Abstract
The insufficiency of compensatory angiogenesis in the heart of hypertension patients contributes to heart failure transition. The HIF1α-VEGF signaling cascade controls responsive angiogenesis. One of the challenges in reprograming the insufficient angiogenesis is to achieve a sustainable tissue exposure to the pro-angiogenic factors, such as HIF1α stabilization. In this study, we identified Rnd3, a small Rho GTPase, as a pro-angiogenic factor participating in the regulation of the HIF1α-VEGF signaling cascade. Rnd3 physically interacted with and stabilized HIF1α, and consequently promoted VEGFA expression and endothelial cell tube formation. To demonstrate this pro-angiogenic role of Rnd3 in vivo, we generated Rnd3 knockout mice. Rnd3 haploinsufficient (Rnd3+/−) mice were viable, yet developed dilated cardiomyopathy with heart failure after transverse aortic constriction stress. The post-stress Rnd3+/− hearts showed significantly impaired angiogenesis and decreased HIF1α and VEGFA expression. The angiogenesis defect and heart failure phenotype were partially rescued by cobalt chloride treatment, a HIF1α stabilizer, confirming a critical role of Rnd3 in stress-responsive angiogenesis. Furthermore, we generated Rnd3 transgenic mice and demonstrated that Rnd3 overexpression in heart had a cardio-protective effect through reserved cardiac function and preserved responsive angiogenesis after pressure overload. Finally, we assessed the expression levels of Rnd3 in the human heart and detected significant downregulation of Rnd3 in patients with end-stage heart failure. We concluded that Rnd3 acted as a novel pro-angiogenic factor involved in cardiac responsive angiogenesis through HIF1α-VEGFA signaling promotion. Rnd3 downregulation observed in heart failure patients may explain the insufficient compensatory angiogenesis involved in the transition to heart failure.
Keywords: Small GTPase, Angiogenesis, hypertrophy, Heart failure, HIF1α-VEGF signaling
Hypertension is one of the most prevalent myocardial stress factors for the development of heart failure. Neovascularization is a compensatory mechanism in the heart in response to hemodynamic stress. The compounding effect of the increased cardiac demand for oxygen and nutrients and responsive cardiac angiogenesis contributes to the transition into pathological adaption, eventually leading to heart failure.1–4 Activation of the cardiac angiogenesis program is a promising therapeutic strategy validated in multiple forms of cardiac disease animal models.5–8 The hypoxia-inducible factor 1α-vascular endothelial growth factor (HIF1α-VEGF) molecular signaling cascade is the most powerful and well-studied signaling pathway in ischemia/hypoxia-induced angiogenesis. Preclinical animal studies using pro-angiogenic factors such as VEGF, HIF1α, and fibroblast growth factor (FGF) have achieved remarkable success.7–12 However, the translation of these animal studies to clinical therapy is intermittent, and the results are far from satisfactory.5, 6, 13, 14 Several important lessons have been demonstrated in clinical trials. One of the challenges in clinical practice is to achieve sustainable tissue exposure to pro-angiogenic factors in order to achieve a prolonged stimulation. One possible solution is through the stabilization of HIF1α, which is unstable and quickly degraded by UPS (ubiquitin proteasome system) under normoxic conditions.5 Another challenge is to develop new patient-relevant animal models for basic research.5 Therefore, identifying a novel factor to stabilize key pro-angiogenic factors as well as establishing a new animal model closely related to human heart failure are critical strategies for the resolution of the serious deficiency in currently available information pertaining to translational studies of angiogenesis involvement in heart failure.
