Abstract
End-to-side nerve coaptation brings regenerating axons from the donor to the recipient nerve. Several techniques have been used to perform coaptation: microsurgical sutures with and without opening a window into the epi(peri)neurial connective tissue; among these, window techniques have been proven more effective in inducing axonal regeneration. The authors developed a sutureless model of end-to-side coaptation in the rat upper limb. In 19 adult Wistar rats, the median and the ulnar nerves of the left arm were approached from the axillary region, the median nerve transected and the proximal stump sutured to the pectoral muscle to prevent regeneration. Animals were then randomly divided in two experimental groups (7 animals each, 5 animals acting as control): Group 1: the distal stump of the transected median nerve was fixed to the ulnar nerve by applying cyanoacrylate solution; Group 2: a small epineurial window was opened into the epineurium of the ulnar nerve, caring to avoid damage to the nerve fibres; the distal stump of the transected median nerve was then fixed to the ulnar nerve by applying cyanoacrylate solution. The grasping test for functional evaluation was repeated every 10–11 weeks starting from week-15, up to the sacrifice (week 36). At week 36, the animals were sacrificed and the regenerated nerves harvested and processed for morphological investigations (high-resolution light microscopy as well as stereological and morphometrical analysis). This study shows that a) cyanoacrylate in end-to-side coaptation produces scarless axon regeneration without toxic effects; b) axonal regeneration and myelination occur even without opening an epineurial window, but c) the window is related to a larger number of regenerating fibres, especially myelinated and mature, and better functional outcomes.
Introduction
A number of experimental [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17] as well as clinical [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] studies have shown that end-to-side nerve coaptation is able to induce collateral sprouting from donor nerve’s axons, allowing for significant repopulation of the distal nerve stump.
The injury to the donor nerve due to microsurgical suture in end-to-side coaptation, seems to be the starter of axonal growth and reinnervation in distal stump of receiving damaged nerve. However, it has been observed that this procedure results in the loss (“escape”) of nerve fibres from the donor nerve, acting as a restricting factor in the surgeon’s mind when choosing the technique for nerve repair [38,39,40,41,42].
In this study we evaluate the possibility that this kind of reinnervation may be obtained without microsurgical procedure and, consequently, without the loss of nerve fibres in the donor nerve.
In an experimental model the receiving (cut) median nerve was coapted to the donor healthy ulnar nerve, by means of only adhesive biocompatible substance, butyl 2-cyanoacrylate.
Cyanoacrylates are a group of substances well-known and tested for their gluing properties and to date available for clinical use [43,44,45,46,47,48]. Restrictions to their use in nerve coaptation have been registered by some authors [43,48], who detected that uncontrolled contamination by the gluing agent of the coapted nerve surfaces could produce local inflammation and a scar wall stopping nerve fibre regeneration. However, their findings have been questioned by other studies, demonstrating that a transient inflammatory effect produced by cyanoacrylate in the coaptation zone is capable to stimulate local ingrowth of Schwann cells and connective tissue, creating the way for axonal sprouting [44,45,46,47].
The aim of this study was thus 1) to develop a sutureless, less traumatic, simple and fast technique for coaptation of the distal stump of a dissected nerve onto a nearby health donor nerve with end-to-side reinnervation model and 2) to prove that the growth of axons is possible only through the biological events associated with the tropism of the receiving damaged nerve and the corresponding target organ.
The chosen experimental model was the rat upper limb, which allows a detailed analysis on functional recovery by grasping test [49,50] followed by morphological evaluation of donor and receiving nerves [51,52].
Materials and Methods
Surgery
For this study, 19 adult female Wistar rats, weighing approximately 200g, were utilized. Experimental surgery was carried out at the microsurgical laboratory of the Ecole de Chirurgie in Paris (Institutional license from the “Direction départementale de la protection des populations”, DDPP number C-75-05-23) according to the French law on experimental animal research (law no. 87–848, October 19, 1987). All the surgeries were carried out in the period between January 2012 and March 2013 by expert surgeons certified by the “Service protection et santé animals de le Ministère de l’Agriculture”.
All animals were housed in plastic cages with a 12/12 light/dark cycle, and water and food were available ad libitum. Adequate and standard measures were taken to minimize pain and discomfort taking into account human endpoints for animal suffering and distress.
After deep anesthesia induced with ketamine (40 mg/250g) and cloropromazine (3.75 mg/250g), the median and the ulnar nerves of the left upper limb were approached under operating microscope magnification (ZEISS OPMI 7, magnification 0.4/0.63/1.0/1.6/2.5) from the axillary region and carefully exposed. A 10-mm long segment of the median nerve was dissected and cut, and the proximal nerve stump was sutured to the pectoral muscle to prevent regeneration. Animals were then randomly divided in two experimental groups:
Group 1 (N-butyl-2-cyanoacrylate w/o epineurial window): the distal stump of the transected median nerve was fixed to the ulnar nerve by applying N-butyl-2-cyanoacrylate solution (7 animals);
Group 2 (N-butyl-2-cyanoacrylate with epineurial window): a small epineurial window was opened into the epineurium of the ulnar nerve, trying to avoid damage to the nerve fibres below; the distal stump of the transected median nerve was then fixed to the ulnar nerve by applying N-butyl-2-cyanoacrylate solution (7 animals).
The skin was then sutured and the animals were allowed to recover.
Five animals were used as un-injured controls (i.e. without sham operation).
Functional assessment
Functional evaluation of median nerve recovery was assessed by the grasping test, as previously described [50]. Briefly, this test consists of holding the rat by its tail and lowering the animal towards the device. Then, when the animal grips the grid, it is pulled upward until it loses it. The balance records the maximum weight that the animal is able to hold up before losing the grip. Animals were tested every 10–11 weeks starting from week-15, up to the sacrifice (week 36); each animal was tested three times and the average value was recorded. The day before surgery, the function of the left median nerve was assessed to have the control values.
Morphology, stereology and morphometry
36 weeks after surgery, animals were subjected to deep anesthesia (ketamine, 40 mg/250g and cloropromazine, 3.75 mg/250g) and the median nerve was approached. From each animal, the regenerated nerve was harvested. Animals were then euthanized with overdose of anesthetic and animal death was confirmed by exsanguination (abdominal aorta resection).
