Abstract
In 2014, the Eighth Joint National Committee revised the target maximum systolic blood pressure (SBP) from 140 to 150 mmHg in persons aged ≥60 years without diabetes mellitus (DM) or chronic kidney disease (CKD). The evidence from cohort studies supporting this change was sparse, particularly among U.S. minority populations. In the Northern Manhattan Study, 1,750 participants aged ≥60 years and free of stroke, DM, and CKD had SBP measured at baseline and were annually followed for incident stroke. Mean age at baseline was 72±8 years; 63% were women; 48% Hispanic, 25% non-Hispanic white, and 25% non-Hispanic black. Among all participants, 40% were on antihypertensive medications, and 43% had SBP <140 mmHg, 20% 140-149 mmHg, and 37% ≥150 mmHg. Over a median follow-up of 13 years, 182 participants developed stroke. The crude stroke incidence was greater among individuals with SBP ≥150 mmHg (10.8 per 1000 person-years) and SBP 140-149 (12.3) compared to those with SBP<140 (6.2). After adjusting for demographics, vascular risk factors, diastolic BP, and medication use, participants with SBP 140-149 mmHg had an increased risk of stroke (HR, 1.7; 95% CI, 1.2-2.6) compared with those with SBP <140 mmHg. The increased stroke risk was most notable among Hispanics and non-Hispanic blacks. Raising the SBP threshold from 140 to 150 mmHg as a new target for hypertension treatment in older individuals without DM or CKD could have a detrimental effect on stroke risk reduction, especially among minority U.S. populations.
Keywords: Systolic blood pressure, stroke, cardiovascular disease, epidemiology
Hypertension is a well-established major risk factor for stroke, one of the leading causes of death and disability in the United States and worldwide.1 Hypertension is also an important contributor to substantial disparities in the mortality and incidence of stroke across race and ethnic subgroups, with greater burdens among blacks and Hispanics.2,3 Given that elevated blood pressure (BP) is a modifiable risk factor amenable to lifestyle interventions and relatively inexpensive medications, evidence-based BP management is an important strategy for the prevention of stroke and the reduction of race-ethnic stroke disparities. Although there is a clinical emphasis on detection of hypertension, most epidemiological studies have shown that BP is a continuous vascular risk factor and even modest elevations are associated with increased stroke risk. 4
In 2014, the Eighth Joint National Committee (JNC8) on prevention, detection, evaluation, and treatment of high BP published an updated guideline for the management of high BP in adults.5 One of the major changes was a revision of the systolic BP target from 140 mmHg to 150 mmHg in persons 60 years or older and without diabetes mellitus (DM) or chronic kidney disease (CKD). Based on a systematic review of available randomized controlled trials, the panel used rigorous evidence-based methods and developed evidence statements and recommendations for BP treatment, but did not consider data from the population-based cohort studies. Members of the JNC8 panel who disagreed with this new recommendation published their minority opinion regarding this controversial revision in the SBP threshold.6 Recently, the Systolic Blood Pressure Intervention Trial (SPRINT) was terminated early and demonstrated that lowering SBP to 120 mmHg reduced rates of major cardiovascular events and all-cause mortality in older or high-risk patients who did not have diabetes, however, no significant reduction was observed for stroke events alone. 7
Given that the evidence leading to the recommendations for hypertension treatment has largely been derived from white cohorts, one concern is the potential differential impact of the increasing systolic BP treatment threshold across race-ethnic groups.8 The Northern Manhattan Study (NOMAS) is a prospective population-based cohort study that represents a multi-ethnic community with a significant proportion of Hispanics, the fastest growing and largest minority group in the United States. Using NOMAS data, we sought to assess this SBP modification by evaluating incident stroke risk for systolic BP levels of 140-149 mmHg among those aged ≥60 years without stroke, DM, or CKD.
METHODS
Study Population
The present study included a subsample of 1,750 participants who were 60 years or older and free of stroke, diabetes mellitus (DM) and chronic kidney disease (CKD) at baseline from the Northern Manhattan Study (NOMAS). Details of the NOMAS study design have been published previously.9-11 The original cohort included 3,298 stroke-free participants enrolled from 1993 to 2001. Written informed consent was obtained from all the participants and the study was approved by the Institutional Review Boards of the University of Miami and Columbia University Medical Center.
