Abstract
Objective:
Early initiation of antiretroviral treatment (ART) at CD4+ cell count ≥500 cells per microliter reduces morbidity and mortality in HIV-infected adults. We determined the proportion of HIV-infected people with high viral load (VL) for whom transmission prevention would be an additional benefit of early treatment.
Design:
A randomly selected subset of a nationally representative sample of HIV-infected adults in Swaziland in 2012.
Methods:
Eight to 12 months after a national survey to determine adult HIV prevalence, 1067 of 5802 individuals identified as HIV-infected were asked to participate in a follow-up cross-sectional assessment. CD4+ cell enumeration, VL measurements, and ART status were obtained to estimate the proportion of currently untreated adults and of the entire HIV-infected population with high VL (≥1000 copies/mL) whose treatment under a test-and-treat or VL threshold eligibility strategy would reduce HIV transmission.
Results:
Of the 927 (87% of 1067) participants enrolled, 466 (50%) reported no ART use. Among them, 424 (91%) had VL ≥1000 copies per milliliter; of these, 148 (35%) were eligible for ART at the then existing CD4+ count threshold of <350 cells per microliter; an additional 107 (25%) were eligible with expanded CD4+ criterion of <500 cells per microliter; and 169 (40%) remained ART ineligible. Thus, 36% of the 466 currently untreated and 18% of the total 927 had high VL yet remained ART ineligible under a CD4+ criterion of <500 cells per microliter.
Conclusions:
A test-and-treat or VL threshold for treatment eligibility is necessary to maximize the HIV transmission prevention benefits of ART.
Key Words: HIV, antiretroviral treatment, HIV treatment eligibility, CD4+ count, viral load, Swaziland
INTRODUCTION
Well into the fourth decade of the global HIV epidemic, an estimated 35 million people are living with HIV. Sub-Saharan Africa accounts for approximately 24.7 million prevalent infections and more than two-third of new annual HIV infections worldwide.1 Although the numbers remain daunting, the high prevalence partially reflects reduced morbidity and mortality through increasing access to antiretroviral treatment (ART). Recent studies have documented that morbidity and mortality can be further reduced by early initiation of ART in asymptomatic persons with high CD4 counts.2,3 In response, the World Health Organization has announced that new treatment guidelines recommending the treatment for all regardless of CD4+ cell count will be forthcoming.4
In addition to the benefits to individual health, in 2011, a randomized controlled trial found 96% reduction in HIV transmission among discordant heterosexual couples, where the HIV-infected partner was started on ART at CD4+ count between 350 and 550 cells per microliter compared with deferral of ART until CD4+ cell count fell to below 250 cells per microliter, establishing the efficacy of treatment as prevention.5 Possibilities of and barriers to expanding HIV treatment for prevention, either by treating those with high viral load (VL) despite high CD4+ cell counts or by treating all HIV-infected individuals irrespective of CD4+ cell count and VL, have been widely discussed.6–8
A major barrier to expanding ART coverage is the complex continuum of HIV care, which Gardner et al9 refer to as the “spectrum of engagement in HIV care.” Each of 6 defined steps along the continuum—HIV diagnosis by testing, linkage to care, retention in care, starting appropriate ART, adherence with ART, and achieving viral suppression—have been demonstrated as programmatic vulnerabilities, where individual patients can and do disengage, ultimately resulting in morbidity and mortality and also uncontrolled VL and increased transmission risk. Initial cost for treatment expansion, despite the long-term cost savings, is another barrier.10
Given the risks of overburdening already stretched infrastructures, and also the additional costs, it is helpful to quantify the degree to which a national ART program would have to expand if it were to include either all HIV-infected adults or those with high VL who are at the greatest risk of transmitting HIV but not “eligible” for treatment based on CD4+ cell count. In this study, we use data from the nationally representative Swaziland HIV Incidence Measurement Survey (SHIMS) to examine the potential prevention impact of current treatment guidelines in Swaziland, a country with one of the highest HIV incidence and prevalence rates in the world.11,12
METHODS
Sampling Scheme and Eligibility Criteria
A detailed description of the SHIMS sampling scheme has been previously described.12 Briefly, a 2-stage cluster sample design selected census or enumeration areas and then households within each enumeration area. Adult household members who agreed to participate underwent HIV testing and those who were HIV-negative were offered enrollment in a seroincidence cohort. Those who were HIV-infected were counseled about the benefits of HIV care and treatment services, provided a referral to facilitate their linkage to those services, and asked for consent to be contacted for future research. A subset of participants who were identified as HIV-infected at baseline and who gave consent to be contacted for future research was randomly selected to participate in a follow-up household visit 8–12 months after their initial visit.
