Abstract
Clinically significant anxiety symptoms are prevalent among the elderly, yet knowledge about the longitudinal course of anxiety symptoms in later life remains scarce. The goals of this study were to (1) characterize age trajectories of state anxiety symptoms in the second half of life, and (2) estimate genetic and environmental contributions to individual differences in the age trajectory of state anxiety. This study was based on data from 1,482 participants in the Swedish Adoption/Twin Study of Aging who were aged 50 and older at their first occasion (512 complete twin pairs, 458 singletons) and had up to six measurement occasions spanning 11 years. Consistent with lifespan developmental theories of age-related emotional change, anxiety symptom levels declined during the transition from midlife to the mid −60s, followed by a mild increase that gradually plateaued in the 80s. There were substantial individual differences in the age trajectory of anxiety. After accounting for effects of sex, cohort, mode of testing and proximity to death, this longitudinal variation was partitioned into biometric sources. Nonshared environmental variance was highest in the late 60s and declined thereafter, whereas genetic variance increased at an accelerated pace from approximately age 60 onward. There was no evidence for effects of rearing or other shared environment on anxiety symptoms in later life. These findings highlight how the etiology of anxiety symptoms changes from midlife to old age.
Keywords: Anxiety, Aging, Trajectory, Twin Study, Longitudinal Study
Anxiety is an important clinical concern in older adults, yet research on the course of anxiety symptoms in later life is scarce and has yielded inconsistent findings. A focus on anxiety symptoms as a dimensional measure is consistent with current thinking about psychopathology, including the recognition that subclinical anxiety is consequential (e.g., Grenier et al., 2011). Another understudied area is whether individual differences in anxiety become more or less prominent with age, and whether genetic and environmental contributions to such individual differences change with age – the latter would suggest potentially different etiological mechanisms underlying anxiety manifestation over age and has treatment implications. For example, a greater role of genetic contributions to individual differences in anxiety with age would motivate molecular genetic inquiry to identify potential targets for intervention. The first goal of this study was to characterize the trajectory of anxiety from age 50 onward. The second goal was to examine biometric sources of individual differences underlying anxiety trajectory in later life.
Theoretical Models of Aging and Anxiety
Based on several key theories on age-related emotional change, we hypothesized that state anxiety would decline from midlife to old age (50s into late 60s / early 70s), followed by an increase in later years. The Socioemotional Selectivity Theory (SST; Carstensen, 1993; Carstensen & Mikels, 2005), Strength and Vulnerability Integration model of emotional well-being across adulthood (SAVI; Charles, 2001), and Selective Optimization with Compensation theory (SOC; Baltes, 1997; Baltes & Smith, 2003) converge in their predictions that emotional well-being improves in later life. SST postulates that a motivational shift from information acquisition to emotional gratification toward the end of life is accompanied by changes in information processing style and problem-solving strategies that facilitate emotion regulation (e.g., Blanchard-Fields, 2007; Scheibe & Carstensen, 2010). SAVI builds on SST and highlights accrued self-knowledge and social experiences as age-related strengths that enhance emotional well-being. SOC proposes that emotional intelligence peaks and individuals become more skilled at adapting to and reconstructing losses as they transition from midlife to old age. Given age-related gains in social competence, problem-solving, and emotion regulation in the context of relatively intact cognition and physical health, we hypothesized that individuals would experience anxiety less frequently and intensely (i.e., a decrease in state anxiety) from midlife to old age.
SAVI and SOC also led us to hypothesize a subsequent increase in state anxiety during the transition into advanced old age, or Baltes' “fourth age” (i.e., from the 70s into the 80s and 90s). SAVI identifies numerous vulnerabilities that become more salient with age and cannot be compensated by age-related strengths. These include greater physiological rigidity that prevents prompt recovery from emotional arousal, loss of social belongingness, chronic and uncontrollable stressors such as caregiving, and neurological dysfunction leading to cognitive impairment. Similarly, SOC holds that cultural forces (e.g., medical advances) become less efficacious in counteracting cognitive and biological declines in advanced old age, leaving individuals with diminishing control over their emotional well-being.
Empirical Research on Aging and Anxiety
State vs. Trait Anxiety
Anxiety is represented by both state- and trait-like constructs in the literature. State anxiety is typically defined as short-term, unpleasant feelings of tension, fear, nervousness, worry, and autonomic arousal (e.g., Beck & Steer, 1990; Spielberger, 1983; Zigmund & Snaith, 1983), with time frames generally ranging from “at the moment” to weeks. Trait anxiety is typically operationalized as liability to anxiety disorders and related behaviors, and may be indexed by higher-order personality traits that feature a prominent anxiety component, such as neuroticism (Costa & McCrae, 1995) or harm avoidance (Cloninger, 1987).
Although state anxiety is by definition transient, emotion states assessed by state measures appear to reflect more stable, “temperament-like” attributes. For example, the state and trait anxiety subscales of the State-Trait Anxiety Inventory (STAI-Y; Spielberger, 1983) correlate about 0.7 in adult samples (Kabacoff, Segal, Hersen, & Van Hasselt, 1997; Grös, Antony, Simms, & McCabe, 2007; Willis, Dodd, & Palermo, 2013). Items on the STAI state anxiety subscale were designed to measure emotions that are higher in stressful situations and lower in relaxing situations. However, a meta-analysis reported an average test-retest reliability of 0.70 (compared to 0.88 for the trait subscale; Barnes, Harp, and Jung, 2002). Assessing state anxiety longitudinally is akin to taking a series of snapshots of one's emotional states over intervals of months or years. A multitude of transient factors can affect emotion state at any given moment, creating significant occasion-specific “noise” that obscures systematic change over time. Nonetheless, empirical evidence suggests a high degree of stability across the snapshots. Thus, systematic change in state anxiety should serve as a sensitive indicator of age changes in emotional well-being, especially when occasion-specific “noise” (i.e., transient influences and random error) can be partialed out statistically.
Age-Related Change in Anxiety
Few studies have examined change in state anxiety over age, with preliminary evidence suggesting an age-related decline in anxiety. Mirowsky and Scheiman (2008) conducted the only longitudinal study to date on the age trajectory of state anxiety. Based on data collected from three occasions across six years, they reported that frequency of experiencing anxiety in the past week decreased with age, with slower rates of decline in older ages, and no cohort effect on the trajectory (Mirowsky & Schieman, 2008). A similar age trend was reported cross-sectionally, with older women but not older men showing lower levels of recent anxiety symptoms than younger adults (Henderson et al., 1998). The prevalence of state anxiety symptoms was highly stable over time in two epidemiologic studies of older adults (de Beurs, Beekman, Deeg, Van Dyck, & Van Tilburg, 2000; Heikkinen & Kauppinen, 2011).
For trait anxiety, a cross-sectional study found that STAI-trait anxiety followed a cubic shape over age, such that it increased with age among younger adults and peaked in middle-adulthood, followed by a decline into the 70s, and rose again in older ages (Teachman et al., 2006). As noted, neuroticism is a personality trait theorized to underlie anxiety disorders (Khan, Jacobson, Gardner, Prescott, & Kendler, 2005; Malouff, Thorsteinsson, & Schutte, 2005) and therefore considered an index of trait anxiety. Longitudinal evidence from three large studies suggests a U-shaped age trajectory with nadirs occurring in the 70s or early 80s (Mroczek & Spiro, 2003; Steunenberg, Twisk, Beekman, Deeg, & Kerkhof, 2005; Terracciano, McCrae, Brant, & Costa, 2005), which is consistent with the cross-sectional findings by Teachman (2006). Cohort differences were reported in two of these studies (Mroczek and Spiro, 2003; Terracciano et al., 2005), which included lower levels of neuroticism in later-born cohorts.
Individual Differences in Anxiety
Research on age-related change in anxiety constructs has focused on characterizing the average trajectory and paid less attention to the extent of individual differences in trajectories. Intercept variance of a trajectory represents individual differences in initial level; slope variance represents individual differences in systematic (i.e., predictable) change over age; and residual variance represents occasion-specific (i.e., within-person) variance not captured by the overall trajectory and includes measurement error. Because aging is associated with tremendous heterogeneity (Baltes & Baltes, 1990), we hypothesized that there would be greater variance in state anxiety as people age.