Nerve specimens were fixed by immediate immersion in 2.5% glutaraldehyde in 0.1 M PBS pH 7.4 for up to 6 h at 4°C, washed in Sorensen phosphate buffer 0.1 M (pH 7.4) with 1.5% sucrose, and post-fixed in 2% osmium tetroxide for 2 h. Samples were then carefully dehydrated in passages in ethanol from 30% to 100%, cleared in propylene oxide and embedded in Glauerts’ embedding mixture of resins consisting in equal parts of Araldite M and Araldite Harter, HY 964 (Merck, Darmstadt, Germany), containing 0.5% of the plasticizer dibutylphthalate and 1–2% of the accelerator 964, DY 064 (Merck, Darmstadt, Germany).
For high-resolution light microscopy, semi-thin transverse sections (2.5 μm thick) were cut starting from the distal stump of each nerve specimen, using an Ultracut UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany) and stained with 1% toluidine blue.
For stereological and morphometrical analysis, design-based quantitative morphology was performed [53,54]. One toluidine-blue section was randomly selected and the cross-sectional area of the whole nerve section was measured. On the same image, 10–12 sampling fields were selected using a systematic random sampling protocol [53,55,56]. In each sampling field, the "edge effect" was avoided by employing a two-dimensional dissector method which is based on counting the "tops" of the myelinated fibers. The total number of myelinated fibers, as well as different size parameters (fiber and axon diameter and myelin thickness) were then calculated.
Statistical analysis
Quantitative data are presented as mean + Standard Error. All data were statistically analyzed using one-way analysis of variance (SPSS software).
Results
Functional assessment
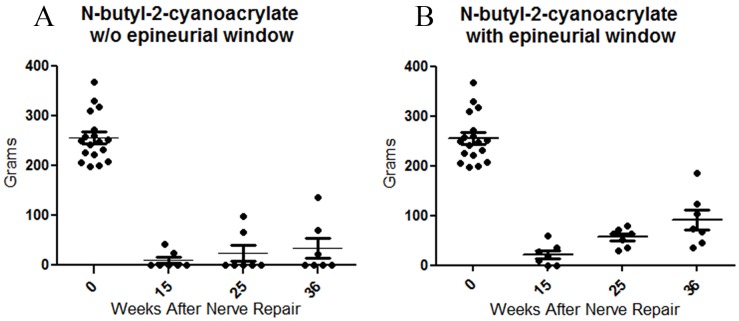
To investigate whether N-butyl-2-cyanoacrylate solution and the presence or not of the epineurial window can interfere with the regeneration, we first evaluated motor functional recovery after end-to-side coaptation, obtained at the time of the first evaluation (pre-operative) and after 15, 25 and 36 weeks from the repair. Results are presented in Fig 1. 15 weeks after nerve repair, only two animals of Group 1 (N-butyl-2-cyanoacrylate w/o epineurial window) started to recover motor function (Fig 1A), whereas in Group 2 (N-butyl-2-cyanoacrylate with epineurial window) already five animals reached this result (Fig 1B). This discrepancy between the two experimental groups is more detectable after 25 and 36 weeks: all the animals of group 2, but only three animals of Group 1, recovered motor function at the end of the experiment (36 weeks). The remaining four animals of Group 1 did not recovered active digit flexion even 36 weeks after nerve repair. However, both experimental groups were statistically different (p ≤ 0.05) compared to control, also after 36 weeks.
Fig 1. Performance of rats in the grasping test following end-to-side neurorrhaphy.
A: N-butyl-2-cyanoacrylate w/o epineurial window group (group 1); B: N-butyl-2-cyanoacrylate with epineurial window group (group 2). A predominately number of animals of group 2 has recovered motor function (five animals after 15 weeks and all the seven animals after 36 weeks), compared to group 1 (only two animals recovered motor function activity after 15 weeks, and three animals after 36 weeks). Data are presented as scatterplots showing individual animal values with integrated mean and variance values.
Morphological analysis
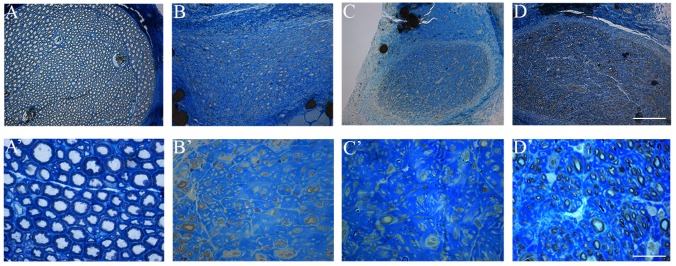
We compared semi-thin sections of the regenerated nerves harvested 36 weeks after end-to-side coaptation. In Fig 2, representative images taken from the distal part of the median nerve are shown. Since Group 1 (N-butyl-2-cyanoacrylate w/o epineurial window) showed only a partial functional recovery (some animals recovered and some animals did not), we displayed representative pictures of both conditions (Fig 2B–2B’, Group 1 with functional recovery; 2C–2C’, Group 1 without functional recovery).
Fig 2. High resolution light microscopic images of a control median nerve (A-A’) and median nerves 36 weeks after end-to-side coaptation.
B-B’: N-butyl-2-cyanoacrylate w/o epineurial window group, animal with functional recovery; C-C’: N-butyl-2-cyanoacrylate w/o epineurial window group, animal without functional recovery; D-D’: N-butyl-2-cyanoacrylate with epineurial window group. Both experimental groups show regenerating fibres, but animals which did not recover functional activity of Group 1 (C-C’) show smaller nerve cross sectional area with fewer and smaller fibres compared to both Group 1_with functional recovery (B-B’) and Group 2 (D-D’). Bars: A-D: 100 μm; A’D’: 10 μm.
Low power images of whole cross-section of regenerated nerves showed that, in accordance with functional results, animals belonging to Group 1 that did not recovered functional activity (Fig 2C), have a smaller cross-sectional area compared to animals of Group 1 which recovered functional activity (Fig 2B) and to animals of Group 2 (N-butyl-2-cyanoacrylate with epineurial window) (Fig 2D).
Moreover, high magnification pictures showed that regenerating fibres are present in all experimental group, but animals belonging to Group 1 without functional recovery (Fig 2C’), have fewer, smaller and with thinner myelin thickness fibres compared to animals of Group 1 which recovered functional activity (Fig 2B’) and to animals of Group 2 (Fig 2D’). Among Group 1, animals with functional recovery showed regenerated fibres comparable to those of animals belonging to Group 2 from a morphological point of view.
