats at different life stages using a physiologically based pharmacokinetic model. Toxicol Appl Pharmacol 270: 45–59. 10.1016/j.taap.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 23.Andersen ME, Clewell HJ 3rd, Gearhart J, Allen BC, Barton HA (1997) Pharmacodynamic model of the rat estrus cycle in relation to endocrine disruptors. J Toxicol Environ Health 52: 189–209. [DOI] [PubMed] [Google Scholar]
- 24.Gearhart JM, Jepson GW, Clewell HJ 3rd, Andersen ME, Conolly RB (1990) Physiologically based pharmacokinetic and pharmacodynamic model for the inhibition of acetylcholinesterase by diisopropylfluorophosphate. Toxicol Appl Pharmacol 106: 295–310. [DOI] [PubMed] [Google Scholar]
- 25.Ploeger B, Mensinga T, Sips A, Deerenberg C, Meulenbelt J, et al. (2001) A population physiologically based pharmacokinetic/pharmacodynamic model for the inhibition of 11-beta-hydroxysteroid dehydrogenase activity by glycyrrhetic acid. Toxicol Appl Pharmacol 170: 46–55. [DOI] [PubMed] [Google Scholar]
- 26.Merrill EA, Clewell RA, Gearhart JM, Robinson PJ, Sterner TR, et al. (2003) PBPK predictions of perchlorate distribution and its effect on thyroid uptake of radioiodide in the male rat. Toxicol Sci 73: 256–269. [DOI] [PubMed] [Google Scholar]
- 27.Tan YM, Butterworth BE, Gargas ML, Conolly RB (2003) Biologically motivated computational modeling of chloroform cytolethality and regenerative cellular proliferation. Toxicol Sci 75: 192–200. [DOI] [PubMed] [Google Scholar]
- 28.Verhaar HJ, Morroni JR, Reardon KF, Hays SM, Gaver DP Jr., et al. (1997) A proposed approach to study the toxicology of complex mixtures of petroleum products: the integrated use of QSAR, lumping analysis and PBPK/PD modeling. Environ Health Perspect 105 Suppl 1: 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tardif R, Charest-Tardif G, Brodeur J, Krishnan K (1997) Physiologically based pharmacokinetic modeling of a ternary mixture of alkyl benzenes in rats and humans. Toxicol Appl Pharmacol 144: 120–134. [DOI] [PubMed] [Google Scholar]
- 30.Parham FM, Portier CJ (1998) Using structural information to create physiologically based pharmacokinetic models for all polychlorinated biphenyls. II. Rates of metabolism. Toxicol Appl Pharmacol 151: 110–116. [DOI] [PubMed] [Google Scholar]
- 31.Vinegar A, Jepson GW, Cisneros M, Rubenstein R, Brock WJ (2000) Setting safe acute exposure limits for halon replacement chemicals using physiologically based pharmacokinetic modeling. Inhal Toxicol 12: 751–763. [DOI] [PubMed] [Google Scholar]
- 32.Poet TS, Kousba AA, Dennison SL, Timchalk C (2004) Physiologically based pharmacokinetic/pharmacodynamic model for the organophosphorus pesticide diazinon. Neurotoxicology 25: 1013–1030. [DOI] [PubMed] [Google Scholar]
- 33.Tan YM, Liao KH, Clewell HJ 3rd (2007) Reverse dosimetry: interpreting trihalomethanes biomonitoring data using physiologically based pharmacokinetic modeling. J Expo Sci Environ Epidemiol 17: 591–603. [DOI] [PubMed] [Google Scholar]
- 34.Poulin P, Krishnan K (1995) An algorithm for predicting tissue: blood partition coefficients of organic chemicals from n-octanol: water partition coefficient data. J Toxicol Environ Health 46: 117–129. [DOI] [PubMed] [Google Scholar]
- 35.Parham FM, Kohn MC, Matthews HB, DeRosa C, Portier CJ (1997) Using structural information to create physiologically based pharmacokinetic models for all polychlorinated biphenyls. Toxicol Appl Pharmacol 144: 340–347. [DOI] [PubMed] [Google Scholar]
