Abstract
Most mosquito control efforts are primarily focused on reducing the adult population size mediated by reductions in the larval population, which should lower risk of disease transmission. Although the aim of larviciding is to reduce larval abundance and thus recruitment of adults, nonlethal effects on adults are possible, including transstadial effects on phenotypes of adults such as survival and pathogen infection and transmission. In addition, the mortality induced by control efforts may act in conjunction with other sources of mosquito mortality in nature. The consequences of these effects and interactions may alter the potential of the population to transmit pathogens. We tested experimentally the combined effects of a larvicide (Bacillus thuringiensis ssp. israelensis, Bti) and competition during the larval stages on subsequent Aedes aegypti (Linnaeus) traits, population performance, and susceptibility to dengue-1 virus infection. Ae. aegypti that survived exposure to Bti experienced accelerated development, were larger, and produced more eggs with increasing amounts of Bti, consistent with competitive release among surviving mosquitoes. Changing larval density had no significant interactive effect with Bti treatment on development and growth to adulthood. Larval density, but not Bti or treatment interaction, had a strong effect on survival of adult Ae. aegypti females. There were sharper declines in cumulative daily survival of adults from crowded than uncrowded larval conditions, suggesting that high competition conditions of larvae may be an impediment to transmission of dengue viruses. Rates of infection and dengue-1 virus disseminated infections were found to be 87±13% and 88±12%, respectively. There were no significant treatment effects on infection measurements. Our findings suggest that larvicide campaigns using Bti may reduce the number of emerged adults, but survivors will have a fitness advantage (growth, development, enhanced production of eggs) relative to conspecifics that are not under larvicide pressure. However, under most circumstances, these transstadial effects are unlikely to outweigh reductions in the adult population by Bti and altered risk of disease transmission.
Author Summary
Effective control of mosquito-borne diseases like dengue fever has historically been achieved by controlling vector populations. The use of larvicides that kill the larval stages of the yellow fever mosquito is a critical component of effective control. However, larvicides may act together with other components of the environment like larval crowding and alter the biology of adult mosquitoes. The authors found that mosquitoes that survived exposure to the larvicide Bacillus thuringiensis spp. israelensis (Bti) experienced faster development, were bigger, and produced more offspring. Larval crowding, but not larvicide Bti, had a strong effect on survival of adult Aedes aegypti (Linnaeus) females. Adult mosquitoes from crowded larval conditions had reduced survival compared to individuals from uncrowded larval conditions. Exposure to the larvicide Bti or larval crowding did not alter the ability of the surviving adult mosquitoes to become infected with dengue virus. Our findings suggest that larvicide campaigns may reduce the number of emerged adults, but survivors will have a fitness advantage (growth, development, enhanced production of offspring) relative to mosquitoes not exposed to the larvicide. However, under most circumstances, these effects are unlikely to outweigh reductions in the adult population by larvicide Bti and altered risk of disease transmission.
Introduction
Aedes aegypti (Linnaeus) is regarded as one of the most important vectors of arboviruses that affect human health, including yellow fever, chikungunya, and dengue. Protecting humans from the diseases transmitted by this mosquito has historically been achieved by controlling mosquito populations. In conjunction with the use of pesticides, development and testing of non-pesticide control strategies and products is ongoing to determine their utility for the control of mosquito vectors of disease (e.g., genetically modified organisms, [1]; Wolbachia symbionts, [2]). Control approaches to reduce populations of Ae. aegypti have focused on the use of larvicides (e.g., temephos, Bacillus thuringiensis spp. israelensis (Bti), methoprene), space spraying (aircraft and vehicle-mounted ULV sprayers and portable thermal foggers of insecticides such as malathion and pyrethroids), source reduction, biological control (larvivorous fish and copepods), and education of the public [3, 4]. Despite the heavy reliance on larval control, there is little understanding of how larval control interventions (insecticide, biological) act in conjunction with other sources of larval mortality in nature to influence population size of adults, characteristics of surviving adult mosquitoes and subsequent consequences for risk of disease transmission.
Density-dependent processes which induce mortality of mosquito vectors during the immature stages also influence recruitment to the adult stage as well as growth and development [5]. Density-dependence attributable to larval competition has been demonstrated for mosquito vectors of pathogens (e.g. Ae. aegypti, [6]; Culex quinquefasciatus Say, [7]; Anopheles gambiae Giles, [8]) and extends to other mosquito species (e.g., [9]). Specifically, there are greater numbers of larvae that die when there are many larvae than when there are few conspecific larvae, attributable in most instances to competition for food resources. Additionally, density-dependent size at metamorphosis may be the basis for population regulation in some mosquito species (Aedes sierrensis Ludlow) [10].
Density-dependent competition may also have other consequences on risk of disease transmission, such as changes in characteristics of the surviving adult mosquitoes. In other words, density-dependence in the larval stages may have transstadial effects that are realized in the adult stage. Alterations in adult traits or population genetics associated with biting behavior, adult survival, and vector competence for pathogens among survivors after density-induced mortality events could influence vectorial capacity. For example, inter- and intraspecific competition among container mosquitoes alter adult survival [11–13] and vector competence for arboviruses [14–16].
It is often assumed that larval control practices which cause mortality among juvenile mosquitoes will act additively with other sources of mortality resulting in lower adult population size. Although this may be the case in most instances, alternative outcomes are possible [9, 17]. For example, larval competition can be severe in container inhabiting mosquitoes, such that many individuals die in the larval stage. If competition is reduced (a lower number of larvae in a container), more individuals may survive to adulthood [18]. Results observed in other systems have demonstrated that competitive stress may enhance [19, 20] or diminish [21] the lethal effects of pesticides [19, 20], hypothesized to be caused in part through changes in the food web. So, sources of mortality may have additive or non-additive effects. Additive refers to the arithmetic sum of individual effects, whereas non-additive refers to departure from additivity. If multiple causes of mortality are additive, total mortality is the sum of mortality from each source. Non-additive mortality may be observed in compensatory mortality, where the total number surviving is not affected by multiple mortality causes, or in overcompensation, where multiple causes of mortality result in lower total mortality than if acting alone [22]. Therefore, control measures aimed at reducing the larval population can have unanticipated outcomes on the adult numbers as well as characteristics of the adult survivors. Assessing the ultimate consequences of changes in larval density to the final population size or potential for pathogen transmission requires knowledge of the individual and combined effects.
Exposure to pesticides during the larval stages can alter immunity which may influence susceptibility to pathogens and infectivity [23–26]. Insecticides like Bti may influence mosquito life history traits and susceptibility to pathogens through changes in microbial communities and nutrient dynamics [27] and by changes in the energy budget and immune system. For example, exposure to an organophosphate during the larval stages altered the expression of immune-related genes in larvae and adult female Ae. aegypti [28]. Additionally, insecticide resistance is energetically costly, including resistance to Bti [29], which may compromise immune responses (e.g. production of detoxification enzymes that result in metabolic resistance [30, 31], and interfere with pathogen infection [32]. The effect of intraspecific competition among larvae and exposure to the organophosphate malathion on Ae. aegypti and Aedes albopictus (Skuse) survivorship to adulthood and life history traits among survivors have been examined using Sindbis virus as a model system [28, 33]. For both species, competition and the presence of malathion reduced survival to adulthood. The pesticide reduced larval density, eliminating the negative effects of competition that otherwise resulted in lengthened development time and small adults. Thus, malathion treatment led to short development time and large adults in high competition. For Ae. aegypti, but not Ae. albopictus, high competition conditions and the presence of malathion led to an increase in the number of mosquitoes with disseminated Sindbis virus infections [33]. These results suggest that competition and pesticides may influence disease transmission directly by altering recruitment to the population of adults and indirectly by altering phenotypes of adults (size, competence for arboviruses). Although these initial studies suggest an indirect role of malathion on mosquito competence using a model arbovirus system, there is a need to evaluate these effects for pathogens important to human health [34]. The current study investigates the relationship between density-dependence and Bti, one of the pesticides currently used to control dengue vectors [35, 36].