Biometric Contributions to Individual Differences in Anxiety over Age
One way to better understand the etiology of anxiety is by examining the extent to which genetic and environmental factors contribute to individual differences in anxiety. Twin studies are based on the premise that higher similarity for twins in monozygotic (MZ) pairs than dizygotic (DZ) pairs suggests genetic contribution to a phenotype, and equal similarity implies common environmental contribution. Twin studies have demonstrated that both genetic influences and environmental factors unique to each person (i.e., nonshared environment) underlie individual differences in anxiety states (Jardine, Martin, & Henderson, 1984) and liability to anxiety disorders (Hettema, Neale, and Kendler, 2001). For example, in a study of 3810 twin pairs aged 18–88 in the Australian National Health and Medical Research Council Twin Register, variance in past-month anxiety symptoms as measured by the Delusions Symptoms Signs Inventory – Anxiety scale (DSSI/sA; Bedford, Foulds, & Sheffield, 1976) was attributable to genetic (37%) and nonshared environmental factors (63%), with no contribution from environmental factors shared among family members (Jardine et al., 1984). Across studies, sex differences in genetic and environmental contributions were either nonsignificant or very small (Hettema et al., 2001).
While cross-sectional twin studies have provided consistent estimates of the heritability (i.e., proportion of anxiety variance attributable to genetic effects) of state anxiety, whether genetic influences on individual differences in state anxiety become more or less prominent with age remain largely unknown. A common but erroneous assumption of genetic effects on phenotypes is that they are stable over time because genes are inherited and genetic sequence remains the same over time. However, genetic effects can increase or decrease over age as a function of gene expression associated with developmental timing or environmental circumstances (Bergen, Gardner, & Kendler, 2007). The limited research to date suggests significant stability in genetic influences and considerably less consistency in nonshared environmental contributions to state anxiety variation over time (Gillespie et al., 2004; Rijsdijk et al., 2003). Transitory environmental factors accounted for a greater proportion of total state anxiety variation than more enduring environmental factors (Gillespie et al., 2004). However, neither study offered more than limited information on changes in genetic contribution to individual differences in anxiety in older ages. Given the association between later-life anxiety and many genetically-influenced processes, including late-onset diseases (van Dongen, Slagboom, Draisma, Martin, & Boomsma, 2012), we hypothesized that genetic influence on individual differences in state anxiety trajectory would increase with age. We also expected an increase in nonshared environmental influence, which would reflect the cumulative influences of enduring environmental factors (e.g., social support) on state anxiety.
The Present Study
The present study draws from a population-based twin sample assessed at six occasions over 11 years. The first goal was to characterize the trajectory of state anxiety change in later life while considering the effects of birth cohort and sex. We hypothesized that state anxiety would decrease from midlife to old age, followed by a subsequent increase in the later years. We expected substantial individual differences in anxiety trajectories, especially in older ages. Extending current knowledge on the heritability of anxiety, our second goal was to evaluate age-related change in the genetic and environmental contributions to state anxiety variation, testing the hypothesis of an age-related increase in state anxiety variation attributable to both genetic and nonshared environmental sources.
METHODS
Participants and Study Design
The Swedish Adoption/Twin Study of Aging (SATSA) comprises a subset of twins from the population-based Swedish Twin Registry (Lichtenstein, Floderus, Svartengren, Svedberg, & Pedersen, 2002). In 1984, same-sex twin pairs who had previously indicated they had been separated from their cotwin before age 11 and reared apart, and a sample of reared-together twins matched on age, sex, and country of birth were recruited into SATSA (Finkel & Pedersen, 2004; Pedersen, McClearn, Plomin, Nesselroade, Berg, & DeFaire, 1991). Data were collected at 3-year intervals. Each data collection wave included a mail questionnaire (Q) and an in-person testing (IPT) component. Because of the varying time gap between Q and IPT within a data collection wave, most questionnaires administered as part of the Qs were mailed to participants again a week before IPTs and collected during IPTs. Only twin pairs who both had responded to the first questionnaire wave (Q1) and were aged 50 or older at a given wave were invited to participate in IPTs. Therefore, sample sizes were smaller for IPTs than Qs. The current study used data from assessments wherein anxiety was measured, including: Q1 (in 1984), Q2 (1987), IPT2 (1989–91), Q3 (1990), IPT3 (1992–94), and Q4 (1993). Because anxiety was not measured again in SATSA until IPT9 and Q7 (both in 2010), we restricted our analyses to the relatively contiguous observations collected between 1984 and 1994. This is the first study using SATSA data to examine the genetic and environmental contributions to state anxiety. Previous publications based on SATSA have examined the cross-lagged association between anxiety and depression (Wetherell, Gatz,& Pedersen, 2001), and the cross-sectional association between state anxiety and cognitive performance during in-person testing (Wetherell, Reynolds, Gatz, & Pedersen, 2002).
Because we were interested in examining anxiety trajectory in later life, only individuals aged 50+ at their first occasion were included. Of 1,571 eligible individuals, 89 were excluded for not having any anxiety data, yielding 1,482 individuals that formed the sample for the phenotypic analysis. Because zygosity was unknown for 72 individuals, biometric analysis was limited to a subset of 1,410 individuals. Of this overall analytic sample, 20.9% had data for all six occasions, 6.9% had data for five occasions, 23.4% had data for four occasions, 14.0% had data for three occasions, 14.2% had data for two occasions, and 20.9% had data for one occasion. The sample was 60.1% female, born between 1891–1942, and on average 65.9 years old (SD = 8.6; range = 50–96) at the first occasion. It included 512 complete twin pairs and 458 singletons. By design, approximately half (n = 725, 48.9%) of the sample were from reared-apart pairs. Table 1 shows the breakdown of the sample by zygosity, rearing status, and pair status. Table 2 shows the mean age and sex composition of the sample by occasion. A description of participant flow across occasions is provided in Supplemental Table 1.
Table 1.
Study Sample by Zygosity, Rearing Status, and Pair Status (Complete Pair vs. Singleton), N = 1482.
| MZ | DZ | Unknown zygosity | Total # families | |
|---|---|---|---|---|
| Complete pairs (# pairs) | 186 | 306 | 20 | 512 |
| Reared apart | 79 | 148 | 12 | 239 |
| Reared together | 107 | 158 | 8 | 273 |
|
| ||||
| Singletons (# individuals) | 120 | 306 | 32 | 458 |
| Reared apart | 55 | 181 | 11 | 247 |
| Reared together | 65 | 125 | 21 | 211 |
|
| ||||
| Total | 970 | |||
Note: MZ = monozygotic twins; DZ = dizygotic twins.
Table 2.
Descriptive statistics for the sample by measurement occasion.
| Q1 (1984) | Q2 (1987) | IPT2 (1989–91) | Q3 (1990) | IPT3 (1992–4) | Q4 (1993) | |
|---|---|---|---|---|---|---|
| Female N (%) | 661 (58.1%) | 670 (59.3%) | 284 (59.5%) | 594 (59.1%) | 267 (61.5%) | 540 (59.5%) |
| Male N (%) | 477 (41.9%) | 460 (40.7%) | 193 (40.5%) | 411 (40.9%) | 167 (38.5%) | 367 (40.5%) |
| Mean age (SD), range | 65.9 (8.6) 50.1 – 91.9 |
68.5 (8.6) 50.7 – 95.8 |
68.5 (6.9) 55.0 – 91.0 |
70.1 (7.7) 50.8 – 92.5 |
71.8 (6.9) 58.0 – 89.3 |
72.1 (7.7) 50.0 – 93.2 |
| Anxiety M (SD) | 19.1 (8.0) | 18.1 (7.7) | 17.9 (7.3) | 18.9 (7.7) | 17.6 (7.2) | 18.9 (7.5) |
| Correlations Between Anxiety Scores by Occasion (men: above diagonal; women: below diagonal): | ||||||
| Q1 | -- | .52 | .64 | .51 | .38 | .44 |
| Q2 | .61 | -- | .61 | .64 | .61 | .63 |
| IPT2 | .47 | .58 | -- | .68 | .63 | .54 |
| Q3 | .47 | .62 | .63 | -- | .68 | .59 |
| IPT3 | .44 | .53 | .55 | .57 | -- | .62 |
| Q4 | .46 | .59 | .54 | .55 | .50 | -- |
Note: Q = questionnaire component of a data collection wave; IPT = in-person testing component of a data collection wave. Illness represents the number of organ systems reported to be affected by a physical illness at Q1. For each person, the date of each measurement occasion was used to organize observations chronologically for longitudinal analysis. The sex ratios and mean ages in the table represent a composite of longitudinal participants who began the study at Q1 and additional participants who joined in successive waves. Anxiety was higher in women than men at each occasion, t = 3.07 to 5.44. All correlations had p ≤.0001 (N ranged from 149 to 661 for each pair of variables).