- 36.Poulin P, Theil FP (2002) Prediction of pharmacokinetics prior to in vivo studies. II. Generic physiologically based pharmacokinetic models of drug disposition. J Pharm Sci 91: 1358–1370. [DOI] [PubMed] [Google Scholar]
- 37.Dennison JE, Andersen ME, Dobrev ID, Mumtaz MM, Yang RS (2004) PBPK modeling of complex hydrocarbon mixtures: gasoline. Environ Toxicol Pharmacol 16: 107–119. 10.1016/j.etap.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 38.Beliveau M, Krishnan K (2005) A spreadsheet program for modeling quantitative structure-pharmacokinetic relationships for inhaled volatile organics in humans. SAR QSAR Environ Res 16: 63–77. [DOI] [PubMed] [Google Scholar]
- 39.Rodgers T, Rowland M (2006) Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci 95: 1238–1257. [DOI] [PubMed] [Google Scholar]
- 40.Foxenberg RJ, Ellison CA, Knaak JB, Ma C, Olson JR (2011) Cytochrome P450-specific human PBPK/PD models for the organophosphorus pesticides: chlorpyrifos and parathion. Toxicology 285: 57–66. 10.1016/j.tox.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knaak JB, Dary CC, Zhang X, Gerlach RW, Tornero-Velez R, et al. (2012) Parameters for pyrethroid insecticide QSAR and PBPK/PD models for human risk assessment. Rev Environ Contam Toxicol 219: 1–114. 10.1007/978-1-4614-3281-4_1 [DOI] [PubMed] [Google Scholar]
- 42.Haddad S, Beliveau M, Tardif R, Krishnan K (2001) A PBPK modeling-based approach to account for interactions in the health risk assessment of chemical mixtures. Toxicol Sci 63: 125–131. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Tsang AM, Okino MS, Power FW, Knaak JB, et al. (2007) A physiologically based pharmacokinetic/pharmacodynamic model for carbofuran in Sprague-Dawley rats using the exposure-related dose estimating model. Toxicol Sci 100: 345–359. [DOI] [PubMed] [Google Scholar]
- 44.Hamelin G, Haddad S, Krishnan K, Tardif R (2010) Physiologically based modeling of p-tert-octylphenol kinetics following intravenous, oral or subcutaneous exposure in male and female Sprague-Dawley rats. J Appl Toxicol 30: 437–449. 10.1002/jat.1515 [DOI] [PubMed] [Google Scholar]
- 45.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3–26. [DOI] [PubMed] [Google Scholar]
- 46.Paolini GV, Shapland RH, van Hoorn WP, Mason JS, Hopkins AL (2006) Global mapping of pharmacological space. Nat Biotechnol 24: 805–815. [DOI] [PubMed] [Google Scholar]
- 47.Obach RS, Lombardo F, Waters NJ (2008) Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos 36: 1385–1405. 10.1124/dmd.108.020479 [DOI] [PubMed] [Google Scholar]
- 48.Lombardo F, Waters NJ, Argikar UA, Dennehy MK, Zhan J, et al. (2013) Comprehensive assessment of human pharmacokinetic prediction based on in vivo animal pharmacokinetic data, part 1: volume of distribution at steady state. J Clin Pharmacol 53: 167–177. 10.1177/0091270012440281 [DOI] [PubMed] [Google Scholar]
- 49.Zhao YH, Le J, Abraham MH, Hersey A, Eddershaw PJ, et al. (2001) Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure-activity relationship (QSAR) with the Abraham descriptors. J Pharm Sci 90: 749–784. [DOI] [PubMed] [Google Scholar]
- 50.Nakao K, Fujikawa M, Shimizu R, Akamatsu M (2009) QSAR application for the prediction of compound permeability with in silico descriptors in practical use. J Comput Aided Mol Des 23: 309–319. 10.1007/s10822-009-9261-8 [DOI] [PubMed] [Google Scholar]
- 51.Iyer M, Tseng YJ, Senese CL, Liu J, Hopfinger AJ (2007) Prediction and mechanistic interpretation of human oral drug absorption using MI-QSAR analysis. Mol Pharm 4: 218–231. [DOI] [PubMed] [Google Scholar]