This paper reports on laboratory investigations to test Bti and density-dependent effects on Ae. aegypti traits, population performance, and infection with dengue-1 virus. Our first goal was to determine whether Bti affects adult survival and reproduction. Secondly, we investigated whether Bti interacts with intraspecific competition to alter adult survival, propensity to blood feed and barriers to infection and dissemination for dengue-1 virus. We explore possible effects using multiple levels within each Bti and density treatment. The estimates obtained from these experiments will facilitate parametrization of models aimed at investigating the interactive effects of larval crowding and Bti on risk of dengue transmission.
Materials and Methods
Mosquitoes
Mosquitoes used were F3 progeny of larvae collected from Key West, Florida. We used Ae. aegypti from Key West as this population vectored dengue virus in 2009 and 2010. Larvae were hatched from eggs submerged in containers with 1.0 L tap water and 0.15 g of larval food consisting of an equal mixture of brewer’s yeast and lactalbumin at 25±1°C and a 14:10 L:D photoregime. First instar larvae were rinsed free of larval food and added to experimental microcosms consisting of 2.5 L cylindrical plastic containers with lids, 2.0 L tap water and 0.2 g larval food. No additional food was added during the experiments. The experimental containers were maintained under the same temperature and photoregime used during larval hatching.
Influence of Bti on immature development and adult survival and reproduction
Each experimental container was stocked with 300 newly hatched Ae. aegypti larvae. Bti was applied to each replicate container on the same day the larvae were added. The concentrations of Bti applied were 0 (control), 0.0009, 0.0025, 0.007, 0.0194, 0.054, 0.15, and 0.25 parts per million (ppm) using our own dilutions from a commercially available formulation with a potency of 3000 International toxic units (ITU) per mg. The levels of Bti used approximate the LD50 (LD50 for a similar Bti product, VectoBac WG: active ingredient: 3,000 Bti ITU per mg, 0.017–0.018 ppm for 3rd instar Ae. aegypti [37], as well as concentrations below and above the LD50. No mosquitoes survived to adulthood in concentrations of 0.0194 ppm or higher. We replicated treatments with exposure to Bti three times, whereas treatments not exposed to Bti (0 ppm, control) were replicated four times. Experimental containers were maintained in a walk-in incubator. On a daily basis experimental containers were examined for pupae which were transferred to plastic vials plugged with cotton to capture emerged adults. The position of treatment containers within the incubator were changed daily. Newly eclosed adult Ae. aegypti were recorded by sex and date and then transferred to 16 ounce (by volume) cylindrical cages. Mosquitoes were provided with a 20% sucrose solution. Adult females aged 7–10 days were allowed to feed on defibrinated bovine blood using an artificial feeding system (Hemotek, Discovery workshops, Accrington, UK). Fully engorged females were separated from unfed and partially fed mosquitoes and held individually in cages. Mosquitoes that took partial blood meals were used in calculation of development and survivorship to adulthood. However, these mosquitoes were not used in calculation of adult survival, fecundity, and size. Each cage contained a plastic cup (30ml volume) filled with water and lined with seed germination paper as an oviposition substrate. Fully engorged females were maintained using the same environmental conditions and access to water and sucrose. Mosquitoes were monitored daily and the date of death was recorded by treatment and replicate. As an indicator of body size, the wings of mosquitoes were dissected and measured by length (axillary incision to wing tip) from a photo using a microscope and image analysis software (Media Cybernetics, Maryland, USA). The number of eggs oviposited by individual females during the first gonotrophic cycle was counted. For each container replicate we used all the mosquitoes available to measure survivorship to adulthood, development time, size, number of eggs oviposited, and adult survival.
Effect of Bti and competition on adult survival and infection with dengue-1 virus
Density and Bti treatments
The experiment employed a factorial combination of five intraspecific larval densities and four Bti concentrations. Each experimental container was stocked with initial densities; 50, 100, 150, 250, and 300 (0.025–0.15 larvae/ml). Larval densities were within natural densities in Florida containers occupied by Ae. aegypti and competitor Ae. albopictus (N = 790, mean±SE, 0.17±0.02, range 0.00083–3.08 larvae/ml [14]. Bti was applied to each replicate container on the same day the larvae were added. The concentrations of Bti applied were 0 (control), 0.0042, 0.0056, and 0.007 ppm. We replicated treatments with exposure to Bti three times and treatments not exposed to Bti (0 ppm, control) were replicated four times. Experimental containers were maintained similarly as in the first experiment. Adult females from this experiment were then used in an infection experiment with dengue-1 virus (DENV-1).
Estimated finite rate of increase (λ')
An estimated finite rate of increase (λ') was calculated for each experimental container;
where N0 is the initial number of females in the cohort (assumed to be 50%); Ax is the number of females eclosing on day x; wx is the mean adult female size on day x, and D is the time from eclosion to reproduction taken as 12 d [38]. The relationship between size and fecundity was f (wx) = 60.730(wx)– 111.76 based on mosquitoes from our first experiment.
Virus
Dengue-1 virus (GenBank accession number JQ675358) was obtained from a human in Key West, FL during an outbreak of dengue and subsequently passaged twice in Vero cells prior to use in the infection study. Viruses and host cells were cultured in media containing medium 199, 10% fetal bovine serum, 100 units/mL of penicillin, and 100 μg/mL of streptomycin.
Oral infection of mosquitoes
Infected blood meals were prepared by harvesting media from monolayers of Vero cells previously inoculated with a multiplicity of infection of 0.01 (viruses/cell) seven days prior. Virus infected media were combined with an equal volume of defibrinated bovine blood. Adult females aged 7–10 days were allowed to feed on DENV-1 infected blood using an artificial feeding system. Fully engorged females were separated from unfed and partially fed mosquitoes and held at 28°C with access to 20% sucrose solution. After 14 days of incubation, mosquitoes were stored at -80°C. Blood feeding rates were calculated as the number of fully engorged mosquitoes from the total of mosquitoes offered blood. Mosquitoes that did not blood feed were maintained under similar conditions and monitored daily until death.
