Abstract
Purpose
Single exon inversions have rarely been described in clinical syndromes and are challenging to detect using Sanger sequencing. We report a 40-year-old woman with adenomatous colon polyps too numerous to count who had a complex inversion spanning the entire exon 10 in the APC gene, causing exon skipping and resulting in a frameshift and premature protein truncation.
Methods
Complete APC gene sequencing using high coverage next-generation sequencing by ColoSeq, analysis with Breakdancer and SLOPE software, and confirmatory transcript analysis.
Results
ColoSeq identified a complex small genomic rearrangement consisting of an inversion that results in translational skipping of exon 10 in the APC gene. This mutation would not have been detected by traditional sequencing or gene dosage methods.
Conclusion
We report a case of adenomatous polyposis resulting from a complex single exon inversion. Our report highlights the benefits of large scale sequencing methods that capture intronic sequences with high enough depth of coverage and informatics tools to enable detection of small pathogenic structural rearrangements.
Keywords: ColoSeq, familial adenomatous polyposis, massively parallel sequencing, inversion, complex genomic rearrangement, next-generation sequencing, APC, exonic skipping, FAP
Introduction
Inherited deleterious mutations in the APC gene cause familial adenomatous polyposis (FAP) and have also been associated with Gardner and Turcot syndromes (1). Sanger sequencing of all 15 coding exons in the APC gene has become the initial standard screening test for APC mutations. Sanger sequencing of APC exons has about 55% sensitivity for mutations in patients with >100 colorectal adenomas (2). Assays for large rearrangement of the APC gene detect mutations in an additional 3% of FAP patients (3, 4). Beyond this, testing for two common mutations in MUTYH will identify 7% of patients with classic polyposis as carriers of biallelic mutations in MUTYH, which has an overlapping phenotype (2, 5). So, current screening for APC and MUTYH using 3 separate tests has a cumulative sensitivity of about 65% for causative mutations in patients with classic polyposis defined as >100 polyps (2). Of the mutations in APC that are detected in current protocols, Sanger sequencing detects frameshift, nonsense, and splice site mutations which represent, respectively, 43%, 42%, and 9% identified mutations as well as detecting missense mutations that have been categorized as pathogenic (2, 3). The remaining 6% of mutations detected with current protocols are detected by multiplex ligation-dependent probe amplification (MLPA) or Fluorescence In Situ Hybridization (FISH)(3, 4).
Several assays have been designed to rapidly screen for mutations in APC that are not detectable with Sanger sequencing or confirm pathogenicity of mutations detected mutations. Assays, such as conformation sensitive denaturing gel electrophoresis or denaturing high-performance liquid chromatography can rapidly scan for variants in amplified exons (6, 7). Some laboratories use the protein truncation test to evaluate pathogenicity of mutations that may not have obvious effects (8). However, many mutations are not detectable with methods that target coding exons. A small proportion of patients with FAP have complex rearrangements or somatic mosaicism; these are also not detected with routine screening (4, 9, 10).
High throughput “next-generation” sequencing technology has dramatically reduced the per-base cost of sequencing, making sequencing of intronic segments in addition to exons at high depth economically practical. Consequently, next-generation detection strategies allow for more comprehensive detection of disruptive mutations, including point mutations, splice site mutations, intronic mutations, deletions, duplications, large rearrangements, and complex structural rearrangements. ColoSeq is a recently validated next-generation sequencing assay that interrogates both the intronic and exonic sequence of 19 genes associated with colon cancer and polyposis (11). Here we describe the identification of a complex genomic inversion spanning APC exon 10.
Materials and Methods
Patient DNA Samples
We tested DNA extracted from peripheral blood leukocytes, and prepared genomic DNA with the Gentra Puregene DNA Isolation Kit (Qiagen, Germantown, MD, catalog no. 158489). Clinical specimens were obtained in accordance with the declaration of Helsinki and the ethics guidelines of Human Subjects Division of the University of Washington.
Next-Generation Deep Sequencing by ColoSeq
ColoSeq solution-based targeted gene capture, genomic library preparation, and massively parallel sequencing methods have been described in detail previously (11). Briefly, genomic DNA was sheared and SureSelect probes were used to capture exonic and intronic sequence of multiple genes associated with Lynch Syndrome and polyposis (Agilent Technologies, Santa Clara, CA). Custom design targets included exonic and intronic sequences in MLH1, MSH2, MSH6, PMS2, EPCAM, APC, MUTYH, CDH1, PTEN, STK11, TP53, SMAD4, BMPR1A, POLE, POLD1, GALNT12, GREM1, AKT1, and PIK3CA. Paired end sequencing of amplified targets was done on an Illumina HiSeq2000 (Illumina, Inc, San Diego, CA) according to standard protocols. SNPs and indels were called as described in previously (11). To evaluate structural variation, reads were mapped to the human reference genome (hg19) using BWA and variants were identified using Breakdancer (12) and CREST (13) as described elsewhere (14). Split reads at inversion breakpoints were identified using SLOPE (15). Inversion breakpoints and exact structure were confirmed using Sanger sequencing (primer sequences available from authors).