Our recent study discovered that Rnd3, a small Rho GTPase also called RhoE, was a cardiac protective factor and protected the heart from pressure overload-induced heart failure. Mice with Rnd3 haploinsufficiency developed severe heart failure after pressure overload challenge.15 However, the associated molecular mechanism remains largely unknown. In this study, we demonstrated Rnd3 as an important proactive factor for adaptive myocardial angiogenesis in response to pressure overload. We revealed the molecular mechanism of Rnd3-mediated angiogenesis regulation through the stabilization of HIF1α, a key pro-angiogenic transcriptional factor. The findings uncovered a new function of this small GTPase in the refinement of the HIF1α-VEGFA regulatory pathway, and provided a potential new target for pharmacological manipulation of HIF1α-VEGF signaling. The assessment of Rnd3 expression levels in patients could be a new reference biomarker for human heart failure.
In summary, this study uncovered a pro-angiogenic function of Rnd3 in the regulation of the HIF1α-VEGFA signaling pathway. The decrease in Rnd3 expression levels contributed to the insufficiency of adaptive coronary angiogenesis in the mouse heart in response to hemodynamic stress. The discovery of a significant downregulation in Rnd3 levels in myocardia of heart failure patients implicated the translational and clinical significance of these findings.
Methods
Methods and associated references were provided in detail in the online supplement of the paper, which included the approval of animal protocol and human heart tissue usage, generation of the mutant mouse lines and assessments of cardiac function and angiogenesis.
Statistics
Data are expressed means ± s.d. In multiple group comparisons, one-way ANOVA followed by Student-Newman-Keuls method was used. In two group comparisons, unpaired, two-tailed student’s t test was used. All of the analyses were conducted by SigmaPlot 11.0 (Systat, San Jose, CA, USA). A value of P<0.05 was considered statistically significant.