Baseline Characteristics
At baseline, all participants underwent a thorough evaluation that included medical history, physical examination, review of medical records, and tests of fasting blood samples. Self-reported race-ethnicity was classified based on a series of questions modeled after the US census. Standardized questions regarding hypertension, diabetes mellitus, and cardiac conditions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System.12 Office systolic blood pressure (SBP) and diastolic blood pressure (DBP) (mean of 2 readings in sitting position) were measured by trained research assistants after a period of rest with a mercury sphygmomanometer. Fasting blood glucose was measured with standard procedures using a glucose dehydrogenase method.13 Diabetes was defined by taking antiglycemic medications or fasting blood glucose>125 mg/dL. Serum creatinine (sCr) was measured using the kinetic alkaline picrate assay (Jaffé reaction). Baseline kidney function was assessed using estimated glomerular filtration rate (eGFR) based on the Modification of Diet in Renal Disease formula as: eGFR = 186.3 × (sCr−1.154) × (age− 0.203) × (0.742 if female) × (1.21 if black).14 CKD was defined as eGFR<60 mL/min/1.73 m2. Smoking status was categorized into never, former, current (within a year) based on self-reported age of starting smoking and age of quitting smoking. Moderate alcohol consumption was defined as 1 drink/month up to 2 drinks/day. Leisure-time physical activity was evaluated using a questionnaire adapted from the National Health Interview Survey.15
Ascertainment of Incident Stroke Events
The primary outcome was any incident stroke. Follow-up procedures and stroke classifications have been published previously.16 In brief, NOMAS participants have been followed up annually by telephone interviews with an average annual contact rate of 99%.17 The outcome surveillance network also includes daily screening of admissions, review of neurology consult lists, hospital admission and discharge data (including screening of ICD-9 codes), emergency room visits, and visits to the ambulatory care network. Persons who screen positive for any potential cardiac or neurological event undergo in-person assessment, chart review, and examination by a study neurologist. Incident stroke events were verified and classified as the first occurrence of stroke by at least two NOMAS vascular neurologists as described in previous reports.10
Data Analysis
The present study included participants who were ≥60 years of age, without stroke, DM or CKD at baseline. The participants were divided into 3 groups based on their baseline SBP level (<140, 140-149, ≥150 mmHg). The primary outcome was first-ever stroke during follow-up. All covariates were measured at baseline.
For the baseline characteristics, we used the F test to examine the differences in continuous variables and the χ2 test to compare the frequencies of categorical variables among the SBP groups. For each participant, we calculated the person-time at risk accrued from baseline to the time of incident stroke, death, loss to follow-up, or the most recent follow-up date up to July 2014, whichever came first. For association between SBP levels and incident stroke risk, we used Kaplan-Meier curves to describe the unadjusted association and Cox proportional hazards models to estimate the multivariable-adjusted hazard ratios (HRs) with 3 sequential models. Model 1 was adjusted for age, sex and race-ethnicity; Model 2 was additionally adjusted for antihypertensive medication use, waist circumference (WC), smoking status, moderate alcohol drinking, leisure-time physical activity, and history of myocardial infarction, atrial fibrillation, and coronary heart disease; and Model 3 was adjusted further for diastolic BP. As secondary analyses, we examined the association by excluding the individuals who were on baseline antihypertensive medication, excluding those who had elevated DBP >90, and by grouping the subjects into three categories based on both baseline SBP and DBP levels (SBP<140 and DBP<90 mmHg, SBP 140-149 and DBP<90 mmHg, SBP≥150 or DBP≥90 mmHg). Finally, given the difference in stroke risk across race-ethnic, sex and age groups,1, 18, 19 we conducted stratified analyses to explore the potential modifications by race-ethnicity, sex and age. We performed all data analyses with SAS statistical software version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
The baseline characteristics of 1,750 NOMAS participants aged ≥60 years without stroke, DM, or CKD are presented in Table 1 by SBP levels and overall. The mean age of the participants was 72±8 years; 63% were women; 48% Hispanic, 25% non-Hispanic white, and 25% non-Hispanic black. Overall, 40% were on antihypertensive medications; 43% had SBP <140 mmHg, 20% 140-149 mmHg, and 37% ≥150 mmHg. Univariate analysis showed that baseline SBP levels were associated with age, race-ethnicity, smoking status, waist circumference, anti-hypertensive medication use, and DBP level, but not with leisure-time physical activity, moderate alcohol drinking, or any history of cardiovascular diseases (CVD).
Table 1.
