Abstract
Bax, a central cell death regulator, is an indispensable gateway to mitochondrial dysfunction and a major pro-apoptotic member of the Bcl-2 family proteins that control apoptosis in normal and cancer cells. Dysfunction of apoptosis renders the cancer cell resistant to treatment as well as promotes tumorigenesis. Bax activation induces mitochondrial membrane permeabilization, thereby leading to the release of apoptotic factor cytochrome c and consequently cancer cell death. A number of drugs in clinical use are known to indirectly activate Bax. Intriguingly, recent efforts demonstrate that Bax can serve as a promising direct target for small-molecule drug discovery. Several direct Bax activators have been identified to hold promise for cancer therapy with the advantages of specificity and the potential of overcoming chemo- and radioresistance. Further investigation of this new class of drug candidates will be needed to advance them into the clinic as a novel means to treat cancer.
Keywords: Bcl-2 family proteins, apoptosis, Bax activators, drug discovery, cancer therapy
1. INTRODUCTION
Cell death1 has numerous vital roles in sculpting tissues and optimizing functions (e.g. in the immune or central nervous systems) during normal human body development.1,2 Apoptosis, a.k.a. programmed cell death, is a major death process of cells that is critical for elimination of unwanted, damaged or infected cells and is associated with diverse biological processes including cell development, differentiation and proliferation.3 Insufficient apoptosis may promote cancer and auto immune diseases, while excessive cell death may augment ischemic conditions and drive neurodegeneration.4 The recognition that apoptosis is crucially involved in the regulation of tumor formation and also critically determines treatment response is one of the most important advances in cancer research in recent years.5-7 The intrinsic and extrinsic signaling pathways are two principal processes leading to apoptosis.8 The intrinsic pathway, also termed as the mitochondrial pathway, is triggered by diverse cytotoxic stimuli including oncogenic stress, chemotherapeutic agents as well as metabolic stress. These stimuli activate related Bcl-2 family proteins leading to mitochondrial outer membrane permeabilization (MOMP).9 Upon disruption of the outer mitochondrial membrane, a set of apoptotic proteins are released including cytochrome c and Smac/DIABLO.3,10,11 Cytosolic cytochrome c recruits apoptosis protease-activating factor 1 (Apaf-1) and procaspase 9 to generate “apoptosome” which activates caspase 9 leading to processing of caspase 3.12-14,10 Caspases are executive proteins of apoptosis. The extrinsic pathway, a.k.a. death receptor pathway, is activated by the interactions between death receptors and their cognate ligands of the tumor necrosis factor (TNF) family. Death ligand stimulation brings about oligomerization of the receptors and recruitment of the adaptor protein FADD and caspase 8, resulting in the formation of a death-inducing signaling complex (DISC). Autoactivation of caspase 8 activates other effector caspases including caspase 3, 6 and 7.15 These two pathways converge on effector caspases and subsequently on other proteases and nucleases that drive cell death. Mitochondrial signaling is critical for normal cellular homeostasis and participates in the pathogenesis of various diseases12,16 including cancer,17 diabetes mellitus,18 obesity,19 and neurodegenerative disorders20,18 such as Parkinson’s disease.21,20 Thus, abnormalities in mitochondrial signaling represent an actively pursued research frontier of the biomedical enterprise.
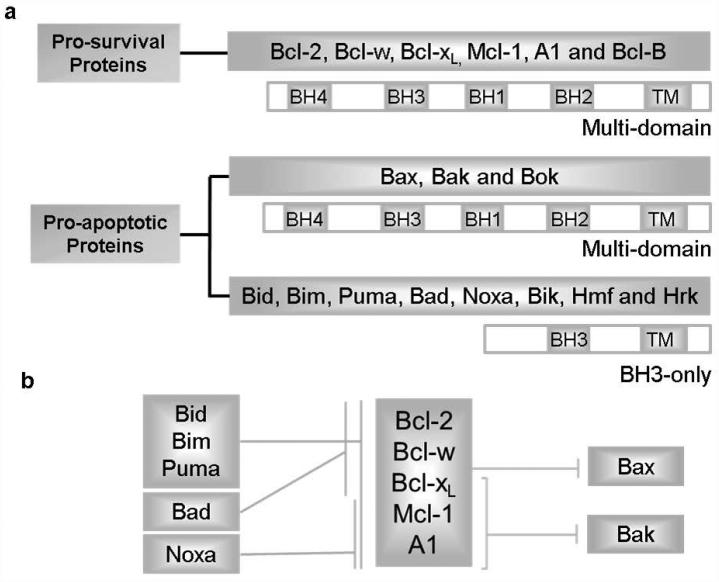
The mitochondrial signaling is primarily regulated by the interactions of B-cell lymphoma 2 (Bcl-2) family proteins.22 Based on their different structures and functions, the Bcl-2 family is classified into two groups (Fig. 1a): anti-apoptotic proteins (e.g. Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1) and pro-apoptotic proteins. Pro-apoptotic proteins are further divided into two sub-classes: multi-domain proteins (e.g. Bax and Bak) and BH3-only proteins (e.g. Bid, Bim, Puma, Bad, Noxa, Bik, Bmf and Hrk) according to the presence of Bcl-2 homology domains (BH1-4 domains).22 Multi-domain pro-apoptotic proteins Bax and Bak are essential executive proteins responsible for MOMP and a requisite gateway to mitochondrial dysfunction as well as cell death.23,16 Cells lacking both Bax and Bak have proven to be completely resistant to truncated Bid (t-Bid)-induced cytochrome c release and apoptosis.23 Bax likely can be inhibited by all the anti-apoptotic proteins,24 whereas Bak is inhibited predominantly by Bcl-xL, Mcl-1 and A125 (Fig. 1b). Bcl-2 anti-apoptotic (or pro-survival) family members sequester BH3-only proteins or neutralize Bax and Bak, thus preventing the allosteric activation of Bax and a subsequent mitochondrial program of apoptosis.26,27 The BH3-only proteins are classified as “activators” and “sensitizers” based on the direct activation model, one of the three generally accepted apoptotic models.28,29 Activator-type BH3-proteins such as Bid,30,24 Bim31 and Puma32,33,24 function by the direct physical binding and activation of Bax to induce apoptosis, while sensitizer-type BH3-proteins such as Bad, Noxa and Bik engage pro-survival proteins to free up activators and induce Bax activation-mediated apoptosis.28,34 Homeostasis is maintained by controlling the ratio of active pro- and anti-apoptotic proteins along with tissue-specific patterns of expression.35
Figure 1. The Bcl-2 family proteins and their interactions.
a, The Bcl-2 family is classified into pro-survival proteins (or anti-apoptotic proteins, including Bcl-2, Bcl-xL, Bcl-w, Mcl-1, etc.) and pro-apoptotic proteins due to their different functions. The pro-apoptotic proteins are further divided into two sub-classes: multi-domain proteins (e.g. Bax, Bak and Bok) that exhibit BH1-4 domains and BH3-only proteins that exhibit sole BH3 domain (e.g. Bim, Bid, Puma, Noxa, Bad, Hrk, Bmf and Bik). Based on the direct activation model, BH3-only proteins consist of “activators” (e.g. Bim, Bid and Puma) that are able to bind and activate Bax directly and “sensitizers” (e.g. Bad and Noxa) that act by releasing activators from pro-survival proteins. b, Bax may be inhibited by all pro-survival proteins, while Bak is inhibited mainly by Bcl-xL, Mcl-1 and A1. Some BH3-only proteins (e.g. Bim, Bid and Puma) are able to neutralize all pro-survival proteins, while some of them (e.g. Bad and Noxa) can only bind a limited subset.
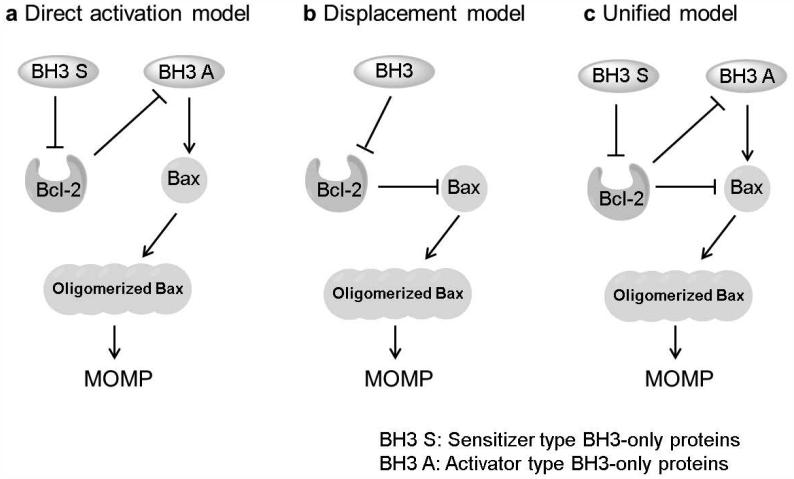
Currently, three models are available as to how Bcl-2 family proteins control apoptosis - the direct activation model, the displacement model and the unified model.4,36,37 The direct activation model27 (Fig. 2a) contends that activator type BH3-only proteins directly associate with Bax/Bak4 and cause the downstream series of events leading to cell death.38,39,20,54 The sensitizer type BH3-only proteins bind to the anti-apoptotic proteins to liberate activators, thereby facilitating Bax activation and MOMP.28 The sole function of anti-apoptotic proteins is to sequester BH3-only proteins instead of Bax or Bak.40 The displacement model (Fig. 2b) concludes that BH3-only proteins cause apoptosis through Bax or Bak indirectly by neutralizing the relevant anti-apoptotic proteins, thereby enabling the activation of Bax and Bak to proceed.24,41,42,163 Bax and Bak are always active and anti-apoptotic proteins constitutively bind them to prevent MOMP in this mode. The sole function of BH3-only proteins is to displace Bax and Bak from pro-survival proteins, rather than bind Bax or Bak. The unified model43 (Fig. 2c) builds on the embedded together model which combines features of both aforementioned models. The unified model assigns dual functions to pro-survival proteins, sequestering not only the activator type BH3-only proteins but also the active forms of Bax and Bak. The embedded together model44 similar to the unified model emphasizes that binding to membranes is essential for interactions between Bcl-2 family members.45,46
Figure 2. Different models of Bax activation-mediated mitochondrial outer membrane permeabilization (MOMP).
a, The direct activation model contends that activator type BH3-only proteins directly bind and activate Bax/Bak. b, The displacement model concludes that BH3-only proteins trigger apoptosis indirectly through Bax or Bak by neutralizing the relevant pro-survival proteins. c, The unified model assigns dual functions to anti-apoptotic proteins, sequestering not only the activator type BH3-only proteins but also the active forms of Bax and Bak.