Stereological and morphometric analysis
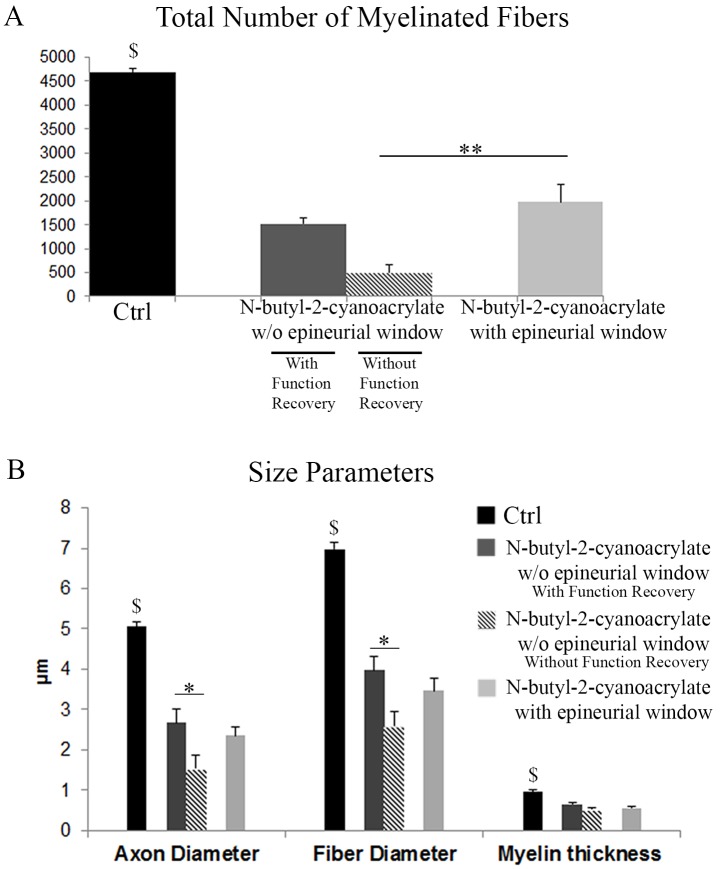
Quantitative stereological evaluations for axon numbers, and morphometrical analysis for axon and fibre diameter and thickness of the myelin sheath, were performed in the distal part of the median nerve in both experimental groups, and compared to control values (Fig 3).
Fig 3. Histograms showing the results of stereological and morphometric evaluations.
Data of Group 1 (N-butyl-2-cyanoacrylate w/o epineurial window) are divided into two parts: animal which displayed functional recovery (n = 3) and animals which did not (n = 4). Group 2 (N-butyl-2-cyanoacrylate with epineurial window) shows more myelinated fibres compared to animals of Group 1 (N-butyl-2-cyanoacrylate w/o epineurial window) without functional recovery. Significant differences are detectable for the analyzed size parameters between animals of Group 1_with functional recovery and animals of Group 1_without functional recovery. Values in the graphics are expressed as mean+standard error. $: p ≤0.001 between control and both the experimental groups; **: p≤0.01; *: p≤0.05.
Also in this case we divided the results of Group 1 (N-butyl-2-cyanoacrylate w/o epineurial window) into two parts: animal which displayed functional recovery (n = 3) and animals which did not (n = 4).
Distal to the end-to-side coaptation, the number of myelinated axons was significantly decreased (p≤0.01) in both experimental groups (Group 1, both conditions and Group 2) compared to control nerve. Moreover, Group 1_without functional recovery showed significantly less (p≤0.01) myelinated fibre number compared to Group 2. Intriguingly, no significant differences were present between Group 1_with functional recovery and Group 2 (Fig 3A).
With regard to size parameters, both experimental groups (Group 1, both conditions and Group 2) showed smaller axon and fibre diameters compared to control (p≤0.01). Moreover, Group 1_without functional recovery, showed significantly smaller (p≤0.05) axon and fibre diameters compared to Group 1_ with functional recovery. No significant differences were present between Group 1_with functional recovery and Group 2. Finally, also myelin thickness was decreased after end-to-side repair compared to control, but the two experimental groups showed comparable values (Fig 3B).
Discussion
1) Why nerve fibers sprout throughout end-to-side coaptation sites?
Several studies have investigated the starting mechanism of end-to-side nerve repair; in their accurate and original review, Bontioti and Dahlin [57] proposed three basic mechanisms consisting in a) contamination from axons regenerating from the proximal stump of the recipient nerve, b) true collateral sprouting from healthy fibres of the donor nerve and c) true axonal regeneration from damaged fibres of the donor nerve (“terminal sprouting”).
a) Contamination can be a problem and must be taken into account, especially when the site of end-to-side coaptation is near the site of recipient nerve lesion: traditional technique of treatment of neuroma can help.
b) Collateral sprouting from healthy fibres does not need donor nerve trauma: simple coaptation with the chemical call from the degenerated distal stump and from the distal effector is capable to induce axonal ingrowth from the donor trunk’s healthy fibres [58,59,60].
The origin of the axons has been demonstrated with double label techniques from Ranvier’s nodes proximal to coaptation site [61], but the level has been questioned, with some authors believing the closest Ranvier nodes to be involved [62] and other papers [11,63] claiming for a role played by more proximal structures. Maybe these different opinions have been produced by different techniques and methods of investigation, as just noted [64]: the former study [62] had been conducted on an epineurial window model (see below c) and using electrophysiological registration, and the latter by simple suture (see below c) to the donor nerve and double retrograde labelling technique [11] or epineurial window ad suturing (see below c), microtearing and histomorphometric analysis [63]. A role of interneuronal signalling in dorsal root ganglia had been claimed [65] and the hypothesis of a Central Nervous System origin has been recently regained [64].
Moreover, the efficacy of simple coaptation has been questioned [57,60] as far as it concerns axon number, myelination and functional results and other concerns have been introduced regarding the influence of axons ingrowing into the recipient nerve, whether motor [66,67,68,69,70], sensory [42,71,72] or even autonomic [73] in this kind of regeneration. Motor axons seem to need injury to start regeneration, whereas sensory axons can sprout spontaneously [42,57,72,74,75,76]. Finally, neuronal plasticity from pruning, including also assessment of agonistic donors to be selected [38,77,78] to brain involvement has also been investigated [57,79,80,81,82].
c) Trauma to the donor nerve is the cause of the third mechanism evoked by Bontioti and Dahlin [57]: terminal sprouting can be produced either opening a window in the trunk connective, or passing classical suture stitches.