Study Procedures
The selected HIV-infected individuals who were located underwent verification of their participation in the earlier survey and were asked to participate in a follow-up cross-sectional assessment. Those who agreed provided written informed consent and then underwent an interviewer-administered survey regarding medical care including current ART use. When available, participants' medical booklet records of ART initiation ascertained ART use at baseline and follow-up. When medical booklets were unavailable, this ART information was based on self-report. Counseling about the benefits of HIV care and treatment services, and information on how to link to them was provided to all participants. Approximately 10 mL of blood was obtained by venipuncture.
Laboratory Methods
Whole blood samples were transported in ethylenediaminetetraacetic acid tubes (Greiner Bio One, Frickenhausen, Germany) in cooler boxes to the National Reference Laboratory in Mbabane within 24 hours of collection.
CD4+ cell enumeration was determined using the Becton Dickinson FACSCalibur automated flow cytometry system according to the manufacturer's instructions. Quality assurance testing was performed on 5% of the whole blood samples. VL quantification was performed using undiluted plasma on the COBAS, AmpliPrep/COBAS, TaqMan, System platform and the COBAS, AmpliPrep/COBAS, TaqMan, and HIV-1 Test (Roche Diagnostics, Indianapolis, IN) version 2.0 assay according to the manufacturer's instructions; the limit of detection for the assay was 20 copies per milliliter.
Those participants who agreed to receive their CD4+ count results received a paper copy of the results within 28 days. The study staff counseled about the benefits of HIV care services and provided information to help those not in care link to these services. They encouraged participants to share the CD4+ test results with their health care provider, noting that it would assist their provider in making decisions about starting treatment. They also counseled participants about the importance of HIV testing of partners.
Data Analyses
CD4+ cell enumeration, VL measurements, and ART treatment status of participants were used to determine the additional number and proportion of untreated adults and of the entire estimated HIV-infected adult population who would be eligible for ART when the then prevailing treatment eligibility criterion of CD4+ cell count < 350 cells per microliter was expanded to include those between 350 and 499 cells per microliter. We also calculated the number and proportion of those with high VL (HIV-1 RNA VL >1000 copies/mL) who remained ART ineligible with the expanded criterion of CD4+ cell count <500 cells per microliter.13 Finally we estimated the remaining number and proportion who would be included in a test-and-treat strategy. By estimating the size of the adult HIV-infected population in Swaziland, we converted the proportions to absolute numbers of persons potentially added to treatment programs. We also assessed demographic and health seeking behavior variables associated with being on ART (for those eligible) and having suppressed VL (for those on ART).
Statistical Methods
Survey weights were applied to the data to account for differential probability of selection by the initial cluster sampling procedure and nonresponse rates across sex, age, and geography during both initial sampling procedure and follow-up assessment of selected HIV-infected individuals. Weighted estimates were scaled to the size of the SHIMS subset of HIV-infected individuals who underwent the CD4+ assessment. Throughout, reports of number and proportions of participants are based on these scaled weighted estimates, and thus do not always add up to 100%. When available, participants' medical booklet records of ART initiation ascertained ART use at baseline and follow-up. Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) were calculated to describe associations from logistic regression models, using binary outcomes for the 2 dependent variables of interest at follow-up, (1) ART use (yes/no) and (2) viral suppression (yes/no), defined as <1000 copies per milliliter. Analyses were performed using Stata (StataCorp. 2013, Stata Statistical Software: Release 13; StataCorp LP, College Station, TX).
Size Estimation of HIV-Infected Adult Population
The total population of Swaziland is projected to be 1.268 million, with near equal proportion male and female (49.4% versus 50.6%).14 From the Swaziland 2007 Demographic and Health Survey, the proportion of adult men and women (defined as aged 15 years and older) was 53.8% and 58.4%, respectively, thus 711,348 persons.15 Applying the SHIMS HIV prevalence estimate of 24% of adult males and 39% of adult females12 yields 226,262 adults estimated to be living with HIV.