Overexpression of anti-apoptotic Bcl-2 or Bcl-xL exists in a large number of human cancers,47 and inactivating mutations of pro-apoptotic proteins occurs in numerous cancers leading to an uncontrolled growth of tumors.48-50 Moreover, overexpression of anti-apoptotic Bcl-2 and its close relatives is a major component of chemoresistance.51 Bcl-2 family proteins are critical checkpoints of apoptotic cell death,52,35 and targeting various Bcl-2 family members is thus one of the most promising therapeutic strategies for dysfunctional apoptosis related diseases including cancer,4,53-59,36 autoimmune diseases and35 neurodegenerative disorders.60,43 In this review, we summarize the current understanding of the structures and physiological functions of Bax protein, and focus on recent advances in the direct targeting of Bax for cancer therapy. Several newly emerged direct activators of Bax including peptides and non-peptide small molecules, and their potential as novel cancer therapeutics are highlighted.
2. STRUCTURE OF BAX
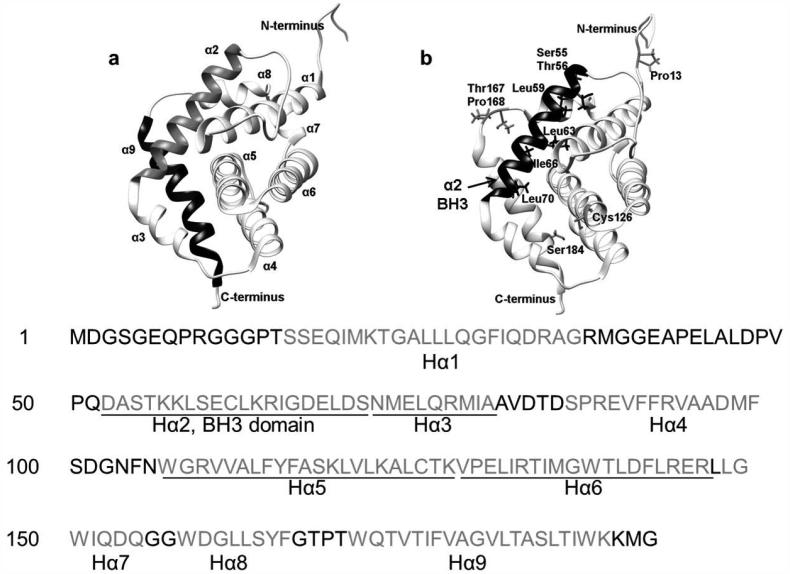
Bax, a tumor suppressor,61 was first identified as a heterodimer with Bcl-2 in 1993.62 It is a 21 kD protein of 192 amino acids possessing 9 α-helices and its three-dimensional structure was resolved by nuclear magnetic resonance (NMR) in 2000.63 Just like other three dimensional structures of Bcl-2 family proteins,35 Bax exhibits a similar tertiary structure (Fig. 3). Helices α5 (Hα5) and Hα6 constitute the core of the protein and are embedded within the other 7 helices which are amphipathic and keep their hydrophilic residues exposed to the exterior.64 Hα5 and Hα6 are recognized as the putative mitochondria pore-forming domain65 and transmembrane domain.66-68 N-terminal Hα1 containing a mitochondrial addressing signal is believed to be essential for the translocation of Bax to mitochondria.69-71 In addition, Hα1 is the interaction site for BH3-only proteins t-Bid and Puma.72 Hα1 controls the engagement of the α9 helix into the dimerization pocket formed by BH1, BH2 and BH3, rendering Bax as a monomer in cytosol. Once Hα1 is attacked by BH3-only proteins (e.g. tBid, Bim and Puma) and exposed, Hα9 will disengage from the hydrophobic groove, leading to mitochondrial insertion.39 There is a long and unstructured loop between Hα1 and Hα2, which is a common feature of Bcl-2 family members. The amino acid sequence of this region is highly variable, and its function remains to be further elucidated. Hα2 comprises the BH3 domain which is requisite for the heterodimerization with other Bcl-2 family members.73 It has been determined by mutational analyses that Bax/Bcl-2 heterodimerization requires the BH1, BH2 and BH3 domains of Bcl-2 but only the BH3 domain of Bax.74,75 Hα2, Hα3, Hα4 and Hα5 form a hydrophobic groove of the protein which is the canonical BH3-binding site. Hα9 is bound to the Bax hydrophobic groove63 and thought to participate in the conformational stability.76 Bax is predominantly an inactive monomer in the cytosol of healthy cells or loosely attached to the mitochondrial, nuclear, or endoplastic reticulum membrane.70,77 The hydrophobic side chains (e.g. Ser55, Thr56, Leu59, Leu63, Ile66 and Leu70) of the BH3 helix point inward toward Hα5/Hα6 and are covered by Hα9. During apoptosis, Bax translocates to the mitochondria in the fully activated form whose Hα9 disengages from the binding groove and Hα2 rotates about its axis to expose the hydrophobic side chains of the BH3 domain.64
Figure 3. Structure of Bax.
a, Ribbon representation of Bax (PDB: 1F16). The 9 α-helices are indicated separately. Hα2 comprises BH3 domain. Hα5 (helices α5) and Hα6 form the core of the protein and are embedded within the other 7 helices. b, Critical amino acid residues (e.g. Cys126, Thr167, Pro168, Pro13 and Ser184) are highlighted including hydrophobic residues on BH3 domain (e.g. Ser55, Thr56, Leu59, Leu63, Ile66 and Leu70).
Some critical amino acid residues have also been identified. Cys62 within Hα2 close to the BH3 domain and Cys126 between the Hα5 and Hα6 are both exposed and potentially form a disulfide bridge for homo- or heterodimerization.78,77,61 Ser184, at the end of C-terminus, and Thr167, between Hα8 and Hα9, are identified as two important phosphorylation sites. Phosphorylation of Ser184 by protein kinase C zeta,79 or AKT80 neutralizes Bax, while its dephosphorylation by protein phosphatase 2A actives Bax.81 Mutation or deletion of Ser184 also influences Bax localization.82,83 Pro168 and Pro13 are also found to be important for Bax activation and localization to mitochondria.84, 85
3. Function of Bax and its associated signaling
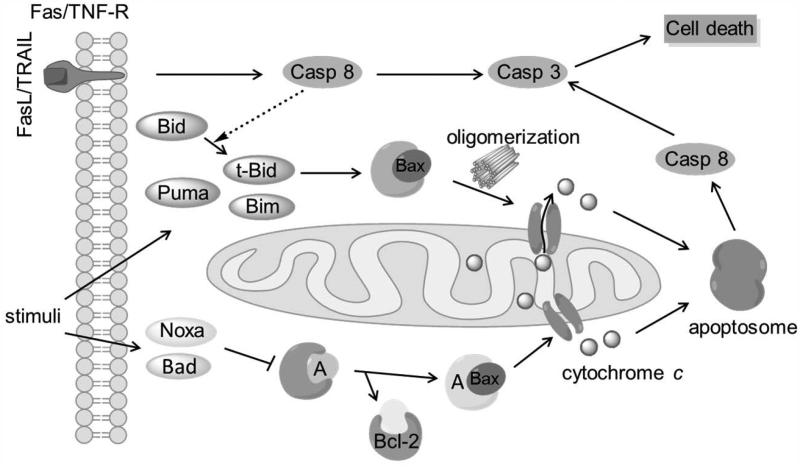
Bax is a unique entry point for intrinsic apoptotic signaling (Fig. 4). The intrinsic pathway is initiated by various stimuli including DNA damage, cytokine deprivation and cytotoxic stress.86-88,61 Under these stresses, BH3-only proteins activate Bax via direct or indirect means according to the direct activation model. The direct mode is characterized by activators (e.g. Bim, t-Bid) that bind and activate Bax, leading to MOMP. The indirect mode is manifested by sensitizers (e.g. Bad, Noxa) that inhibit anti-apoptotic protein’s ability to bind activators and thereby induce subsequent Bax-mediated apoptosis. Bax function is tightly regulated through a series of changes including conformational switching (inactive to active conformation),89 trafficking (from cytosol to mitochondria),90 and aggregation status changes (from monomer to dimer and multimer).91 Oligomerized Bax facilitates mitochondrial membrane permeabilization and promotes the formation of pores which enables the release of cytochrome c and Smac/DIABLO from the intermembrane space into cytosol.10,92-94 Together with apoptotic protease activating factor 1 (Apaf-1), dATP and procaspase-9, cytochrome c forms a complex “apoptosome”, which activates caspase 9 followed by downstream activation of other executioner caspases and ultimately results in cell death.12
Figure 4. The apoptotic pathways mediated by interactions between Bax and other Bcl-2 family members based on the direct activation model.
Once initiated by stimuli, BH3-only proteins activate Bax directly or indirectly. Oligomerized Bax leads to mitochondrial membrane permeabilization/pore formation and cytochrome c release. Cytochrome c, Apaf-1, dATP and procaspase-9 form the apoptosome which activates caspase 9 and other downstream executioner caspases, thereby ultimately leading to cell death. Casp: Caspase. A: Activator type BH3-only proteins.