Results
The expression of Rnd3 was downregulated in end-stage human failing hearts
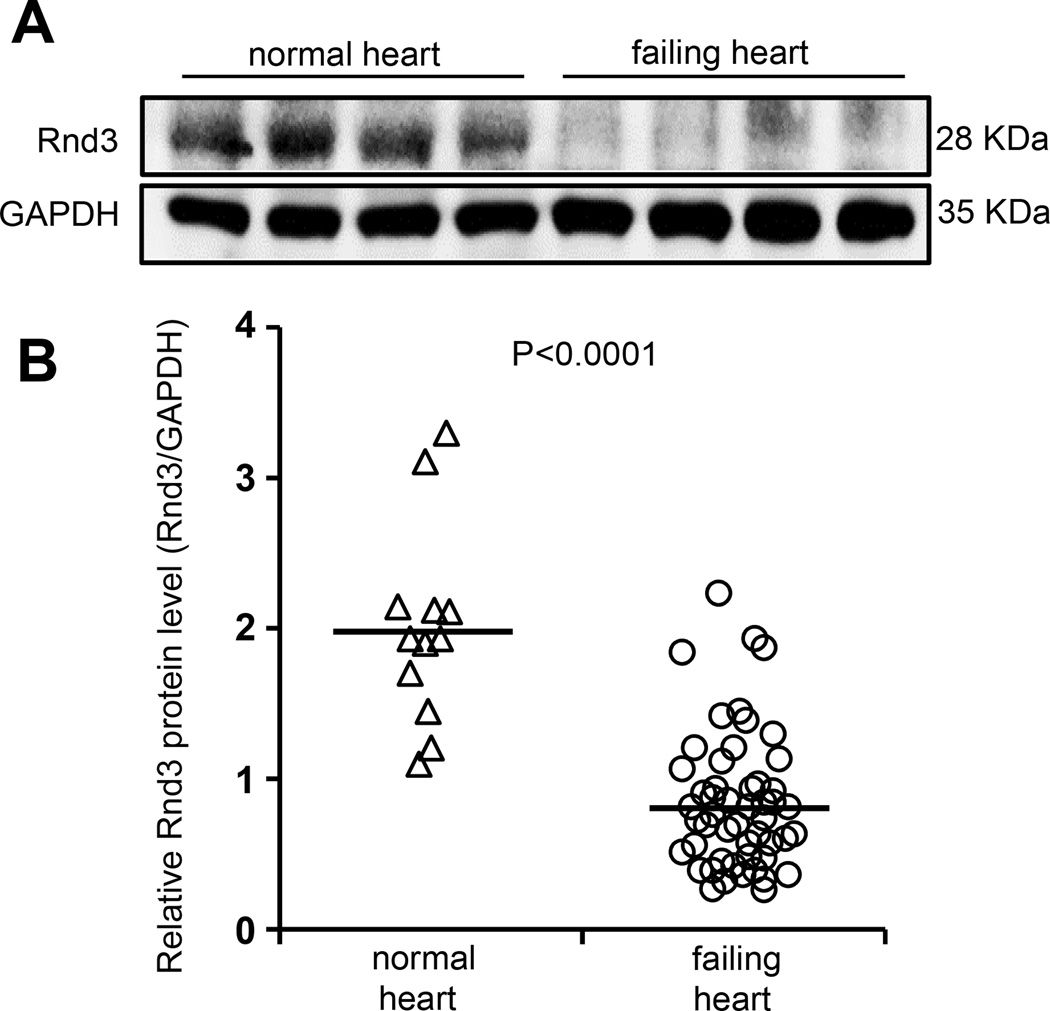
The study of the role of Rnd3 in heart failure was initiated by analyzing human heart tissue samples. Rnd3 protein levels were measured in 12 normal and 51 human failing hearts. In patients with end-stage heart failure, we found a 57.9% decrease in the Rnd3 expression levels (Fig. 1). This clinical observation suggested that Rnd3 may act as a potential new regulator in the etiology of the transition to heart failure. Systemic and comprehensive experiments based on genetic Rnd3 knockout mice were conducted for the understanding of the underlying molecular mechanisms.
Figure 1. Rnd3 protein levels were decreased in human failing hearts.
(a) Representative Rnd3 protein expression by Western blot analysis. (b) Quantification of Rnd3 protein expression levels from 12 normal and 51 failing myocardia.
Rnd3 deficiency resulted in an angiogenesis defect in the mouse heart in response to pressure overload
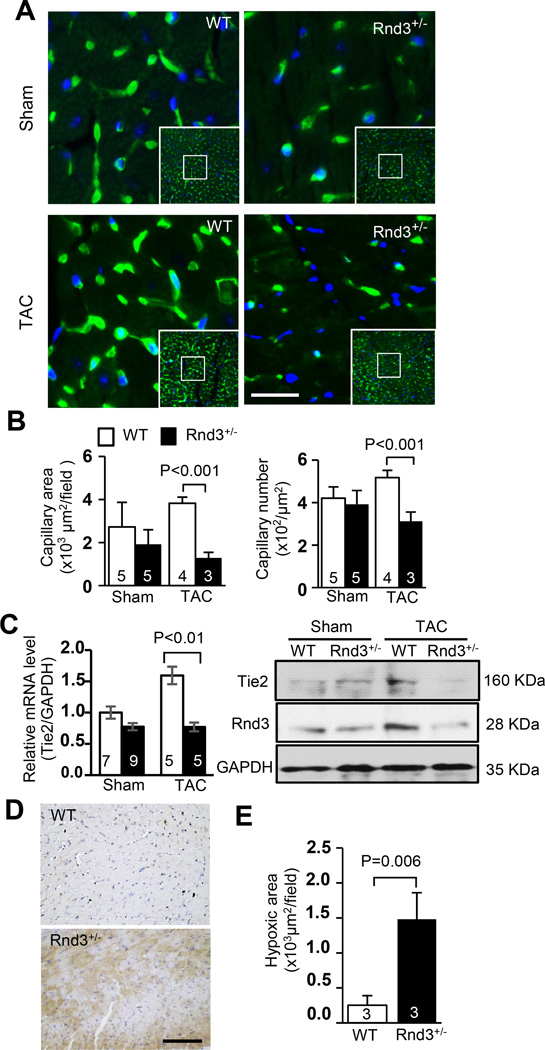
Our recent study found that Rnd3 haploinsufficient (Rnd3+/−) mice were hypersensitive to pressure overload and developed heart failure after transverse aortic constriction (TAC) challenge.15 However, the severity of cardiac fibrosis and hypertrophy showed no significant differences between the wild-type mice and Rnd3 mutant mice (supplemental figure S1). To investigate the underlying molecular mechanisms, we conducted a series of experiments. We first analyzed cardiac capillary generation in the mouse hearts after TAC. Compensatory increases in the capillary area and capillary number were found in WT mice in response to TAC stress as expected. However, we failed to detect the responsive