As regards opening a window, several papers claim a window to be opened in the donor trunk; in particular, some clinical studies [38] show that a more complex and harder connective structure envelopes the nerve trunks and an epiperineurial window is needed to start end-to-side axonal regeneration; these data have confirmed previous experiences in rats and rabbits respectively [83,84]. On the other hand, in rats, a simple epineurial window seems enough [85,86,87]. A variant opening a larger epineurial window has also been proposed by Yan et al [88,89].
As regards suture without window, several authors suggest that coaptation without a window is capable to attract axons from the intact donor nerve trunk [1,4,6,11]. Interestingly, some authors [90] described a model of end-to-side coaptation without any window but with perineurial suture as more effective in producing axon regeneration than the same model with epineurial suture. Kelly et al [59] have also risen the question for a role played by pressure produced by the sutures as well as associated bleeding and inflammation.
The most popular technique, however, consists in passing stitches after opening an epineurial or an epiperineurial window (that is, coupling the two modalities of donor nerve trauma above mentioned) and results with this last technique are recognized by most authors as the best as far as it concerns number and myelination of axons and functional efficaciousness [10,14,59,72,80,91,92,93,94,95,96,97].
2) Glues in nerve reconstruction
Glues deserve separate considerations; in basic experiences, fibrin glue has been shown to be a good sealant in end-to-end nerve repair [98,99,100], and starting from Palazzi’s data [98], it has also been investigated as an interesting conduit for nerve regeneration, but its role has been questioned as not effective in end-to-side nerve regeneration [5]; however, these data have been questioned and fibrin’s role reconsidered [101,102].
Introduction of cyanoacrylates in both experimental and clinical practice has stimulated researchers’ curiosity, and has been applied to end-to-side nerve repair, but with debatable outcomes, whether questioned [43,48] or not [44,45,46,47]. Some recent experiences [103] are based on the debatable premise that cyanoacrylate produce inflammation and scar in the coaptation site.
a) Why end-to-side coaptation with glue?
All the experiences with glues have reported coupling gluing to opening an epineurial window. Indeed, even if some observations and hypotheses coming from basic sciences have been coupled with all these technique, no data but morphological analyses have been added to the biology of end-to-side nerve repair.
In fact, the role of Schwann cells and chemical signals (growth factors, cytokines from the distal stump products of Wallerian degeneration and reorganization, and also exocytosis products from the distal effector) has been suggested in attracting axons through a nerve injury or a gap [60], but not yet investigated in case of pure end-to side coaptation.
Both functional and morphological outcomes in our study confirm that end-to-side repair is followed by axonal regeneration in each group of animals, showing that axonal regeneration as well as myelination occurs both after opening an epineurial window and after simple coaptation of the distal stump of the cut nerve to the trunk of the healthy donor nerve. That is, from a qualitative point of view speaking, the event “regeneration” occurs whether the epineurium is disrupted or not. These data confirm the previous hypothesis from Viterbo [1] and Lundborg [6] that an important call comes from the distal stump to attract axons and can be explained with recent data from in vitro and experimental studies, as this same role speculated in case of end-to-end and/or graft or tubule repair [60] could be applied to our model.
Our study, however, demonstrates a marked and statistically significant difference between end-to-side axonal regeneration with epineurial window with respect to the group without window; indeed, not all the animals in the group without window showed functional recovery, whereas all the animals in the group with window recovered; this was also reflected from a morphological and morphoquantitative point of view, where the epineurial window spread out more axons and fibres. These data confirm previous evidences [10,14,59,72,80,91,92,93,94,95,96,97] that a trauma such as opening a window in the nerve trunk connective and passing a suture stitch stimulates axonal growth into the distal stump.
b) Is gluing with cyanoacrylates safe and does it produce nerve regeneration in the end-to-side model?
Our study shows that gentle applying of small quantities of cyanoacrylates on the coaptation site is enough to produce regeneration through this site, according to other experiences [44,45,46,47]. We did not experience either toxic local effects nor scar impeding regeneration, reported in other papers [43,48]. Indeed, neither fibrous tissue reaction nor other harmful effects on axonal regeneration were observed from a histologic evaluation. Dealing with toxicity through blood-nerve barrier, cyanoacrylate has recently been used in a rat model in a specific nanoparticle form to vehicle peptides into brain targets; no toxicity neither inflammation have been shown [104].
In our opinion, safe gluing with cyanoacrylates can be simply and carefully obtained and there is no need for isolating the coaptation site with a biological chamber [103], nor the stimulus by suture stitches is needed to obtain regeneration through end-to-side coaptation [90]. In this last case, axons sprouting occurs in a sutureless model, and our results demonstrate that it is detectable, even if it looks poorer, with a simple coaptation, but more evident and significant when coaptation is coupled to opening a window in the donor trunk, even if with the “escape” effect [36,38,39,40,41,42] that is with a reduction of axons, fibres and myelination in the donor trunk. This important side effect seems to occur whatever the coaptation method after opening a window in the nerve connective, even that it has been questioned by some important clinical papers [94,105], it still represents to date a major concern for clinical application of end-to-side coaptation [42,72].
Conclusions
We can conclude that although regeneration per se applies to end-to-side repair after sutureless coaptation, an epineurial window is needed to achieve a significant number and quality of myelinated fibres as well as effective functional recovery. The use of cyanoacrylate glue provides to the microsurgeon a valid alternative to suturing for end-to-side nerve coaptation.
Data Availability
Relevant data are within the paper. Row data about Grasping test and morphoquantitative analysis are available on the "Open Science Framework" Repository (doi: osf.io/p9n52).
Funding Statement
The authors have no support or funding to report.