Confirmatory experimental analysis of splicing errors due to a genomic inversion
Splicing errors due to gene rearrangements (i.e. deletions, duplication and inversions, involving one or more exons) lead to transcripts of abnormal length. To detect these events, we isolated total RNA from the patient’s whole blood within 24 hours of collection using TRIzol® LS Reagent (Invitrogen, Life Technologies) and generated complementary DNA (cDNA) by oligo(dT) priming using SuperScript® First-Strand Synthesis System (Invitrogen, Life Technologies). The cDNA was amplified with a primer pair spanning exons 7 and 13 of APC (primer sequences available from authors). Products of cDNA RT-PCR were electrophoresed on 2% agarose gels. Products of PCR that were of aberrant size were gel extracted using QIAquick (Qiagen) and sequenced in both directions.
Results
Case presentation
The proband is a 40-year-old woman of self-reported Irish and Scottish ancestry who presented to medical genetics due to a history of polyposis of the colon. A colonoscopy performed at 35 years of age was remarkable for five tubular adenomas. A repeat colonoscopy at 39 years of age noted multiple subcentimeter polyps in the terminal ileum, cecum, and transverse colon. There were too many polyps to be able to ascertain an accurate number. At the hepatic flexure there were at least 15 subcentimeter polyps. Biopsies obtained from the ileum, cecum, and transverse colon confirmed tubular adenomas. An esophagogastroduodenoscopy at 40 years of age was unremarkable. The proband is an otherwise healthy individual with a negative review of systems. Her mother was diagnosed with an invasive colorectal cancer at 54 years of age, and the proband’s maternal grandmother had a niece (1st cousin once removed of proband) with colorectal cancer at 50 years of age. Consanguinity was denied. Relatives were unavailable for testing.
ColoSeq identifies an APC exon 10 inversion
A multi-gene panel screen of 13 genes associated with colon cancer and polyposis was performed. Average read depth across all genes was 335x, with average read depth of 324x across the APC gene. Breakdancer software identified 17 discordant paired-end reads consistent with an estimated 445 basepair inversion between chr5:112154543 and chr5:112155245, as well as 5 reads with an estimated 676 basepair inversion between chr5:11215434 and chr5:112155245. Breakdancer estimates feature size by comparing differences between expected and actual mapping location of paired end reads, highlighting candidate changes without giving accurate breakpoint locations or precise size estimates (12). So, we used other methods to characterize actual inversion breakpoints. Orthologous analysis using SLOPE revealed a total of 19 split reads consistent with an inversion between chr5:112154359 and chr5:11215008 with an additional 9 split reads between chr5:112154359 and sequence near chr5:112155232 (15, 16), supporting the presence of a complex disruptive rearrangement.
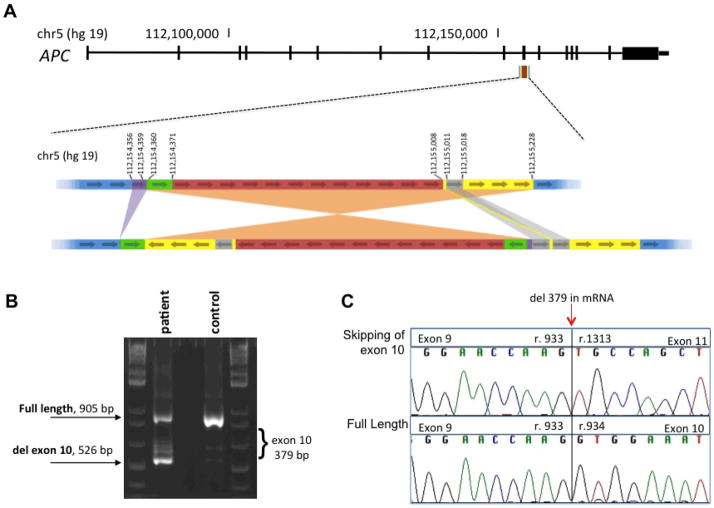
The breakpoint of the genomic inversion was confirmed and the exact complex rearrangement was defined using Sanger sequencing. The inversion is complex enough that determining the correct HGVS nomenclature is challenging (Figure 1a). In genome build hg19, a large sequence from chr5:112154359-112155228 was inverted with chr5:112154360-112154371 and chr5:112155008-112155228 duplicated before the inversion event. Near the inversion insertion point, at chr5:112154356-112154360, 5 base pairs (CTTAT) were deleted and at the other inversion insertion point, chr5:112155008, 8 base pairs (GAACCAGG) were inserted or duplicated from chr5:112155011-112155018 (see Figure 1a).