Sample Baseline Characteristics
| Characteristics | All (n=1,750) |
Baseline SBP, mmHg |
|||
|---|---|---|---|---|---|
| <140 (n=761) |
140-149 (n=354) |
≥ 150 (n=635) |
P | ||
| Age (y), mean ± SD | 72±8 | 71±8 | 72±8 | 72±8 | 0.0356 |
| Male, % | 36.9 | 38.9 | 39.0 | 33.2 | 0.0596 |
| Race-ethnicity, % | 0.0007 | ||||
| NH-white | 24.6 | 28.6 | 25.1 | 19.4 | |
| NH-black | 25.1 | 21.8 | 27.4 | 27.7 | |
| Hispanic | 47.8 | 46.4 | 44.9 | 51.2 | |
| NH-other | 2.5 | 3.2 | 2.5 | 1.7 | |
| Smoking, % | 0.0002 | ||||
| Never | 47.8 | 44.3 | 42.4 | 55.1 | |
| Former | 36.7 | 38.6 | 41.2 | 31.8 | |
| Current | 15.4 | 16.8 | 16.4 | 13.1 | |
| Physical activity, % | 60.0 | 59.1 | 61.6 | 60.2 | 0.6376 |
| Moderate alcohol drinking, % | 35.2 | 37.6 | 35.9 | 32.0 | 0.0876 |
| Waist circumference (inch), mean ± SD |
36±5 | 35±5 | 37±5 | 37±5 | <0.0001 |
| History of cardiovascular disease*, % |
21.2 | 20.4 | 22.3 | 21.6 | 0.7287 |
| Antihypertension medication use, % |
40.3 | 24.0 | 46.3 | 56.5 | <0.0001 |
| DBP≥90 mmHg | 30.6 | 10.2 | 29.1 | 55.7 | <0.0001 |
including myocardial infarct, atrial fibrillation or coronary artery disease.
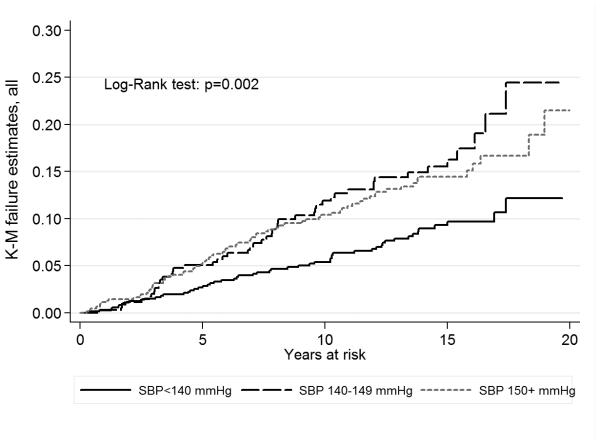
A total of 182 incident strokes (159 ischemic, 18 hemorrhagic, 3 Subarachnoid and 2 unknown) occurred during a median follow-up of 13 years (interquartile range: 7-15 years). The overall crude incidence rate was 9.1/1000 person-years (Table 2). The crude stroke incidence was greater (p for log-rank test =0.002) among those with SBP ≥150 mmHg (10.8/1000 person-years) and SBP 140-149 (12.3) compared to those with SBP<140 (6.2). The Kaplan-Meier curves for the individuals with SBP ≥150 mmHg and 140-149 were closer to each other than they were to the curve for those with SBP <140 mmHg (Figure 1).
Table 2.
Association between SBP Levels and Incident Stroke Risk among Subjects Aged 60 or Older without Diabetes Mellitus or Chronic Kidney Disease
| Sample | SBP, mmHg |
N | No. cases |
Crude rate |
Model 1* | Model 2† | Model 3 ‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | PHet§ | |||||
| All | <140 | 761 | 55 | 6.2 | Ref. | Ref. | Ref. | ||||
| 140-149 | 354 | 50 | 12.3 | 1.83 (1.24-2.68) | 0.002 | 1.79 (1.20-2.65) | 0.004 | 1.72 (1.15-2.57) | 0.008 | ||
| ≥150 | 635 | 77 | 10.8 | 1.56 (1.10-2.21) | 0.013 | 1.53 (1.06-2.22) | 0.024 | 1.41 (0.95-2.10) | 0.092 | ||
| Total | 1750 | 182 | 9.1 | ||||||||
| Hispanic | <140 | 353 | 17 | 3.9 | Ref. | Ref. | Ref. | ||||
| 140-149 | 159 | 20 | 10.3 | 2.57 (1.34-4.90) | 0.004 | 2.49 (1.29-4.80) | 0.006 | 2.61 (1.35-5.07) | 0.005 | 0.039 | |
| ≥150 | 325 | 43 | 11.3 | 2.57 (1.46-4.52) | 0.001 | 2.59 (1.43-4.67) | 0.002 | 2.85 (1.52-5.34) | 0.001 | 0.004 | |
| Total | 439 | 56 | 11.6 | ||||||||