Determination of susceptibility to virus infection and dissemination
Mosquitoes were dissected to remove legs and a single wing from the remainder of the body. Bodies and legs were triturated separately in centrifuge tubes with 0.9 mL BA-1 (10x medium 199, 1% bovine serum albumin, 0.05 M TRIS, 100 units/mL of penicillin, 100 μg/mL of streptomycin, 1 μg/mL of mycostatin). Nucleic acid was extracted from 250-μL samples and eluted in 50 μL buffer using the MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche Diagnostics, Indianapolis, IN). Viral RNA in samples was determined using the Superscript III One-Step Quantitative RT-PCR System (Invitrogen, Carlsbad, CA) with a Light Cycler 480 system (Roche, Mannheim, Germany) [39]. A standard curve method was used to relate the amount of DENV RNA present in mosquito samples to serial dilutions of virus stock with known concentrations expressed in plaque forming unit equivalents (pfue)/ml [40, 15]. Infection rate was calculated as the percent of mosquitoes with DENV-1 RNA present in their bodies from the total number that fed. Dissemination rate was calculated as the percent of mosquitoes with infected bodies that have DENV-1 RNA present in their legs. A total of 755 adult females were used in testing for DENV-1.
Statistical analysis
Treatment effects on survivorship to adulthood, female size, and female development were analyzed using ANOVA and multivariate analysis of variance (MANOVA). Standardized canonical coefficients (SCC) were used to determine the relative contribution of survivorship, size, and development when significant treatment effects were detected (PROC GLM, SAS 9.22). Blood feeding rates, number of eggs oviposited, and λ' were analyzed by ANOVA. When significant treatment effects were detected, pair-wise contrasts of means were performed (PROC GLM, SAS 9.22). Regression analysis was used to relate mosquito wing length to number of eggs oviposited (PROC REG, SAS 9.22).
Treatment effects on survival of adults were compared using a regression analysis of survival data based on the Cox proportional hazards model (PROC PHREG, SAS 9.22). Treatment effects on susceptibility to DENV-1 infection and dissemination were analyzed using MANOVA and SCC. Treatment effects on DENV-1 titer in the bodies and legs of mosquitoes were analyzed using ANOVA.
Results
Influence of Bti on immature development and adult survival and reproduction
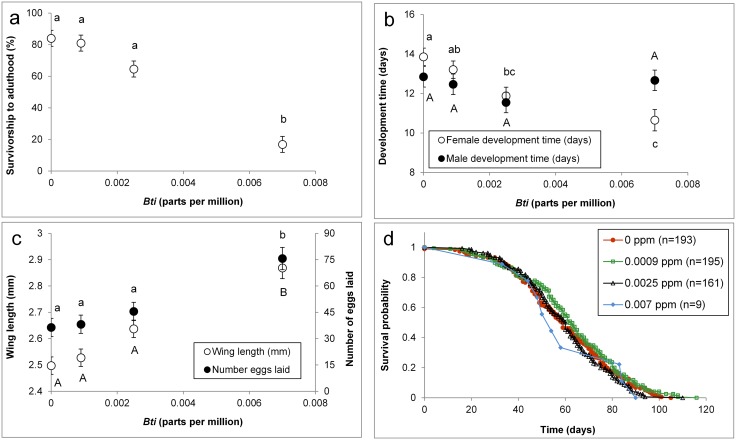
Mean survivorship to adulthood was significantly affected by the concentration of Bti (F7,16 = 58.01, p<0.0001), with no survivors at concentrations ≥ 0.0194. Increasing concentrations of Bti were associated with significantly lower survivorship to adulthood (Fig 1a). Development time of females, from treatments with survivors, was significantly affected by the presence of Bti (F3,7 = 8.48, p = 0.009). In the absence of Bti, development times were significantly longer than treatments with Bti, except at the lowest level of Bti. In the presence of Bti, there was a consistent decrease in development time of females with increases in the concentration of Bti (Fig 1b). Development time of males were unaffected by the presence of Bti (F3,8 = 1.20, p = 0.36; Fig 1b). Regression analysis showed a significant positive relationship between Ae. aegypti wing length (mm) and number of eggs oviposited (y = 60.737x-111.76, r2 = 0.35, n = 212, p<0.0001; where y = number of eggs and x = wing length in mm). Significantly longer wing lengths and more eggs were oviposited by Ae. aegypti from the highest Bti treatment than all other treatments (F3,7 = 9.11, p = 0.008; Fig 1c). The relationship between wing length and number of eggs oviposited did not differ between Bti treatments (test for equal slopes, F3 = 1.17, p = 0.32). Thus, the slopes were not different between Bti treatments. There was an approximate 46% increase in mean number of eggs oviposited by females exposed to the highest amount of Bti relative to the control. A caveat to this interpretation is that females were not dissected and examined for retained eggs, thus potentially underestimating fecundity. A total of 558 adult females were monitored daily to record date of death, which was used to establish treatment dependent survival distributions. Survival analyses showed no significant differences in adult survival among Bti treatments (χ2 = 5.13, df = 3, p = 0.16; Fig 1d).
Fig 1. Bacillus thuringiensis israelensis treatment effects on Aedes aegypti a) survivorship to adulthood, b) female and male development time, c) female wing length and number of eggs oviposited, and d) adult female survival.
Symbols associated with different letters show significant differences. The number of mosquitoes tested is denoted in parentheses. There were no survivors at Bti concentrations ≥0.0194.
Effect of Bti and competition on adult survival and infection with dengue-1 virus
Size, development, survivorship and λ'
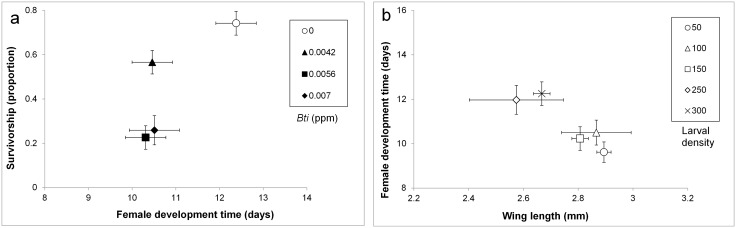
MANOVA showed significant effects of pesticide and density treatments on survivorship to adulthood, development, and size. The interaction between these two factors was not significant (Table 1). SCCs showed that differences in survivorship to adulthood and female development time contributed the most to the significant effect of pesticide. Increases in pesticide were associated with decreased survivorship and shorter development times (Fig 2a). SCCs showed that differences in wing length and female development time contributed the most to the significant effect of density (Table 1). Increases in density were associated with shorter wing lengths and lengthened development times (Fig 2b).
Table 1. Multivariate analysis of variance and standardized canonical coefficients (SCC) for treatment effects of density, presence of pesticide (Bti) and interaction on survivorship to adulthood, female development time and female wing length.
| Source | df (num., denom.) | Pillai’s trace | p1 | SCC | ||
|---|---|---|---|---|---|---|
| survivorship | development | wing length | ||||
| Pesticide (P) | 9, 108 | 0.68 | 0.0007 | 1.19 | 0.52 | -0.10 |
| Density (D) | 12, 108 | 0.94 | <0.0001 | 0.27 | -0.47 | 1.48 |
| P x D | 30, 108 | 0.53 | 0.77 | 0.13 | -0.25 | 1.57 |
1Significant p-values are in bold.
Fig 2. Bivariate plots of least-squares means (±SE) for three dependent variables for Aedes aegypti females.
a) The effects of pesticide treatment on survivorship to adulthood and female development time. b) The effects of density treatment on wing length and female development time.