References
- 1.Viterbo F, Trindade JC, Hoshino K, Mazzoni Neto A (1992) Latero-terminal neurorrhaphy without removal of the epineural sheath. Experimental study in rats. Rev Paul Med 110: 267–275. [PubMed] [Google Scholar]
- 2.Gurney ME, Yamamoto H, Kwon Y (1992) Induction of motor neuron sprouting in vivo by ciliary neurotrophic factor and basic fibroblast growth factor. J Neurosci 12: 3241–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viterbo F, Trindade JC, Hoshino K, Mazzoni Neto A (1994) End-to-side neurorrhaphy with removal of the epineurial sheath: an experimental study in rats. Plast Reconstr Surg 94: 1038–1047. [DOI] [PubMed] [Google Scholar]
- 4.McCallister WV, Tang P, Trumble TE (1999) Is end-to-side neurorrhaphy effective? A study of axonal sprouting stimulated from intact nerves. J Reconstr Microsurg 15: 597–603; discussion 603–594. [DOI] [PubMed] [Google Scholar]
- 5.Bertelli JA, dos Santos AR, Calixto JB (1996) Is axonal sprouting able to traverse the conjunctival layers of the peripheral nerve? A behavioral, motor, and sensory study of end-to-side nerve anastomosis. J Reconstr Microsurg 12: 559–563. [DOI] [PubMed] [Google Scholar]
- 6.Lundborg G, Zhao Q, Kanje M, Danielsen N, Kerns JM (1994) Can sensory and motor collateral sprouting be induced from intact peripheral nerve by end-to-side anastomosis? J Hand Surg Br 19: 277–282. [DOI] [PubMed] [Google Scholar]
- 7.Mennen U (1998) End-to-side nerve suture in the primate (chacma baboon). Hand Surg 3: 1–6. [Google Scholar]
- 8.Tham SK, Morrison WA (1998) Motor collateral sprouting through an end-to-side nerve repair. J Hand Surg Am 23: 844–851. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto M, Hirata H, Nishiyama M, Morita A, Sasaki H, Uchida A (1999) Schwann cells can induce collateral sprouting from intact axons: experimental study of end-to-side neurorrhaphy using a Y-chamber model. J Reconstr Microsurg 15: 281–286. [DOI] [PubMed] [Google Scholar]
- 10.Giovanoli P, Koller R, Meuli-Simmen C, Rab M, Haslik W, Mittlböck M, et al. (2000) Functional and morphometric evaluation of end-to-side neurorrhaphy for muscle reinnervation. Plast Reconstr Surg 106: 383–392. [DOI] [PubMed] [Google Scholar]
- 11.Kanje M, Arai T, Lundborg G (2000) Collateral sprouting from sensory and motor axons into an end to side attached nerve segment. Neuroreport 11: 2455–2459. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T, Maeda M, Tamai S, Tamai M, Yajima H, Takakura Y, et al. (2000) Collateral sprouting mechanism after end-to-side nerve repair in the rat. Med Electron Microsc 33: 151–156. [DOI] [PubMed] [Google Scholar]
- 13.McCallister WV, Tang P, Smith J, Trumble TE (2001) Axonal regeneration stimulated by the combination of nerve growth factor and ciliary neurotrophic factor in an end-to-side model. J Hand Surg Am 26: 478–488. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi A, Yanai A, Komuro Y, Nishida M, Inoue M, Seki T (2004) Collateral sprouting occurs following end-to-side neurorrhaphy. Plast Reconstr Surg 114: 129–137. [DOI] [PubMed] [Google Scholar]
- 15.Cederna PS, Kalliainen LK, Urbanchek MG, Rovak JM, Kuzon WM Jr (2001) "Donor" muscle structure and function after end-to-side neurorrhaphy. Plast Reconstr Surg 107: 789–796. [DOI] [PubMed] [Google Scholar]
- 16.Papalia I, Geuna S, D'Alcontres FS, Tos P (2007) Origin and history of end-to-side neurorrhaphy. Microsurgery 27: 56–61. [DOI] [PubMed] [Google Scholar]
- 17.Geuna S, Papalia I, Tos P (2006) End-to-side (terminolateral) nerve regeneration: a challenge for neuroscientists coming from an intriguing nerve repair concept. Brain Res Rev 52: 381–388. [DOI] [PubMed] [Google Scholar]
- 18.Mennen U (1999) End-to-side nerve suture—a technique to repair peripheral nerve injury. S Afr Med J 89: 1188–1194. [PubMed] [Google Scholar]
- 19.Kostakoglu N (1999) Motor and sensory reinnervation in the hand after an end-to-side median to ulnar nerve coaptation in the forearm. Br J Plast Surg 52: 404–407. [DOI] [PubMed] [Google Scholar]
- 20.Battiston B, Lanzetta M (1999) Reconstruction of high ulnar nerve lesions by distal double median to ulnar nerve transfer. J Hand Surg Am 24: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 21.Kayikcioglu A, Karamursel S, Agaoglu G, Kecik A, Celiker R, Cetin A (2000) End-to-side neurorrhaphies of the ulnar and median nerves at the wrist: report of two cases without sensory or motor improvement. Ann Plast Surg 45: 641–643. [DOI] [PubMed] [Google Scholar]
- 22.Al-Qattan MM (2001) Terminolateral neurorrhaphy: review of experimental and clinical studies. J Reconstr Microsurg 17: 99–108. [DOI] [PubMed] [Google Scholar]
- 23.Al-Qattan MM (2002) End-to-side nerve repair. J Hand Surg Am 27: 739; author reply 739–740. [DOI] [PubMed] [Google Scholar]
- 24.Tung TH, Mackinnon SE (2001) Flexor digitorum superficialis nerve transfer to restore pronation: two case reports and anatomic study. J Hand Surg Am 26: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 25.Mennen U, van der Westhuizen MJ, Eggers IM (2003) Re-innervation of M. biceps by end-to-side nerve suture. Hand Surg 8: 25–31. [DOI] [PubMed] [Google Scholar]
- 26.Tung TH, Novak CB, Mackinnon SE (2003) Nerve transfers to the biceps and brachialis branches to improve elbow flexion strength after brachial plexus injuries. J Neurosurg 98: 313–318. [DOI] [PubMed] [Google Scholar]
- 27.Frey M, Giovanoli P (2003) End-to-side neurorrhaphy of motor nerves: reinnervation of free muscle transplants—first clinical application. Eur J Plast Surg 26: 89–94. [Google Scholar]
- 28.Frey M, Giovanoli P (2003) End-to-side neurorrhaphy of sensory nerves. Eur J Plast Surg 26: 85–88. [Google Scholar]