| NH-black | <140 | 166 | 16 | 8.7 | Ref. | Ref. | Ref. | ||||
| 140-149 | 97 | 18 | 17.3 | 1.93 (0.98-3.80) | 0.056 | 2.22 (1.07-4.57) | 0.032 | 1.93 (0.93-4.03) | 0.078 | 0.148 | |
| ≥150 | 176 | 22 | 11.3 | 1.21 (0.63-2.32) | 0.560 | 1.40 (0.70-2.82) | 0.339 | 1.01 (0.47-2.15) | 0.983 | 0.328 | |
| Total | 439 | 56 | 11.6 | ||||||||
| NH-white | <140 | 218 | 20 | 8.3 | Ref. | Ref. | Ref. | ||||
| 140-149 | 89 | 10 | 10.1 | 1.01 (0.47-2.16) | 0.987 | 0.88 (0.39-2.00) | 0.760 | 0.85 (0.37-1.95) | 0.700 | ||
| ≥150 | 123 | 9 | 7.3 | 0.81 (0.37-1.78) | 0.593 | 0.62 (0.27-1.43) | 0.261 | 0.56 (0.23-1.38) | 0.208 | ||
| Total | 430 | 39 | 8.4 | ||||||||
| Women | <140 | 465 | 34 | 6.1 | Ref. | Ref. | Ref. | ||||
| 140-149 | 2.12 (1.32-3.41) | 0.002 | 2.04 (1.25-3.31) | 0.004 | 1.98 (1.22-3.24) | 0.006 | 0.369 | ||||
| ≥150 | 424 | 51 | 10.3 | 1.49 (0.96-2.30) | 0.077 | 1.40 (0.88-2.22) | 0.162 | 1.31 (0.80-2.15) | 0.291 | 0.552 | |
| Total | 1105 | 120 | 9.2 | ||||||||
| Men | <140 | 296 | 21 | 6.3 | Ref. | Ref. | Ref. | ||||
| 140-149 | 138 | 15 | 9.7 | 1.44 (0.74-2.79) | 0.284 | 1.44 (0.71-2.89) | 0.310 | 1.33 (0.65-2.72) | 0.442 | ||
| ≥150 Total | 211 645 | 26 62 | 11.8 8.8 | 1.75 (0.98-3.13) | 0.057 | 1.93 (1.04-3.60) | 0.038 | 1.69 (0.86-3.33) | 0.131 | ||
| Age<80 | <140 | 621 | 37 | 4.7 | Ref. | Ref. | Ref. | ||||
| 140-149 | 289 | 36 | 10.3 | 1.97 (1.24-3.12) | 0.004 | 1.94 (1.21-3.14) | 0.006 | 1.90 (1.17-3.08) | 0.009 | 0.507 | |
| ≥150 | 514 | 62 | 10.0 | 1.81 (1.20-2.73) | 0.005 | 1.83 (1.18-2.86) | 0.008 | 1.74 (1.08-2.78) | 0.022 | 0.102 | |
| Total | 1424 | 135 | 7.7 | ||||||||
| Age ≥80 | <140 | 140 | 18 | 16.8 | Ref. | Ref. | Ref. | ||||
| 140-149 | 65 | 14 | 24.3 | 1.50 (0.74-3.03) | 0.260 | 1.52 (0.73-3.19) | 0.267 | 1.40 (0.65-3.00) | 0.392 | ||
| ≥150 | 121 | 15 | 16.3 | 0.99 (0.50-1.97) | 0.974 | 0.92 (0.44-1.94) | 0.835 | 0.80 (0.36-1.79) | 0.591 | ||
| Total | 326 | 47 | 18.3 | ||||||||
Model 1: adjusted for age, and applicable, for sex and race-ethnicity
Model 2: model 1 additionally adjusted for BP medication use, waist circumference, smoking, moderate alcohol drinking, physical activity, and history of myocardial infarct, atrial fibrillation or coronary artery disease.
Model 3: model 2 additionally adjusted for DBP.
PHet: p value for heterogeneity for effect comparison between Hispanic and white, between black and white, between men and women, between age<80 and age≥80.
Figure 1.
Kaplan-Meier curves of stroke risk for systolic blood pressure (SBP) in persons aged 60 years or older without diabetes mellitus or chronic kidney disease. Kaplan-Meier curves were constructed by SBP levels at <140, 140-149 and ≥ 150 mmHg and compared with Log-Rank test, NOMAS (Northern Manhattan Study), 1993-2014.