ANOVA showed significant effects of pesticide, density, and interactions on λ' (all F≥6.50, p≤0.001). The interaction was attributable to significantly lower λ'-values from the highest Bti treatment and a density of 250 initial larvae (mean±SE, 0.33±0.07) than all other treatments. All remaining pairwise comparisons of treatments did not significantly differ from one another. λ' in the highest Bti treatment was significantly lower than in all other treatments (Fig 3). λ' values were not significantly different between the other Bti treatments. λ' in the 250 initial larvae treatment was significantly lower than in all other density treatments (Fig 3). The reason for the relatively low values of λ' associated with the highest Bti treatment and a density of 250 initial larvae is because two of the three replicates failed to produce adult females, thus yielding zero values for λ' for these replicates.
Fig 3. Least-squares means (±SE) for λ' for Aedes aegypti from Bacillus thuringiensis israelensis and density treatments.
Adult blood feeding and survival
Treatment effects of density, pesticide, and their interaction on the immature stages did not significantly affect blood feeding rates of adult females (all F<1.51, p>0.18; Table 2, mean±SE, 59.8±17.5%).
Table 2. Analysis of variance for larval density and pesticide treatment effects on rate of blood feeding.
Regression analysis of survival of adult Aedes aegypti females in response to treatment effects of density, pesticide (Bti) and their interaction.
| Blood feeding | Survival of adults | |||||
|---|---|---|---|---|---|---|
| Source | df | F | p1 | df | χ2 | p1 |
| Density (D) | 3 | 1.37 | 0.27 | 4 | 37.20 | <0.0001 |
| Pesticide (P) | 4 | 0.70 | 0.60 | 3 | 5.77 | 0.12 |
| D x P | 10 | 1.51 | 0.18 | 11 | 15.71 | 0.15 |
1Significant p-values are in bold.
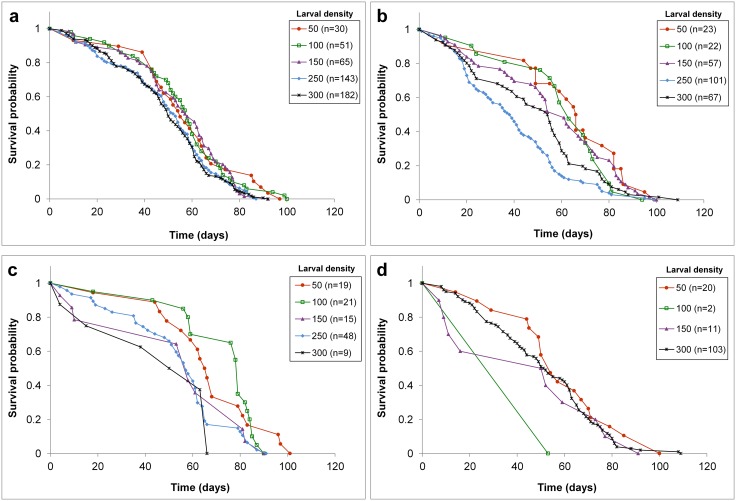
A total of 969 adult females were used to establish treatment dependent survival distributions. Survival of adult females depended on density but not pesticide or the treatment interaction (Table 2). The treatment 250 larvae and 0.007 ppm Bti yielded very few survivors, thus limiting our ability to make pairwise comparisons with the analysis that included both Bti and density treatments. Because the effects of Bti were not significant, we reduced the model to test only for density, enabling us to perform all possible pair-wise comparisons for the density treatment. Comparisons of survival over time showed significantly steeper declines at initial densities of 300, 250, and 150 compared to 50 and 100 Ae. aegypti larvae (Table 3 and Fig 4). Survival did not significantly differ among adult females produced from the three highest density larval environments at various Bti concentrations.
Table 3. Pairwise comparisons for significant density treatment effect on adult Aedes aegypti female survival.
P-values were corrected for multiple comparisons using the Tukey-Kramer method.
| Comparison | Estimate | Std. Error | z value | Adjusted p1 |
|---|---|---|---|---|
| 100 vs 150 | -0.14 | 0.13 | -1.08 | 0.816 |
| 100 vs 250 | -0.53 | 0.12 | -4.45 | <0.0001 |
| 100 vs 300 | -0.41 | 0.11 | -3.53 | 0.0038 |
| 100 vs 50 | 0.07 | 0.14 | 0.52 | 0.985 |
| 150 vs 250 | -0.39 | 0.10 | -3.81 | 0.0013 |
| 150 vs 300 | -0.27 | 0.09 | -2.72 | 0.050 |
| 150 vs 50 | 0.22 | 0.13 | 1.63 | 0.477 |
| 250 vs 300 | 0.12 | 0.07 | 1.51 | 0.554 |
| 250 vs 50 | 0.61 | 0.12 | 4.97 | <0.0001 |
| 300 vs 50 | 0.49 | 0.12 | 4.09 | 0.0004 |
1Significant p-values are in bold.
Fig 4. Density and treatment effects on Aedes aegypti adult female survival (969 mosquitoes tested) derived from immature environments with Bacillus thuringiensis israelensis concentrations of a) 0 parts per million (ppm), b) 0.0042 ppm, c) 0.0056 ppm, and d) 0.007 ppm.
Larval densities range from 50–300 and the number of mosquitoes tested is denoted in parentheses. The survival of females is not shown for 0.007 ppm Bti and 250 density of Ae. aegypti because there were too few survivors.
Susceptibility of Ae. aegypti for dengue-1 virus
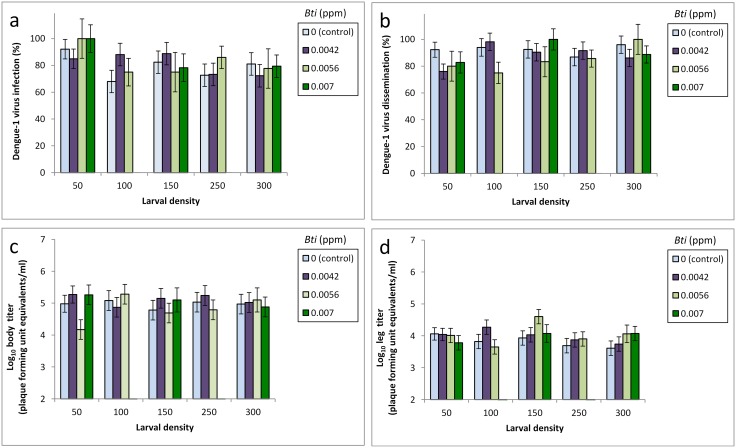
The dengue viral titers in blood meals were within the range of viremia in humans (mean±SD, 7.2±0.8 PFU/ml [41, 42]. A total of 755 adult females were tested for susceptibility to infection and viral dissemination for dengue-1 virus. Rates of infection and viral dissemination were 87±13% and 88±12%, respectively (Fig 5a and 5b). Dengue-1 virus body and leg titers were 4.9±0.5 and 3.9±0.2 log10 plaque forming unit equivalents/ml (Fig 5c and 5d). Treatment effects of density, pesticide, and their interaction experienced by the immature stages did not significantly affect infection and viral dissemination rates as well as viral titer of adult females (Table 4).