Bax also participates in the extrinsic apoptotic pathway that is mediated by transmembrane death receptors.21 Bax reinforces the extrinsic pathway when caspase 8 cleaves Bid to generate the activated t-Bid (Fig. 4).95,22 It is also revealed that Bax-deficient human colon carcinoma cells are resistant to death-receptor ligands, while Bax-expressing sister clones are sensitive,96 indicating that Bax is essential for death receptor-mediated apoptosis. Moreover, it has been reported that sensitization of melanoma for TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis appears to be particularly dependent on Bax.97 Knockdown of Bax prevents release of Smac from the mitochondria and thereby blocking TRAIL-induced apoptosis.97,98 Suppressed Bax activity is one of the major reasons of TRAIL resistance in melanoma.11,99,100
Bax is also involved in the endoplasmic reticulum (ER) signaling pathway which plays a decisive role in many cellular events especially in cell death via crosstalk with mitochondrial pathways.101,102 Bax not only increases ER Ca2+ load and enhances Ca2+ release,103,104 but also modulates the unfolded-protein response (UPR) by directly interacting with inositol-requiring 1α (IRE1α).48 Bax is also believed to regulate mitochondrial dynamics in healthy cells and to be required for normal fusion of mitochondria into elongated tubules.105
Bax is expressed in essentially all organs,106 indicating that it may be a regulator of apoptosis in various cell types.107,108 Bax-deficient mice display selective expansion of cell population,62 selective hyperplasias, and resistance to certain apoptotic stimuli.109 Although mice lacking Bax are viable with limited phenotypic abnormalities, Bax−/−Bak−/−mice show various developmental defects.110
Down-regulation and mutation of Bax plays an important role in tumor resistance to apoptosis.111 Reduced Bax expression was found to be associated with Cisplatin resistance in ovarian carcinoma cells.112 The Bax gene is down-regulated in tumor colorectal cancer cell lines acquiring resistance to 5-FU compared to wild type HT-29 cells, suggesting that Bax down-regulation may serve as a key factor during both colorectal carcinogenesis and cell resistance to 5-FU.113 Down-regulation of Bax also plays an important role in Zoledronat-resistant lung cancer cell lines.114 Decreased Bax/Bcl-2 ratio and caspase activity serves as the main mechanism of temozolomide (TMZ)-induced chemoresistance in U87MG cells115 and paclitaxel resistant MCF-7 cells.116 Inhibition of Bax conformational change by Akt also contributes to chemoresistance.117,118 Loss of function mutation of Bax was reported to be found in hematopoietic malignancies31 and results in TRAIL resistance in mitochondria dependent type II cancer cells.119 Acquired point mutation of Bax G179E confers resistance to ABT-299 by abrogating Bax translocation to mitochondria. In addition, G179E Bax mutation also induces partial cross-resistance to other antineoplastic drugs.120 Phosphorylation (Ser184) of Bax inactivates its pro-apoptotic function by maintaining Bax in the cytoplasm and heterodimerizing with anti-apoptotic Bcl-2 proteins, and thus contributes to increased survival and chemoresistance of human lung cancer cells.83,121,118 Interestingly, Bax dephosphorylation (T172, T174 and T186) of wild-type p53-induced phosphatase 1 (Wip1) suppresses Bax-mediated apoptosis in response to γ-irradiation in prostate cancer cells and the effect can be reversed by a Wip1 inhibitor.122,121 Taken together, abnormalities regarding Bax including down-regulation, inactivated mutation, phosphorylation and dephosphorylation affect the ratio of Bax/Bcl-2,62,123 and confer resistance to cell death as well as overexpression of Bcl-2.124 Therefore, Bax activators may be used to promote pro-apoptotic activity with the potential to overcome resistance, and serve as a surrogate in lieu of enhanced of Bax expression as a means to augment apoptotic stimuli and decrease tumor enlargement.125
Given the critical role of Bax in apoptosis, it is not surprising that various anticancer agents that induce apoptosis of cancer cells involve the participation of the pro-apoptotic protein Bax. Examples include Hsp90 inhibitor 17-AAG126 and histone deacetylase (HDAC) inhibitors.127 Hsp90 inhibitors promote p53-dependent apoptosis through Puma and Bax.128 Cells lacking Bax and Bak prevent apoptosis mediated by HDAC inhibitors.129 Human colorectal cancer cells that lack functional Bax genes are partially resistant to the apoptotic effects of chemotherapeutic agent 5-fluofouracil, and completely abolish the apoptotic response to the chemopreventive agent sulindac and other nonsteroidal antiinflammatory drugs (NSAIDs).130 Selected drugs which exert their effect with respect to Bax activation are listed in TABLE 1.
Table 1.
Selected drugs or drug candidates involving indirect activation of Bax
| Name | Structure | Action mode | Status | Ref. |
|---|---|---|---|---|
| Bortezomib |
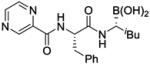
|
Proteasome inhibitor | Launched | 131-133 |
| Ixabepilone |
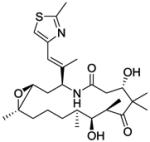
|
Tubulin modulator | Launched | 134 |
| Sorafenib |
|
Flt3, Kit etc. tyrosine kinase inhibitor |
Launched | 135 |
| Atorvastatin |
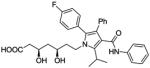
|
HMG CoA reductase inhibitor |
Launched | 136 |
| Erlotinib |
|
EGFR inhibitor | Launched | 137 |
| Sirolimus |
|
mTOR inhibitor | Launched | 138 |
| Simvastatin |
|
Hypercholesterolemia | Launcheda | 139 |
| Paclitaxel |
|
Mitotic inhibitor | Launched | 140 |
| Panobinostat |
|
Histone deacetylase inhibitor |
Pre-registration | 129 |
| Venetoclax (ABT 199) |
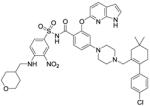
|
Bcl-2 inhibitor | Phase III (breakthrough therapy) |
141 |
| Navitoclax (ABT 263) |
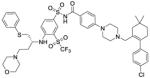
|
Bcl-2 inhibitor | Phase II | 142 |
Simvastatin was approved as a lipid lowering medication rather than an agent for cancer therapy. The recently reported anticancer effect of simvastatin is correlated to Bax activation.
4. Small molecules as direct Bax activators
While a number of anticancer drugs in the clinic induce Bax activation indirectly to facilitate apoptosis, none of them directly activates Bax. Accumulating evidence suggests that direct activation of Bax can be viewed as a novel and specific approach for cancer therapy. First, Bax is often differentially expressed in cancer cell lines versus normal cells. It is reported that human lung cancer cells expressing higher levels of total Bax also contain relatively higher levels of pBax (e.g. H292 and H1975).143 Approximately 21% of human hematopoietic malignancies possess loss-of-function mutations of Bax, perhaps most commonly in the acute lymphoblastic leukemia subset.49 Additionally, Bax has unique and critical sites that are not shared with other Bcl-2 family members, providing the molecular basis for an ideal targeted approach for cancer treatment with the desired outcome of a decreased side effect profile. Given that Bax plays a primary role in the intrinsic apoptosis pathway and participates in the extrinsic pathway, which is not the case for BH-3 only proteins as evidenced by their dependence on Bax/Bak,23,144 small-molecule activation of Bax represents a novel approach to promote the pro-apoptotic function of this centrally acting protein. It has been shown that the expression of Bax appears to play an important role in suppressing cancer development,80,145 and decreased Bax levels contribute to chemoresistance in a number of cancers including lung cancer, chronic lymphocytic leukemia (CLL), prostate cancer and others which may be ameliorated by small-molecule Bax activators.62, 127 Intriguingly, several small-molecule activators of Bax have been identified to induce cell death in a Bax-dependent fashion via direct binding to Bax in vitro and in vivo. By leveraging the requisite conformational change of Bax from its inactive to active state via several binding sites and key amino acid residues, the potential to explore the rational development of Bax small-molecule activators may prove fruitful towards the discovery of novel cancer therapeutics. Given that many anticancer agents induce cancer cell death via Bax activation, direct activation of Bax may also provide powerful agents for novel drug combinations. Taken together, direct activation of Bax represents a promising approach for cancer therapy.
4.1 Peptides Bid SAHBA and Bim SAHB
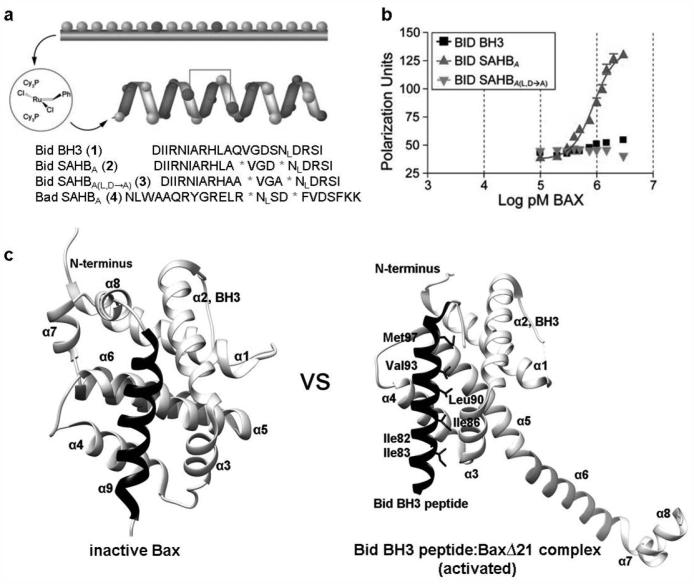
Direct involvement by selected BH3 domains of BH3-only proteins has been implicated in initiating Bax activation.38,146,147 Not only BH3-only proteins such as Bim, Bid and Puma, but also the tumor suppressor p53,148 are found to act as direct activators of Bax, resulting in cytochrome c release and apoptosis.149,150 A convenient and quick option to access Bax activators is through BH3 domain mimetics. Given that traditional peptides typically have poor uptake and are often comprised by their loss of secondary structures, several new approaches have emerged including a chemical strategy termed hydrocarbon stapling.151-154 The stabled Bid BH3 (1; Fig. 5a) mimetic Bid SAHBA (stabilized α helices of Bcl-2 domains, 2) is the first peptide identified to directly associate with Bax.147 The intramolecular all-hydrocarbon “staples”139 are generated by inserting non-natural amino acids bearing olefin tethers into the BH3 sequence, followed by ruthenium-catalyzed olefin metathesis (Fig. 5a). The peptide displays dramatically stabilized Bid BH3 α helicity compared to the random coil in solution.155 Fluorescein isothiocyanate (FITC)-derivatized Bid SAHBA demonstrates a direct binding interaction with full-length Bax, exhibiting an EC50 of 885 nM measured by a solution-phase fluorescence polarization assay (FPA) (Fig. 5b).156,157 In contrast, Bid SAHBA (L,D-A) (3) mutant known to impair the biological activity of Bid BH3 with enhanced α helicity,28 displays no binding activity at Bax or Bid BH3. At the same time, Bid SAHBA also displays binding activity for full-length Bcl-xL with an EC50 of 230 nM. In vitro mitochondrial cytochrome c release assays were also performed using Bak-deficient mouse liver mitochondria. Dosing with Bid SAHBA and Bax resulted in the release of cytochrome c in a dose-dependent manner. Addition of Bcl-xL inhibits Bid SAHBA induced Bax activation. Bid SAHBA(L,D/A) mutant and Bad SAHBA (4) reveal no significant cytochrome c release. These results suggest that direct binding interaction between Bid SAHBA and Bax is sufficient to activate Bax and the interaction is specific. Similarly, Bax coimmunoprecipitated with Bid SAHBA, but not with mutant Bid SAHBAs, indicating that cell-permeable Bid SAHBA can interact with Bax in cells. Bid SAHBA may ultimately be a valuable pro-apoptotic agent as it has bifunctional properties by directly engaging both pro-apoptotic and pro-survival multi-domain proteins.
Figure 5. Bid SAHBA binds Bax and activates Bax directly.
a, Structure and sequence of Bid SAHBA. The native methionine of Bid BH3 was replaced with norleucine (NL) in Bid SAHBA due to the incompatibility of sulfur with the metathesis reaction. b, Fluorescence polarization binding assays were carried out using FITC-labeled peptides (50 nM) and full-length Bax. Direct interaction between Bid SAHBA and Bax was observed. c, Comparison of the inactive form of Bax (PDB: 1F16) and activated form of BaxΔ21 (PDB: 4BD2). The crystal structure of Bid BH3 peptide (SESQEDIIRNIARHLAQVGDSMDRSIPPGL) complexed with BaxΔ21 shows that Hα1-Hα5 are released from Hα6-Hα8. Reproduced, with permission, from REF. 147© the Elsevier Inc. (2006).