Measures
State Anxiety
State anxiety symptoms were measured at each occasion using the 10-item State Anxiety subscale of the State-Trait Personality Inventory (STPI; Spielberger, 1979). The measure was translated into Swedish by a bilingual translator, then back-translated into English by another translator. Discrepancies were resolved and corrected to ensure accurate representation of the original measure by the Swedish version. Items assessed how participants felt at the moment and were worded in both positive (e.g., “I feel calm”) and negative (e.g., “I feel tense”) directions. Responses ranged from 1=does not fit at all to 5=fits exactly. Item-level missingness was handled with mean substitution if a participant had no more than one missing item (<6% of participants at each wave). The total score was considered missing if two or more items were missing. Two observations were considered invalid and excluded because the participant answered all 1's or 5's to every item. After reverse-coding the positively-worded items, item scores were summed to yield a total score (ANX) that ranges from 10 to 50, with higher scores reflecting higher anxiety. In the present sample, the STPI showed good internal consistency in the present sample (Cronbach's α ≥.91 for all occasions) and a moderate to high degree of temporal stability, with correlations across occasions between .38 and .68 (Table 2).
Covariates
Covariates included sex, mode of testing, birth year, age, imminent death, and non-death related drop-out. Sex was coded as 1=female and 0=male. Mode of testing was coded as 1=IPT and 0=Q to adjust for potential differences in ANX due to different entry criteria and procedures for IPT and Q.
Birth year was included to adjust for birth cohort differences in ANX intercept and slope. Age was a time-varying covariate to examine ANX change over age. Both terms were needed to separate within-person age change from between-person age differences in ANX trajectory, as these effects are confounded when only age or birth year is included (Hoffman, 2012; Sliwinski, Hoffman, & Hofer, 2010).
Two time-varying covariates were used to adjust for the potential impact of attrition on anxiety trajectory. Imminent death represented circumstances surrounding the very end of life, such as severe illness or disability, which may be linked to higher anxiety (Neimeyer, Wittkowski, & Moser, 2004). It was coded as 1 if a participant died within two years following a measurement occasion and 0 otherwise. Drop-out represented other circumstances leading up to non-participation and potentially related to anxiety. For a given occasion, it was coded as 1 if a participant was known to be alive at the subsequent occasion but did not participate and 0 otherwise.
Baseline physical health status was included as a covariate in post-hoc analysis. It is based on answers to 51 health items at Q1, which ask whether a person has, or ever had, particular health problems or diagnoses. Most of the items are from the OARS health battery (Duke University, 1978). Following prior work in SATSA (Harris, Pedersen, McClearn, Plomin, & Nesselroade, 1992), the 51 items were coded into a count variable that represents the number of organ systems affected by at least one health problem or a cancer diagnosis (range: 0–13).
Zygosity determination
Twin pairs were initially classified as MZ or DZ based on information from the Swedish Twin Registry and supplemented with Q1 questionnaire items regarding physical resemblance of twins in a pair. Zygosity was subsequently updated with blood and DNA samples, which validated 92% of the original classifications.
Analytic Strategy
Phenotypic models of longitudinal anxiety symptoms
ANX change over age was characterized using a series of mixed-effects multilevel models (Raudenbush & Bryk, 2002; Singer & Willett, 2003), which can accommodate nested data. Our dataset included 3 levels of nesting: ooccasion-specific data (Level 1) from individuals (Level 2) who are members of twin pairs (Level 3). We modeled ANX variation as a combination of fixed and random effects at each level. Fixed effects are regression weights applied to measured variables. Level 1 fixed effects operate at the occasion level and included mode of testing, imminent death, drop-out, and polynomial age terms (age, age2, age3). We did not examine any Level 2 fixed effects. Level 3 fixed effects included sex and birth year (centered at 1920). Variation in ANX that was not predictable from the fixed effects was partitioned into random variance at each level. Level 3 random variation reflects deviation of pair trajectories from the sample mean trajectory, Level 2 random variation reflects deviation of individual trajectories from the pair's estimated trajectory. The remaining, Level 1 random variation is due to a combination of measurement error and occasion-specific unmeasured sources.
Models were built sequentially, beginning with an unconditional model to examine the amount of within-person, between-person, and between-pair variation in ANX across observations. Next, main effects of covariates were entered to adjust for their influence on ANX level. Because mortality rates were higher in men and drop-out rates were higher among women (Supplemental Table 1), we created two interaction terms (sex × imminent death, gender × drop-out) to evaluate sex differences in the impact of attrition on ANX level. Fixed and random effects of linear, quadratic, and cubic age were sequentially added to quantify mean ANX change over age and the amount of between-person variation in such change. Finally, we created fixed effect interaction terms to explore whether age-related ANX change was modified by sex, mode of testing, birth year, imminent death and drop-out. Age was centered at 67 (sample mean age at participants' first observation) and birth year was centered at 1920.
Data analysis was conducted with SAS PROC MIXED (SAS Institute Inc., 2012) using full maximum likelihood estimation. Nested models were evaluated with likelihood ratio tests (Δ −2LL /Δ parameters). Fixed effects were also assessed by the statistical significance of the regression coefficient. Non-nested models were compared using the Akaike Information Criterion (AIC; Akaike, 1974) and the Bayesian Information Criterion (BIC; Schwarz, 1978), for which smaller values indicate better fit. Explained variance was calculated based on the reduction of unexplained variance after adding predictors (Singer & Willet, 2003).
Biometric Decomposition of Anxiety Variance
Twin correlations were examined first. Based on the results (described later), ANX variance was partitioned into five sources in biometric analyses: (1) additive genetic effects (A) are genetic allelic effects that combine additively; (2) dominant genetic effects (D) are allelic effects that combine non-additively; (3) rearing environment effects (S) result in shared variance between cotwins reared in the same environment; (4) correlated environment effects (C) refer to experiences shared by cotwins that are unrelated to rearing status (e.g., in-utero environments, adult contact with cotwin); and 5) nonshared environmental effects (E) are unique to one twin in a pair.
The first task of our longitudinal, biometric analyses was to estimate genetic and environmental contributions to individual differences in ANX trajectories over age. The second task was to evaluate biometric sources of occasion-specific ANX variance, that is, fluctuation of each person's ANX score about his/her estimated trajectory. McArdle and Prescott (2005) first demonstrated that the phenotypic mixed-effects multilevel models described earlier can be re-parameterized to partition variance due to A, C, and E effects. Extending this method, we parameterized variance in anxiety intercept and slope as orthogonal components and partitioned them into uncorrelated A, C, E, D and S subcomponents. We assigned weights to each biometric subcomponent according to expectations of shared variance between cotwins given their zygosity and rearing status (Supplementary Table 2). Models were estimated with SAS PROC MIXED using TYPE=TOEP(1) (for A, D and S) and TYPE=VC (for C and E). We partitioned occasion-specific ANX variance into biometric sources in a similar manner. Whereas the E component of ANX trajectory does not contain random error, occasion-specific E is a combination of error and occasion-specific environmental variance. For each model, we calculated the intercept and slope variances due to each biometric component. Negative variance estimates suggest a poorly identified solution. In these cases, the negative parameters were sequentially set to zero, starting with the smallest parameter and checking for no significant deterioration in model fit, until non-zero estimates were obtained. Models were compared using the procedures described earlier.
RESULTS
Descriptive statistics are shown in Table 2. The moderate to high correlations of ANX across study intervals provide support for examining systematic change over time. As expected, correlations were lower across longer intervals. Women reported higher ANX than men across all occasions, but the correlations over time were comparable between sexes. Among 503 individuals who participated in at least one IPT occasion, ANX was slightly lower during IPT (M=17.72, SD=6.69) than questionnaire occasions (M=18.34, SD=6.09; t=2.99, df=502, p=.003).
Phenotypic Models of Anxiety Change over Age
In the Baseline model (not shown), we estimated how much variation in ANX was attributable to each of the three levels of nesting in our design: within-person, occasion-specific fluctuation (40.8%); variation between members of a pair (i.e., between-person, 38.2%); and across twin pairs (21.0%). Next, we estimated a Covariates-Only model, including main effects of sex, birth year, mode of testing, imminent death and drop-out. Significant effects included higher ANX for: women than men, earlier-born than more recently-born individuals, and when assessed in questionnaire than IPT occasions. There were no main effects for imminent death or drop-out; however, the imminent death × sex interaction was significant: among individuals who died within two years after an assessment occasion, women had higher ANX than men. Compared to the baseline model, including sex, birth year, mode of testing, imminent death, and sex × imminent death improved the model fit substantially (Δ−2LL=−51.7 /+5 parameters; Table 3, model a), and these covariates were retained in all subsequent models
Table 3.