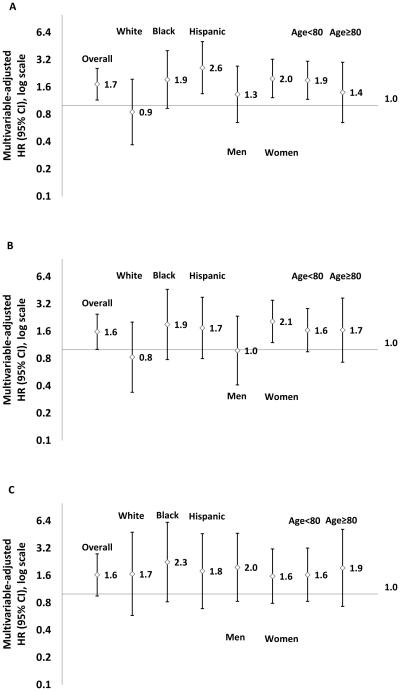
After adjusting for demographics, vascular risk factors, DBP, and anti-hypertensive medication use (Model 3, Table 2), participants with SBP 140-149 mmHg had increased risk of stroke (HR, 1.72; 95% CI, 1.15-2.57; p=0.008) compared to those with SBP <140mmHg. No significant interactions were detected by race-ethnicity, sex, and age. For SBP 140-149 compared to <140 mmHg, however stratified analysis using Model 3 showed an increased stroke risk in Hispanics (2.61; 1.35-5.07) and non-Hispanic blacks (1.93; 0.93-4.03), but not in non-Hispanic whites (0.85; 0.37-1.95) (Table 2, Figure 2A).
Figure 2.
Multivariable-adjusted hazards ratio of stroke for systolic blood pressure 140-149 verse <140 mmHg, overall or by race-ethnicity, sex and age, in persons aged 60 years or older without diabetes mellitus or chronic kidney disease at baseline for all (A), for those without diastolic blood pressure ≥ 90 mmHg (B), and for those without antihypertensive medication use (C). The model was adjusted for age, waist circumference, smoking, moderate alcohol drinking, physical activity, and history of myocardial infarction, atrial fibrillation and coronary artery disease, and applicable, for sex, race-ethnicity, antihypertensive medication use, and diastolic blood pressure, NOMAS (Northern Manhattan Study), 1993-2014.
Two sensitivity analyses were performed to confirm the robustness of the findings. To minimize the effect of DBP, we excluded 535 subjects with baseline DBP ≥ 90 mmHg and examined the association with the same adjustment as in Model 3 (Figure 2B). Compared to those with SBP <140, participants with SBP 140-149 mmHg had increased risk of stroke overall (1.58; 1.01-2.47) and tended to increase the risk among Hispanics (1.74; 0.80-3.79) and non-Hispanic blacks (1.90; 0.78-4.64) but not in non-Hispanic whites (0.83; 0.34-2.02); in women (2.05; 1.20-3.52) but not in men (0.98; 0.41-2.35); and in both those aged <80 years (1.64, 0.95-2.85) and those aged ≥80 years (1.65, 0.73-3.72).
In another sensitivity analysis, we excluded 706 subjects with baseline antihypertensive medication use (Figure 2C).In this group, compared to those with SBP <140, participants with SBP 140-149 mmHg had increased risk of stroke with an HR ranging from 1.6 to 2.3 across race-ethnic, sex and age groups. In addition, we regrouped the subjects into three categories based on their baseline SBP and DBP levels. The relative risk for SBP 140-149 compared to SBP<140 mmHg remained similar to the relative risk found by grouping the subjects based on baseline SBP levels and adjusting for DBP levels in both overall and stratified analyses (Table S1).
DISCUSSION
In the present study conducted in a sample from the NOMAS multi-ethnic cohort with a median follow-up of 13 years, we demonstrated that the recent JNC8 recommendation for revising the SBP threshold from 140 to 150 mmHg for hypertension treatment could have a detrimental effect on stroke risk and may contribute to stroke disparities across race-ethnicity and sex. The increased stroke risk for SBP between 140-149 mmHg compared with SBP below 140 mmHg tended to be more prominent among Hispanics and non-Hispanic blacks than in non-Hispanic whites, was mainly observed among women, and also appeared among those over age 80 where cautions have been raised about aggressive management of hypertension. When excluding all persons on any antihypertensive medications at baseline, there was a clear and consistent increased risk of stroke for those with SBP of 140-149 compared those with SBP below 140 across age, sex and race-ethnic subgroups.