- 29.Bertelli JA, Ghizoni MF (2003) Nerve repair by end-to-side coaptation or fascicular transfer: a clinical study. J Reconstr Microsurg 19: 313–318. [DOI] [PubMed] [Google Scholar]
- 30.Yuksel F, Peker F, Celikoz B (2004) Two applications of end-to-side nerve neurorrhaphy in severe upper-extremity nerve injuries. Microsurgery 24: 363–368. [DOI] [PubMed] [Google Scholar]
- 31.Amr SM, Moharram AN (2005) Repair of brachial plexus lesions by end-to-side side-to-side grafting neurorrhaphy: experience based on 11 cases. Microsurgery 25: 126–146. [DOI] [PubMed] [Google Scholar]
- 32.Millesi H, Schmidhammer R (2008) Nerve fiber transfer by end-to-side coaptation. Hand Clin 24: 461–483, vii. 10.1016/j.hcl.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 33.Viterbo F, Amr AH, Stipp EJ, Reis FJ (2009) End-to-side neurorrhaphy: past, present, and future. Plast Reconstr Surg 124: e351–358. 10.1097/PRS.0b013e3181bf8471 [DOI] [PubMed] [Google Scholar]
- 34.Tos P, Geuna S, Papalia I, Conforti LG, Artiaco S, Battiston B (2011) Experimental and clinical employment of end-to-side coaptation: our experience. Acta Neurochir Suppl 108: 241–245. 10.1007/978-3-211-99370-5_37 [DOI] [PubMed] [Google Scholar]
- 35.Terzis JK, Tzafetta K (2009) "Babysitter" procedure with concomitant muscle transfer in facial paralysis. Plast Reconstr Surg 124: 1142–1156. 10.1097/PRS.0b013e3181b2b8bc [DOI] [PubMed] [Google Scholar]
- 36.Magdi Sherif M, Amr AH (2010) Intrinsic hand muscle reinnervation by median-ulnar end-to-side bridge nerve graft: case report. J Hand Surg Am 35: 446–450. 10.1016/j.jhsa.2009.10.033 [DOI] [PubMed] [Google Scholar]
- 37.Colonna M, Russo A, Galeano M, Delia G, Pajardi G, d'Alcontres FS (2016) “Baby sitting” procedures in proximal nerve trunk injuries: A review of graft bridging techniques at the level of forearm and a personal approach. Plast Aesth Res. In Press. [Google Scholar]
- 38.Mackinnon SE, Dellon AL, O'Brien JP (1991) Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve 14: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 39.Cederna PS, Youssef MK, Asato H, Urbanchek MG, Kuzon WM Jr (2000) Skeletal muscle reinnervation by reduced axonal numbers results in whole muscle force deficits. Plast Reconstr Surg 105: 2003–2009; discussion 2010–2001. [DOI] [PubMed] [Google Scholar]
- 40.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: 41–51. [DOI] [PubMed] [Google Scholar]
- 41.Myckatyn TM, Mackinnon SE, Hunter DA, Brakefield D, Parsadanian A (2004) A novel model for the study of peripheral-nerve regeneration following common nerve injury paradigms. J Reconstr Microsurg 20: 533–544. [DOI] [PubMed] [Google Scholar]
- 42.Dvali LT, Myckatyn TM (2008) End-to-side nerve repair: review of the literature and clinical indications. Hand Clin 24: 455–460, vii. 10.1016/j.hcl.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 43.Wieken K, Angioi-Duprez K, Lim A, Marchal L, Merle M (2003) Nerve anastomosis with glue: comparative histologic study of fibrin and cyanoacrylate glue. J Reconstr Microsurg 19: 17–20. [DOI] [PubMed] [Google Scholar]
- 44.Choi BH, Kim BY, Huh JY, Lee SH, Zhu SJ, Jung JH, et al. (2004) Microneural anastomosis using cyanoacrylate adhesives. Int J Oral Maxillofac Surg 33: 777–780. [DOI] [PubMed] [Google Scholar]
- 45.Landegren T, Risling M, Brage A, Persson JK (2006) Long-term results of peripheral nerve repair: a comparison of nerve anastomosis with ethyl-cyanoacrylate and epineural sutures. Scand J Plast Reconstr Surg Hand Surg 40: 65–72. [DOI] [PubMed] [Google Scholar]
- 46.Landegren T, Risling M, Persson JK (2007) Local tissue reactions after nerve repair with ethyl-cyanoacrylate compared with epineural sutures. Scand J Plast Reconstr Surg Hand Surg 41: 217–227. [DOI] [PubMed] [Google Scholar]
- 47.Landegren T, Risling M, Persson JK, Sonden A (2010) Cyanoacrylate in nerve repair: transient cytotoxic effect. Int J Oral Maxillofac Surg 39: 705–712. 10.1016/j.ijom.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 48.Gencer ZK, Ozkiris M, Saydam L, Daglioglu YK, Sakallioglu O, Kuyucu Y, et al. (2014) The comparison of histological results of experimentally created facial nerve defects repaired by 2 different anastomosis techniques: classic suture technique or tissue adhesives for nerve anastomosis? J Craniofac Surg 25: 652–656. 10.1097/SCS.0000000000000605 [DOI] [PubMed] [Google Scholar]
- 49.Bertelli JA, Mira JC (1995) The grasping test: a simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J Neurosci Methods 58: 151–155. [DOI] [PubMed] [Google Scholar]
- 50.Papalia I, Tos P, Stagno d'Alcontres F, Battiston B, Geuna S (2003) On the use of the grasping test in the rat median nerve model: a re-appraisal of its efficacy for quantitative assessment of motor function recovery. J Neurosci Methods 127: 43–47. [DOI] [PubMed] [Google Scholar]
- 51.Fox IK, Brenner MJ, Johnson PJ, Hunter DA, Mackinnon SE (2012) Axonal regeneration and motor neuron survival after microsurgical nerve reconstruction. Microsurgery 32: 552–562. 10.1002/micr.22036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovacic U, Tomsic M, Sketelj J, Bajrovic FF (2007) Collateral sprouting of sensory axons after end-to-side nerve coaptation—a longitudinal study in the rat. Exp Neurol 203: 358–369. [DOI] [PubMed] [Google Scholar]
- 53.Geuna S, Tos P, Battiston B, Guglielmone R (2000) Verification of the two-dimensional disector, a method for the unbiased estimation of density and number of myelinated nerve fibers in peripheral nerves. Ann Anat 182: 23–34. [DOI] [PubMed] [Google Scholar]