Utilizing BH3 peptides including Bid BH3 as chemical probes, new progress has been made on the mechanism of Bax activation based on the crystal structures of BaxΔC21 with detergents and BH3 peptides.158 Compared to the inactive form of Bax (Fig. 5c, left panel), Hα1-Hα5 are released from Hα6-Hα8 in the active form of BaxΔC21 (Fig. 5c, right panel) induced by the engagement of Bid BH3 into canonical hydrophobic groove of Bax and BH3 domain of Bax is dislodged. The freed Bax BH3 domain then competes with activator BH3-only proteins for binding Bax to form stable homodimers, which is the fundamental unit of the Bax oligomers. Several hydrophobic residues of Bid BH3 interacting with Bax are highlighted as h0 (resides Ile82 and Ile 83), h1 (Ile86), h2 (Leu90), h3 (Val93) and h4 (Met97). Mutations at h0 position (I82A/I83A) abolish its activity of Bax activation in all assays. An isoleucine or a glutamate in h0 favors activator function on Bax. The destabilizing cavity is formed at the Bax-Bid BH3 intersurface to promote the liberty of Bax BH3 domain and trigger Bax oligomerization. Nevertheless, no cavities can be formed when BH3 peptides interact with pro-survival proteins. These findings based on the crystal structures of Bax and BH3 peptide complexes provide new insight on the mechanism of Bax activation and make it feasible to design BH3 sequences targeting Bax or pro-survival proteins selectively.
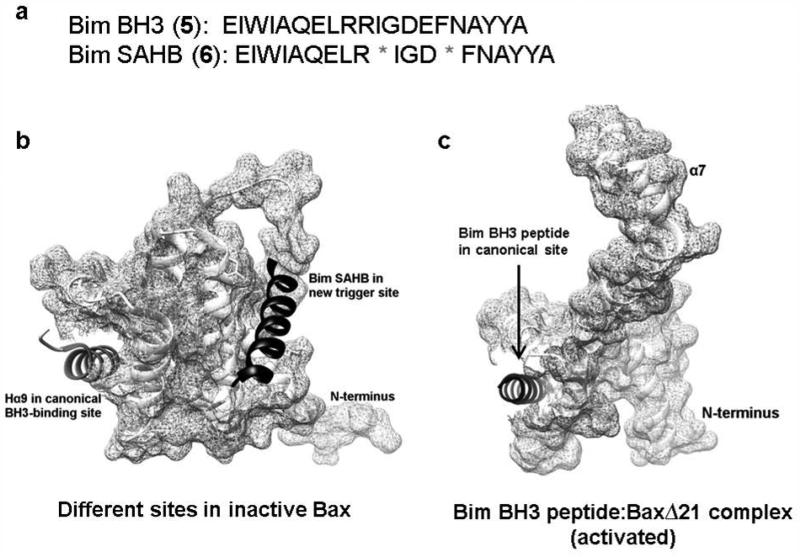
Bim BH3 (5) mimetic Bim SAHB (6, Fig. 6a), obtained in a same fashion as Bid SAHBA, has also been investigated. It shows 35-fold greater potency than Bid SAHBA, with an EC50 value of 23.7 nM. The direct Bim SAHB binding to Bax was confirmed by using NMR spectroscopy techniques and a new triggered binding site (Fig. 6b, right site), at which Bim SABH binds Bax leading to a battery of events including its direct activation and Bax-mediated mitochondrial apoptosis, was revealed.159 This Bax trigger site is defined by helices α1 and α6, on the opposite face of canonical BH3 binding site (Fig. 6b, left site) of anti-apoptotic proteins. However, more recent studies on the crystal structure of Bim BH3 peptide complexed with BaxΔ21 (Fig. 6c) did not address Bax activation at this site directly, while supporting the canonical binding site.160 The apoptotic response of Bax−/− Bak−/− mouse embryonic fibroblasts (DKO MEFs) reconstituted with Bax or Bax (K21E) and Bim SAHB or Bim SAHB (R153D) was examined. Neither Bim SAHB bearing a single amino acid mutation within the core BH3 consensus sequence nor Bax mutagenesis at the α1-α6 interaction site induces time-dependent apoptosis. Specificity of Bim SAHB-induced Bax activation was further evidenced by the “staple scan” and mutagenesis studies. Different in vitro assays (oligomerization, 6A7 immunoprecipitation, liposomal and mitochondrial assays) that measure ligand-induced Bax activation indicate that Bim SAHB directly and dose dependently activates Bax. The identification of the novel binding sites to interact with and activate Bax, and the elucidations of the crystal structures of BH3 peptides complexed with BaxΔ21 represent exciting breakthroughs that support the direct targeting of Bax for the therapeutic modulation of apoptosis.
Figure 6. Different interaction sites of Bim BH3 peptides with Bax.
a, Sequence of peptides Bim BH3 and Bim SAHB. b, Canonical BH3-binding site and the Bax trigger site located on the opposite sides of each other (PDB: 2K7W). c, The crystal structures of Bim BH3 peptide (RPEIWIAQELRRIGDEFNAYYA) complexed with Bax (PDB: 4ZIE) showing that the interaction site lies in the hydrophobic groove.
4.2 Non-peptide small molecule activators
Low bioavailability, poor membrane permeability and metabolic instability are the most common issues regarding the development of peptides as therapeutic agents.161-163 Although the described “stapled” peptides have relatively increased stability, cell-permeability, and the ability to induce apoptosis via direct binding and activation of Bax, non-peptide small molecules are preferred given the capacity of medicinal chemistry campaigns to fine tune molecular architecture towards the optimization of desired drug-like traits. Recently, several screen campaigns have been carried out to discover non-peptide small molecules that can directly bind and activate Bax to induce the apoptosis of cancer cells based on the Bax trigger site and critical amino acids residues.
4.2.1 BAM7 and its analogue BTC-8
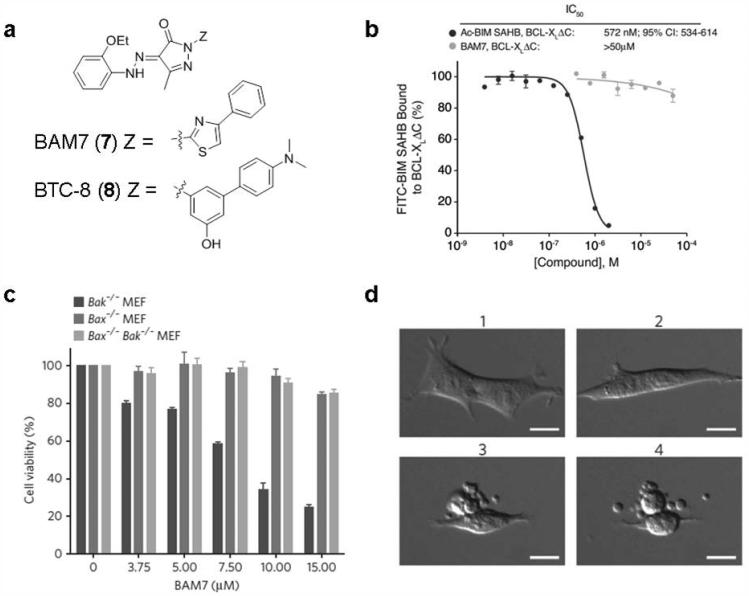
BAM7 is a non-peptide small-molecule direct activator of Bax which was identified in 2012 by Walensky et al.164 Based upon the newly recognized triggered site of Bax by Bim SAHB, a diverse in silico screen of 750,000 small molecules was conducted using Glide 4.0. The top 100 hits were selected for experimental analysis using competitive FPA involving FITC-BIM SAHB and Bax. BAM7 (7; Fig. 7a), a pyrazolone core substituted with an ethoxyphenylhydrazono, methyl, and phenylthiazole moieties, was identified as the most effective small-molecule binder of Bax among the compounds tested in this series, with an IC50 value of 3.3 μM. NMR analysis of [15N]Bax upon BAM7 titration shows that BAM7 and Bax interact at the very surface used by the BIM BH3 helix to trigger Bax activation. Different from N-terminal acetylated Bim SAHB (Ac-Bim SAHB), which can effectively compete with FITC-Bim SAHB for binding to the diversity of Bcl-2 family multi-domain protein members, BAM7 shows little or no competitive binding interactions with other Bcl-2 family targets including C-terminal deleted Bcl-xL (Bcl-xLΔC), Mcl-1ΔNΔC and BakΔC even at 50 μM concentration (Fig. 7b). Thus, BAM7 exhibits a high selectivity for Bax. The interaction between BAM7 and Bax induces characteristic structural changes yielding functional Bax oligomerization and Bax-dependent cell death (Fig. 7c) according to structural, biochemical and cellular studies. In vitro, BAM7 only impairs the viability of Bak−/− MEFs which rely on Bax in a time- and dose-dependent manner, and BAM7 has no effect on Bax−/− or DKO MEFs. BAM7-treated Bak−/− MEFs display characteristic microscopic features of apoptosis including cellular shrinkage (Fig. 7d). These results suggest that directly targeting Bax with a non-peptide small-molecule activator is feasible to trigger its pro-apoptotic activity. BAM7 is a selective small molecular Bax activator that binds to the Bax trigger site, representing a new approach toward combating human cancer.
Figure 7. BAM7 specifically binds Bax and induces Bax-dependent cell death.
a, The structure of BAM7 and its analog BTC-8. b, The specificity of BAM7 for the BH3 binding site on Bax was examined by competitive FPA employing FITC-BIM SAHB and Bcl-xLΔC (Only the result of BCL-xL is shown here). c, BAM7 selectively impairs the viability of Bak−/− MEFs but has no effect on MEFs lacking Bax (Bax−/−) or both Bax and Bak (Bax−/− Bak−/−). d. Bak−/− MEFs demonstrate the morphologic features of apoptosis in response to BAM7 treatment at the concentration of 15 μM. 1, 20 min; 2, 6 h; 3, 12 h; 4, 12.5 h. Scale bars, 15 μm. Reproduced, with permission, from REF. 164 © the Macmillan Publishers Limited (2012).
Recently, a structure-based lead optimization of BAM7 led to the discovery of BTC-8 (8; Fig. 7a), which induces MOMP with an EC50 of 700 nM, approximately one order of magnitude more potent than BAM7 in cultured HuH7 cells.165 BTC-8 was obtained through replacement of thiazole with phenyl ring and introduction of exocyclic basic group. BTC-8 can induce translocation of Bax to mitochondria leading to the release of cytochrome c, activation of caspase 3 and formation of apoptotic nuclei. Moreover, it is selectively toxic for cancer cells (HuH7, NB4, SHSY-5Y and LLC1) and immortalized cells (MEF) while having little effects on healthy resting cells (healthy splenocytes). BTC-8 shows in vivo efficacy in a murine Lewis lung carcinoma mouse model at a low intraperitoneal injection dose of 1 mg/kg. After only 4 days of treatment, a significant tumor mass reduction was observed to reach 50% compared with control with no gross toxicity.165
4.2.2 Compound 106 (ZINC 14750348)
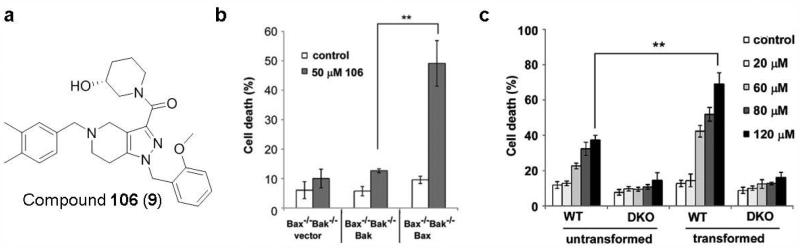
Structure-based drug design was used to discover agents capable of activating Bax, and compound 106 (9; Fig. 8a) was predicted to bind the Bax hydrophobic groove by a virtual screening approach.166 A total of approximately 10 million small molecules in the ZINC drug-like database were screened in the classic carboxyl-terminal transmembrane helix binding site based on the NMR structure of Bax (PDB code: 1F16) to identify compounds that can bind Bax. Among 46 high-scoring molecules that can fit into the hydrophobic groove, compound 106 was found to exhibit the highest Bax- and dose-dependent cytotoxicity.