Ethical Considerations
All study participants provided written informed consent before the collection of data and blood samples. Ethical approval was obtained from the Swaziland Scientific and Ethics Committee, Columbia University Institutional Review Board (IRB), and the US Centers for Disease Control and Prevention IRB before initiation of field work.
RESULTS
Participants
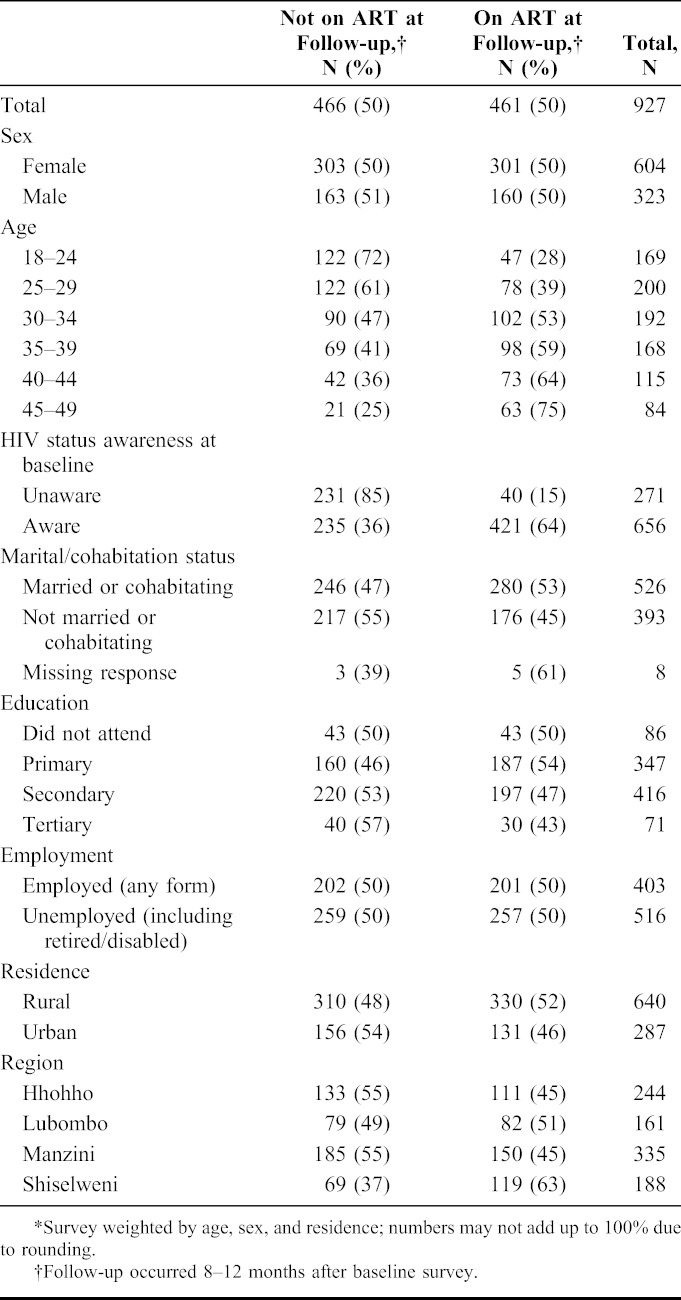
Of 5802 participants identified as HIV-infected at SHIMS baseline 8–12 months earlier, 1067 (18%) were randomly selected for this follow-up study; 138 of the 1067 (13%) either could not be located or refused to participate, and 3 (0.3%) did not have CD4+ enumeration results available. From February to May 2012, 927 participants (87% of those selected for participation) were enrolled, interviewed, and underwent CD4+ cell enumeration and VL assessment. Of these 927, 65% were women, 69% lived in rural areas, 57% reported being married or partner cohabitation, 50% were on ART, and 71% were aware of their HIV-infected status at the time of the baseline survey 8–12 months earlier (Table 1).
TABLE 1.