The JNC85 recommendation for raising the threshold 10 mmHg above that in the previous guideline for SBP treatment in the general population who are 60 years or older and do not have DM or CKD raised a major concern. Although a recent study suggested that this recommendation would be cost-effective and may reduce side effects of medications and adverse events,20 the JNC8 members who did not agree with this recommendation6 argued that the increased SBP target of 150 could reduce the intensity of antihypertensive treatment in a large population at high risk for CVD and stroke, especially among high-risk populations such as African Americans.21
In the US population, BP is adequately controlled only in 36% of men and 28% of women between ages 60–79 years and in 38% of men and 23% of women aged 80 years and older.21 A cross-sectional study22 conducted in 16,372 subjects from the National Health and Nutrition Examination Survey demonstrated that the proportion of older adults (≥60 years) receiving BP-lowering medication and meeting the more stringent JNC7 targets [68.9% (95% CI, 66.9%−70.8%)] was greater compared to JNC8 guidelines [61.2% (95% CI, 59.3%−63.0%)]. Following these results, other investigators raised the concern that JNC8 will lead to a higher incidence of cardiovascular events and mortality especially in more vulnerable populations.23 Previous published data from INVEST24 showed that among hypertensive patients with coronary artery disease (CAD) who are 60 year of age or older, achieving a BP of <150 mmHg as recommended by JCN8 was associated with less benefit than the target of <140 mmHg in terms of all-cause mortality, cardiovascular mortality, stroke, and myocardial infarct. Moreover, results from SPRINT also demonstrated that lowering SBP to 120 mmHg reduces rates of major cardiovascular events and all-cause mortality, suggesting that for SBP the lower goal is better to improve the health in older or high-risk patients.7
A main finding of our study is the race-ethnic differences in the increased risk for SBP of 140-149 mmHg compared to SBP below 140 mmHg, showing an increase by 2.6 times in Hispanics and 1.9 times in non-Hispanic blacks, but not in non-Hispanic whites (p=0.039 for heterogeneity between Hispanics and whites; p=0.148 for heterogeneity between blacks and whites, Table 2). Our results are in agreement with the concerns already reported from the Association of Black Cardiologists (ABC),7 on the race-ethnicity gaps of the JCN8. The ABC supported the need for a more appropriate and stringent control of BP, especially in high-risk groups such as African Americans where life expectancy is 5.4 years shorter than Caucasians, and hypertension is the single largest contributor to this disparity.25 In NOMAS, we previously demonstrated that overall the incidence of stroke was higher in Hispanic and Black populations compared to whites,18, 19 and similarly the levels of BP were greater in these race-ethnic subgroups.3 Moreover, less effective BP medications may be more frequently used in ethnic minority populations, and this can be one of the reasons of the higher levels of BP and higher incidence of stroke in Hispanics and Blacks.26 Interestingly, among those without any antihypertensive medications at baseline, our study showed a similar increased risk of stroke for those with SBP of 140-149 across race-ethnic subgroups (p values > 0.93 for heterogeneity, Table S1), suggesting that disparity in antihypertensive treatment may be an important contributor to the observed race-ethnic difference in the stroke risk. Given that the incidence of hypertension and stroke in Hispanics is high compared to other race-ethnicities, a major concern could be that the most recent JNC8 guidelines did not take into consideration the specific needs of these minority populations.
Another concern regarding the JNC 8 recommendation is that women could be differentially affected by the recommendation to relax the SBP threshold for initiating treatment to 150 mmHg. In the present study, women with SBP of 140-149 mmHg had a 97% greater risk of stroke compared to women with SBP<140 mmHg, and these risks tended to be more notable than among men. A similar concern regarding the JNC 8 recommendation and risk for CVD mortality among women with hypertension was already reported by the Working Group on Women’s Cardiovascular Health.7 The Group raised this concern since most patients ≥60 years of age with hypertension are women.20 . Moreover, older women generally have poorly controlled BP and among those a high percentage are African-American women.27, 28
Compared to JNC8, the European Society of Hypertension (ESH) guidelines maintained the target BP of <140/90 mmHg for the general population, and recommended initiation of BP reduction therapy when SBP is above 150 mmHg only in patients older than 80 years of age.29 Some studies and meta-analyses, conducted in very old patients, suggested that achieving a SBP goal below 140 mmHg may increase the risk of side effects, like orthostatic hypotension,30 and increase risks for all-cause of mortality.31, 32 However, results from the Hypertension in the Very Elderly Trial (HYVET)33 demonstrated that more aggressive treatment for hypertension in older people may have a benefit in terms of lower mortality for CVD, especially for stroke. A meta-analysis performed on randomized controlled trials examining antihypertensive use in octogenarians concluded that hypertensive patients who are healthy and functionally independent should be treated according to current recommendations for people older than 65 years.34 In the present study, we found that both subjects aged <80 years old and those aged ≥80 years with SBP of 140-149 mmHg had greater risk of stroke compared to those with SBP<140 mmHg, suggesting that similar control of hypertension, even in elderly people, may result in better stroke prevention. For elderly patients, therapeutic BP goals are always less restrictive, depending on other comorbid conditions, frailty, and cognitive functions, but the reason to systematically increase the threshold for treatment of hypertension in all elderly patients is still debated.