Results of Selected Phenotypic Models of Anxiety Trajectory over Age, Based on 5091 observations from 1482 twins aged 50 and older.
| (a) No Age Change | (b) Linear Age | (c) Quadratic Age | (d) Cubic Age | |||||
|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | |
| Fixed Effects: | ||||||||
| Intercept | 17.72 | 0.30 | 17.68 | 0.30 | 17.53 | 0.32 | 17.59 | 0.33 |
| Female (1=Y, 0=N) | 1.92 | 0.39 | 1.91 | 0.39 | 1.78 | 0.39 | 1.80 | 0.39 |
| Mode (1=IPT, 0=Q) | −0.67 | 0.20 | −0.70 | 0.21 | −0.65 | 0.20 | −0.64 | 0.20 |
| Birth year | −0.05 | 0.02 | −0.04 | 0.03 | −0.02 | 0.03 | −0.05 | 0.04 |
| Die ≤ 2 yrs (1=Y, 0=N) | −0.38 | 0.60 | −0.47 | 0.61 | −0.69 | 0.61 | −0.74 | 0.61 |
| Female * Die ≤ 2 yrs | 2.02 | 0.92 | 2.03 | 0.93 | 1.98 | 0.93 | 2.16 | 0.93 |
| Age | -- | -- | 0.01 | 0.03 | 0.01 | 0.03 | 0.03 | 0.04 |
| Age2 | -- | -- | -- | -- | 0.003 | 0.001 | 0.01 | 0.003 |
| Age3 | -- | -- | -- | -- | -- | -- | −0.0003 | 0.0001 |
| Age * Birth year | -- | -- | -- | -- | 0.01 | 0.005 | ||
|
| ||||||||
| Random Effects: | ||||||||
| Level 3 (Between-Pair): | ||||||||
| Intercept | 11.36 | 1.97 | 11.01 | 1.95 | 11.24 | 1.98 | 11.05 | 1.97 |
| Level 2 (Within-Pair): | ||||||||
| Intercept | 23.43 | 1.95 | 21.97 | 2.07 | 27.19 | 2.46 | 27.25 | 2.47 |
| Linear slope | -- | -- | 0.19 | 0.10 | 0.02 | 0.14 | 0.03 | 0.14 |
| Quadratic slope | -- | -- | -- | -- | 0.10 | 0.02 | 0.10 | 0.02 |
| Intercept-linear slope covariance | -- | -- | −0.14 | 0.10 | 0.14 | 0.14 | 0.12 | 0.14 |
| Intercept-quadratic slope covariance | -- | -- | -- | -- | −0.10 | 0.02 | −0.10 | 0.02 |
| Linear-quadratic slope covariance | -- | -- | -- | -- | −0.002 | 0.001 | − 0.002 | 0.001 |
| Level 1 (Within-Person, Across-Time) | ||||||||
| Residual | 24.99 | 0.59 | 24.24 | 0.61 | 22.27 | 0.61 | 22.26 | 0.61 |
|
| ||||||||
| Model Fit Compared to Baseline†: | ||||||||
| Δ−2LL (Δ parameters) | −51.7 | (+5) | −66.1 | (+8) | −124.3 | (+12) | −137.3 | (+14) |
| ΔAIC | −41.7 | −50.1 | −100.3 | −109.3 | ||||
| ΔBIC | −17.3 | −11.1 | −41.7 | −41.0 | ||||
Note: Bold indicates p ≤.05. Italics indicates p ≤.10. −2LL = −2 log-likelihood; df = degrees of freedom. Age was centered at 67; birth year was centered at 1920.
Model fit was compared against a baseline model with a fixed intercept and random intercepts at L2 and L3 (−2LL = 33287.9 for 4 parameters, AIC = 33295.9, BIC = 33315.4).
Results from models of linear, quadratic, and cubic age change in ANX are presented in Table 3, columns b-d. The predicted age slope of ANX in the linear model was not significantly different from zero, but including the random effect of linear age improved the model fit, indicating notable individual differences in the linear age slope of ANX (Δ−2LL=−14.4 /+3 parameters; model b). Compared to the random linear model (b), model fit was improved by adding the fixed effect (Δ−2LL=−9.2 /+1 parameter; not shown) and random effects of quadratic age (Δ−2LL=−49.0 /+3 parameters compared to the fixed quadratic model; model c). Adding the fixed effect of cubic age to the random quadratic model further improved model fit (Δ−2LL=−8.3 /+1 parameter; not shown). Random effects of cubic age were extremely small and not included.
Next, we evaluated a series of fixed effect interactions between the polynomial age terms (age, age2, age3) and other covariates. Only the birth year × age interaction was significant and improved the fit of the fixed cubic model (Δ−2LL=−4.7 /+1 parameter), thus this was retained as the final phenotypic model (model d). In this model, 18.2% of the unexplained variation in ANX was attributed to between-pair differences in trajectory, 45.2% to between-person differences, and 36.6% to within-person fluctuation across measurement occasions. We calculated pseudo-R2 (Singer & Willett, 2003) to examine the amount of explained variance in ANX across models: Compared to the Baseline model, the Covariates-Only model explained <1% of residual variance in ANX, whereas the final model accounted for an additional 11.1%.
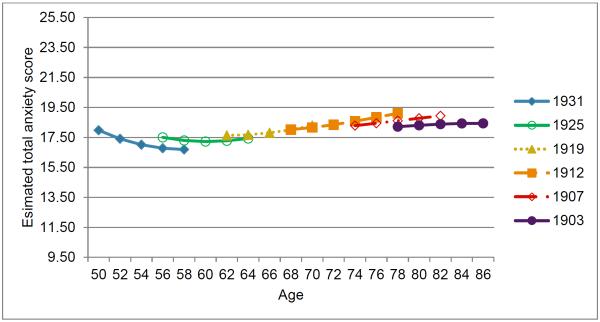
Based on the final model, we plotted the estimated ANX trajectories for five example birth years across age ranges corresponding to the study interval (Figure 1). Overall, the results indicate little systematic change in ANX with age. On average, ANX declined from the 50s until the mid-60s, followed by a subsequent increase that eventually plateaued in the 80s. Cohort differences in the average ANX trajectory were also very small. For individuals born every 10 years later than 1920, ANX at the intercept of age 67 was estimated to be 0.5 points lower (−0.5*10) and the linear rate of ANX increase at age 67 was 0.1 higher (0.01*10). Importantly, this average trajectory does not represent each person's unique trajectory. There were substantial individual differences in the intercept, and to a smaller extent, the slopes. For example, the estimated 95% confidence interval for a person's ANX intercept was broad, 7.35 to 27.83 (i.e., 17.59 ± 1.96*√27.27).
Figure 1.
Plot of estimated ANX over age based on best-fitting phenotypic model (Table 3, column d). Results for six example birth years are shown to demonstrate age cohort differences in anxiety trajectory. Estimated ANX are shown for the observed age range for individuals born in a given year. The range of the Y-axis covers the range of the model-based intercept (17.59) +/− 1 standard deviation of ANX (SD based on Q1 data).
Longitudinal Biometric Analyses
Twin correlations of state anxiety are presented in Table 4. MZ twin correlations were generally twice the magnitude of DZ twin correlations or higher, consistent with A and D effects and providing little evidence for C effects. Correlations tended to be higher between reared-together twins than reared-apart twins, suggesting possible similarity due to S effects.
Table 4.
Twin pair correlations (# complete pairs) of anxiety scores by occasion and pair status.
| Q1 | Q2 | Q3 | Q4 | IPT2 | IPT3 | |
|---|---|---|---|---|---|---|
| MZT | .44 (79) | .49 (77) | .36 (68) | .39 (57) | .45 (49) | .23 (41) |
| MZA | .29 (51) | .36 (45) | .25 (38) | .06 (35) | .50 (25) | .18 (19) |
| DZT | .10 (11) | −.01 (103) | .26 (95) | .28 (78) | .18 (58) | .17 (57) |
| DZA | .03 (10) | .12 (117) | .22 (95) | .04 (85) | .03 (75) | .04 (63) |
Note: Q = questionnaire occasion; IPT = in-person testing occasion; MZT = monozygotic twins reared together; MZA = monozygotic twins reared apart; DZT = dizygotic twins reared together; DZA = dizygotic twins reared apart. Bold indicates p ≤ .05.