Demographics and Characteristics of 927 Adults Sampled for CD4+ Cell Count and Viral Load Stratified by the Use of Antiretroviral Treatment (ART)*
Distribution of CD4+ Cell Count and ART Eligibility Among 466 Participants Not on ART
Eight to 12 months after the SHIMS baseline survey, about one-third (158 of 466, or 34%) had CD4+ count <350 cells per microliter and were eligible for ART by national CD4 criteria at the time of the survey; an additional 110 (24%) had CD4+ count between 350 and 499 cells per microliter and were eligible for ART by new initiation criteria adopted by Swaziland in the summer of 2014 (Table 2). Thus, expanding eligibility criteria from CD4+ count <350 to <500 cells per microliter added 12% of the adult HIV-infected population (110 of the sampled 927) to those eligible. With the estimate of 226,262 HIV-infected adults nationally, 26,849 more adults became treatment eligible with the higher CD4+ cell count criteria. By 2013 WHO guidelines, 43% of those not on ART (198 of 466) remain ineligible due to a CD4+ cell count of ≥500 cells per microliter.
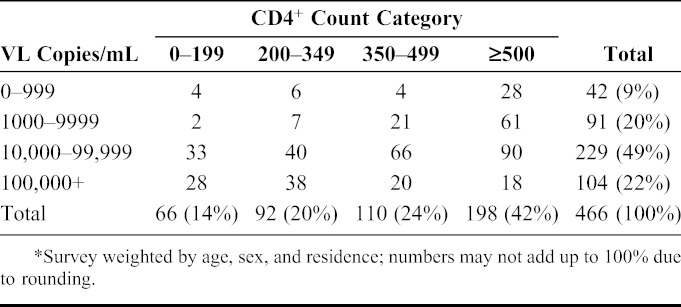
TABLE 2.
Distribution of Viral Load (VL) by CD4+ Cell Count Strata Among 466 HIV-Positive Individuals Not on ART*
Distribution of VL, CD4+ Cell Count, and ART Eligibility
In terms of VL, 91% (424 of 466) participants not on ART had a VL ≥1000 copies per milliliter (Table 2). Among these 40% (169 of 424) had a CD4+ count ≥500 cells per microliter and thus would remain ART ineligible with a CD4+ threshold of <500 cells per microliter despite having a high VL 8–12 months after documented HIV-infected status. These 169 individuals represent 18% of the total 927 HIV-positive participants in the population. With the estimated 226,262 HIV-infected adults nationally, 40,727 more adults would become treatment eligible if a VL threshold of ≥1000 copies per milliliter was added to CD4+ count criteria of <500 cells per microliter. A test-and-treat approach would add the remaining 3% (28 of 927) of persons, those with CD4+ count ≥500 cells per microliter and VL <1000 copies per milliliter, or 6788 adults.