Some studies demonstrated an inverse relationship, J-shaped, or U-shaped association between DBP and outcomes in elderly.35, 36 Therefore, to further confirm our results we excluded subjects with baseline DBP ≥90 mmHg in order to avoid bias related to DBP. After this exclusion, we confirmed the role of SBP in prediction of stroke and found similar results. When we reclassified our subgroups taking into account DBP levels, we also found similar trends of an increased stroke incidence for SBP 140-149 mmHg among those with DBP <90 mmHg (Table S1).
Strengths of our study include the community-based random-sampling method, the inclusion of a tri-ethnic cohort with a sizeable number of Hispanics from the same community that allows comparisons and helps minimize socioeconomic confounding, the availability of comprehensive data on health behaviors, and the excellent retention of the cohort with follow-up for as long as 20 years. Nevertheless, several limitations also deserve mention. This is an observational study, therefore biases present in observational data for generating hypothesis need to be considered. The sample size for whites and blacks, as well as for those over 80 years of age, was relatively small, limiting the study’s ability to prove differences in risk among subgroups. Also, SBP readings and patients’ use of blood pressure medications were based on a single baseline assessment and not time-dependent variables. Our cohort represents a lower socioeconomic, multi-ethnic, urban community and may not be representative of other populations.
Perspectives
We report a significantly greater incidence of stroke in subjects over age 60 years without diabetes and chronic kidney disease who had a systolic blood pressure of 140-149 mmHg. This effect was more pronounced among minorities and women. Our data suggests that raising the systolic threshold for treatment of BP from 140 to 150 mmHg as suggested by the JNC8 could lead to an increase in stroke risk and likely widen race, ethnic and sex disparities for stroke. Our data support adherence to the current AHA recommendations that consistently recommend treatment for BP above 140-90 mmHg in order to improve cardiovascular health and reduce stroke.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first report to examine the effect of raising the systolic blood pressure (SBP) threshold from 140 to 150 mmHg for hypertension treatment on stroke risk in a prospective population-based cohort that represents a multi-ethnic community with a significant proportion of Hispanics.
What Is Relevant?
Our findings indicate that SBP between 140-149 mmHg, compared with SBP below 140 mmHg, increases the risk of incident stroke in persons aged 60 years or older without diabetes mellitus (DM) or chronic kidney disease (CKD).
Summary
Raising the SBP threshold from 140 to 150 mmHg as a new target for hypertension treatment in older individuals without DM or CKD could have a detrimental effect on stroke risk reduction, especially among minority U.S. populations. Our data support adherence to the current AHA recommendations that recommend treatment for BP above 140-90 mmHg in order to reduce stroke.
Acknowledgments
The authors thank study participants for their collaboration and all staff of the Northern Manhattan Study for their dedication to the study especially Janet DeRosa.
Funding Sources
This work was supported by a grant from the National Institute of Neurological Disorders and Stroke (R01 NS 29993) and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Dong: Systolic Blood Pressure and Stroke Risk
Disclosures: None
Contributor Information
Chuanhui Dong, Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL.
David Della-Morte, Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL; Department of Systems Medicine, School of Medicine, University of Rome 'Tor Vergata', 00133 Rome, Italy; IRCCS San Raffaele Pisana, Rome, Italy.
Tatjana Rundek, Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL; Department of Public Health Sciences, Miller School of Medicine, University of Miami, Miami, FL.
Clinton B. Wright, Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL; Department of Public Health Sciences, Miller School of Medicine, University of Miami, Miami, FL.
Mitchell S. V. Elkind, Departments of Neurology and Epidemiology, Columbia University, New York, NY.
