We have previously shown that angiotensin receptor blockers (ARB) use is associated with improved cognitive function in those with mild cognitive impairment (MCI) and with lower amyloid and Tau content on autopsy studies.1,2 The impact of ARBs on cerebrospinal fluid (CSF) amyloid and tau biomarkers is unknown. We analyzed data from the longitudinal Alzheimer’s disease Neuroimaging Initiative (ADNI) to assess the impact of ARB use on CSF amyloid and Tau markers.
Methods
ADNI is a longitudinal study of clinical, biochemical, and neuroimaging data collected from participants with normal cognition, MCI or dementia enrolled from 59 centers in the US. Lumbar punctures (LP) were performed using standardized protocols and biomarker measurements were conducted at the University of Pennsylvania. Amyloid-β 1-42 peptide (Aβ), total tau (Tau), and tau phosphorylated at the threonine 181 (p-Tau) were measured using the multiplex xMAP Luminex platform with Innogenetics immunoassay.3 Antihypertensive medication information was coded into one of 5 classes (ARB, angiotensin converting enzyme inhibitors (ACEI), diuretics, beta blockers, calcium channel blockers and others).Those receiving multiple classes were categorized into each class separately. Blood pressure was measured at baseline and follow-up. Participants were hypertensive if their blood pressure ≥ 140/90 mm Hg or receiving antihypertensive medication. Since switching class during the follow-up period is possible, our independent variable was use of ARBs during at least one visit prior to the CSF measurement and our comparison groups were those treated with other antihypertensive classes or those with untreated hypertension (additional comparisons were done including non-hypertensive participants). Mixed models with repeated measures were used. Our parsimonious models included age, gender, systolic blood pressure, and baseline measure for corresponding marker (adding additional covariates did not alter the results or improve the model-fit statistics). We included those with 2, 3 or 4 CSF collections and conducted analyses in normal cognitive controls, MCI and those with dementia.
Results
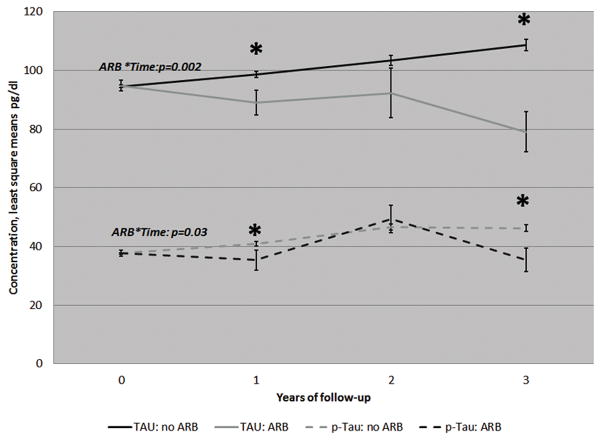
Our sample (Table 1) was 319: 45% with HTN, 8% on ARBs, 47% MCI, 30% healthy controls, 23% with Alzheimer’s disease (AD), mean age=73 years (standard deviation=7.5), 56% women, range of number of visits:2–4. In those with MCI (figure 1) after adjusting for the covariates and baseline marker measure, use of ARBs during at least one visit prior to LP was associated with a decrease in both total Tau (p=0.002) and p-Tau (p=0.01) but not Aβ (p=0.12) relative to those treated with non-ARB classes and untreated hypertension. In normal cognitive controls, ARB use was associated with lower decline in Aβ (p=0.03) but no impact on Tau markers (p=0.3 for Tau and 0.7 for p-tau). In AD, ARB use was not associated with any changes (all p>0.5). Results did not change when we included normotension in the comparison group.
Table 1.