Predictors of Being on ART at Follow-up 8–12 Months After SHIMS Baseline Survey Among Those Eligible by CD4+ Threshold of <350 Cells Per Microliter at Follow-up
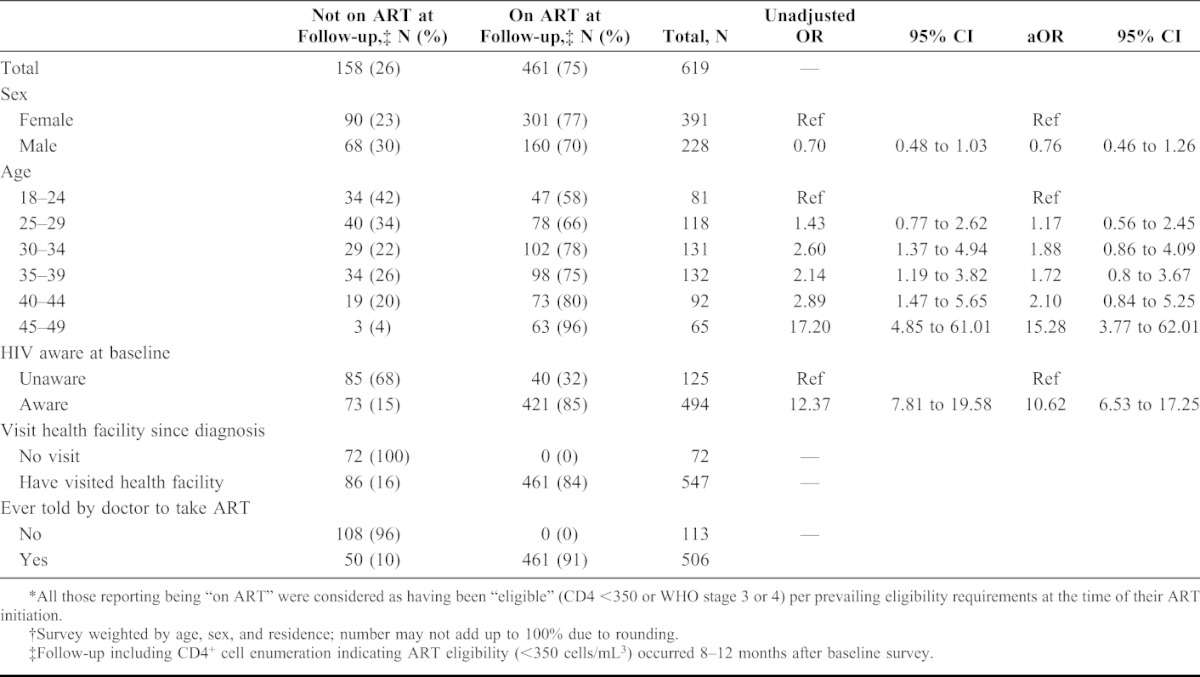
Approximately one-fourth (158 of 619, or 26%) of participants eligible for ART based on CD4+ count threshold of <350 cells per microliter reported not being on treatment at the time of the follow-up survey. In multivariate analysis, participants on treatment, compared with those eligible but not on treatment, were more likely to be older than 44 years of age [adjusted odds ratio (aOR): 15.28; 95% CI: 3.77 to 62.01] and to have already been aware of their HIV infection before the SHIMS baseline survey (aOR: 10.62, CI: 6.53 to 17.25) (Table 3). Sex, urban versus rural residence, marital status, education level, region, and employment status were not associated with ART treatment (data not shown). At follow-up, almost half (72/158 or 46%) of those eligible but not on treatment reported never having visited a health facility for HIV-related medical care since diagnosis.
TABLE 3.
Predictors of Taking Antiretroviral treatment (ART) Among 619 Adults Eligible* by CD4+ Cell Count <350 Cells Per Cubic Millimeter†
Predictors of Viral Suppression at Follow-up 8–12 Months After SHIMS Baseline Survey Among Those Reporting ART Use at Follow-up
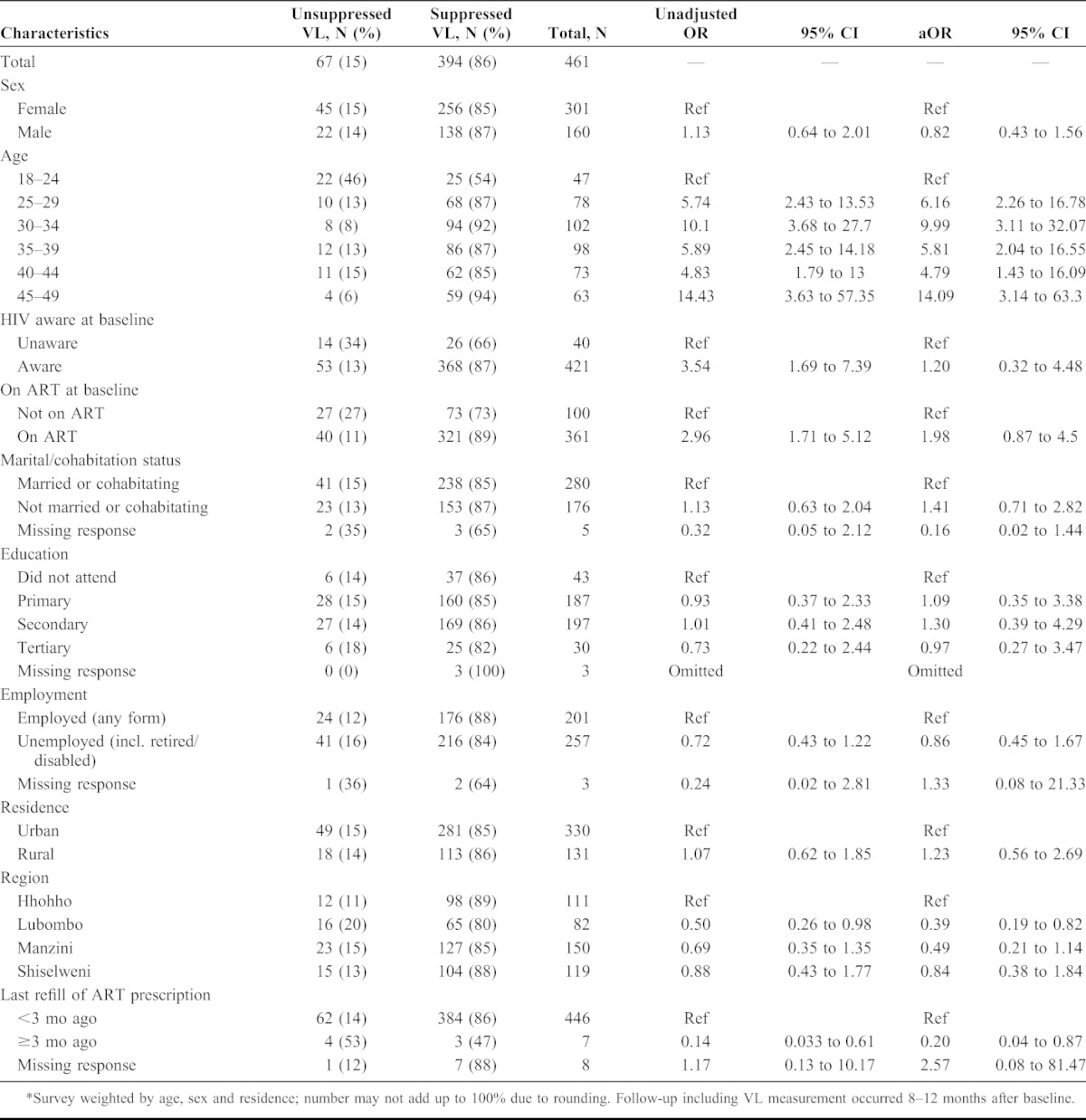