- 52.Gombar VK, Hall SD (2013) Quantitative structure-activity relationship models of clinical pharmacokinetics: clearance and volume of distribution. J Chem Inf Model 53: 948–957. 10.1021/ci400001u [DOI] [PubMed] [Google Scholar]
- 53.Gao H, Steyn SJ, Chang G, Lin J (2010) Assessment of in silico models for fraction of unbound drug in human liver microsomes. Expert Opin Drug Metab Toxicol 6: 533–542. 10.1517/17425251003671022 [DOI] [PubMed] [Google Scholar]
- 54.Zhivkova Z, Doytchinova I (2012) Quantitative structure—plasma protein binding relationships of acidic drugs. J Pharm Sci 101: 4627–4641. 10.1002/jps.23303 [DOI] [PubMed] [Google Scholar]
- 55.Terfloth L, Bienfait B, Gasteiger J (2007) Ligand-based models for the isoform specificity of cytochrome P450 3A4, 2D6, and 2C9 substrates. J Chem Inf Model 47: 1688–1701. [DOI] [PubMed] [Google Scholar]
- 56.Lewis DF, Jacobs MN, Dickins M (2004) Compound lipophilicity for substrate binding to human P450s in drug metabolism. Drug Discov Today 9: 530–537. [DOI] [PubMed] [Google Scholar]
- 57.Lombardo F, Waters NJ, Argikar UA, Dennehy MK, Zhan J, et al. (2013) Comprehensive assessment of human pharmacokinetic prediction based on in vivo animal pharmacokinetic data, part 2: clearance. J Clin Pharmacol 53: 178–191. 10.1177/0091270012440282 [DOI] [PubMed] [Google Scholar]
- 58.Haddad S, Charest-Tardif G, Tardif R, Krishnan K (2000) Validation of a physiological modeling framework for simulating the toxicokinetics of chemicals in mixtures. Toxicol Appl Pharmacol 167: 199–209. [DOI] [PubMed] [Google Scholar]
- 59.Andersen ME, Clewell HJ 3rd, Gargas ML, MacNaughton MG, Reitz RH, et al. (1991) Physiologically based pharmacokinetic modeling with dichloromethane, its metabolite, carbon monoxide, and blood carboxyhemoglobin in rats and humans. Toxicol Appl Pharmacol 108: 14–27. [DOI] [PubMed] [Google Scholar]
- 60.Sweeney LM, Kirman CR, Gannon SA, Thrall KD, Gargas ML, et al. (2009) Development of a physiologically based pharmacokinetic (PBPK) model for methyl iodide in rats, rabbits, and humans. Inhal Toxicol 21: 552–582. 10.1080/08958370802601569 [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Zhou Q, Gallo JM (2009) Demonstration of the equivalent pharmacokinetic/pharmacodynamic dosing strategy in a multiple-dose study of gefitinib. Mol Cancer Ther 8: 1438–1447. 10.1158/1535-7163.MCT-09-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vossen M, Sevestre M, Niederalt C, Jang IJ, Willmann S, et al. (2007) Dynamically simulating the interaction of midazolam and the CYP3A4 inhibitor itraconazole using individual coupled whole-body physiologically-based pharmacokinetic (WB-PBPK) models. Theor Biol Med Model 4: 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonate PL, Swann A, Silverman PB (1996) Preliminary physiologically based pharmacokinetic model for cocaine in the rat: model development and scale-up to humans. J Pharm Sci 85: 878–883. [DOI] [PubMed] [Google Scholar]
- 64.Kambayashi A, Blume H, Dressman J (2013) Understanding the in vivo performance of enteric coated tablets using an in vitro-in silico-in vivo approach: case example diclofenac. Eur J Pharm Biopharm 85: 1337–1347. 10.1016/j.ejpb.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 65.Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, et al. (2004) Physiological modeling of formulated and crystalline 3,3'-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos 32: 632–638. [DOI] [PubMed] [Google Scholar]