Figure 1.
Chromosome 5 inversion spanning APC exon 10 causing skipping of APC exon 10 in patient RNA. a) Schematic APC gene with location and detail of complex genomic rearrangement, all positions are on hg19 chromosome 5. b) Gel electrophoresis of cDNA products consistent with exon 10 skipping. c) cDNA sequence of resulting APC protein product illustrating cDNA sequence of exon 9 spliced to exon 11.
Confirmatory cDNA analysis of splicing errors due to a genomic inversion
Analysis of cDNA successfully identified a mutant message in APC containing a premature stop codon due to the genomic inversion. The detection of an abnormal length message suggested that the inversion did not lead to complete transcript degradation due to nonsense-mediated decay. The cDNA product was consistent with skipping of APC exon 10 in patient mRNA: r. 934_1312 del 379 with predicted stop at position 327 of 2844 (Figure 1 b and c).
Discussion
We are not aware of previous reports of any single exon inversions in APC causing FAP, and this is the first report of an isolated APC exon 10 inversion. However, there are several reports of different small APC rearrangements. One study that examined cDNA transcripts to identify small rearrangements in 8% of FAP families screened (17) and another study that used multiple methods to screen for APC mutations reached the conclusion that tests for splicing defects and larger genomic changes should be included in all diagnostic screening (4). The important distinction between these studies and our report is that previous work identified altered transcripts using processed nucleic acid, and followed this with additional studies to identify the underlying genomic alteration. In contrast, our next-generation sequencing assay detected the small rearrangement at the genomic level in the course of primary clinical testing, and we confirmed findings in the altered transcript. Had the rearrangement not been so complex, with small deletions and insertions at the breakpoints, we may have been able to identify inversion breakpoints using split reads.
Sanger sequencing that interrogates exonic sequence and intron/exon boundaries followed by deletion/duplication analysis where sequencing is negative has become standard of care of FAP. Rearrangements, such as the one we report, would not normally be detected by either of these methods. The breakpoints of this inversion are such that published primers for exonic sequencing would provide reliable data from the normal copy of the affected exon, but would fail to detect the inversion (18), and it is unlikely that the less than 50 base pairs of duplicated exonic sequence in this complex rearrangement would be detected by MLPA probes. Only a few investigators routinely perform the transcript based APC analyses that would be expected to detect this complex rearrangement. A next-generation sequencing approach offers significant advantages in allowing identification of sequence variants, deletion/duplication, and structural rearrangements through the use of a single test. Our report demonstrates how deep next-generation sequencing may obviate the need for multiple screening tests, by enabling detection of small rearrangements at the genomic level and illustrates several analytic tools that can be used to identify these variants.