Fig 5. Least-squares means (±SE) for treatment effects of Bacillus thuringiensis israelensis and initial larval density on Aedes aegypti a) body infection, b) leg infection (viral dissemination), c) body viral titer (plaque forming unit equivalents/ml), and d) leg viral titer (plaque forming unit equivalents/ml) following exposure to dengue-1 virus.
Table 4. Multivariate analysis of variance and standardized canonical coefficients (SCC) for treatment effects of density, presence of pesticide (Bti) and interaction on infection and viral dissemination rates, and viral titer in mosquito bodies and legs.
Mean±SD provided for infection (%), dissemination (%), body titer (B), leg titer (L). Titers are expressed as log10 plaque forming unit equivalents/ml. A total of 755 adult females were used in testing for dengue-1 virus.
| Source | df (num., denom.) | Pillai’s trace | p | SCC | |||
|---|---|---|---|---|---|---|---|
| Infection | Dissemination | Titer (B) | Titer (L) | ||||
| Pesticide (P) | 12, 90 | 0.31 | 0.58 | 0.38 | -0.43 | -0.06 | 1.02 |
| Density (D) | 16, 124 | 0.51 | 0.37 | 0.96 | -0.51 | -0.36 | 0.16 |
| P x D | 40, 124 | 0.97 | 0.48 | 0.25 | 0.46 | -0.08 | 0.89 |
| Mean±SD | 87±13% | 88±12% | 4.9±0.5 | 3.9±0.2 |
Discussion
Our results demonstrate that the outcome of mortality and phenotypic traits induced by Bti control efforts would not be expected to change at different amounts of larval crowding of Ae. aegypti. Female mosquitoes that survived exposure to Bti experienced accelerated development, were larger, and produced more eggs during the first gonotrophic cycle after being exposed to the highest concentration of Bti. Superior reproductive potential among survivors may facilitate recovery of local populations of Ae. aegypti following larviciding [18, 43]. Mosquito larvae in nature may be exposed to sublethal concentrations of larvicide attributable to recolonization of container habitats with sublethal amounts of larvicide or exposure to less than the “full” lethal dose at time of widespread application of larvicides (low-volume area-wide strategy) [44]. For example, changes in life history traits in our experiments occurred at high exposure to Bti that resulted in ~80% mortality (inhibition of adult emergence) which approximates observations of rates of mortality (87%) of area-wide ground applications of Bti to control dengue vector Ae. albopictus in residential neighborhoods [44]. Larvicide resistance in many populations of Ae. aegypti is further evidence for the occurrence of exposure to sublethal concentrations of larvicides in nature [45–48].
Our observations suggest that Ae. aegypti larvae may respond to reductions in the number of conspecifics attributable to Bti killing a fraction of the larvae. It is possible that selective mortality due to Bti allowed for survival of some individuals with rapid growth and development (e.g., lethal concentration increases with larval development) [49]. However, it seems more likely that the mechanism relates to changes in density and availability of nutrition (microbial biomass) because consumption by mosquito larvae reduces digestible microorganisms [50]. Although we did not directly measure microbial populations, reductions in the number of larvae with associated increases in per capita nutrient resources are consistent with observed changes in growth and development. Other studies have shown strong and complex interactive effects of Bti on microbial communities and nutrient dynamics in microcosms [27]. Our observations support previous studies showing similar effects of pesticides on growth and developmental responses in Ae. aegypti, including fungal larvicide Metarhizium anisopliae (Metschn.) Sorokīn [43], neurotoxin spinosad [51], organophosphate malathion [28, 33, 52, 53], and botanical insecticides [54].
Most mosquitoes are sexually dimorphic and protandrous, where the latter here refers to the eclosion of males before females into a seasonal breeding population. Sex-specific development and size may explain why the developmental response of males (demonstrating canalization) differed from females along a gradient of Bti. Males are often smaller than females and develop more quickly. Competitive release among surviving larvae accelerated metamorphosis among females (21% reduction in development time in the highest amount of Bti than controls), whereas the same benefit was negligible among males with inherently shorter developmental time. Sex-specific reaction norms (development, size, survival, nutrient reserves) have been observed among several container dwelling mosquito species exposed to predators [55, 56] competitors [57, 58], and varying nutrition [59] during the immature stages.
Mosquitoes experienced a 46% increase in the number of eggs produced at the highest amount of Bti than controls, which covaried with size. This means that mosquitoes exposed to sublethal amounts of Bti did not incur a physiological cost of reproduction. Alternatively, any physiological cost of reproduction was outweighed by enhanced size (13% increase) following exposure to Bti, presumably associated with reductions in the number of larvae. A caveat to this interpretation is that we did not measure cumulative lifetime fecundity (net reproductive rate) and so we cannot assess whether there are costs of reproduction later in life (e.g., daily fecundity through lifespan) [60]. However, substantially fewer individuals would survive the second gonotrophic cycle assuming a mean probability of daily survival of 0.6–0.89 [61–63] and approximate duration of the gonotrophic cycle of three days in nature [64]. Size of Ae. aegypti females in our study were within the range and approximated the mean size observed in the field [65–67]. Fecundity increases with size of adult Ae. aegypti females [68–70] as in most insects [71]. However, the current study is one of a few demonstrating an indirect role of a pesticide enhancing the number of eggs produced by mosquitoes (e.g.,[51]).
The presence of Bti did not alter survival of adults and so Bti appears to selectively influence traits of adults. Lifespan and fecundity determine fitness and so the lasting effects of sublethal exposure to Bti are likely to minimally influence (perhaps increase) net reproductive rate. The influence of Bti on mosquito life history traits acted independently of those effects attributable to larval crowding, suggesting that competitively stressed mosquitoes respond similarly to this pesticide as do mosquitoes from less stressful conditions. This suggests that the efficacy of Bti in nature should be robust to spatial and temporal heterogeneity in the number of larvae in containers. Responses in population performance to treatments were less distinct than those observed for life history traits. λ' is heavily influenced by changes in survivorship to adulthood. Despite using a 6-fold difference in the initial density of larvae, survivorship to adulthood contributed less than that of development and growth effects, which in part, explains the lack of many treatment differences in λ'. Curiously, two of the three treatments at the highest Bti concentration and a density of 250 initial larvae failed to produce adult females which largely contributed to observed treatment differences. Although it is unclear what accounts for the observed effect, non-linearities in the relationship between density and mosquito responses have been observed in other container-dwelling Aedes species [11, 72]. The highest concentration of Bti significantly reduced λ', but lower concentrations did not. This result suggests that larvicidal controls that achieve less than about 75% mortality may not be effective at achieving substantial declines in Ae. aegypti populations. This interpretation agrees in general with transmission models of dengue that have found that substantial levels of larval control such as source reduction would be needed to reduce Ae. aegypti pupae per person in an environment [73].