Figure 8. Compound 106 induces Bax-dependent apoptotic cell death.
a, The chemical structure of compound 106 (ZINC 14750348). b, Compound 106 preferentially induces cell death of Bak−/−Bax−/− MEFs expressing Bax at the concentration of 50 μM. c, The viability of the untransformed MEFs and their isogenic counterparts (transformed by expression of the K-Ras and E1A oncogenes) was determined 48 h following treatment with compound 106 at various concentrations (20-120 μM). Reproduced, with permission, from REF. 166 © 2014 by the American Society for Microbiology.
Compound 106, a pyrazolo[4,3-c]pyridine core substituted with 3,4-dimethylbenzyl, 2-methoxybenzyl and (R)-1-(3-hydroxypiperidin-1-yl)ethanone moieties can fit well into the cavity in the Bax hydrophobic groove according to the virtual screening experiments. Compound 106 can trigger cell apoptosis in a Bax-dependent fashion. It selectively induces cell death of Bak−/−Bax−/− MEFs expressing Bax rather than vector or Bak (Fig. 8b). Compound 106 activates Bax in vitro by altering the proteins conformation, inducing Bax insertion into mitochondria and subsequent cytochrome c release. Compound 106 kills various tumor cell lines including murine Lewis lung carcinoma (LLC) cells, A549 human non-small cell lung carcinoma cells, and PANC-1 human pancreatic carcinoma cells in a Bax- and dose-dependent fashion. Moreover, compound 106 inhibits lung tumor growth and induces tumor cell apoptosis in vivo on the female C57BL/6 mice implanted LLC cells at the dose of 40 mg/kg/day by intraperitoneal injection. Compound 106 functions synergistically with carboplatin167 or ABT-737168 to induce human tumor cell death, indicating that Bax activators may serve as a component of combination chemotherapy regimens. Intriguingly, compound 106 is preferentially toxic to transformed MEFs cells (transformed by expression of the K-Ras and E1A oncogenes) compared to their normal counterparts with similar Bax expression (Fig. 8c). This finding suggests that tumor cells may respond more acutely than normal cells to stressful conditions such as Bax activation because of an overwhelmed antiapoptotic reserve, and Bax activators may thus have a superior selectivity for cancerous cells over normal cells.
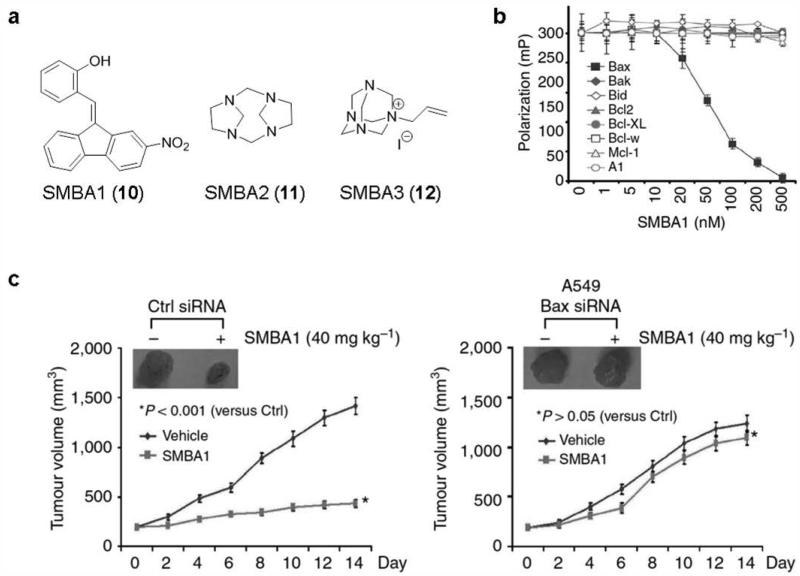
4.2.3 SMBA COMPOUNDS
Recently, several new small molecular ligands as direct activators of Bax have been identified by our team.143 Based on the previous finding that nicotine-induced Bax phosphorylation at serine 184 (S184) inactivates the pro-apoptotic function of Bax,80 it was reasoned that the pocket around the S184 site is an attractive target for structure-based drug discovery. A total of about 300,000 molecules by using the NCI compound library were docked into the structural pocket around S184 residue with the DOCK program suite virtual screening software. Further investigation of selected hits on their apoptotic effects against human lung cancer A549 and H1299 cells led to the discovery of three structurally diverse lead compounds SMBA1 (10), SMBA2 (11) and SMBA3 (12) (Fig. 9a). All three compounds exhibit significant suppression effects on nicotine-induced Bax phosphorylation in A549 cells. The competition fluorescence polarization assay169 demonstrates that SMBAs selectively bind to the Bax protein and display excellent binding affinities with Ki values of 43.3 ± 3.25 nM, 57.2 ± 7.29 nM and 54.1 ± 9.77 nM for SMBA1, SMBA2 and SMBA3, respectively, but fail to bind to other Bcl-2 family proteins such as Bcl-2, Bcl-xL, Mcl-1, Bcl-w, BFL-1/A1, Bid and Bak at the concentration of up to 0.5 μM (Fig. 9b). SMBAs selectively impair the viability of Bak−/− MEFs, but exhibit no effects on MEFs lacking Bax (Bax−/−) or both Bax and Bak (Bax−/− Bak−/−), indicating that Bax not Bak is the required essential target for SMBAs to induce apoptosis. Structural modeling with these chemical leads reveals that SMBAs can fit well into the Ser184 binding pocket. Further mechanistic investigation has validated that SMBAs indeed alter various apoptotic biomarkers and induce conformational changes of Bax, Bax oligomerization, mitochondrial insertion and cytochrome c release by blocking S184 phosphorylation.143
Figure 9. SMBA1 suppresses lung cancer in vivo by specifically targeting Bax.
a, The chemical structures of SMBA1~3. b, Bax agonist SMBA1 binds selectively with Bax rather than other relevant Bcl-2 family members at the increasing concentration (0~500 nM). c, Mice with xenografts derived from A549 expressing Bax siRNA, or Ctrl siRNA were treated with SMBA1 (40 mg/kg) or vehicle for 14 days. Reproduced, with permission, from REF. 143 © the Macmillan Publishers Limited (2014).
The anticancer activity of SMBA1 was further evaluated in vivo using nude mice bearing subcutaneous lung cancer xenograft derived from A549 cells. Significant antitumor efficacy was observed at the doses of 40 mg/kg and 60 mg/kg with treatment for 14 days. Body weight change of mice was monitored once every other day during treatment with increasing doses of SMBA1. No significant body weight loss and normal tissue toxicity in vivo were observed at all the tested doses, indicating that SMBA1 represents a new and safe class of anticancer agents. Intriguingly, SMBA1 shows target specificity, and almost displays no antitumor effect in Bax-deficient lung cancer xenograft derived from A549 expressing Bax siRNA at the effective dose of 40 mg/kg, demonstrating that Bax expression is essential for SMBA1 suppression of tumor growth in vivo (Fig. 9c).
5. Conclusions and future directions
Since its discovery in 1993, Bax has attracted an increasing amount of attention due to its central role in the regulation of apoptosis. Bax is critical to maintain homeostasis while it tends to be disordered in cancerous cells. Based on the solid foundation of cancer genetics and cell biology studies, direct binding and activation of Bax as a promising approach for cancer therapy is not only feasible, but also proven to be effective both in vitro and in vivo. Direct Bax activators have demonstrated a variety of advantages including potential superiority to overcome radio- and chemoresistance as well as to selectively induce apoptosis of cancer cells with low toxicity in normal cells.
To date, several classes of novel direct Bax activators including Bid SAHBA, Bim SAHB, BAM7, BTC-8, compound 106 and SMBAs have been identified to effectively induce Bax-mediate apoptosis in vitro and in vivo. Peptides Bid SAHBA and Bim SAHB exhibit an improved capability of overcoming the traditional limitations of peptides such as poor cellular permeability, bioavailability, solubility and metabolic stability as well as triggering Bax-mediated apoptosis. Bid SAHBA treatment consistently suppresses leukemia growth in vivo on immunodeficient mice bearing established human leukemia xenografts.155 However, it shows a relatively lower selectivity of Bax over other related Bcl-2 family members. Inspired by the designation of venetoclax, a Bcl-2 BH3 mimetic, as a breakthrough therapy for the treatment of 17p deletion relapsed-refractory CLL granted by FDA, these stapled peptides as BH3 mimetics of activator BH3-proteins may have great potential to be further optimized as unique chemical probes and peptide-based drugs.170,171 Both BAM7/BTC-8 and SMBAs directly bind Bax with high selectivity over other Bcl-2 family members and induce Bax-dependent cell death in a genetically defined context. Intriguingly, BTC-8 is highly efficacious in vivo in a murine Lewis lung carcinoma mouse model even at a low dose of 1 mg/kg. SMBA1 displays nanomolar binding affinity to the unique Ser184 site and suppresses tumor growth in the lung tumor xenograft mouse model at the dose of 40 mg/kg with no overt toxicity. More extensive structure-activity relationship (SAR) studies are imperative to improve efficacy and drug-likeness to yield optimized drug candidates for human clinical trials. Given the tremendous market for novel anticancer agents, these target-specific molecules that directly activate Bax offer great potential and hold promise for cancer treatment.