We based the fixed effects portion of the longitudinal biometric model on the best-fitting phenotypic model (Table 3, model d), and estimated biometric contributions to variation in ANX trajectory over age. Table 5 displays the fit indices for models representing different biometric contributions to variation in ANX trajectory. In the E-Only model shown under Table 5(a), individual differences in anxiety trajectory are entirely attributed to individual-specific sources. The alternative AE, CE, and SE models fit the data better than the E-only model. The AE model had superior fit compared with the SE and CE models, as evidenced by its lower AIC and BIC, suggesting little to no contribution of rearing or correlated environments on systematic anxiety change over age. Compared with the AE model, the ADE and ASE models1 had higher AIC and BIC, suggesting neither dominant genetic effects nor shared rearing explained variation in anxiety trajectory. The ACE model could not be identified without constraining all parameters representing C effects to zero, suggesting a lack of shared environmental influences.
Table 5.
Comparison of Fit Indices from Longitudinal Biometric Models of ANX trajectory, Based on 4924 Observations from 1410 Twins (492 Complete Pairs and 426 Singletons).
| (a) Biometric models of longitudinal ANX variance (i.e., intercept and slope variance): | |||
| Longitudinal E-only model | −2LL = 32067.6 (17 parameters) | AIC = 32101.6 | BIC = 32190.9 |
| Compared to: | Δ−2LL / Δ parameters | Δ AIC | Δ BIC |
| Longitudinal AE | −46.5 / +6 | −34.5 | −12.9 |
| Longitudinal CE | −37.2 / +6 | −25.2 | −3.6 |
| Longitudinal SE | −38.1 / +6 | −26.1 | −4.5 |
| Longitudinal AE model | −2LL = 32021.1 (23 parameters) | AIC = 32067.1 | BIC = 32178.0 |
| Compared to: | Δ−2LL / Δ parameters | Δ AIC | Δ BIC |
| Longitudinal ADE | (not nested) | +11.4 | +40.3 |
| Longitudinal ACE | (not well-identified) | -- | -- |
| Longitudinal ASE | (not nested) | +1.2 | +30.1 |
|
| |||
| (b) Biometric models of occasion-specific ANX variance (i.e., occasion-specific fluctuation around each person's estimated trajectory). All models below are based on the longitudinal AE model: | |||
| Compared to occasion-specific E-only◇ model: | Δ−2LL / Δ parameters | Δ AIC | Δ BIC |
| Occasion-specific AE | −2.9 / +1 | −0.9 | +4.0 |
| Occasion-specific CE | −1.1 / +1 | +0.9 | +5.8 |
| Occasion-specific SE | −2.9 / +1 | −0.9 | +3.9 |
| Occasion-specific ADE | (not well-identified due to negative parameter estimate) | ||
| Occasion-specific ACE | (not well-identified due to negative parameter estimate) | ||
| Occasion-specific ASE | −3.4 / +2 | +0.6 | +10.2 |
Note: Bold indicates p ≤.05. −2LL = −2 log-likelihood; A = additive genetic variance, D = dominant genetic variance, S = variance due to shared rearing environment between twins, C = variance due to shared environment between twins independent of rearing status (e.g., in-utero environments), E = non-shared environmental influences. Across all models, fixed effects parameters included gender, mode of testing, birth year, dying in 2 years, female * die in 2 years, age, age2, age3, and birth year * age.
For all models under section (a), occasion-specific variance was E-only. Therefore, the occasion-specific E-only model shown here is identical to the longitudinal AE model shown under (a).
Next, we examined the biometric sources that contributed to occasion-specific ANX variance (Table 5(b)). Based on the longitudinal AE model wherein occasion-specific variance was entirely attributed to nonshared environmental sources (i.e., an occasion-specific E-only model), neither the occasion-specific AE, CE, SE, nor ASE model resulted in better fit. The occasion-specific ADE and ACE models yielded negative parameter estimates, rejecting the effects of genetic dominance and correlated environments on anxiety fluctuation at each occasion. Thus, after adjusting for the effects of sex, mode, birth year, imminent death, sex × imminent death, and age × birth year on the mean trajectory, occasion-specific ANX variance was entirely attributable to nonshared environmental factors (including random error).
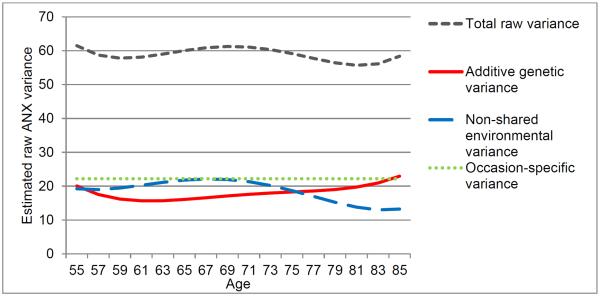
Based on the longitudinal AE model with E-only occasion-specific variance (see Table 6 for estimates of variance components), we calculated the contribution of genetic and environmental factors to individual differences in ANX trajectory over age. Total ANX variation was relatively stable over age, but the manner in which genetic factors and nonshared environment contributed differed with age (Figure 2). Genetic contribution to variation in ANX trajectory increased from approximately age 60 onward and this increase became steeper in the 80s, whereas nonshared environmental variance increased from the 50s to the late 60s, but declined thereafter.
Table 6.
Estimated Raw ANX Aariance due to Genetic and Environmental factors According to the Best-Fitting Longitudinal Biometric Model; Based on 4924 Observations from 1410 Twins (492 Complete Pairs and 426 Singletons); Only Random Effects are Shown.
| Longitudinal AE, Occasion-Specific E-only Model | Est. | SE |
|---|---|---|
| Intercept variance due to: | ||
| Additive genetic effects | 16.46 | 3.43 |
| Nonshared environmental effects | 22.14 | 3.30 |
| Linear slope variance due to: | ||
| Additive genetic effects | −0.06 | 0.22 |
| Nonshared environmental effects | 0.05 | 0.21 |
| Quadratic slope variance due to: | ||
| Additive genetic effects | 0.04 | 0.03 |
| Nonshared environmental effects | 0.07 | 0.03 |
|
| ||
| Covariance parameters: | ||
| Intercept-linear slope covariance due to additive genetic effects | 0.14 | 0.21 |
| Intercept-quadratic slope covariance due to additive genetic effects | −0.03 | 0.03 |
| Linear slope-quadratic slope covariance due to additive genetic effects | −0.001 | 0.001 |
| Intercept-linear slope covariance due to nonshared environmental effects | 0.03 | 0.20 |
| Intercept-quadratic slope covariance due to nonshared environmental effects | −0.07 | 0.03 |
| Linear slope-quadratic slope covariance due to nonshared environmental effects | −0.001 | 0.001 |
|
| ||
| Occasion-Specific Variance due to: | ||
| Nonshared environmental effects + error | 22.19 | 0.62 |
Note: The longitudinal AE model here refers to the identically-named model in Table 5(a). Bold indicates p ≤.05. Est. = model estimate, SE = standard error. Fixed effects parameters included gender, mode of testing, birth year, dying in 2 years, female * die in 2 years, age, age2, age3, and birth year * age.
Figure 2.
Raw estimates of total variance in ANX trajectory over age (grey, short dashed line) was partitioned into: additive genetic variance (red line), non-shared environmental variance (blue, long dashed line), and occasion-specific variation (green dotted line).
Post-hoc Analyses
Post-hoc analyses were conducted to address two potential concerns, namely, whether our findings would differ: (a) by the valence of the ANX items, and (b) adjusting for health status. Regarding the first issue, a growing body of literature suggests that emotional aging entails changes as a function of affective valence and arousal (Ross and Mirowsky, 2008; Kessler & Staudinger, 2009). Our state anxiety measure includes both positive and negative items, raising the concern that the overall anxiety trajectory was the sum of heterogeneous trajectories. Of note, valence and arousal are confounded in the STPI, such that positive items were low-arousal (e.g., I feel calm) and negative items were high-arousal (e.g., I feel nervous). We repeated the phenotypic analysis using the total score of the negative items as the outcome. The best-fitting trajectory for the negative STPI items closely mirrored that for the total score (i.e., ANX), except for a more pronounced increase in anxiety in age 80+ (Supplemental Figure 1). This suggests that the ANX trajectory described earlier (Table 3d and Figure 1) was not the combination of two dissimilar trajectories based on positively- and negatively-valenced items. Relatedly, the pattern of twin correlations was similar between ANX and the total score for the negative items (Supplemental Table 3), suggesting little difference in their biometric variance components.