Lengthened development and reduced growth were largely responsible for the density effect, suggesting that larval crowding and nutrition were not limiting factors that substantially influenced survivorship to adulthood. We observed strong transstadial effects of density with steeper declines in survival of adult Ae. aegypti females from crowded larval conditions, perhaps attributable to changes in larval biosynthesis of nutritive reserves [69, 74–76] or an additional stressor on the mosquito’s physiology [28, 77, 78]. Our observations support findings from laboratory studies that used manipulations of larval nutrition and crowding and measured survival of adult Ae. aegypti females [11, 13, 76]. Measurements of daily survival rates of Ae. aegypti in nature and the laboratory have used size as an indicator of relative nutrition and crowding experienced during the immature stages. [66] analyzed laboratory results in which adult survival of Ae. aegypti increases with size, but decreases at the largest sizes (i.e., non-linear relationship between size and longevity). Similarly, the relationship between adult parous rate, used to measure mosquito survival, and size among wild-caught Ae. sierrensis females was curvilinear [79]. Using the same approach, field studies in Thailand and Puerto Rico found no relationship between parity status and size of Ae. aegypti [67]. A mark-release-recapture field study in Brazil showed that laboratory reared large adult Ae. aegypti males, but not females, had a survival advantage relative to smaller individuals from a low food diet [62]. Field studies often assume that heterogeneity in size of mosquitoes is largely determined by nutrition or larval crowding, however other factors may be responsible as well (environmental temperature, genetics). Collectively, these studies suggest that ecological conditions that larvae experience may have a strong transstadial effect on daily survival rates of adult Ae. aegypti females.
In the current study, the heterogeneity in sizes of adult females was comparable to those observed in Ae. aegypti in the laboratory [80, 81], but narrower than the range in the field [66], where differences in dengue virus infection were observed. The reason for lack of heterogeneity in dengue virus infection in the current study is unclear. However, infection and viral dissemination rates in Ae. aegypti females were much higher than those observed in other studies [15, 80, 81]. Thus, dose-dependent effects of virus exposure to Ae. aegypti females may obscure more subtle effects attributable to ecological conditions experienced by larvae. For example, our ability to detect a treatment-induced increase, but not reduction, in infection and viral dissemination is limited due to the relatively high rates observed. Also, survivorship to adulthood was higher in the current study (41–57%) compared to other studies (31–36%) [15] that have observed density-dependent heterogeneity in dengue virus infection, suggesting less competitive stress. Previous studies have demonstrated that intra and interspecific larval competition and availability of nutrients enhanced susceptibility to dengue virus infection [15, 81, 82]. However these effects appear to be stronger for Ae. albopictus than Ae. aegypti [15]. Similarly, larval competition enhanced infection and viral dissemination of Sindbis virus in Ae. albopictus, but not Ae. aegypti [14], suggesting species-specific differences in barrier(s) to infection and tolerance for stress.
Given the prominent role that larvicides serve in Ae. aegypti control programs, there is a compelling need to understand how larvicides act in concert with other sources of mortality in larval habitats in nature. Here we show that low concentrations of a common larvicide had direct lethal effects on Ae. aegypti immatures and indirect effects on select life history traits. We would expect that larvicide campaigns may reduce the number of emerged adults, but survivors will have a lifetime fitness advantage (growth, development, number of eggs oviposited) relative to conspecifics. However, we did not find any evidence to suggest that exposure to Bti changes survival of adult females or their rates of infection with dengue virus, both components of vectorial capacity. Under most circumstances, these transstadial effects are unlikely to outweigh reductions in the adult population by Bti and altered risk of disease transmission.
Acknowledgments
Dengue virus (strain BOL-KW010) was kindly provided by the Florida Department of Health Bureau of Laboratories. The authors thank S. Ortiz, D. Bettinardi, and P. DeChant for assistance with experiments, and N. Burkett-Cadena, D. Duguma, and L.P. Lounibos for reviewing earlier versions of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study is supported by the Florida Department of Agriculture and Consumer Services grant number 00083933. http://www.freshfromflorida.com/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alphey L (2014) Genetic Control of Mosquitoes In: Berenbaum MR, editor. Annual Review of Entomology. pp. 205–224. 10.1146/annurev-ento-011613-162002 [DOI] [PubMed] [Google Scholar]
- 2.Iturbe-Ormaetxe I, Walker T, Neill SLO (2011) Wolbachia and the biological control of mosquito-borne disease. EMBO Reports 12: 508–518. 10.1038/embor.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization WHO (2006) Report of the scientific working group on dengue 1–5.
- 4.Nathan MB (1993) Critical review of Aedes aegypti control programs in the Caribbean and selected neighboring countries. Journal of the American Mosquito Control Association 9: 1–7. [PubMed] [Google Scholar]
- 5.Service MW. Population dynamics and mortalities of mosquito preadults In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes; 1985; Vero Beach, FL: Florida Medical Entomology Laboratory; pp. 185–201. [Google Scholar]
- 6.Dye C (1984) Models for the population dynamics of the yellow fever mosquito, Aedes aegypti. Journal of Animal Ecology 53: 247–268. [Google Scholar]
- 7.Ahumada JA, Lapointe D, Samuel MD (2004) Modeling the population dynamics of Culex quinquefasciatus (Diptera: Culicidae), along an elevational gradient in Hawaii. Journal of Medical Entomology 41: 1157–1170. [DOI] [PubMed] [Google Scholar]
- 8.Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, et al. (2002) Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. Journal of Medical Entomology 39: 162–172. [DOI] [PubMed] [Google Scholar]
- 9.Juliano SA (2007) Population dynamics In: Floore TG, editor. Biorational Control of Mosquitoes Supplement to the Journal of the American Mosquito Control Association: American Mosquito Control Association Bulletin No.7. pp. 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawley WA (1985) The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: epidemiological consequences. Animal Ecology 54: 955–964. [Google Scholar]
- 11.Reiskind MH, Lounibos LP (2009) Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Medical and Veterinary Entomology 23: 62–68. 10.1111/j.1365-2915.2008.00782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alto BW (2011) Interspecific larval competition between invasive Aedes japonicus and native A. triseriatus (Diptera: Culicidae) and adult longevity. Journal of Medical Entomology 48: 232–242. [DOI] [PubMed] [Google Scholar]
- 13.Alto BW, Bettinardi DJ, Ortiz S (2015) Interspecific larval competition differentially impacts adult survival in dengue vectors. Journal of Medical Entomology 52: 163–170. 10.1093/jme/tju062 [DOI] [PubMed] [Google Scholar]