Both challenges and opportunities remain regarding the development of direct Bax activators. First, only a very limited number of small molecules have been reported that directly bind and activate Bax leaving the door open for the discovery of small molecules with diverse scaffolds suitable for preclinical development, representing an endeavor that is urgently needed. With the assistance of modern drug discovery technologies and multidisciplinary approaches including high-throughput screening (HTS), structure-based drug design, and computer-aided drug research, identifying new chemical entities directly targeting Bax likely can be facilitated. The discovery of BTC-8 appears to be a good example of structure-based drug design. Due to the well established in vitro and in vivo assays, HTS is feasible to yield more potent and otherwise undiscovered chemical scaffolds as direct Bax activators. Meanwhile, virtual screening with classic drug discovery guidelines properly applied is also a wise option given the crystal structures of Bax and relevant complexes as well as several different binding pockets are well characterized. Furthermore, HTS of fragments with relatively weaker binding and fragment-based drug design (FBDD)172,173 may also facilitate the discovery and enhance the structural diversity of Bax activators. Given the importance of several specific amino acid residues of Bax (e.g. Ser184) and the role of the associated phosphorylation, developing appropriate phosphonate probes174,175 might be very useful for elucidating the mechanisms of Bax activation and assisting the target-specific drug discovery.175
As discussed, the currently available structural studies on Bax and BH3 peptides complexed with BaxΔ21 have provided critical insights into the molecular mechanism of Bax activation and interactions between Bax and other Bcl-2 family members. If co-crystal structures of Bax and its non-peptide small molecular activator complexes are revealed, we envision that the rational drug design will be significantly facilitated to yield new insights for directly targeting Bax. It is the opinion of the authors that developing novel and efficient small molecules as specific and direct Bax activators will find an important place in both biopharmaceutical industry and academic settings, and open new avenues for understanding the fundamental mechanisms of Bax in multiple cellular contexts and eventually lead to the development of viable therapeutic regimens that may benefit cancer patients. Given the important role of Bax in apoptosis and drug resistance, combination therapies of Bax activators and chemotherapeutic drugs can also take prominence in the years to come.
ACKNOWLEDGEMENTS
This work was supported by grants P30 DA028821 and R01 DA038446 from the National Institutes of Health, Cancer Prevention Research Institute of Texas (CPRIT) award, R. A. Welch Foundation Chemistry and Biology Collaborative Grant from the Gulf Coast Consortia (GCC), and a training fellowship from the Keck Center for Interdisciplinary Bioscience Training of the GCC (T32 GM089657), John Sealy Memorial Endowment Fund, Institute for Translational Sciences (ITS), Sealy Center for Molecular Medicine, and the Center for Addiction Research (CAR) at UTMB.
Abbreviations
- Bcl-2
B-cell lymphoma 2
- MOMP
mitochondrial outer membrane permeabilization
- Smac/DIABLO
second mitochondria-derived activator of caspase/direct IAP-binding protein with low PI
- TNF
tumor necrosis factor
- FADD
Fas-associated death domain protein
- TRAIL
TNF-related apoptosis-inducing ligand
- ER
endoplasmic reticulum
- HDAC
histone deacetylase
- CLL
chronic lymphocytic leukemia
- SAHB
stabilized α helices of Bcl-2 domains
- DISC
death-inducing signaling complex
- BH
Bcl-2 homology domains
- NMR
nuclear magnetic resonance
- UPR
unfolded-protein response
- IRE1α
inositol-requiring 1α
- TMZ
temozolomide
- Wip1
wild-type p53-induced phosphatase 1
- NSAIDs
nonsteroidal antiinflammatory drugs
- FITC
fluorescein isothiocyanate
- FPA
fluorescence polarization assay
- SAR
structure-activity relationship
- HTS
high-throughput screening
- FBDD
fragment-based drug design
Biographies
Zhiqing Liu received her B.S. degree in Pharmaceutical Engineering from Shandong Normal University in 2009. She obtained her Ph.D. degree from Shanghai Institute of Materia Medica, Chinese Academy of Sciences in 2014 under the supervision of Professor Ao Zhang. She is currently a postdoctoral research fellow in Professor Jia Zhou’s Chemical Biology Program at University of Texas Medical Branch (UTMB). Her research interests include the rational design and chemical synthesis of small molecules as novel pharmacological probes and therapeutics for CNS disorders and human cancers.
Ye Ding obtained his Ph.D. in Medicinal Chemistry from China Pharmaceutical University under the tutelage of Dr. Yihua Zhang in 2011. During the subsequent two years, he worked as a Research Scientist at Research & Development Center, Nanjing Sanhome Pharmaceutical Co., Ltd. Dr. Ding is currently pursuing his postdoctoral training at the Chemical Biology Program, Department of Pharmacology and Toxicology at University of Texas Medical Branch under the supervision of Professor Jia Zhou. His research interests currently focus on drug discovery and development of target-based small molecules and natural product-inspired analogues as potential therapeutics for CNS disorders, cancer, and other human diseases.
Na Ye graduated in Basic Pharmacy from Shenyang Pharmaceutical University in 2008 and then earned her Ph.D. in Medicinal Chemistry from Shanghai Institute of Materia Medica, Chinese Academy of Sciences, under the supervision of Professor Ao Zhang in 2013. She is currently pursuing her postdoctoral training under the direction of Professor Jia Zhou at UTMB. Her research topics focus on the target-based drug design and chemical synthesis of bioactive small molecules as pharmacological probes and drug candidates for the treatment of cancer, CNS disorders, and other human diseases.
Christopher Wild studied biology and chemistry at the California State University, Northridge, where he received his Bachelor and Master of Science degrees under the direction of Professor Gagik Melikyan. Subsequently, he worked as a program chemist for ChemicoMays, CA and a research chemist at Celenese, TX where he was a member of the acetyl catalyst development team under the tutelage of Dr. Michael Nutt. Currently, Christopher is a member of the chemistry faculty at San Jacinto College, TX, and is pursuing a Ph.D. as a Keck Research Fellow at UTMB under the supervision of Professor Jia Zhou. His research focus is on the design and synthesis of small molecules as pharmacological probes and potential therapeutics.
Haiying Chen received her Bachelor of Science degree in Engineering from Tianjin University (Branch) in 1995. She worked as an engineer in Tianjin Research Institute of Construction Machinery associated with designing and programming computer testing systems. She joined Professor Jia Zhou’s drug discovery program in 2014 as a Research Associate. Her research interests focus on computer-assisted rational drug design of small molecules for CNS disorders, cancer and other human diseases.
Jia Zhou received his Ph.D. in organic chemistry in 1997 from Nankai University, China. Then he joined the chemistry faculty in the same university and was promoted to Associate Professor there. In 1999, he started his postdoctoral training in organic chemistry with Dr. Sidney M. Hecht at the University of Virginia. After further postdoctoral training in medicinal chemistry with Dr. Alan P. Kozikowski at Georgetown University, he has conducted research at Acenta Discovery, and PsychoGenics, Inc. as a Senior Principal Scientist for 7 years. Dr. Zhou is currently an Associate Professor (tenured) at the Chemical Biology Program, Department of Pharmacology and Toxicology at UTMB, leading a drug discovery research group. He is also a faculty member of Center for Addiction Research, Center for Biodefense and Emerging Infectious Diseases, and Sealy Center for Molecular Medicine at UTMB. He is an author of more than 90 papers, 3 book chapters and an inventor of 12 patents.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interest.
References
- 1.Golstein P. Cell death in us and others. Science. 1998;281(5381):1283. doi: 10.1126/science.281.5381.1283. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361(16):1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15(1):49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 5.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 6.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 7.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 8.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 9.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 10.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95(9):4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16(1):33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 13.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90(3):405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 14.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2(8):469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 15.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Kass GE, Szegezdi E, Joseph B. The mitochondrial death pathway: a promising therapeutic target in diseases. J Cell Mol Med. 2009;13(6):1004–1033. doi: 10.1111/j.1582-4934.2009.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang MH, Reynolds CP. Bcl-2 Inhibitors: Targeting Mitochondrial Apoptotic Pathways in Cancer Therapy. Clin Cancer Res. 2009;15(4):1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szendroedi J, Schmid AI, Meyerspeer M, Cervin C, Kacerovsky M, Smekal G, Graser-Lang S, Groop L, Roden M. Impaired mitochondrial function and insulin resistance of skeletal muscle in mitochondrial diabetes. Diabetes Care. 2009;32(4):677–679. doi: 10.2337/dc08-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Xie C, Spencer HJ, Zuo C, Higuchi M, Ranganathan G, Kern PA, Chou MW, Huang Q, Szczesny B, Mitra S, Watson AJ, Margison GP, Fan CY. Obesity and hepatosteatosis in mice with enhanced oxidative DNA damage processing in mitochondria. Am J Pathol. 2011;178(4):1715–1727. doi: 10.1016/j.ajpath.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 21.Vila M, Ramonet D, Perier C. Mitochondrial alterations in Parkinson's disease: new clues. J Neurochem. 2008;107(2):317–328. doi: 10.1111/j.1471-4159.2008.05604.x. [DOI] [PubMed] [Google Scholar]
- 22.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 23.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 25.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19(11):1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369(6478):321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 27.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 28.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2(3):183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 29.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111(3):331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 31.Czabotar PE, Colman PM, Huang DC. Bax activation by Bim? Cell Death Differ. 2009;16(9):1187–1191. doi: 10.1038/cdd.2009.83. [DOI] [PubMed] [Google Scholar]
- 32.Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, Geneste O, Cartron PF, Vallette FM, Manon S, Juin P. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185(2):279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letai A. Puma strikes Bax. J Cell Biol. 2009;185(2):189–191. doi: 10.1083/jcb.200903134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giam M, Huang DC, Bouillet P. BH3-only proteins and their roles in programmed cell death. Oncogene. 2008;27(Suppl 1):S128–136. doi: 10.1038/onc.2009.50. [DOI] [PubMed] [Google Scholar]
- 35.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2004;1644(2–3):83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18(4):157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leber B, Geng F, Kale J, Andrews DW. Drugs targeting Bcl-2 family members as an emerging strategy in cancer. Expert Rev Mol Med. 2010;12:e28. doi: 10.1017/S1462399410001572. [DOI] [PubMed] [Google Scholar]