The second set of post-hoc analysis evaluated whether adjusting for physical health status would alter the findings, given that individuals with poor health are more likely to drop out. We first created residualized ANX scores by partialing an index of baseline physical health status from ANX. Next, we repeated the phenotypic analysis by using the residualized ANX scores as the outcome variable. As shown in Supplemental Figure 2, the best-fitting model was highly similar to that for the unadjusted ANX score (i.e., Table 3d and Figure 1), suggesting there is little incremental value in including baseline physical health status as a covariate beyond the covariates already included in the main phenotypic analysis. Additionally, the pattern of twin correlations for the unadjusted and residualized ANX scores were very similar (Supplemental Table 3) and suggests that adjusting for baseline physical health status is unlikely to alter the biometric findings.
DISCUSSION
This study characterized the trajectory of anxiety symptoms in later life based on repeated assessments of more than 1400 twins across 11 years. Using biometric latent curve analysis, we estimated genetic and environmental contributions to individual differences in anxiety over age. Average anxiety declined with age from the 50s to the mid-60s, followed by a subsequent increase that eventually plateaued in the 80s. Compared to earlier-born cohorts (i.e., older individuals at baseline), later-born cohorts had lower levels of anxiety in their late 60s, but their anxiety increased more steeply in older ages. Women reported higher anxiety than men. Mode effects were present, such that anxiety was lower when the questionnaire was collected by study staff than when it was returned by mail. Importantly, while the magnitude of mean anxiety change over age was small, there were substantial individual differences in the age trajectory of anxiety. A novel finding is that genetic and environmental contributions to individual differences in anxiety varied with age. Nonshared environmental variance in anxiety trajectory was highest in the late 60s and declined in older ages, whereas genetic variance became increasingly higher from approximately age 60 onward. Rearing and other experiences shared by twins did not account for individual differences in anxiety trajectory. Occasion-specific fluctuation in anxiety was entirely attributable to nonshared environmental factors and random error.
Anxiety in the Second Half of Life: Mean Trajectory and Individual Differences
Although state anxiety is by definition transient, our findings indicate that of total state anxiety variation, 60–74% reflects individual differences in trajectory (i.e., systematic differences in intercept and slopes), whereas 26–40% was occasion-specific. The proportion of systematic variance in our state anxiety measure was comparable to estimates for recent anxiety symptoms (60–73%; Gillespie et al., 2004) and neuroticism (67–72%; Mroczek & Spiro, 2003; Steunenberg et al., 2005), suggesting that the state-trait distinction is blurrier than widely believed or intended by such measures.
The observed decrease in state anxiety during the transition from midlife into early old age conforms to predictions based on lifespan developmental theories. SST, SOC, and SAVI posit that emotion regulation improves during this time because of greater self-knowledge (Baltes & Smith, 2003), expertise in making social inferences (Hess, Osowski, & Leclerc, 2005), culling of less desirable relationships (Lang & Carstensen, 1994), and mastery of problem-solving strategies (Blanchard-Fields, 2007). Acquisition of social competence and use of emotion regulation strategies are strongly influenced by individual circumstances and life experiences, which may explain our finding of increasing nonshared environmental variance in state anxiety variation from midlife to early old age.
Our hypothesis of an increase in state anxiety from the 70s into “fourth age” was partially supported. Average anxiety scores increased slightly during the mid-60s and 70s, and plateaued in the 80s. The trend of increasing anxiety in advanced old age parallels findings an upturn in negative affect (e.g., Charles, Reynolds, & Gatz, 2001) and neuroticism (e.g., Mroczek & Spiro, 2003) in the 70s and 80s age range. An age-related increase in anxiety in older ages is also compatible with the position of the SOC and SAVI theories, but not the SST, that age-related strengths in emotional functioning may be overcome by other vulnerabilities as individuals enter “fourth age” (Baltes, 1997).
While the amount of total anxiety variation remained relatively stable over age, there were age-related changes in the sources of such variation: Nonshared environmental contributions to individual differences in anxiety trajectory peaked around age 67 and then declined, while genetic contributions began to increase in the early 60s and showed an uptick in the 80s. What might explain the shift toward a diminishing role of nonshared environment in older ages? One possibility is that culling of undesirable relationships (Lang & Carstensen, 1994), functional impairment, and mobility limitation in advanced old age render a narrower social environment with fewer environmental triggers of anxiety, thus resulting in reduced nonshared environmental contributions to individual differences in anxiety.
We propose several reasons for why genetic contributions to individual differences in anxiety trajectory may become stronger in later life. Genetic influences include factors on which identical twins of a pair are similar. To the extent that twins are concordant on late-onset conditions that bring about anxiety, it would contribute to genetic influence on anxiety in older ages. Coronary heart disease and specific types of cancer (e.g., lung, colorectal) are examples of highly heritable physical health conditions with late onset ages (Frank, 2007; Go et al., 2013; van Dongen, Slagboom, Draisma, Martin, & Boomsma, 2012) and often trigger anxiety reactions. They may represent new sources of genetic variation in anxiety in older ages. Second, age-changes in physiological stress response may be another new source of genetic variance for anxiety for people in their 70s and 80s. Aging is associated with elevated systolic blood pressure reactivity and cortisol response to psychological stressors (Otte et al., 2005; Uchino, Birmingham, & Berg, 2010) and slow recovery from sustained physiological arousal (Charles et al., 2001), which possibly mediate stronger and more prolonged anxiety states in old age. Twin studies have established a strong genetic component for physiological stress response (Wu, Snieder, & de Geus, 2010). To the extent that twins of a pair are concordant on these physiological changes and the changes play a role in anxiety, they represent a new source of genetic influence in anxiety variation in old age. Third, as noted earlier, the transition from midlife to early old age is characterized by age-related strengths in emotion regulation and social competence, as well as sociocultural resources (e.g., medical, social, and technological advances) that enable effective coping with emotion distress. While these coping resources may suppress genetic propensity for anxiety in younger ages, they tend to lose efficacy in very old age while age-related losses (e.g., in health, autonomy, and social connectedness) become more prominent (Baltes & Smith, 2003). To the extent that twins are concordant on the depletion of coping resources, which in turn give rise to greater anxiety in very old age, this will manifest as an increase in genetic variance in anxiety. While these speculations require empirical support, they are compatible with the proposition by SOC and SAVI that the role of biological vulnerabilities (e.g., genetic risk for anxiety or anxiety-linked conditions) on emotional well-being become more prominent in “fourth age.”
Whereas nonshared environmental influences on anxiety trajectory reflect more enduring factors that explain between-person differences in initial status and systematic change over age, nonshared environmental influences on occasion-specific anxiety fluctuation index factors that explain a person's deviation from his/her estimated trajectory at a given time. As hypothesized, our results indicate that such deviation can be explained by environmental factors unique to each person and have short-term effects that do not alter one's overall trajectory. Examples may include physician visits or a distressing argument with someone that occurred shortly before the assessment occasion. Measurement errors are also estimated under this category.
Of individual differences in anxiety levels from age 50 onward, 42–63% were attributable to genetic and 37–57% to nonshared environmental factors. This is consistent with earlier findings for state anxiety (Jardine et al., 1984) and anxiety disorder liability (Hettema et al., 2001) based on cross-sectional assessments.
Several other features of our findings on the age trajectory of anxiety are worth highlighting: Our observation of higher anxiety levels among women than men is consistent with earlier findings on sex differences in state anxiety (de Beurs et al., 2000; Knight, Waal-Manning, & Spears, 1983; Mirowsky & Schieman, 2008). Sex differences in anxiety change over age have been examined infrequently in the literature. Mirowsky and Schieman (2008) reported a sharper decrease in anxiety frequency for men than women. In our sample, however, the magnitude of sex difference in anxiety did not change over age, but as we discuss later, women were more likely to have higher anxiety shortly before death, which may partially account for the difference in findings between the two studies.