- 14.Alto BW, Lounibos LP, Higgs S, Juliano SA (2005a) Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology 86: 3279–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alto BW, Lounibos LP, Mores CN, Reiskind MH (2008a) Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proceedings of the Royal Society B-Biological Sciences 275: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bevins SN (2008) Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae). Biological Invasions 10: 1109–1117. [Google Scholar]
- 17.Yakob L, Alphey L, Bonsall MB (2008) Aedes aegypti control: the concomitant role of competition, space and transgenic technologies. Journal of Applied Ecology 45: 1258–1265. [Google Scholar]
- 18.Agudelo-Silva F, Spielman A (1984) Paradoxical effects of simulated larviciding on production of adult mosquitoes. American Journal of Tropical Medicine and Hygiene 33: 1267–1269. [DOI] [PubMed] [Google Scholar]
- 19.Relyea RA, Diecks N (2008) An unforeseen chain of events: Lethal effects of pesticides on frogs at sublethal concentrations. Ecological Applications 18: 1728–1742. [DOI] [PubMed] [Google Scholar]
- 20.Jones DK, Hammond JI, Relyea RA (2011) Competitive stress can make the herbicide Roundup more deadly to larval amphibian. Environmental Toxicology and Chemistry 30: 446–454. 10.1002/etc.384 [DOI] [PubMed] [Google Scholar]
- 21.Boone MD, Semlitsch RD (2002) Interactions of an insecticide with competition and pond drying in amphibian communities. Ecological Applications 12: 307–316. [Google Scholar]
- 22.Washburn JO (1995) Regulatory factors affecting larval mosquito populations in container and pool habitats—implications for biological control. Journal of the American Mosquito Control Association 11: 279–283. [PubMed] [Google Scholar]
- 23.Kiesecker JM (2002) Synergism between trematode infection and pesticide exposure: A link to amphibian limb deformities in nature? Proceedings of the National Academy of Sciences of the United States of America 99: 9900–9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbertson MK, Haffner GD, Drouillard KG, Albert A, Dixon B (2003) Immunosuppression in the northern leopard frog (Rana pipiens) induced by pesticide exposure. Environmental Toxicology and Chemistry 22: 101–110. [PubMed] [Google Scholar]
- 25.Relyea R, Hoverman J (2006) Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecology Letters 9: 1157–1171. [DOI] [PubMed] [Google Scholar]
- 26.Davidson C, Benard MF, Shaffer HB, Parker JM, O'Leary C, et al. (2007) Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow-legged frogs. Environmental Science & Technology 41: 1771–1776. [DOI] [PubMed] [Google Scholar]
- 27.Duguma D, Hall MW, Rugman-Jones P, Stouthamer R, Neufeld JD, et al. (2015) Microbial communities and nutrient dynamics in experimental microcosms are altered after the application of a high dose of Bti. Journal of Applied Ecology 52: 763–773. [Google Scholar]
- 28.Muturi EJ, Kim HC, Alto BW, Berenbaum MR, Schuler MA (2011a) Larval environmental stress alters adult mosquito fitness and competence for Sindbis virus. Tropical Medicine and International Health 16: 955–964. [DOI] [PubMed] [Google Scholar]
- 29.Paris M, David JP, Despres L (2011) Fitness costs of resistance to Bti toxins in the dengue vector Aedes aegypti. Ecotoxicology 20: 1184–1194. 10.1007/s10646-011-0663-8 [DOI] [PubMed] [Google Scholar]
- 30.Hemingway J, Ranson H (2000) Insecticide resistance in insect vectors of human disease. Annual Review of Entomology 45: 371–391. [DOI] [PubMed] [Google Scholar]
- 31.Tantely ML, Tortosa P, Alout H, Berticat C, Berthomieu A, et al. (2010) Insecticide resistance in Culex pipiens quinquefasciatus and Aedes albopictus mosquitoes from La Reunion Island. Insect Biochemistry and Molecular Biology 40: 317–324. 10.1016/j.ibmb.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 32.McCarroll L, Paton MG, Karunaratne SHPP, Jayasuryia HTR, Kalpage KSP, et al. (2000) Insecticides and mosquito-borne disease. Nature 407: 961–962. [DOI] [PubMed] [Google Scholar]
- 33.Muturi EJ, Constanzo K, Kesavaraju B, Alto BW (2011b) Can insecticides and larval competition alter susceptibility of Aedes mosquitoes to arbovirus infection? Journal of Medical Entomology 48: 429–436. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization WHO (2014) A global brief on vector-borne diseases. WHO/DCO/WHD/2014.1: 54.
- 35.Tan AWA, Loke SR, Benjamin S, Lee HL, Chooi KH, et al. (2012) Spray application of Bacillus thuringiensis israelensis (Bti strain AM65-52) against Aedes aegypti (L.) and Ae. albopictus Skuse populations and impact on dengue transmission in a dengue endemic residential site in Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health 43: 296–310. [PubMed] [Google Scholar]
- 36.Boyce R, Lenhart A, Kroeger A, Velayudhan R, Roberts B, et al. (2013) Bacillus thuringiensis israelensis (Bti) for the control of dengue vectors: systematic literature review. Tropical Medicine & International Health 18: 564–577. [DOI] [PubMed] [Google Scholar]
- 37.Russell TL, Brown MLD, Purdie DM, Ryan PA, Kay BH (2003) Efficacy of VectoBac (Bacillus thuringiensis variety israelensis) formulations for mosquito control in Australia. Journal of Economic Entomology 96: 1786–1791. [DOI] [PubMed] [Google Scholar]
- 38.Grill CP, Juliano SA (1996) Predicting species interactions based on behavior: predation and competition in container-dwelling mosquitoes. Journal of Animal Ecology 65: 63–76. [Google Scholar]
- 39.Callahan JD, Wu S-JL, Dion-Schultz A, Mangold BE, Peruski LF, et al. (2001) Development and evaluation of serotype- and group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. Journal of Clinical Microbiology 39: 4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. Journal of Molecular Endocrinology 25: 169–193. [DOI] [PubMed] [Google Scholar]
- 41.Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A (1981) Viremia in patients with naturally acquited dengue infection. Bulletin of the World Health Organization 59: 623–630. [PMC free article] [PubMed] [Google Scholar]
- 42.Stramer SL, Linnen JM, Carrick JM, Foster GA, Krysztof DE, et al. (2012) Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion 52: 1657–1666. 10.1111/j.1537-2995.2012.03566.x [DOI] [PubMed] [Google Scholar]
- 43.Wilson ML, Agudelo-Silva F, Spielman A (1990) Increased abundance, size, and longevity of food-deprived mosquito populations exposed to a fungal larvicide. American Journal of Tropical Medicine and Hygiene 43: 557–566. [DOI] [PubMed] [Google Scholar]
- 44.Williams GM, Faraji A, Unlu I, Healy SP, Farooq M, et al. (2014) Area-wide ground applications of Bacillus thuringiensis var. israelensis for the control of Aedes albopictus in residential neighborhoods: From optimization to operation. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcombe S, Poupardin R, Darriet F, Reynaud S, Bonnet J, et al. (2009) Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies). BMC Genomics 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez MM, Bisset J, de Fernandez DM, Lauzan L, Soca A (2001) Detection of insecticide resistance in Aedes aegypti (Diptera: Culicidae) from Cuba and Venezuela. Journal of Medical Entomology 38: 623–628. [DOI] [PubMed] [Google Scholar]
- 47.Vaughan A, Chadee DD, Ffrench-Constant R (1998) Biochemical monitoring of organophosphorus and carbamate insecticide resistance in Aedes aegypti mosquitoes from Trinidad. Medical and Veterinary Entomology 12: 318–321. [DOI] [PubMed] [Google Scholar]
- 48.Mebrathu YB, Norem J, Taylor M (1997) Inheritance of larval resistance to permethrin in Aedes aegypti and association with sex ratio distortion and life history variation. American Journal of Tropical Medicine and Hygiene 56: 456–465. [DOI] [PubMed] [Google Scholar]