- 38.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17(4):525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36(3):487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8(12):1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 41.Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17(6):617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17(3):393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 43.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44(4):517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12(5):897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bogner C, Leber B, Andrews DW. Apoptosis: embedded in membranes. Curr Opin Cell Biol. 2010;22(6):845–851. doi: 10.1016/j.ceb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Leber B, Lin J, Andrews DW. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene. 2010;29(38):5221–5230. doi: 10.1038/onc.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60(21):6101–6110. [PubMed] [Google Scholar]
- 48.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275(5302):967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 49.Meijerink JP, Mensink EJ, Wang K, Sedlak TW, Sloetjes AW, de Witte T, Waksman G, Korsmeyer SJ. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91(8):2991–2997. [PubMed] [Google Scholar]
- 50.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27(50):6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 51.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danial NN. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007;13(24):7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- 53.Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci U S A. 2004;101(43):15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinkmann K, Kashkar H. Targeting the mitochondrial apoptotic pathway: a preferred approach in hematologic malignancies? Cell Death Dis. 2014;5:e1098. doi: 10.1038/cddis.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai L, Wang S. Targeting apoptosis pathways for new cancer therapeutics. Annu Rev Med. 2014;65:139–155. doi: 10.1146/annurev-med-010713-141310. [DOI] [PubMed] [Google Scholar]
- 56.Thomas S, Quinn BA, Das SK, Dash R, Emdad L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M, Sarkar D, Fisher PB. Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther Targets. 2013;17(1):61–75. doi: 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7(12):989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- 58.Jeng PS, Cheng EH. Cancer therapeutics: Pulling the plug on BCL-X(L) Nat Chem Biol. 2013;9(6):351–352. doi: 10.1038/nchembio.1256. [DOI] [PubMed] [Google Scholar]
- 59.Han B, Park D, Li R, Xie M, Owonikoko TK, Zhang G, Sica GL, Ding C, Zhou J, Magis AT, Chen ZG, Shin DM, Ramalingam SS, Khuri FR, Curran WJ, Deng X. Small-Molecule Bcl2 BH4 Antagonist for Lung Cancer Therapy. Cancer Cell. 2015;27(6):852–863. doi: 10.1016/j.ccell.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merino D, Bouillet P. The Bcl-2 family in autoimmune and degenerative disorders. Apoptosis. 2009;14(4):570–583. doi: 10.1007/s10495-008-0308-4. [DOI] [PubMed] [Google Scholar]
- 61.Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385(6617):637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 62.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103(4):645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 64.Lalier L, Cartron PF, Juin P, Nedelkina S, Manon S, Bechinger B, Vallette FM. Bax activation and mitochondrial insertion during apoptosis. Apoptosis. 2007;12(5):887–896. doi: 10.1007/s10495-007-0749-1. [DOI] [PubMed] [Google Scholar]
- 65.Heimlich G, McKinnon AD, Bernardo K, Brdiczka D, Reed JC, Kain R, Kronke M, Jurgensmeier JM. Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. Biochem J. 2004;378:247–255. doi: 10.1042/BJ20031152. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24(12):2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Saez AJ, Coraiola M, Serra MD, Mingarro I, Muller P, Salgado J. Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J. 2006;273(5):971–981. doi: 10.1111/j.1742-4658.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- 68.Schendel SL, Montal M, Reed JC. Bcl-2 family proteins as ion-channels. Cell Death Differ. 1998;5(5):372–380. doi: 10.1038/sj.cdd.4400365. [DOI] [PubMed] [Google Scholar]
- 69.Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM. The N-terminal end of Bax contains a mitochondrial-targeting signal. J Biol Chem. 2003;278(13):11633–11641. doi: 10.1074/jbc.M208955200. [DOI] [PubMed] [Google Scholar]
- 70.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143(1):207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cartron PF, Moreau C, Oliver L, Mayat E, Meflah K, Vallette FM. Involvement of the N-terminus of Bax in its intracellular localization and function. FEBS Lett. 2002;512(1-3):95–100. doi: 10.1016/s0014-5793(02)02227-5. [DOI] [PubMed] [Google Scholar]
- 72.Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16(5):807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 73.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8(8):324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 74.Hanada M, Aime-Sempe C, Sato T, Reed JC. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem. 1995;270(20):11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- 75.Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;271(13):7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- 76.Bleicken S, Zeth K. Conformational changes and protein stability of the pro-apoptotic protein Bax. J Bioenerg Biomembr. 2009;41(1):29–40. doi: 10.1007/s10863-009-9202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol. 2000;149(2):431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D'Alessio M, De Nicola M, Coppola S, Gualandi G, Pugliese L, Cerella C, Cristofanon S, Civitareale P, Ciriolo MR, Bergamaschi A, Magrini A, Ghibelli L. Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J. 2005;19(11):1504–1506. doi: 10.1096/fj.04-3329fje. [DOI] [PubMed] [Google Scholar]
- 79.Xin M, Gao F, May WS, Flagg T, Deng X. Protein kinase Czeta abrogates the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2007;282(29):21268–21277. doi: 10.1074/jbc.M701613200. [DOI] [PubMed] [Google Scholar]
- 80.Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280(11):10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- 81.Xin M, Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J Biol Chem. 2006;281(27):18859–18867. doi: 10.1074/jbc.M512543200. [DOI] [PubMed] [Google Scholar]
- 82.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18(9):2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279(20):21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 84.Upton JP, Valentijn AJ, Zhang L, Gilmore AP. The N-terminal conformation of Bax regulates cell commitment to apoptosis. Cell Death Differ. 2007;14(5):932–942. doi: 10.1038/sj.cdd.4402092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cartron PF, Arokium H, Oliver L, Meflah K, Manon S, Vallette FM. Distinct domains control the addressing and the insertion of Bax into mitochondria. J Biol Chem. 2005;280(11):10587–10598. doi: 10.1074/jbc.M409714200. [DOI] [PubMed] [Google Scholar]
- 86.Brenner D, Mak TW. Mitochondrial cell death effectors. Curr Opin Cell Biol. 2009;21(6):871–877. doi: 10.1016/j.ceb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 87.Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci U S A. 2005;102(50):17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoo KC, Yoon CH, Kwon D, Hyun KH, Woo SJ, Kim RK, Lim EJ, Suh Y, Kim MJ, Yoon TH, Lee SJ. Titanium dioxide induces apoptotic cell death through reactive oxygen species-mediated Fas upregulation and Bax activation. Int J Nanomedicine. 2012;7:1203–1214. doi: 10.2147/IJN.S28647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144(5):891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139(5):1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276(15):11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 92.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17(14):3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20(3):929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem. 1999;274(4):2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 95.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 96.LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A. Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8(3):274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 97.Quast SA, Berger A, Plotz M, Eberle J. Sensitization of melanoma cells for TRAIL-induced apoptosis by activation of mitochondrial pathways via Bax. Eur J Cell Biol. 2014;93(1-2):42–48. doi: 10.1016/j.ejcb.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 98.Berger A, Quast SA, Plotz M, Kammermeier A, Eberle J. Sensitization of melanoma cells for TRAIL-induced apoptosis by BMS-345541 correlates with altered phosphorylation and activation of Bax. Cell Death Dis. 2013;4:e477. doi: 10.1038/cddis.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ravi R, Bedi A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L) Cancer Res. 2002;62(6):1583–1587. [PubMed] [Google Scholar]
- 100.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5(11):876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 101.Su J, Zhou L, Xia MH, Xu Y, Xiang XY, Sun LK. Bcl-2 family proteins are involved in the signal crosstalk between endoplasmic reticulum stress and mitochondrial dysfunction in tumor chemotherapy resistance. Biomed Res Int. 2014;2014:234370. doi: 10.1155/2014/234370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez D, Rojas-Rivera D, Hetz C. Integrating stress signals at the endoplasmic reticulum: The BCL-2 protein family rheostat. Biochim Biophys Acta. 2011;1813(4):564–574. doi: 10.1016/j.bbamcr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 103.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300(5616):135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 104.Chami M, Prandini A, Campanella M, Pinton P, Szabadkai G, Reed JC, Rizzuto R. Bcl-2 and Bax exert opposing effects on Ca2+ signaling, which do not depend on their putative pore-forming region. J Biol Chem. 2004;279(52):54581–54589. doi: 10.1074/jbc.M409663200. [DOI] [PubMed] [Google Scholar]
- 105.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443(7112):658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 106.Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994;145(6):1323–1336. [PMC free article] [PubMed] [Google Scholar]
- 107.Han J, Sabbatini P, White E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol Cell Biol. 1996;16(10):5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kiefer MC, Brauer MJ, Powers VC, Wu JJ, Umansky SR, Tomei LD, Barr PJ. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374(6524):736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- 109.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270(5233):96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 110.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6(6):1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 112.Perego P, Giarola M, Righetti SC, Supino R, Caserini C, Delia D, Pierotti MA, Miyashita T, Reed JC, Zunino F. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res. 1996;56(3):556–562. [PubMed] [Google Scholar]
- 113.Manoochehri M, Karbasi A, Bandehpour M, Kazemi B. Down-Regulation of BAX Gene During Carcinogenesis and Acquisition of Resistance to 5-FU in Colorectal Cancer. Pathol Oncol Res. 2013 doi: 10.1007/s12253-013-9695-0. [DOI] [PubMed] [Google Scholar]
- 114.Aoyagi T, Morii T, Ohtsuka K, Ohnishi H, Tajima T, Yoshiyama A, Mochizuki K, Satomi K, Ichimura S. Lung cancer cell line sensitivity to Zoledronic acid is BAX-dependent. Anticancer Res. 2013;33(12):5357–5363. [PubMed] [Google Scholar]
- 115.Shi L, Chen J, Yang J, Pan T, Zhang S, Wang Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010;1352:255–264. doi: 10.1016/j.brainres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 116.Sharifi S, Barar J, Hejazi MS, Samadi N. Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of breast cancer cells to paclitaxel. Asian Pac J Cancer Prev. 2014;15(20):8617–8622. doi: 10.7314/apjcp.2014.15.20.8617. [DOI] [PubMed] [Google Scholar]
- 117.Yamaguchi H, Wang HG. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene. 2001;20(53):7779–7786. doi: 10.1038/sj.onc.1204984. [DOI] [PubMed] [Google Scholar]
- 118.Yuan ZQ, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ. AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1: implication of AKT2 in chemoresistance. J Biol Chem. 2003;278(26):23432–23440. doi: 10.1074/jbc.M302674200. [DOI] [PubMed] [Google Scholar]