angiogenesis in Rnd3+/− mice (Fig. 2a–2b). Significantly smaller capillary areas and fewer capillary numbers were observed in the Rnd3 haploinsufficient mouse heart compared to the WT control after TAC (Fig. 2a–2b). The impaired angiogenesis was further indicated by the decrease in capillary/cardiomyocyte ratio (supplemental figure S2). In line with the morphological changes, the upregulation of endothelial-specific receptor tyrosine kinase 2 (Tie2) was detected in the WT mouse heart in response to TAC but not in the Rnd3+/− heart (Fig. 2c). To assess coronary microvascular circulation, CFR was measured. Rnd3+/− hearts displayed no abnormality in the microvascular function compared with WT hearts prior to stress (supplemental figure S3, Sham), however, a 20.8% reduction of CFR was detected in the Rnd3+/− heart after TAC (supplemental figure S3, TAC). Cardiac tissue hypoxia staining displayed a 5.9-fold increase in hypoxic areas in the Rnd3+/− heart compared to the WT control heart (Fig. 2d–2e). These data strongly suggested that the adaptive coronary angiogenesis was severely impaired in the Rnd3+/− heart in response to hemodynamic challenge.
Figure 2. Rnd3 deficiency resulted in an angiogenesis defect in the mouse heart in response to TAC.
(a) Fewer capillaries were observed in the post-TAC Rnd3+/− heart by isolectin staining shown in green. Blue indicated nuclear DAPI staining. Scale bar represents 12.5 µm. (b) Cardiac capillaries were quantified by LAS V4.0 software (Germany). Smaller capillary areas and fewer capillary numbers were observed in the Rnd3+/− mouse heart compared to the WT control after TAC. (c) The responsive increases in angiogenic marker Tie2 mRNA and protein levels were attenuated in the post-TAC Rnd3+/− heart. (d) Larger hypoxic areas were detected in the Rnd3+/− heart compared to the WT heart after TAC. Hypoxyprobe™-1 staining (brown) showed hypoxic myocardium. Scale bar represents 50 µm. (e) Quantification of hypoxic areas by LAS 4.0 software (Germany). The numbers in the columns represented the number of mice in each group.