Cohort differences in anxiety level and slope were small. The finding of lower anxiety intercept among more recently born individuals is consistent with the trend observed for neuroticism reported in two large U.S. studies (Mroczek & Spiro, 2003; Terracciano et al., 2005). However, in contrast with Mroczek and Spiro (2003), we found that state anxiety increased more steeply among people in their 60s for later-born cohorts than earlier-born cohorts. It is unclear to what extent differences in construct, measure, and sample characteristics account for the discrepant finding. In our sample, lower anxiety levels and steeper age-related increase may suggest a larger range of emotional expression in later-born cohorts, a conjecture that warrants further investigation.
For mode of testing, anxiety was slightly higher when questionnaires were returned by mail than when they were collected by nurses who conducted in-person visits. Our observation is consistent with findings based on SATSA and another sample that participants tend to report lower levels of negative psychosocial functioning in data collection modes with greater direct contact with interviewers than modes with less direct contact (Luong, Charles, Rook, Reynolds, & Gatz, 2014). Participants might be driven to report lower anxiety in IPTs than Qs because they were gladly expecting a visit by the study nurses, or for reasons of social desirability.
Recent studies have shown participants report a precipitous decline in life satisfaction within a five-year period prior to death (Gerstorf et al., 2008a; Gerstorf, Ram, Röcke, Lindenberger, & Smith, 2008b). Given the frequency of our measurements occasions (≤3 years), we tested whether there was a detectable effect on anxiety scores of dying within two years following a measurement occasion. We found that anxiety was higher prior to death, but only in women. Gerstorf et al. (2008a) reported a nominal finding of lengthier and steeper terminal decline in life satisfaction for women than men in a population-based German sample, but these sex differences were not observed in a higher-functioning sample with fewer assessment occasions (Gerstorf et al., 2008b). Whether and why imminent death is related to an anxiety increase in women warrant further evaluation.
Post-hoc analyses demonstrated that our findings regarding the age trajectories of state anxiety did not differ by the affective valence of items. While we were unable to further divide positively and negatively valenced items by arousal and examine their age trajectories, we also note that anxiety is by definition a negative emotional state marked by high arousal, thus we consider our findings to be a good representation of how anxiety changed with age in our sample.
Limitations
As with any longitudinal research, a potentially important limitation is the impact of non-random attrition. Our analyses relied on the missing-at-random assumption, that is, the observed data allowed us to adequately account for the missing data. To address concerns regarding the effect of non-random attrition, we evaluated proximity to death and non-death related drop-out, as well as their interactions with sex and age slopes, as potential factors influencing anxiety trajectories.
Second, this study is based on largely homogeneous group of Caucasian twins born between 1891 and 1943 in Sweden. Our findings may not generalize to individuals of other ethnic or cultural backgrounds, or later-born cohorts. However, this limitation is ameliorated by the benefits of a large scale, population-based twin sample with longitudinal follow-up, which has provided the opportunity to address knowledge gaps about age-related changes in anxiety. Our findings indicate that cohort differences in anxiety are very small, alleviating some of the concerns about the generalizability of our finding to later-born cohorts.
Third, we were unable to directly compare the anxiety scores of our sample to other (normative or clinical) samples due to measurement differences. Other studies have used a version of the STPI with a 4-point response scale (Spielberger, 1979), but our version has a 5-point response scale.
Fourth, we were unable to account for all possible covariates. Post-hoc analyses established that differences in baseline physical health status did not account for additional variance in state anxiety and were unlikely to alter the biometric findings. However, we could not evaluate cognition as a covariate because questionnaire occasions did not include cognitive measures.
Conclusion and Future Directions
In this longitudinal investigation of state anxiety symptoms, we reported that anxiety declined from midlife into the 60s, followed by an increase that leveled off in older ages. The magnitude of average change in anxiety over age was small, but there were tremendous individual differences in the longitudinal course of anxiety in later life. While the amount of total variance in anxiety trajectory was stable, nonshared environmental variance for anxiety peaked for individuals in their late 60s and declined thereafter, whereas genetic variance showed an increase from approximately age 60 onward that became more prominent in the 80s. Placing our findings in the context of lifespan developmental theories on emotional well-being, we proposed that the diminishing role of nonshared environmental factors in the 70s and 80s may be attributable to shrinkage in one's social environment, which has relatively fewer environmental triggers that can systematically alter one's overall anxiety trajectory than in earlier ages. An increase in genetic contributions to anxiety variation in advanced old age can be interpreted with respect to the SOC and SAVI theories. Specifically, biological declines in the “fourth age” may introduce new sources of genetic influence on anxiety via late-onset diseases and physiological changes. Moreover, resources that enable effective coping with anxiety in younger ages become less efficacious and depleted at this time, rendering individuals more vulnerable to any pre-existing genetic predisposition to anxiety.
In light of the accumulating evidence for a decline in emotional well-being (or greater emotional ill-being, such as anxiety and negative affect) in advanced old age, there is a need to identify ways to optimize the quality of life as individuals move toward the end of life. Our findings also call for additional research to determine whether late-onset diseases, such as cardiovascular diseases, may account for the increasing contribution of genetic factors to anxiety in advanced old age.
Supplementary Material
Acknowledgments
This study was supported by National Institute on Aging (NIA) grant F31 AG031691. The Swedish Adoption/Twin Study of Aging (SATSA) is supported by NIA grants R01 AG04563 and AG10175, the MacArthur Foundation Research Network on Successful Aging, and the Swedish Research Council (Grant 97:0147:1B). The views expressed in this paper are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs or other support institutions.
Footnotes
The longitudinal ADE and ASE models contained negative random effect estimates. Following the steps described in the Analytic Strategy, the negative parameters were constrained to 0 to yield reduced ADE and ASE models and aid model identification. Consequently the reduced models were not nested in the AE model and could not be compared via chi-square difference test. The fit indices in Table 5a were calculated based on 29 parameters in each model (i.e., parameters constrained to 0 were counted as fitted parameters because they were part of the hypothesized model).
REFERENCES
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American Psychologist. 1997;52:366–380. doi: 10.1037//0003-066x.52.4.366. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM. Psychological perspectives on successful aging: The model of selective optimization with compensation. In: Baltes PB, editor; Baltes MM, editor. Successful aging: Perspectives from the Behavioral Sciences. Cambridge University Press; New York, NY: 1990. pp. 1–34. http://dx.doi.org/10.1017/CBO9780511665684. [Google Scholar]
- Baltes PB, Smith J. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology. 2003;49:123–35. doi: 10.1159/000067946. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Harp D, Jung WS. Reliability generalization of scores on the Spielberger state-trait anxiety inventory. Educational and Psychological Measurement. 2002;62:603–618. [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- Bedford A, Foulds GA, Sheffield BF. A new personal disturbance scale (DSSI/sAD) British Journal of Social and Clinical Psychology. 1976;15:387–394. doi: 10.1111/j.2044-8260.1976.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F. Everyday problem solving and emotion: An adult developmental perspective. Current Directions in Psychological Science. 2007;16:26–31. [Google Scholar]
- Carstensen LL. Motivation for social contact across the life span: A theory of socioemotional selectivity. In: Jacobs J, editor. Nebraska Symposium on Motivation: Vol. 40. Developmental perspectives on motivation. University of Nebraska Press; Lincoln: 1993. pp. 209–254. [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Charles ST. Strength and Vulnerability Integration (SAVI): A Model of Emotional Well-Being in Later Adulthood. Psychological Bulletin. 2001;136:1068–1091. doi: 10.1037/a0021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Reynolds C, Gatz M. Age-related differences and change in positive and negative affect over twenty-five years. Journal of Personality and Social Psychology. 2001;80:136–151. [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants: a proposal. Archives of General Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Domains and facets: Hierarchical personality assessment using the Revised NEO Personality Inventory. Journal of Personality Assessment. 1995;64:21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- De Beurs E, Beekman ATF, Deeg DJH, Van Dyck R, Van Tilburg W. Predictions of change in anxiety symptoms of older persons: results from the Longitudinal Aging Study Amsterdam. Psychological Medicine. 2000;30:515–527. doi: 10.1017/s0033291799001956. [DOI] [PubMed] [Google Scholar]