- 119.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12(3):228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 120.Fresquet V, Rieger M, Carolis C, Garcia-Barchino MJ, Martinez-Climent JA. Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood. 2014;123(26):4111–4119. doi: 10.1182/blood-2014-03-560284. [DOI] [PubMed] [Google Scholar]
- 121.Deng X. Bcl2 Family Functions as Signaling Target in Nicotine-/NNK-Induced Survival of Human Lung Cancer Cells. Scientifica (Cairo) 2014;2014:215426. doi: 10.1155/2014/215426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song JY, Ryu SH, Cho YM, Kim YS, Lee BM, Lee SW, Choi J. Wip1 suppresses apoptotic cell death through direct dephosphorylation of BAX in response to gamma-radiation. Cell Death Dis. 2013;4:e744. doi: 10.1038/cddis.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stoetzer OJ, Nussler V, Darsow M, Gullis E, Pelka-Fleischer R, Scheel U, Wilmanns W. Association of bcl-2, bax, bcl-xL and interleukin-1 beta-converting enzyme expression with initial response to chemotherapy in acute myeloid leukemia. Leukemia. 1996;10(Suppl 3):S18–S22. [PubMed] [Google Scholar]
- 124.Pietenpol JA, Papadopoulos N, Markowitz S, Willson JK, Kinzler KW, Vogelstein B. Paradoxical inhibition of solid tumor cell growth by bcl2. Cancer Res. 1994;54(14):3714–3717. [PubMed] [Google Scholar]
- 125.Bargou RC, Wagener C, Bommert K, Mapara MY, Daniel PT, Arnold W, Dietel M, Guski H, Feller A, Royer HD, Dorken B. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996;97(11):2651–2659. doi: 10.1172/JCI118715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Powers MV, Valenti M, Miranda S, Maloney A, Eccles SA, Thomas G, Clarke PA, Workman P. Mode of cell death induced by the HSP90 inhibitor 17-AAG (tanespimycin) is dependent on the expression of pro-apoptotic BAX. Oncotarget. 2013;4(11):1963–1975. doi: 10.18632/oncotarget.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu C, Friday BB, Yang L, Atadja P, Wigle D, Sarkaria J, Adjei AA. Mitochondrial Bax translocation partially mediates synergistic cytotoxicity between histone deacetylase inhibitors and proteasome inhibitors in glioma cells. Neuro Oncol. 2008;10(3):309–319. doi: 10.1215/15228517-2007-063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He K, Zheng X, Zhang L, Yu J. Hsp90 inhibitors promote p53-dependent apoptosis through PUMA and Bax. Mol Cancer Ther. 2013;12(11):2559–2568. doi: 10.1158/1535-7163.MCT-13-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ierano C, Chakraborty AR, Nicolae A, Bahr JC, Zhan Z, Pittaluga S, Bates SE, Robey RW. Loss of the proteins Bak and Bax prevents apoptosis mediated by histone deacetylase inhibitors. Cell Cycle. 2013;12(17):2829–2838. doi: 10.4161/cc.25914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290(5493):989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 131.Premkumar DR, Jane EP, DiDomenico JD, Vukmer NA, Agostino NR, Pollack IF. ABT-737 synergizes with bortezomib to induce apoptosis, mediated by Bid cleavage, Bax activation, and mitochondrial dysfunction in an Akt-dependent context in malignant human glioma cell lines. J Pharmacol Exp Ther. 2012;341(3):859–872. doi: 10.1124/jpet.112.191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Unterkircher T, Cristofanon S, Vellanki SH, Nonnenmacher L, Karpel-Massler G, Wirtz CR, Debatin KM, Fulda S. Bortezomib primes glioblastoma, including glioblastoma stem cells, for TRAIL by increasing tBid stability and mitochondrial apoptosis. Clin Cancer Res. 2011;17(12):4019–4030. doi: 10.1158/1078-0432.CCR-11-0075. [DOI] [PubMed] [Google Scholar]
- 133.Liu FT, Agrawal SG, Gribben JG, Ye H, Du MQ, Newland AC, Jia L. Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood. 2008;111(5):2797–2805. doi: 10.1182/blood-2007-08-110445. [DOI] [PubMed] [Google Scholar]
- 134.Yamaguchi H, Paranawithana SR, Lee MW, Huang Z, Bhalla KN, Wang HG. Epothilone B analogue (BMS-247550)-mediated cytotoxicity through induction of Bax conformational change in human breast cancer cells. Cancer Res. 2002;62(2):466–471. [PubMed] [Google Scholar]
- 135.Panka DJ, Wang W, Atkins MB, Mier JW. The Raf inhibitor BAY 43-9006 (Sorafenib) induces caspase-independent apoptosis in melanoma cells. Cancer Res. 2006;66(3):1611–1619. doi: 10.1158/0008-5472.CAN-05-0808. [DOI] [PubMed] [Google Scholar]
- 136.Schweitzer M, Mitmaker B, Obrand D, Sheiner N, Abraham C, Dostanic S, Chalifour LE. Atorvastatin mediates increases in intralesional BAX and BAK expression in human end-stage abdominal aortic aneurysms. Can J Physiol Pharmacol. 2009;87(11):915–922. doi: 10.1139/y09-085. [DOI] [PubMed] [Google Scholar]
- 137.Ling YH, Lin R, Perez-Soler R. Erlotinib induces mitochondrial-mediated apoptosis in human H3255 non-small-cell lung cancer cells with epidermal growth factor receptorL858R mutation through mitochondrial oxidative phosphorylation-dependent activation of BAX and BAK. Mol Pharmacol. 2008;74(3):793–806. doi: 10.1124/mol.107.044396. [DOI] [PubMed] [Google Scholar]
- 138.Tirado OM, Mateo-Lozano S, Notario V. Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax : Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity. Oncogene. 2005;24(20):3348–3357. doi: 10.1038/sj.onc.1208471. [DOI] [PubMed] [Google Scholar]
- 139.Spampanato C, De Maria S, Sarnataro M, Giordano E, Zanfardino M, Baiano S, Carteni M, Morelli F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int J Oncol. 2012;40(4):935–941. doi: 10.3892/ijo.2011.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Strobel T, Swanson L, Korsmeyer S, Cannistra SA. BAX enhances paclitaxel-induced apoptosis through a p53-independent pathway. Proc Natl Acad Sci U S A. 1996;93(24):14094–14099. doi: 10.1073/pnas.93.24.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 142.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 143.Xin M, Li R, Xie M, Park D, Owonikoko TK, Sica GL, Corsino PE, Zhou J, Ding C, White MA, Magis AT, Ramalingam SS, Curran WJ, Khuri FR, Deng X. Small-molecule Bax agonists for cancer therapy. Nat Commun. 2014;5:4935. doi: 10.1038/ncomms5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15(12):1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pepper C, Hoy T, Bentley DP. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br J Cancer. 1997;76(7):935–938. doi: 10.1038/bjc.1997.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marani M, Tenev T, Hancock D, Downward J, Lemoine NR. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22(11):3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24(2):199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 148.Marcel V, Catez F, Diaz JJ. p53, a translational regulator: contribution to its tumour-suppressor activity. Oncogene. 2015 doi: 10.1038/onc.2015.25. [DOI] [PubMed] [Google Scholar]
- 149.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10(22):2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 150.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303(5660):1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 151.Shangary S, Johnson DE. Peptides derived from BH3 domains of Bcl-2 family members: a comparative analysis of inhibition of Bcl-2, Bcl-x(L) and Bax oligomerization, induction of cytochrome c release, and activation of cell death. Biochemistry. 2002;41(30):9485–9495. doi: 10.1021/bi025605h. [DOI] [PubMed] [Google Scholar]
- 152.Li R, Boehm AL, Miranda MB, Shangary S, Grandis JR, Johnson DE. Targeting antiapoptotic Bcl-2 family members with cell-permeable BH3 peptides induces apoptosis signaling and death in head and neck squamous cell carcinoma cells. Neoplasia. 2007;9(10):801–811. doi: 10.1593/neo.07394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schafmeister CE, Po J, Verdine GL. An All-Hydrocarbon Cross-Linking System for Enhancing the Helicity and Metabolic Stability of Peptides. J Am Chem Soc. 2000;122(24):5891–5892. [Google Scholar]
- 154.Blackwell HE, Grubbs RH. Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis. Angew Chem Int Ed. 1998;37(23):3281–3284. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 155.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305(5689):1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97(13):7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang H, Nimmer P, Rosenberg SH, Ng SC, Joseph M. Development of a high-throughput fluorescence polarization assay for Bcl-x(L) Anal Biochem. 2002;307(1):70–75. doi: 10.1016/s0003-2697(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 158.Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, Huang DC, Kluck RM, Adams JM, Colman PM. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152(3):519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 159.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455(7216):1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Robin AY, Krishna Kumar K, Westphal D, Wardak AZ, Thompson GV, Dewson G, Colman PM, Czabotar PE. Crystal structure of Bax bound to the BH3 peptide of Bim identifies important contacts for interaction. Cell Death Dis. 2015;6:e1809. doi: 10.1038/cddis.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81(1):136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 162.Adessi C, Soto C. Converting a peptide into a drug: strategies to improve stability and bioavailability. Curr Med Chem. 2002;9(9):963–978. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 163.Biron E, Chatterjee J, Ovadia O, Langenegger D, Brueggen J, Hoyer D, Schmid HA, Jelinek R, Gilon C, Hoffman A, Kessler H. Improving oral bioavailability of peptides by multiple N-methylation: somatostatin analogues. Angew Chem Int Ed Engl. 2008;47(14):2595–2599. doi: 10.1002/anie.200705797. [DOI] [PubMed] [Google Scholar]
- 164.Gavathiotis E, Reyna DE, Bellairs JA, Leshchiner ES, Walensky LD. Direct and selective small-molecule activation of proapoptotic BAX. Nat Chem Biol. 2012;8(7):639–645. doi: 10.1038/nchembio.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Stornaiuolo M, La Regina G, Passacantilli S, Grassia G, Coluccia A, La Pietra V, Giustiniano M, Cassese H, Di Maro S, Brancaccio D, Taliani S, Ialenti A, Silvestri R, Martini C, Novellino E, Marinelli L. Structure-Based Lead Optimization and Biological Evaluation of BAX Direct Activators as Novel Potential Anticancer Agents. J Med Chem. 2015;58(5):2135–2148. doi: 10.1021/jm501123r. [DOI] [PubMed] [Google Scholar]
- 166.Zhao G, Zhu Y, Eno CO, Liu Y, Deleeuw L, Burlison JA, Chaires JB, Trent JO, Li C. Activation of the proapoptotic Bcl-2 protein Bax by a small molecule induces tumor cell apoptosis. Mol Cell Biol. 2014;34(7):1198–1207. doi: 10.1128/MCB.00996-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Azzoli CG, Kris MG, Pfister DG. Cisplatin versus carboplatin for patients with metastatic non-small-cell lung cancer--an old rivalry renewed. J Natl Cancer Inst. 2007;99(11):828–829. doi: 10.1093/jnci/djk222. [DOI] [PubMed] [Google Scholar]
- 168.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 169.Bruncko M, Oost TK, Belli BA, Ding H, Joseph MK, Kunzer A, Martineau D, McClellan WJ, Mitten M, Ng SC, Nimmer PM, Oltersdorf T, Park CM, Petros AM, Shoemaker AR, Song X, Wang X, Wendt MD, Zhang H, Fesik SW, Rosenberg SH, Elmore SW. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J Med Chem. 2007;50(4):641–662. doi: 10.1021/jm061152t. [DOI] [PubMed] [Google Scholar]
- 170.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov Today. 2010;15(1-2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 171.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20(1):122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 172.Chen H, Zhou X, Wang A, Zheng Y, Gao Y, Zhou J. Evolutions in fragment-based drug design: the deconstruction-reconstruction approach. Drug Discov Today. 2015;20(1):105–113. doi: 10.1016/j.drudis.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jhoti H, Williams G, Rees DC, Murray CW. The 'rule of three' for fragment-based drug discovery: where are we now? Nat Rev Drug Discov. 2013;12(8):644–645. doi: 10.1038/nrd3926-c1. [DOI] [PubMed] [Google Scholar]
- 174.Arrendale A, Kim K, Choi JY, Li W, Geahlen RL, Borch RF. Synthesis of a phosphoserine mimetic prodrug with potent 14-3-3 protein inhibitory activity. Chem Biol. 2012;19(6):764–771. doi: 10.1016/j.chembiol.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Panigrahi K, Nelson DL, Berkowitz DB. Unleashing a "true" pSer-mimic in the cell. Chem Biol. 2012;19(6):666–667. doi: 10.1016/j.chembiol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]