Rnd3 deficiency led to the downregulation of key pro-angiogenic factors, HIF1α and VEGFA, in the mouse heart in response to pressure overload
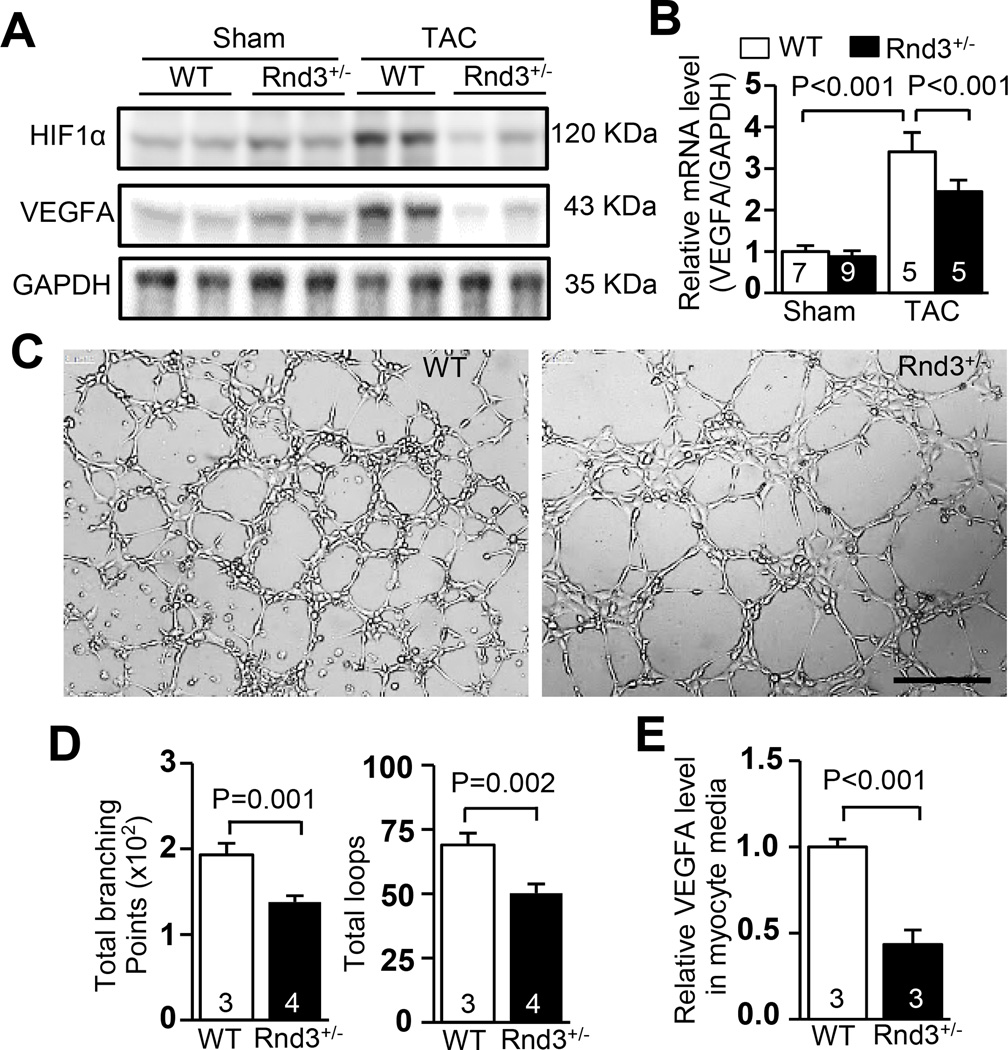
HIF1α and its downstream target VEGFA are critical for responsive angiogenesis in the heart after stress. These factors were evaluated in order to better understand the effect of Rnd3 deficiency in angiogenesis. As expected, the responsive increase in levels of HIF1α and VEGFA proteins was detected in WT animal hearts after TAC (Fig. 3a). However, the responsive increase in levels of HIF1α and VEGFA proteins was not detected in Rnd3 deficient hearts after pressure overload (Fig. 3a), which was consistent with the observation of impaired angiogenesis. Since VEGFA was transcriptionally regulated by transcription factor HIF1α, we measured VEGFA transcripts in the hearts. Again, the responsive increase in VEGFA by TAC was attenuated by Rnd3 deficiency (Fig. 3b).
Figure 3. HIF1α-VEGFA pathway was downregulated in the Rnd3+/− heart in response to TAC.
(a) Downregulation of HIF1α and VEGFA protein in the Rnd3+/− heart in response to TAC was shown by Western blot analysis. (b) The responsive increase in VEGFA mRNA levels was significantly attenuated in the Rnd3+/− heart after TAC. (c) HUVEC cell tube formation was promoted by the media from the WT cardiomyocytes, while fewer tubes were developed in the culture media from the Rnd3+/− cardiomyocytes. Scale bar represents 500 µm. (d) The tube formation was quantified by WimTube software. (e) Cardiomyocytes from the Rnd3+/− heart released less VEGFA compared to control WT cardiomyocytes, indicating weak VEGFA paracrine activity. The numbers in the columns represented the number of mice in each group.