Among 461 participants who reported being on ART, VL was <1000 copies per milliliter in 394 (86%) (Table 4). In multivariate analysis, viral suppression among those who reported being on ART was only associated with being older than 24 years of age but not with having already been aware of their HIV infection or on ART at the time of the SHIMS baseline survey nor with other demographic variables, including sex. Those who reported not filling their ART prescription in the previous 3 months were significantly less likely to have suppressed VL (aOR: 0.20, CI: 0.04 to 0.87).
TABLE 4.
Predictors of Viral Load (VL) Suppression (<1000 Copies/mL) Among 461 Adults Who Report Taking Antiretroviral Treatment (ART) at Follow-up*
DISCUSSION
In Swaziland, the country with the most severe national HIV epidemic globally, our analysis determined that 8–12 months after SHIMS baseline survey had diagnosed or reconfirmed HIV-infected status, almost all participants not on ART had a VL ≥1000 copies per milliliter, and 40% of these were ART ineligible by the 2013 WHO treatment eligibility guidelines (ie, CD4 <500/μL). This population of ART-ineligible adults due to high CD4 count represents 18% of the HIV-infected population, or an estimated 40,727 persons with a VL high enough to be a risk factor for HIV transmission.
Swaziland has implemented a strong HIV treatment program, as evidenced by our study finding that 74% of those participants who were ART eligible based on the prevailing CD4+ count threshold of <350 cells per microliter reported using ART, and 85% of those who reported using ART had viral suppression. The proportion of those eligible who had started treatment had increased by 22% during the time between SHIMS baseline and follow-up surveys, and 73% of those starting ART use after SHIMS baseline had achieved viral suppression, further demonstrating the feasibility of effective treatment programs in a low resource setting. Despite these successes, the annual HIV incidence remains high,16 which may be explained in part by the gap in transmission prevention when ART initiation guidelines are based solely on CD4+ cell count.