- 54.Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130: 813–831. [DOI] [PubMed] [Google Scholar]
- 55.Larsen JO (1998) Stereology of nerve cross sections. J Neurosci Methods 85: 107–118. [DOI] [PubMed] [Google Scholar]
- 56.Piskin A, Kaplan S, Aktas A, Ayyildiz M, Raimondo S, Aliç T, et al. (2009) Platelet gel does not improve peripheral nerve regeneration: an electrophysiological, stereological, and electron microscopic study. Microsurgery 29: 144–153. 10.1002/micr.20599 [DOI] [PubMed] [Google Scholar]
- 57.Bontioti E, Dahlin LB (2009) Chapter 12: Mechanisms underlying the end-to-side nerve regeneration. Int Rev Neurobiol 87: 251–268. 10.1016/S0074-7742(09)87012-8 [DOI] [PubMed] [Google Scholar]
- 58.Chen YG, Brushart TM (1998) The effect of denervated muscle and Schwann cells on axon collateral sprouting. J Hand Surg Am 23: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 59.Kelly EJ, Jacoby C, Terenghi G, Mennen U, Ljungberg C, Wiberg M (2007) End-to-side nerve coaptation: a qualitative and quantitative assessment in the primate. J Plast Reconstr Aesthet Surg 60: 1–12. [DOI] [PubMed] [Google Scholar]
- 60.Wood MD, Mackinnon SE (2015) Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp Neurol 265: 171–175. 10.1016/j.expneurol.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang F, Fischer KA (2002) End-to-side neurorrhaphy. Microsurgery 22: 122–127. [DOI] [PubMed] [Google Scholar]
- 62.Tanigawa N, Saito T, Ogawa K, Iida H (2005) Origin of regenerated axons in nerve bypass grafts. J Neurotrauma 22: 605–612. [DOI] [PubMed] [Google Scholar]
- 63.Zhu QT, Zhu JK, Chen GY (2008) Location of collateral sprouting of donor nerve following end-to-side neurorrhaphy. Muscle Nerve 38: 1506–1509. 10.1002/mus.21116 [DOI] [PubMed] [Google Scholar]
- 64.Kim JK, Chung MS, Baek GH (2011) The origin of regenerating axons after end-to-side neurorrhaphy without donor nerve injury. J Plast Reconstr Aesthet Surg 64: 255–260. 10.1016/j.bjps.2010.04.033 [DOI] [PubMed] [Google Scholar]
- 65.Bajrovic F, Kovacic U, Pavcnik M, Sketelj J (2002) Interneuronal signalling is involved in induction of collateral sprouting of nociceptive axons. Neuroscience 111: 587–596. [DOI] [PubMed] [Google Scholar]
- 66.Brushart TM (1988) Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci 8: 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon T, Yang JF, Ayer K, Stein RB, Tyreman N (1993) Recovery potential of muscle after partial denervation: a comparison between rats and humans. Brain Res Bull 30: 477–482. [DOI] [PubMed] [Google Scholar]
- 68.Brushart TM (1993) Motor axons preferentially reinnervate motor pathways. J Neurosci 13: 2730–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bontioti EN, Kanje M, Dahlin LB (2003) Regeneration and functional recovery in the upper extremity of rats after various types of nerve injuries. J Peripher Nerv Syst 8: 159–168. [DOI] [PubMed] [Google Scholar]
- 70.Bontioti E, Kanje M, Lundborg G, Dahlin LB (2005) End-to-side nerve repair in the upper extremity of rat. J Peripher Nerv Syst 10: 58–68. [DOI] [PubMed] [Google Scholar]
- 71.Tam SL, Gordon T (2003) Neuromuscular activity impairs axonal sprouting in partially denervated muscles by inhibiting bridge formation of perisynaptic Schwann cells. J Neurobiol 57: 221–234. [DOI] [PubMed] [Google Scholar]
- 72.Pannucci C, Myckatyn TM, Mackinnon SE, Hayashi A (2007) End-to-side nerve repair: review of the literature. Restor Neurol Neurosci 25: 45–63. [PubMed] [Google Scholar]
- 73.Chung K, Chung JM (2001) Sympathetic sprouting in the dorsal root ganglion after spinal nerve ligation: evidence of regenerative collateral sprouting. Brain Res 895: 204–212. [DOI] [PubMed] [Google Scholar]
- 74.Tarasidis G, Watanabe O, Mackinnon SE, Strasberg SR, Haughey BH, Hunter DA (1997) End-to-side neurorrhaphy resulting in limited sensory axonal regeneration in a rat model. Ann Otol Rhinol Laryngol 106: 506–512. [DOI] [PubMed] [Google Scholar]
- 75.Tarasidis G, Watanabe O, Mackinnon SE, Strasberg SR, Haughey BH, Hunter DA (1998) End-to-side neurorraphy: a long-term study of neural regeneration in a rat model. Otolaryngol Head Neck Surg 119: 337–341. [DOI] [PubMed] [Google Scholar]
- 76.Beck-Broichsitter BE, Becker ST, Lamia A, Fregnan F, Geuna S, Sinis N (2014) Sensoric protection after median nerve injury: babysitter-procedure prevents muscular atrophy and improves neuronal recovery. Biomed Res Int 2014: 724197 10.1155/2014/724197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lutz BS, Chuang DC, Hsu JC, Ma SF, Wei FC (2000) Selection of donor nerves—an important factor in end-to-side neurorrhaphy. Br J Plast Surg 53: 149–154. [DOI] [PubMed] [Google Scholar]
- 78.Papalia I, Cardaci A, d'Alcontres FS, Lee JM, Tos P, Geuna S (2007) Selection of the donor nerve for end-to-side neurorrhaphy. J Neurosurg 107: 378–382. [DOI] [PubMed] [Google Scholar]
- 79.Sanapanich K, Morrison WA, Messina A (2002) Physiologic and morphologic aspects of nerve regeneration after end-to-end or end-to-side coaptation in a rat model of brachial plexus injury. J Hand Surg Am 27: 133–142. [DOI] [PubMed] [Google Scholar]
- 80.Witzel C, Rohde C, Brushart TM (2005) Pathway sampling by regenerating peripheral axons. J Comp Neurol 485: 183–190. [DOI] [PubMed] [Google Scholar]
- 81.Samal F, Haninec P, Raska O, Dubovy P (2006) Quantitative assessment of the ability of collateral sprouting of the motor and primary sensory neurons after the end-to-side neurorrhaphy of the rat musculocutaneous nerve with the ulnar nerve. Ann Anat 188: 337–344. [DOI] [PubMed] [Google Scholar]