References
- 1.Jasperson KW, Burt RW. APC-Associated Polyposis Conditions. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. Gene Reviews. 2010. Seattle, Wa: University of Washington; 1993. [Google Scholar]
- 2.Grover S, Kastrinos F, Steyerberg EW, Cook EF, Dewanwala A, Burbidge LA, Wenstrup RJ, Syngal S. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. Jama. 2012;308:485–92. doi: 10.1001/jama.2012.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr SE, Thomas CB, Thibodeau SN, Ferber MJ, Halling KC. APC germline mutations in individuals being evaluated for familial adenomatous polyposis: a review of the Mayo Clinic experience with 1591 consecutive tests. J Mol Diagn. 2013;15:31–43. doi: 10.1016/j.jmoldx.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Mihalatos M, Apessos A, Dauwerse H, Velissariou V, Psychias A, Koliopanos A, Petropoulos K, Triantafillidis JK, Danielidis I, Fountzilas G, Agnantis NJ, Nasioulas G. Rare mutations predisposing to familial adenomatous polyposis in Greek FAP patients. BMC Cancer. 2005;5:40. doi: 10.1186/1471-2407-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morak M, Laner A, Bacher U, Keiling C, Holinski-Feder E. MUTYH-associated polyposis - variability of the clinical phenotype in patients with biallelic and monoallelic MUTYH mutations and report on novel mutations. Clin Genet. 2010;78:353–63. doi: 10.1111/j.1399-0004.2010.01478.x. [DOI] [PubMed] [Google Scholar]
- 6.Fodde R, van der Luijt R, Wijnen J, Tops C, van der Klift H, van Leeuwen-Cornelisse I, Griffioen G, Vasen H, Khan PM. Eight novel inactivating germ line mutations at the APC gene identified by denaturing gradient gel electrophoresis. Genomics. 1992;13:1162–8. doi: 10.1016/0888-7543(92)90032-n. [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Wu W, Hegde M, Fawkner M, Chong B, Love D, Su LK, Lynch P, Snow K, Richards CS. Detection of sequence variations in the adenomatous polyposis coli (APC) gene using denaturing high-performance liquid chromatography. Genet Test. 2001;5:281–90. doi: 10.1089/109065701753617408. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Ishioka C, Kato S, Mitachi Y, Shimodaira H, Sakayori M, Shimada A, Asamura M, Kanamaru R. Detection of APC mutations by a yeast-based protein truncation test (YPTT) Genes Chromosomes Cancer. 1998;21:290–7. [PubMed] [Google Scholar]
- 9.Rohlin A, Wernersson J, Engwall Y, Wiklund L, Bjork J, Nordling M. Parallel sequencing used in detection of mosaic mutations: comparison with four diagnostic DNA screening techniques. Hum Mutat. 2009;30:1012–20. doi: 10.1002/humu.20980. [DOI] [PubMed] [Google Scholar]
- 10.Hes FJ, Nielsen M, Bik EC, Konvalinka D, Wijnen JT, Bakker E, Vasen HF, Breuning MH, Tops CM. Somatic APC mosaicism: an underestimated cause of polyposis coli. Gut. 2008;57:71–6. doi: 10.1136/gut.2006.117796. Epub 2007 Jun 29. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard CC, Smith C, Salipante SJ, Lee MK, Thornton AM, Nord AS, Gulden C, Kupfer SS, Swisher EM, Bennett RL, Novetsky AP, Jarvik GP, Olopade OI, Goodfellow PJ, King MC, Tait JF, Walsh T. ColoSeq provides comprehensive lynch and polyposis syndrome mutational analysis using massively parallel sequencing. J Mol Diagn. 2012;14:357–66. doi: 10.1016/j.jmoldx.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K, Wallis JW, McLellan MD, Larson DE, Kalicki JM, Pohl CS, McGrath SD, Wendl MC, Zhang Q, Locke DP, Shi X, Fulton RS, Ley TJ, Wilson RK, Ding L, Mardis ER. BreakDancer: an algorithm for high-resolution mapping of genomic structural variation. Nat Methods. 2009;6:677–81. doi: 10.1038/nmeth.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Mullighan CG, Easton J, Roberts S, Heatley SL, Ma J, Rusch MC, Chen K, Harris CC, Ding L, Holmfeldt L, Payne-Turner D, Fan X, Wei L, Zhao D, Obenauer JC, Naeve C, Mardis ER, Wilson RK, Downing JR, Zhang J. CREST maps somatic structural variation in cancer genomes with base-pair resolution. Nat Methods. 2011;8:652–4. doi: 10.1038/nmeth.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritchard CC, Salipante SJ, Koehler K, Smith C, Scroggins S, Wood B, Wu D, Lee MK, Dintzis S, Adey A, Liu Y, Eaton KD, Martins R, Stricker K, Margolin KA, Hoffman N, Churpek JE, Tait JF, King MC, Walsh T. Validation and Implementation of Targeted Capture and Sequencing for the Detection of Actionable Mutation, Copy Number Variation, and Gene Rearrangement in Clinical Cancer Specimens. J Mol Diagn. 2013;2:00217–1. doi: 10.1016/j.jmoldx.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abel HJ, Duncavage EJ, Becker N, Armstrong JR, Magrini VJ, Pfeifer JD. SLOPE: a quick and accurate method for locating non-SNP structural variation from targeted next-generation sequence data. Bioinformatics. 2010;26:2684–8. doi: 10.1093/bioinformatics/btq528. [DOI] [PubMed] [Google Scholar]
- 16.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–6. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su LK, Steinbach G, Sawyer JC, Hindi M, Ward PA, Lynch PM. Genomic rearrangements of the APC tumor-suppressor gene in familial adenomatous polyposis. Hum Genet. 2000;106:101–7. doi: 10.1007/s004399900195. [DOI] [PubMed] [Google Scholar]
- 18.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]