Ralph L. Sacco, Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL; Department of Public Health Sciences, Miller School of Medicine, University of Miami, Miami, FL; John T. McDonald Department of Human Genetics, Miller School of Medicine, University of Miami, Miami, FL.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA. 2013;173:46–51. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995;45:659–663. doi: 10.1212/wnl.45.4.659. [DOI] [PubMed] [Google Scholar]
- 4.Palmer AJ, Bulpitt CJ, Fletcher AE, Beevers DG, Coles EC, Ledingham JG, O'Riordan PW, Petrie JC, Rajagopalan BE, Webster J, Dollery CT. Relation between blood pressure and stroke mortality. Hypertension. 1992;20:601–605. doi: 10.1161/01.hyp.20.5.601. [DOI] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 6.Wright JT, Jr., Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. doi: 10.7326/M13-2981. [DOI] [PubMed] [Google Scholar]
- 7.The SPRINT Research Group. Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. The New England Journal of Medicine. 2015;273:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krakoff LR, Gillespie RL, Ferdinand KC, Fergus IV, Akinboboye O, Williams KA, Walsh MN, Bairey Merz CN, Pepine CJ. 2014 hypertension recommendations from the eighth joint national committee panel members raise concerns for elderly black and female populations. J Am Coll Cardiol. 2014;64:394–402. doi: 10.1016/j.jacc.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 10.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the Northern Manhattan Study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 11.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry EM, Kalsbeek WD, Hogelin GC, Jones JT, Gaines KL, Forman MR, Marks JS, Trowbridge FL. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. Am J Prev Med. 1985;1:9–14. [PubMed] [Google Scholar]
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Wright CB, Dong C, Stark M, Silverberg S, Rundek T, Elkind MS, Sacco RL, Mendez A, Wolf M. Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS) Neurology. 2014;82:1700–1706. doi: 10.1212/WNL.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss AJ, Parsons VL. Current estimates from the National Health Interview Survey. United States, 1985. Vital Health Stat 10. 1986:1–182. [PubMed] [Google Scholar]
- 16.Boden-Albala B, Cammack S, Chong J, Wang C, Wright C, Rundek T, Elkind MS, Paik MC, Sacco RL. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS) Diabetes care. 2008;31:1132–1137. doi: 10.2337/dc07-0797. [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, Paik MC, Shea S. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 19.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 20.Moran AE, Odden MC, Thanataveerat A, Tzong KY, Rasmussen PW, Guzman D, Williams L, Bibbins-Domingo K, Coxson PG, Goldman L. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med. 2015;372:447–455. doi: 10.1056/NEJMsa1406751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 22.Navar-Boggan AM, Pencina MJ, Williams K, Sniderman AD, Peterson ED. Proportion of US adults potentially affected by the 2014 hypertension guideline. JAMA. 2014;311:1424–1429. doi: 10.1001/jama.2014.2531. [DOI] [PubMed] [Google Scholar]
- 23.Banach M, Aronow WS, Serban C, Sahabkar A, Rysz J, Voroneanu L, Covic A. Lipids, blood pressure and kidney update 2014. Pharmacol Res. 2015;96:111–125. doi: 10.1016/j.phrs.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Bangalore S, Gong Y, Cooper-DeHoff RM, Pepine CJ, Messerli FH. 2014 Eighth Joint National Committee panel recommendation for blood pressure targets revisited: results from the INVEST study. J Am Coll Cardiol. 2014;64:784–793. doi: 10.1016/j.jacc.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 26.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 27.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51:1142–1148. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 28.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 29.Stephan D, Gaertner S, Cordeanu EM. A critical appraisal of the guidelines from France, the UK, Europe and the USA for the management of hypertension in adults. Arch Cardiovasc Dis. 2015;108:453–459. doi: 10.1016/j.acvd.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP, Zhou B, Lin H. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA. 2014;174:588–595. doi: 10.1001/jamainternmed.2013.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oates DJ, Berlowitz DR, Glickman ME, Silliman RA, Borzecki AM. Blood pressure and survival in the oldest old. J Am Geriatr Soc. 2007;55:383–388. doi: 10.1111/j.1532-5415.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 32.Gueyffier F, Bulpitt C, Boissel JP, Schron E, Ekbom T, Fagard R, Casiglia E, Kerlikowske K, Coope J. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. INDANA Group. Lancet. 1999;353:793–796. doi: 10.1016/s0140-6736(98)08127-6. [DOI] [PubMed] [Google Scholar]
- 33.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 34.Benetos A, Rossignol P, Cherubini A, Joly L, Grodzicki T, Rajkumar C, Strandberg TE, Petrovic M. Polypharmacy in the Aging Patient: Management of Hypertension in Octogenarians. JAMA. 2015;314:170–180. doi: 10.1001/jama.2015.7517. [DOI] [PubMed] [Google Scholar]
- 35.Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, LaRosa JC, Treating to New Targets Steering C and Investigators J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31:2897–2908. doi: 10.1093/eurheartj/ehq328. [DOI] [PubMed] [Google Scholar]
- 36.Denker MG, Cohen DL. What is an appropriate blood pressure goal for the elderly: review of recent studies and practical recommendations. Clin Interv Aging. 2013;8:1505–1517. doi: 10.2147/CIA.S33087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.