- 82.Sananpanich K, Galea MP, Morrison WA, Messina A (2007) Quantitative characterization of regenerating axons after end-to-side and end-to-end coaptation in a rat brachial plexus model: a retrograde tracer study. J Neurotrauma 24: 864–875. [DOI] [PubMed] [Google Scholar]
- 83.Spencer PS, Weinberg HJ, Raine CS, Prineas JW (1975) The perineurial window—a new model of focal demyelination and remyelination. Brain Res 96: 323–329. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z, Johnson EO, Vekris MD, Zoubos AB, Bo J, Beris AE, et al. (2006) Long-term evaluation of rabbit peripheral nerve repair with end-to-side neurorrhaphy in rabbits. Microsurgery 26: 262–267. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z, Soucacos PN, Beris AE, Bo J, Ioachim E, Johnson EO (2000) Long-term evaluation of rat peripheral nerve repair with end-to-side neurorrhaphy. J Reconstr Microsurg 16: 303–311. [DOI] [PubMed] [Google Scholar]
- 86.Zhao JZ, Chen ZW, Chen TY (1997) Nerve regeneration after terminolateral neurorrhaphy: experimental study in rats. J Reconstr Microsurg 13: 31–37. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Z, Soucacos PN, Bo J, Beris AE, Malizos KN, Ioachim E, et al. (2001) Reinnervation after end-to-side nerve coaptation in a rat model. Am J Orthop (Belle Mead NJ) 30: 400–406; discussion 407. [PubMed] [Google Scholar]
- 88.Yan JG, Matloub HS, Sanger JR, Zhang LL, Riley DA, Jaradeh SS (2002) A modified end-to-side method for peripheral nerve repair: large epineurial window helicoid technique versus small epineurial window standard end-to-side technique. J Hand Surg Am 27: 484–492. [DOI] [PubMed] [Google Scholar]
- 89.Yan YH, Yan JG, Matloub HS, Zhang LL, Hettinger P, Sanger J, et al. (2011) Helicoid end-to-side and oblique attachment technique in repair of the musculocutaneous nerve injury with the phrenic nerve as a donor: an experimental study in rats. Microsurgery 31: 122–129. 10.1002/micr.20840 [DOI] [PubMed] [Google Scholar]
- 90.al-Qattan MM, al-Thunyan A (1998) Variables affecting axonal regeneration following end-to-side neurorrhaphy. Br J Plast Surg 51: 238–242. [DOI] [PubMed] [Google Scholar]
- 91.Noah EM, Williams A, Jorgenson C, Skoulis TG, Terzis JK (1997) End-to-side neurorrhaphy: a histologic and morphometric study of axonal sprouting into an end-to-side nerve graft. J Reconstr Microsurg 13: 99–106. [DOI] [PubMed] [Google Scholar]
- 92.Liu K, Chen LE, Seaber AV, Goldner RV, Urbaniak JR (1999) Motor functional and morphological findings following end-to-side neurorrhaphy in the rat model. J Orthop Res 17: 293–300. [DOI] [PubMed] [Google Scholar]
- 93.Okajima S, Terzis JK (2000) Ultrastructure of early axonal regeneration in an end-to-side neurorrhaphy model. J Reconstr Microsurg 16: 313–323; discussion 323–316. [DOI] [PubMed] [Google Scholar]
- 94.Rovak JM, Cederna PS, Macionis V, Urbanchek MS, Van Der Meulen JH, Kuzon WM Jr (2000) Termino-lateral neurorrhaphy: the functional axonal anatomy. Microsurgery 20: 6–14. [DOI] [PubMed] [Google Scholar]
- 95.Walker JC, Brenner MJ, Mackinnon SE, Winograd JM, Hunter DA (2004) Effect of perineurial window size on nerve regeneration, blood-nerve barrier integrity, and functional recovery. J Neurotrauma 21: 217–227. [DOI] [PubMed] [Google Scholar]
- 96.Akeda K, Hirata H, Matsumoto M, Fukuda A, Tsujii M, Nagakura T, et al. (2006) Regenerating axons emerge far proximal to the coaptation site in end-to-side nerve coaptation without a perineurial window using a T-shaped chamber. Plast Reconstr Surg 117: 1194–1203; discussion 1204–1195. [DOI] [PubMed] [Google Scholar]
- 97.Hilliard MA (2009) Axonal degeneration and regeneration: a mechanistic tug-of-war. J Neurochem 108: 23–32. 10.1111/j.1471-4159.2008.05754.x [DOI] [PubMed] [Google Scholar]
- 98.Palazzi S, Vila-Torres J, Lorenzo JC (1995) Fibrin glue is a sealant and not a nerve barrier. J Reconstr Microsurg 11: 135–139. [DOI] [PubMed] [Google Scholar]
- 99.Ornelas L, Padilla L, Di Silvio M, Schalch P, Esperante S, Infante RL, et al. (2006) Fibrin glue: an alternative technique for nerve coaptation—Part II. Nerve regeneration and histomorphometric assessment. J Reconstr Microsurg 22: 123–128. [DOI] [PubMed] [Google Scholar]
- 100.Choi BH, Han SG, Kim SH, Zhu SJ, Huh JY, Jung JH, et al. (2005) Autologous fibrin glue in peripheral nerve regeneration in vivo. Microsurgery 25: 495–499. [DOI] [PubMed] [Google Scholar]
- 101.Silva DN, Silva AC, Aydos RD, Viterbo F, Pontes ER, Odashiro DN, et al. (2012) Nerve growth factor with fibrin glue in end-to-side nerve repair in rats. Acta Cir Bras 27: 325–332. [DOI] [PubMed] [Google Scholar]
- 102.Silva DN, Coelho J, Frazilio Fde O, Odashiro AN, Carvalho Pde T, Pontes ER, et al. (2010) End-to-side nerve repair using fibrin glue in rats. Acta Cir Bras 25: 158–162. [DOI] [PubMed] [Google Scholar]
- 103.Liang X, Cai H, Hao Y, Sun G, Song Y, Chen W (2014) Sciatic nerve repair using adhesive bonding and a modified conduit. Neural Regen Res 9: 594–601. 10.4103/1673-5374.130099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kolter M, Ott M, Hauer C, Reimold I, Fricker G (2015) Nanotoxicity of poly(n-butylcyano-acrylate) nanoparticles at the blood-brain barrier, in human whole blood and in vivo. J Control Release 197: 165–179. 10.1016/j.jconrel.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 105.Mennen U (2004) End-to-side nerve suturing technique. J Hand Surg Br 29: 514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Relevant data are within the paper. Row data about Grasping test and morphoquantitative analysis are available on the "Open Science Framework" Repository (doi: osf.io/p9n52).