All participants in the Swaziland analysis had documented HIV infection 8–12 months earlier, at SHIMS baseline survey, and most, 71%, reported having already been aware of their infection at that time. Thus, the high VL levels documented in this survey are unlikely to be due to recent infection.17 Our data suggest that for HIV treatment to contribute to prevention in this high incidence setting, eligibility criteria for initiation on ART need to be expanded beyond CD4+ cell count cutoff to include a VL threshold or a test-and-treat approach. Only 3% of the adult HIV-infected population would not be eligible with the combined criteria of CD4 count <500 cells per microliter and/or VL ≥1000 copies per milliliter, and some would be eligible by other criteria already adopted by Swaziland, including pregnancy, tuberculosis, or being in a serodiscordant relationship. Thus, test-and-treat seems the most straightforward strategy, avoiding VL measurement as a potential default point during enrollment in treatment, and in line with recent data from the TEMPRANO and START trials demonstrating individual clinical benefits from starting ART at CD4+ counts higher than 500 cells per microliter.2,3 Tanser et al18 have demonstrated in routine programmatic conditions at the community level that increased ART coverage results in population-level reductions in risk. In Swaziland, it is clear that coverage must extend beyond those eligible based on a CD4+ count threshold.
Challenges with starting people on ART at higher CD4+ counts, when they are generally healthy, have been poor adherence with associated persistent viremia and outright refusal to take ART.19–22 However, Jain et al23 have recently demonstrated high rates of adherence, retention in care, viral suppression, and safety when treating patients with high CD4+ counts in Uganda [median: 569 cells/μL (interquartile range: 451–716)]. They pointed out that patient attitudes may be changing as they learn more about clinical benefits and reduced transmission to others when starting ART earlier. Just, as knowledge of HIV-infected status has been documented to reduce HIV risk-related behavior after HIV testing,24,25 it is possible that the knowledge of high VL status, and its associated risk for HIV transmission, may change other behaviors such as retention in care and adherence with treatment.
To our knowledge, this study is the first to assess nationally representative population data to estimate the proportion and number of the HIV-infected adult population with high VL that would remain ineligible for ART with expanding CD4+ count criteria. However, our study has limitations. The association between VL and CD4+ count in untreated adults in a population will vary depending on the incidence rate in recent years, the age of the epidemic, the predominant viral subtype, and the viral set point in the population.26,27 Thus, the results from Swaziland, which has a mature generalized epidemic with one of the highest HIV incidence rates in the world, may not apply to all settings. Additionally, we were unable to verify ART treatment status beyond self-report for one-third of the participants, as they did not have their ART clinic record available at the time of interview, and we considered all those reporting being on ART as having been eligible per prevailing eligibility requirements at the time of their ART initiation but did not have clinical verification of their eligibility status at that time. We used data from census and surveys conducted by different methods and with different age ranges for adults to arrive at an estimate of the size of the HIV-infected adult population, then used those estimates to calculate estimated numbers of adults who would be treatment eligible from the proportions determined in our substudy. Finally, the rates of treatment coverage and viral suppression were measured 8–12 months after home-based HIV testing or retesting during SHIMS baseline survey and may not reflect the general HIV-infected population in the absence of home-based testing.
Access to VL measurement has been limited in resource-constrained settings because of the cost, and point-of-care quantification technologies, although in development, are not imminent.28,29 Determining eligibility based on VL would require routine VL assessment, which would increase program costs and add an additional step along the treatment cascade, where patients could be lost to follow up. Implementing a test-and-treat approach would not require VL measurements for eligibility determination, and in Swaziland would result in an additional 21% of the HIV-infected adult population added to the treatment rolls, most of whom, 86%, have VL high enough to be a risk factor for HIV transmission. The treatment for them would provide both an individual and public health benefit.
Footnotes
Supported by Cooperative Agreement #5U2GPS002005 from the U.S. Centers for Disease Control and Prevention.
Presented in part as: Naomi Bock, Ruth Emerson, Azih Charles Ikechi, Deborah Donnell, George Bicego, Velephi Okello, Peter Ehrenkranz, Neena Philip, Rejoice Nkambule, Yen Duong, and Jessica Justman. Potential Impact of Viral Load on ART Eligibility Criteria in Swaziland. Abstract 2258. Conference on Retroviruses and Opportunistic Infections, March 4, 2014, Boston, MA.
The authors have no conflicts of interest to disclose.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). The Gap Report 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. Accessed January 5, 2014. [PubMed] [Google Scholar]
- 2.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asumptomatic HIV infection. N Engl J Med. 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The TEMPRANO ANRS 12136 Study Group. A trial of early antiretorivirals and isoniazid preventive therapy in Africa. N Eng J Med. 2015;373:808–822. [DOI] [PubMed] [Google Scholar]
- 4.Doherty M. New directions in the 2015 WHO consolidated ARV guidelines. Presented at: Eighth International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2015); July 19, 2015; Vancouver, Canada, presentation SUSA0608.