- 66.Peng B, Andrews J, Nestorov I, Brennan B, Nicklin P, et al. (2001) Tissue distribution and physiologically based pharmacokinetics of antisense phosphorothioate oligonucleotide ISIS 1082 in rat. Antisense Nucleic Acid Drug Dev 11: 15–27. [DOI] [PubMed] [Google Scholar]
- 67.Buur JL, Baynes RE, Riviere JE (2008) Estimating meat withdrawal times in pigs exposed to melamine contaminated feed using a physiologically based pharmacokinetic model. Regul Toxicol Pharmacol 51: 324–331. 10.1016/j.yrtph.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 68.Louis B, Agrawal VK (2012) Quantitative structure-pharmacokinetic relationship (QSPkP) analysis of the volume of distribution values of anti-infective agents from J group of the ATC classification in humans. Acta Pharm 62: 305–323. 10.2478/v10007-012-0024-z [DOI] [PubMed] [Google Scholar]
- 69.Zhivkova Z, Doytchinova I (2013) Quantitative structure—clearance relationships of acidic drugs. Mol Pharm 10: 3758–3768. 10.1021/mp400251k [DOI] [PubMed] [Google Scholar]
- 70.Ghafourian T, Amin Z (2013) QSAR models for the prediction of plasma protein binding. Bioimpacts 3: 21–27. 10.5681/bi.2013.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whiting B, Kelman AW, Grevel J (1986) Population pharmacokinetics. Theory and clinical application. Clin Pharmacokinet 11: 387–401. [DOI] [PubMed] [Google Scholar]
- 72.Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13: 407–484. [DOI] [PubMed] [Google Scholar]
- 73.Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51: 45–73. 10.1146/annurev-pharmtox-010510-100540 [DOI] [PubMed] [Google Scholar]
- 74.Egeghy PP, Vallero DA, Cohen Hubal EA (2011) Exposure-based prioritization of chemicals for risk assessment. Environmental Science & Policy 14: 950–964. [Google Scholar]
- 75.Willett P, Barnard JM, Downs GM (1998) Chemical Similarity Searching. Journal of Chemical Information and Computer Sciences 38: 983–996. [Google Scholar]
- 76.Choi S-S, Cha S-H, Tappert CC (2010) A survey of binary similarity and distance measures. Journal of Systemics, Cybernetics and Informatics 8: 43–48. [Google Scholar]
- 77.Bultinck P, Kuppens T, Gironés X, Carbó-Dorca R (2003) Quantum similarity superposition algorithm (QSSA): a consistent scheme for molecular alignment and molecular similarity based on quantum chemistry. Journal of chemical information and computer sciences 43: 1143–1150. [DOI] [PubMed] [Google Scholar]
- 78.Hert J, Keiser MJ, Irwin JJ, Oprea TI, Shoichet BK (2008) Quantifying the relationships among drug classes. Journal of chemical information and modeling 48: 755–765. 10.1021/ci8000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flower DR (1998) On the Properties of Bit String-Based Measures of Chemical Similarity. Journal of Chemical Information and Computer Sciences 38: 379–386. [Google Scholar]
- 80.Kearsley SK, Sallamack S, Fluder EM, Andose JD, Mosley RT, et al. (1996) Chemical Similarity Using Physiochemical Property Descriptors. Journal of Chemical Information and Computer Sciences 36: 118–127. [Google Scholar]
- 81.Agatonovic-Kustrin S, Beresford R (2000) Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. Journal of pharmaceutical and biomedical analysis 22: 717–727. [DOI] [PubMed] [Google Scholar]
- 82.Hoskins JC, Himmelblau D (1988) Artificial neural network models of knowledge representation in chemical engineering. Computers & Chemical Engineering 12: 881–890. [Google Scholar]
- 83.Yap CW, Li H, Ji ZL, Chen YZ (2007) Regression methods for developing QSAR and QSPR models to predict compounds of specific pharmacodynamic, pharmacokinetic and toxicological properties. Mini Rev Med Chem 7: 1097–1107. [DOI] [PubMed] [Google Scholar]