Baseline characteristics in the ADNI cohort by exposure to Angiotensin Receptor Blockers
| ARBs | Non ARBS* | p-value** | |
|---|---|---|---|
| 26 | 293 | ||
| Age, mean (SE) years | 72 (1) | 75 (0.3) | 0.8 |
| %women | 54% | 61% | 0.51 |
| % white | 92% | 96% | 0.34 |
| years of education | 15 (1) | 16 (0.2) | 0.11 |
| % with hypertension | 100% | 41% | <0.0001 |
| % on ACEI | 4% | 19% | 0.07 |
| % on Diuretics | 21% | 15% | 0.42 |
| % on CCB | 42% | 8% | <0.0001 |
| % on Beta-Blockers | 17% | 12% | 0.53 |
| % Untreated | 0% | 59% | <0.0001 |
| Systolic Blood pressure | 131 (3) | 133 (1) | 0.54 |
| Controls, N (%) | 11 (42%) | 85 (29%) | 0.31 |
| Mild Cognitive Impairment, N (%) | 9 (33%) | 144 (49%) | 0.31 |
| Alzheimer’s disease, N (%) | 6 (23%) | 64 (22%) | 0.31 |
| Aβ, pg/ml | 180.2 (11.2) | 167.3 (3.3) | 0.28 |
| Tau, pg/ml | 101 (13.1) | 98.4 (3.0) | 0.82 |
| p-Tau, pg/ml | 37.3 (4.8) | 33.8 (1.0) | 0.35 |
| MMSE | 27 (0.6) | 27 (0.2) | 0.92 |
| CDR-SOB | 1.5 (0.4) | 1.7 (0.1) | 0.55 |
ARB: Angiotensin receptor blockers; ACEI: angiotensin converting enzyme inhibitor; CCB: calcium channel blockers; MCI: mild cognitive impairment; MMSE: Mini-Mental Status Exam; CDRSOB: Clinician dementia rating scale-sum-of-boxes.
non ARBs include treated and untreated hypertension and normotension.
p-value calculated using chi-square or t-test
Figure 1.
Progression of Total and phosphorylated Tau by exposure to angiotensin receptor blockers in those with Mild Cognitive Impairment in the ADNI cohort.
Values are least square means adjusted for age, gender, systolic blood pressure and the baseline measures of Tau and p-Tau respectively. N at baseline=319, year 1=317, year 2=144, year 3=80. P-value for trend/change over the follow-up obtained from the ARB*time parameter. * indicates a p-value<0.05 for the difference between ARB and no ARB within that follow-up visit/year.
Discussion
ARB use in those with MCI may be associated with longitudinal declines in Tau and p-Tau. These data extend our previous favorable ARB findings. Tau markers, more so than Aβ, correlate with severity of clinical symptoms.4 This may provide an explanation of why ARBs may specifically impact symptoms in MCI. Although not investigated in our report, recent animal studies have suggested that ARBs inhibit GSK which is involved in Tau production and neurodegeneration. 5,6 This may offer an underlying biological plausibility for this observation. Despite the limitation of our small sample size and small number of individuals on ARBs, our study offers further evidence of a favorable effect of ARBs on brain markers of Alzheimer’s disease in the pre-dementia stages. Clinical trials are needed to further confirm these effects.
Acknowledgments
Dr. Hajjar is funded by the National Institute of Aging (R01AG042127) AND Dr. Levey is funded by the Emory Alzheimer’s Disease Research Center (P50 AG025688).
Contributor Information
Ihab Hajjar, Email: ihajjar@emory.edu, Associate Professor, Departments of Medicine and Neurology.
Allan Levey, Betty Gage Holland Chair, Professor and Chair, Dept of Neurology, Director, Emory Alzheimer’s Disease Research Center.
References
- 1.Hajjar I, Brown L, Mack WJ, Chui H. Impact of Angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Archives of neurology. 2012;69(12):1632–1638. doi: 10.1001/archneurol.2012.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajjar I, Hart M, Chen YL, et al. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: a double-blind randomized clinical trial. Archives of internal medicine. 2012;172(5):442–444. doi: 10.1001/archinternmed.2011.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta neuropathologica. 2011;121(5):597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierer LM, Hof PR, Purohit DP, et al. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Archives of neurology. 1995;52(1):81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- 5.Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS letters. 1998;436(1):28–34. doi: 10.1016/s0014-5793(98)01090-4. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal D, Dange RB, Raizada MK, Francis J. Angiotensin II causes imbalance between pro- and anti-inflammatory cytokines by modulating GSK-3beta in neuronal culture. Br J Pharmacol. 2013;169(4):860–874. doi: 10.1111/bph.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]