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proceedings from the 3rd International HIV Treatment as Prevention Workshop. 2013. Available at: http://www.treatmentaspreventionworkshop.org/conference/2013-treatment-as-prevention-workshop. Accessed April 14, 2014. [Google Scholar]
- 7.Wilson DP. HIV treatment as prevention: natural experiements highlight limits of antiretroviral treatment as HIV prevention. PLoS Med. 2012;9:e1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novitsky V, Essex M. Using HIV viral load to guide treatment-for-prevention interventions. Curr Opin HIV AIDS. 2012;7:117–124. [DOI] [PubMed] [Google Scholar]
- 9.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention nof HIV infection. Clin Infect Dis. 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granich R, Muraguri N, Doyen A, et al. Achieving universal access for human immunodeficiency virus and tuberculosis: potential prevention impact of an integrated multi-disease prevention campaign in Kenya. AIDS Res Treat. 2012;2012:412643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. “UNAIDS/JC2502/1E,” Revised and Reissued, 2013. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed April 14, 2014. [Google Scholar]
- 12.Bicego GT, Nkambule R, Peterson I, et al. Recent patterns in population-based HIV prevalence in Swaziland. PLoS One. 2013;8:e77101 eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 2010;5:e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Population Review. Available at: http://worldpopulationreview.com/countries/swaziland-population/. Accessed December 22, 2014. [Google Scholar]
- 15.Central Statistical Office (CSO) [Swaziland], and Macro International Inc. Swaziland Demographic and Health Survey 2006-07. Mbabane, Swaziland: Central Statistical Office and Macro International Inc; 2008. Available at: http://dhsprogram.com/pubs/pdf/fr202/fr202.pdf. Accessed October 1, 2015. [Google Scholar]
- 16.Reed JB, Justman J, Bicego G, et al. Estimating national HIV incidence from directly observed seroconversions in the Swaziland HIV Incidence Measurement Survey (SHIMS) longitudinal cohort. Presented at: XIX International AIDS Conference; July 2012; Washington, DC.
- 17.Cohen MS, Shaw GM, McMichael AJ, et al. Acute HIV-1 infection. N Engl J Med. 2011;364:1943–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanser F, Bärnighausen T, Grapsa E, et al. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adakun S, Siedner M, Muzoora C, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viiremia in patients initiating arvs in rural Uganda. J Acquir Immune Defic Syndr. 2013;62:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz I, Essien T, Marinda E, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25:2177–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geng EH, Bwana MB, Muyindike W, et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr. 2013;63:e64–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain V, Byonanebye D, Amanyire G, et al. Successful antiretroviral therapy delivery and retention in care among asymptomatic individuals with high CD4+ T-cell counts above 350 cells/mL in rural Uganda. AIDS. 2014;28:2241–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonner VA, Denison J, Kennedy CE, et al. Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev. 2012;9:CD001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenburg NE, Pettifor AE, De Bruyn G, et al. HIV testing and counseling leads to immediate consistent condom use among South African stable HV-discordant couples. J Acquir Immune Defic Syndr. 2013;62:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoro MM, Perno CF. HIV-1 genetic variability and clinical implications. Microbiol. 2013;2013:481314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackelprang RD, Carrington M, Thomas KK, et al. Host genetic and viral determinants of HIV-1 RNA set-point among HIV-1 seroconverters from sub-Saharan Africa. J Virol. 2015;89:2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murtagh M. Viral Load: Current Technologies and the Pipeline, Including Point-of-care Assays. Consultation on Viral Load Monitoring for African HIV Treatment Programmes. Cape Town, South Africa: African Society for Laboratory Medicine; 2013:18–20. Available at: http://www.aslm.org/?wpdmdl=95. Accessed November 2, 2014. [Google Scholar]
- 29.Sollis K, Smit P, Fiscus S, et al. Systematic review of the performance of HIV viral load technologies on plasma samples. PLoS One. 2014;0085869. [DOI] [PMC free article] [PubMed] [Google Scholar]