- Duke University Center for the Study of Aging and Human Development . Multidimensional functional assessment: The OARS methodology. Duke University Medical Center; Durham, NC: 1978. [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging Neuropsychology and Cognition. 2004;11:325–345. [Google Scholar]
- Frank SA. Dynamics of cancer: incidence, inheritance, and evolution. Princeton University Press; Princeton, NJ: 2007. [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Estabrook R, Schupp J, Wagner GG, Lindenberger U. Life satisfaction shows terminal decline in old age: Longitudinal evidence from the German Socio-Economic Panel Study (SOEP) Developmental Psychology. 2008a;44:1148–1159. doi: 10.1037/0012-1649.44.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Röcke C, Lindenberger U, Smith J. Decline in life satisfaction in old age: Longitudinal evidence for links to distance-to-death. Psychology and Aging. 2008b;23:154–168. doi: 10.1037/0882-7974.23.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Kirk KM, Evans DM, Heath AC, Hickie IB, Martin NG. Do the genetic or environmental determinants of anxiety and depression change with age? A longitudinal study of Australian twins. Twin Research. 2004;7:39–53. doi: 10.1375/13690520460741435. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier S, Préville M, Boyer R, O'Connor K, Béland SG, Potvin O, Scientific Committee of the ESA Study The Impact of DSM-IV symptom and clinical significance criteria on the prevalence estimates of subthreshold and threshold anxiety in the older adult population. American Journal of Geriatric Psychiatry. 2011;19:316–326. doi: 10.1097/JGP.0b013e3181ff416c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros DF, Simms LJ, Antony MM, McCabe RE. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA) in friendship dyads. Behavior Therapy. 2010;41:277–284. doi: 10.1016/j.beth.2009.07.001. PubMed: 20569777. [DOI] [PubMed] [Google Scholar]
- Harris JR, Pedersen NL, McClearn GE, Nesselroade JR, Plomin R. Age differences in genetic and environmental influences for health from the Swedish Adoption/Twin Study of Aging. Journal of Gerontology: Psychological Sciences. 1992;47:P213–P220. doi: 10.1093/geronj/47.3.p213. [DOI] [PubMed] [Google Scholar]
- Heikkinen RL, Kauppinen M. Mental well-being: A 16-year follow-up among older residents in Jyväskylä. Archives of Gerontology and Geriatrics. 2011;52:33–39. doi: 10.1016/j.archger.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Henderson AS, Jorm AF, Korten AE, Jacomb P, Christensen H, Rodgers B. Symptoms of depression and anxiety during adult life: evidence for a decline in prevalence with age. Psychological Medicine. 1998;28:1321–1328. doi: 10.1017/s0033291798007570. [DOI] [PubMed] [Google Scholar]
- Hess TM, Osowski NL, Leclerc CM. Age and experience influences on the complexity of social inferences. Psychology and Aging. 2005;20:447–459. doi: 10.1037/0882-7974.20.3.447. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–78. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hoffman L. Considering alternative metrics of time: Does anybody really know what “time” is? In: Harring JR, Hancock GR, editors. Advances in Longitudinal Methods in the Social and Behavioral Sciences. Information Age Publishing; Charlotte, NC: 2012. pp. 255–287. [Google Scholar]
- Jardine R, Martin NG, Henderson AS. Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genetic Epidemiology. 1984;1:89–107. doi: 10.1002/gepi.1370010202. [DOI] [PubMed] [Google Scholar]
- Kabacoff RI, Segal DL, Hersen M, Van Hasselt VB. Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with older adult psychiatric outpatients. Journal of Anxiety Disorders. 1997;11:33–47. doi: 10.1016/s0887-6185(96)00033-3. [DOI] [PubMed] [Google Scholar]
- Kessler E-M, Staudinger UM. Affective experience in adulthood and old age: The role of arousal and perceived affect regulation. Psychology and Aging. 2009;24:349–362. doi: 10.1037/a0015352. [DOI] [PubMed] [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. Personality and comorbidity of common psychiatric disorders. British Journal of Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. British Journal of Clinical Psychology. 1983;22:245–249. doi: 10.1111/j.2044-8260.1983.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Lang FR, Carstensen LL. Close emotional relationships in late life: Further support for proactive aging in the social domain. Psychology and Aging. 1994;9:315–324. doi: 10.1037//0882-7974.9.2.315. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Luong G, Charles ST, Rook KS, Reynolds CA, Gatz M. Age Differences and Longitudinal Change in the Effects of Data Collection Mode on Self-Reports of Psychosocial Functioning. Psychology and Aging. 2014 doi: 10.1037/a0038502. Advance online publication. http://dx.doi.org/10.1037/a0038502. [DOI] [PMC free article] [PubMed]
- Malouff JM, Thorsteinsson EB, Schutte NS. The relationship between the five-factor model of personality and symptoms of clinical disorders: a meta-analysis. Journal of Psychopathology and Behavioral Assessment. 2005;27:101–114. [Google Scholar]
- McArdle JJ, Prescott CA. Mixed effects variance components models for biometric family analyses. Behavior Genetics. 2005;35:631–652. doi: 10.1007/s10519-005-2868-1. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Schieman S. Gender, age, and the trajectories and trends of anxiety and anger. Advances in Life Course Research. 2008;13:45–73. [Google Scholar]
- Mroczek DK, Spiro A. Modeling intraindividual change in personality traits: Findings from the Normative Aging Study. Journal of Gerontology: Psychological Sciences. 2003;58B:P153–P165. doi: 10.1093/geronb/58.3.p153. III. [DOI] [PubMed] [Google Scholar]
- Neimeyer RA, Wittkowski J, Moser RP. Psychological research on death attitudes: An overview and evaluation. Death Studies. 2004;28:309–340. doi: 10.1080/07481180490432324. [DOI] [PubMed] [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, DeFaire U. The Swedish Adoption/Twin Study of Aging: An update. Acta Geneticae Medicae et Gemellologiae: Twin Research. 1991;40:7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- Rijsdijk FV, Snieder H, Ormel J, Sham P, Goldberg DP, Spector TD. Genetic and environmental influences on psychological distress in the population: General Health Questionnaire analyses in UK twins. Psychological Medicine. 2003;33:793–801. doi: 10.1017/s0033291703007451. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. Age and the balance of emotions. Social Science and Medicine. 2008;66:2391–2400. doi: 10.1016/j.socscimed.2008.01.048. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. Version 9.3. SAS Institute Inc.; Cary, NC: 2012. [Google Scholar]
- Scheibe S, Carstensen LL. Emotional aging: Recent findings and future trends. Journal of Gerontology: Psychological Sciences. 2010;65:135–144. doi: 10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- Sliwinski M, Hoffman L, Hofer SM. Evaluating convergence of within-person change and between-person age differences in age-heterogeneous longitudinal studies. Research in Human Development. 2010;7:45–60. doi: 10.1080/15427600903578169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Preliminary manual for the State-Trait Personality Inventory (STPI) University of South Florida; Tampa: 1979. [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Rev. ed. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Steunenberg B, Twisk JW, Beekman AT, Deeg DJ, Kerkhof AJ. Stability and change of neuroticism in aging. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60:P27–P33. doi: 10.1093/geronb/60.1.p27. [DOI] [PubMed] [Google Scholar]
- Teachman BA. Aging and negative affect: the rise and fall and rise of anxiety and depression symptoms. Psychology and Aging. 2006;21:201–207. doi: 10.1037/0882-7974.21.1.201. [DOI] [PubMed] [Google Scholar]
- Terracciano A, McCrae RR, Brant LJ, Costa PT., Jr Hierarchical linear modeling analyses of the NEO-PI-R scales in the Baltimore Longitudinal Study of Aging. Psychology and Aging. 2005;20:493–506. doi: 10.1037/0882-7974.20.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Birmingham W, Berg CA. Are older adults less or more physiologically reactive? A meta-analysis of age-related differences in cardiovascular reactivity to laboratory tasks. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2010;65:154–162. doi: 10.1093/geronb/gbp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell JL, Gatz M, Pedersen NL. A longitudinal analysis of anxiety and depressive symptoms. Psychology and Aging. 2001;16:187–195. doi: 10.1037//0882-7974.16.2.187. [DOI] [PubMed] [Google Scholar]
- van Dongen J, Slagboom PE, Draisma HH, Martin NG, Boomsma DI. The continuing value of twin studies in the omics era. Nature Reviews Genetics. 2012;13:640–653. doi: 10.1038/nrg3243. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance, and cognitive decline in normal aging. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57:P246–P255. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- Willis ML, Dodd HF, Palermo R. The relationship between anxiety and the social judgements of approachability and trustworthiness. PloS One. 2013;8:e76825. doi: 10.1371/journal.pone.0076825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Snieder H, de Geus E. Genetic influences on cardiovascular stress reactivity. Neuroscience & Biobehavioral Reviews. 2010;35:58–68. doi: 10.1016/j.neubiorev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;6:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.