- 84.Helton DR, Osborne DW, Pierson SK, Buonarati MH, Bethem RA (2000) Pharmacokinetic profiles in rats after intravenous, oral, or dermal administration of dapsone. Drug Metab Dispos 28: 925–929. [PubMed] [Google Scholar]
- 85.Prah J, Ashley D, Blount B, Case M, Leavens T, et al. (2004) Dermal, oral, and inhalation pharmacokinetics of methyl tertiary butyl ether (MTBE) in human volunteers. Toxicological Sciences 77: 195–205. [DOI] [PubMed] [Google Scholar]
- 86.Sams C, Loizou GD, Cocker J, Lennard MS (2004) Metabolism of ethylbenzene by human liver microsomes and recombinant human cytochrome P450s (CYP). Toxicology letters 147: 253–260. [DOI] [PubMed] [Google Scholar]
- 87.Kniemeyer O, Heider J (2001) Ethylbenzene dehydrogenase, a novel hydrocarbon-oxidizing molybdenum/iron-sulfur/heme enzyme. Journal of Biological Chemistry 276: 21381–21386. [DOI] [PubMed] [Google Scholar]
- 88.Geypens B, Claus D, Evenepoel P, Hiele M, Maes B, et al. (1997) Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut 41: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Csanády GA, Filser J (2001) The relevance of physical activity for the kinetics of inhaled gaseous substances. Archives of toxicology 74: 663–672. [DOI] [PubMed] [Google Scholar]
- 90.Tardif R, Charest-Tardif G, Brodeur J (1996) Comparison of the influence of binary mixtures versus a ternary mixture of inhaled aromatic hydrocarbons on their blood kinetics in the rat. Archives of toxicology 70: 405–413. [DOI] [PubMed] [Google Scholar]
- 91.Welsch F, Blumenthal GM, Conolly RB (1995) Physiologically based pharmacokinetic models applicable to organogenesis: extrapolation between species and potential use in prenatal toxicity risk assessments. Toxicology letters 82: 539–547. [DOI] [PubMed] [Google Scholar]
- 92.Williams AJ (2008) Public chemical compound databases. Current Opinion in Drug Discovery and Development 11: 393 [PubMed] [Google Scholar]
- 93.Jamei M, Marciniak S, Edwards D, Wragg K, Feng K, et al. (2013) The simcyp population based simulator: architecture, implementation, and quality assurance. In Silico Pharmacol 1: 9 10.1186/2193-9616-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheeti S, Budha NR, Rajan S, Dresser MJ, Jin JY (2013) A physiologically based pharmacokinetic (PBPK) approach to evaluate pharmacokinetics in patients with cancer. Biopharm Drug Dispos 34: 141–154. 10.1002/bdd.1830 [DOI] [PubMed] [Google Scholar]
- 95.Sinha VK, Snoeys J, Osselaer NV, Peer AV, Mackie C, et al. (2012) From preclinical to human—prediction of oral absorption and drug-drug interaction potential using physiologically based pharmacokinetic (PBPK) modeling approach in an industrial setting: a workflow by using case example. Biopharm Drug Dispos 33: 111–121. 10.1002/bdd.1782 [DOI] [PubMed] [Google Scholar]
- 96.Wetmore BA, Allen B, Clewell HJ 3rd, Parker T, Wambaugh JF, et al. (2014) Incorporating population variability and susceptible subpopulations into dosimetry for high-throughput toxicity testing. Toxicol Sci 142: 210–224. 10.1093/toxsci/kfu169 [DOI] [PubMed] [Google Scholar]
- 97.Goldsmith M- R, Grulke CM, Chang DT, Transue TR, Little SB, et al. (2014) DockScreen: A Database of In Silico Biomolecular Interactions to Support Computational Toxicology. Dataset Papers in Science 2014: 5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TXT)
(CSV)
(CSV)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.