- 49.de Andrande CFS, Modolo M (1991) Susceptibility of Aedes aegypti larvae to temephos and Bacillus thuringiensis var israelensis in integrated conrtol. Revista de Saude Publica 25: 184–187. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman MG, Goodfriend W, Kohler-Garrigan A, Walker ED, Klug MJ (2002) Soluble nutrient effects on microbial communities and mosquito production in Ochlerotatus triseriatus habitats. Aquatic Microbial Ecology 29: 73–88. [Google Scholar]
- 51.Antonio GE, Sanchez DO, Williams T, Marina CF (2009) Paradoxical effects of sublethal exposure to the naturally derived insecticide spinosad in the dengue vector mosquito, Aedes aegypti. Pest Management Science 65: 323–326. 10.1002/ps.1683 [DOI] [PubMed] [Google Scholar]
- 52.Muturi EJ, Alto BW (2011) Larval environmental temperature and insecticide exposure alters Aedes aegypti competence for arboviruses. Vector-Borne and Zoonotic Diseases 11: 1157–1163. 10.1089/vbz.2010.0209 [DOI] [PubMed] [Google Scholar]
- 53.Alto BW, Bettinardi D (2013) Temperature and dengue virus infection in mosquitoes: Independent effects on the immature and adult stages. American Journal of Tropical Medicine and Hygiene 88: 497–505. 10.4269/ajtmh.12-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaalan EAS, Canyon DV, Younes MWF, Abdel-Wahab H, Mansour AH (2005) Effects of sub-lethal concentrations of synthetic insecticides and Callitris glaucophylla extracts on the development of Aedes aegypti. Journal of Vector Ecology 30: 295–298. [PubMed] [Google Scholar]
- 55.Alto BW, Griswold MW, Lounibos LP (2005b) Habitat complexity and sex-dependent predation of mosquito larvae in containers. Oecologia 146: 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wormington JD, Juliano SA (2014) Hunger-dependent and sex-specific antipredator behaviour of larvae of a size-dimorphic mosquito. Ecological Entomology 39: 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alto BW, Yanoviak SP, Lounibos LP, Drake BG (2005c) Effects of elevated atmospheric CO2 on water chemistry and mosquito (Diptera: Culicidae) growth under competitive conditions in container habitats. Florida Entomologist 88: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bedhomme S, Agnew P, Sidobre C, Michalakis Y (2003) Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. Journal of Evolutionary Biology 16: 721–730. [DOI] [PubMed] [Google Scholar]
- 59.Telang A, Wells MA (2004) The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. Journal of Insect Physiology 50: 677–685. [DOI] [PubMed] [Google Scholar]
- 60.Leisnham PT, Towler L, Juliano SA (2011) Geographic variation of photoperiodic diapause but not adult survival or reproduction of the invasive mosquito Aedes albopictus (Diptera: Culicidae) in North America. Annals of the Entomological Society of America 104: 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald PT (1977) Population characteristics of domestic Aedes aegypti (Diptera: Culicidae) in villages on the Kenya coast. I. Adult survivorship and population size. Journal of Medical Entomology 14: 42–48. [DOI] [PubMed] [Google Scholar]
- 62.Maciel De Freitas R, Codego CT, Lourenco De Oliveira R (2007) Body size-associated survival and dispersal rates of Aedes aegypti in Rio de Janeiro. Medical and Veterinary Entomology 21: 284–292. [DOI] [PubMed] [Google Scholar]
- 63.David MR, Lourenco-de-Oliveira R, de Freitas RM (2009) Container productivity, daily survival rates and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighbourhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Memorias do Instituto Oswaldo Cruz 104: 927–932. [DOI] [PubMed] [Google Scholar]
- 64.Pant CP, Yasuno M (1973) Fiela studies on the gonotrophic cycle of Aedes aegypti in Bangkok, Thailand. Journal of Medical Entomology 10: 219–223. [DOI] [PubMed] [Google Scholar]
- 65.Nasci RS (1990) Relationship of wing length to adult dry weight in several mosquito species (Diptera:Culicidae). Journal of Medical Entomology 27: 716–719. [DOI] [PubMed] [Google Scholar]
- 66.Juliano SA, Ribeiro GS, Maciel-De-Freitas R, Castro MG, Codeco C, et al. (2014) She's a femme fatale: low-density larval development produces good disease vectors. Memorias do Instituto Oswaldo Cruz 109: 1070–U1112. 10.1590/0074-02760140455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, et al. (2000) Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. Journal of Medical Entomology 37: 77–88. [DOI] [PubMed] [Google Scholar]
- 68.Steinwascher K (1982) Relationship between pupal mass and adult survivorship and fecundity for Aedes aegypti Environmental Entomology 11: 150–153. [Google Scholar]
- 69.Briegel H (1990) Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti Journal of Insect Physiology 36: 165–172. [Google Scholar]
- 70.Leisnham PT, Juliano SA (2010) Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion by a superior competitor. Oecologia 164: 221–230. 10.1007/s00442-010-1624-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Honek A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66: 483–492. [Google Scholar]
- 72.Livdahl TP (1984) Interspecific interactions and the r-K continuum: laboratory comparisons of geographic strains of Aedes triseriatus Oikos 42: 193–202. [Google Scholar]
- 73.Focks DA, Brenner RJ, Hayes J, Daniels E (2000) Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. American Journal of Tropical Medicine and Hygiene 62: 11–18. [PubMed] [Google Scholar]
- 74.Timmermann SE, Briegel H (1993) Water depth and larval density affect development and accumulation of reserves in laboratory populations of mosquitoes. Bulletin of the Society of Vector Ecology 18: 174–187. [Google Scholar]
- 75.Timmermann SE, Briegel H (1999) Larval growth and biosynthesis of reserves in mosquitoes. Journal of Insect Physiology 45: 461–470. [DOI] [PubMed] [Google Scholar]
- 76.Briegel H, Knusel I, Timmermann SE (2001) Aedes aegypti: size, reserves, survival, and flight potential. Journal of Vector Ecology 26: 21–31. [PubMed] [Google Scholar]
- 77.Telang A, Qayum AA, Parker A, Sacchetta BR, Byrnes GR (2012) Larval nutritional stress affects vector immune traits in adult yellow fever mosquito Aedes aegypti (Stegomyia aegypti). Medical and Veterinary Entomology 26: 271–281. 10.1111/j.1365-2915.2011.00993.x [DOI] [PubMed] [Google Scholar]
- 78.Kim CH, Muturi EJ (2013) Effect of larval density and Sindbis virus infection on immune responses in Aedes aegypti. Journal of Insect Physiology 59: 604–610. 10.1016/j.jinsphys.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 79.Hawley WA. Population dynamics of Aedes sierrensis In: Lounibos LP, Rey JR, Frank JH, editors; 1985; Vero Beach, FL: Florida Medical Entomology Laboratory; pp. 167–184. [Google Scholar]
- 80.Sumanochitrapon W, Strickman D, Sithiprasasna R, Kittayapong P, Innis BL (1998) Effect of size and geographic origin of Aedes aegypti on oral infection with Dengue-2 virus. American Journal of Tropical Medicine and Hygiene 58: 283–286. [DOI] [PubMed] [Google Scholar]
- 81.Alto BW, Reiskind MH, Lounibos LP (2008b) Size alters susceptibility of vectors to dengue virus infection and dissemination. American Journal of Tropical Medicine and Hygiene 79: 688–695. [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang S, He G, Xu L, Lin Q, Zhang S (1993) Effects of larval nutrition on susceptibility of Aedes albopictus to dengue 2 virus. Arbovirus Research in Australia Arbovirus Research in Australia 6: 44–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.