Cardiomyocytes are the major source of VEGFA as a paracrine effector for angiogenesis in the heart.16, 17 To investigate the role of Rnd3 in relation with pro-angiogenic factors, we conducted HUVEC cell tube formation experiments by treating the cells with different cardiomyocyte culture media. We found that fewer tubes were developed in the culture media from Rnd3+/− cardiomyocytes, while the tube formation was stimulated by the media from the WT cardiomyocytes (Fig. 3c–3d). Significantly lower levels of VEGFA were detected in the Rnd3+/− cardiomyocyte culture media compared with the WT group, indicating downregulation of the HIF1α-VEGFA pathway in cardiomyocytes with Rnd3 deficiency (Fig. 3e).
Rnd3 stabilized HIF1α protein and facilitated VEGFA expression
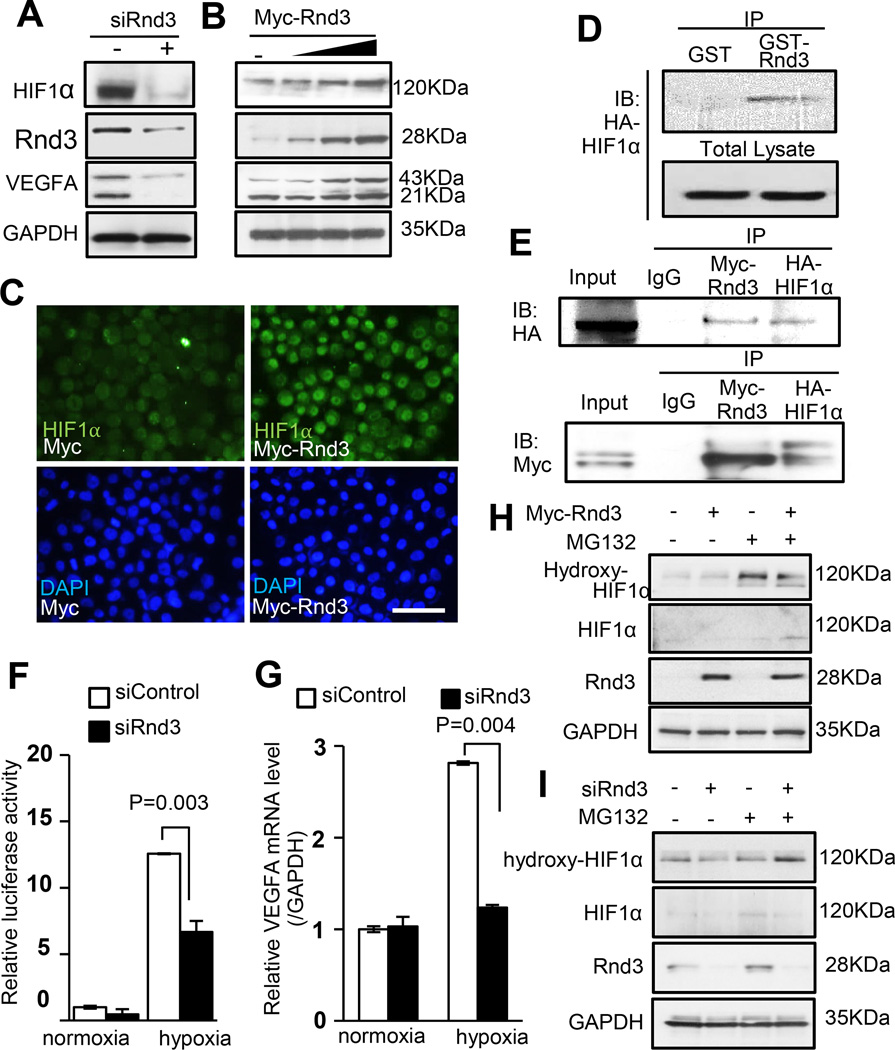
To investigate if HIF1α protein levels could be directly regulated by Rnd3, we knocked down and overexpressed Rnd3 in cell cultures. We found that HIF1α protein levels were decreased when Rnd3 was knocked down (Fig. 4a). Meanwhile, gradual increases in the expression levels of HIF1α were detected corresponding with incremental increases in Rnd3 expression levels (Fig. 4b), and obvious nuclear accumulation of HIF1α protein was also observed (Fig. 4c). Consistent with the change in HIF1α protein levels, we detected increased levels of VEGFA protein when Rnd3 was overexpressed (Fig. 4b), and the opposite trend was detected when Rnd3 was knocked down (Fig. 4a). To further explore the potential mechanism involving the Rnd3-induced HIF1α stability, we conducted an in vitro GST-Rnd3 pull-down assay from cell lysates. As shown in Fig. 4d, Rnd3 protein bound to HIF1α protein, suggesting physical interaction between the two proteins. We then performed mutual coimmunoprecipitation assays to further illustrate the physical interaction of Myc-Rnd3 and HA-HIF1α in vivo (Fig. 4e). Next, since HIF1α is a transcription factor, we conducted a luciferase experiment in order to determine if Rnd3 had any effects on its transcriptional activity. The luciferase reporter was driven by a VEGFA promoter containing HIF1 response elements (HREs). Hypoxic conditions were applied to induce HIF1α expression. Relative luciferase activity was measured under both normoxic and hypoxic conditions. Rnd3 knockdown resulted in an approximate 50% decline in luciferase activity relative to the control level in response to hypoxia (Fig. 4f). Consistent with the change in the HIF1α transcriptional activity, a responsive decrease in the VEGFA mRNA levels was detected as well (Fig. 4g), indicating that Rnd3 regulated responsive HIF1α transcriptional activity. Downregulation of Rnd3 weakened HIF1α transcriptional activity, leading to a decrease in its target gene transcripts in response to hypoxia.
Figure 4. Rnd3 bound to HIF1α, prevented HIF1α protein from hydroxylation and stabilized the protein, which in turn promoted VEGFA expression.
(a) Downregulation of Rnd3 resulted in lower HIF1α and VEGFA protein expression. (b) The incremental increases in the expression levels of HIF1α and VEGFA protein were observed when Rnd3 was gradually overexpressed. (c) HIF1α nuclear accumulation was displayed when cells overexpressed Rnd3. Scale bar represents 50 µm. (d) HIF1α was pulled down by GST-Rnd3 protein in vitro. (e) Myc-Rnd3 physically interacted with HA-HIF1α in vivo evidenced by mutual coimmunoprecipitations. (f) Rnd3 deficiency weakened HIF1α transcriptional activity demonstrated by luciferase assays. (g) The qPCR analysis showed that VEGFA mRNA levels did not increase in the cells with Rnd3 deficiency in response to hypoxia. (h) Rnd3 overexpression in cells reduced hydroxylation of HIF1α. (i) Knockdown of Rnd3 led to an increase in Hydroxy-HIF1α levels. MG132: a proteasome inhibitor.
Hydroxylation of HIF1α at two conserved proline residues by HIF1α prolyl hydroxylases is the key step to control HIF1α protein levels. Upon hydroxylation of HIF1α, it interacts with von-Hippel Lindau tumor-suppressor protein (VHL), therefore leading to HIF1α protein ubiquitination and degradation under normoxic conditions. We next evaluated the effects of Rnd3 on HIF1α hydroxylation. Since hydroxylated HIF1α degradation is dynamic and extremely fast, cell cultures with Rnd3 overexpression (Myc-Rnd3) and Rnd3 knockdown by siRNA (siRnd3) were first treated with MG132, a cell-permeable proteasome inhibitor, to block degradation of ubiquitin-conjugated proteins. Then hydroxylated HIF1α (Hydroxy-HIF1α) levels were analyzed by Western blot. Lower levels of Hydroxy-HIF1α were detected in cells with Rnd3-overexpression (Fig. 4h). Consistently, increased Hydroxy-HIF1α levels were observed under Rnd3 knockdown conditions (Fig. 4i). These data strengthened our hypothesis and suggested that Rnd3 stabilized HIF1α by preventing its hydroxylation.
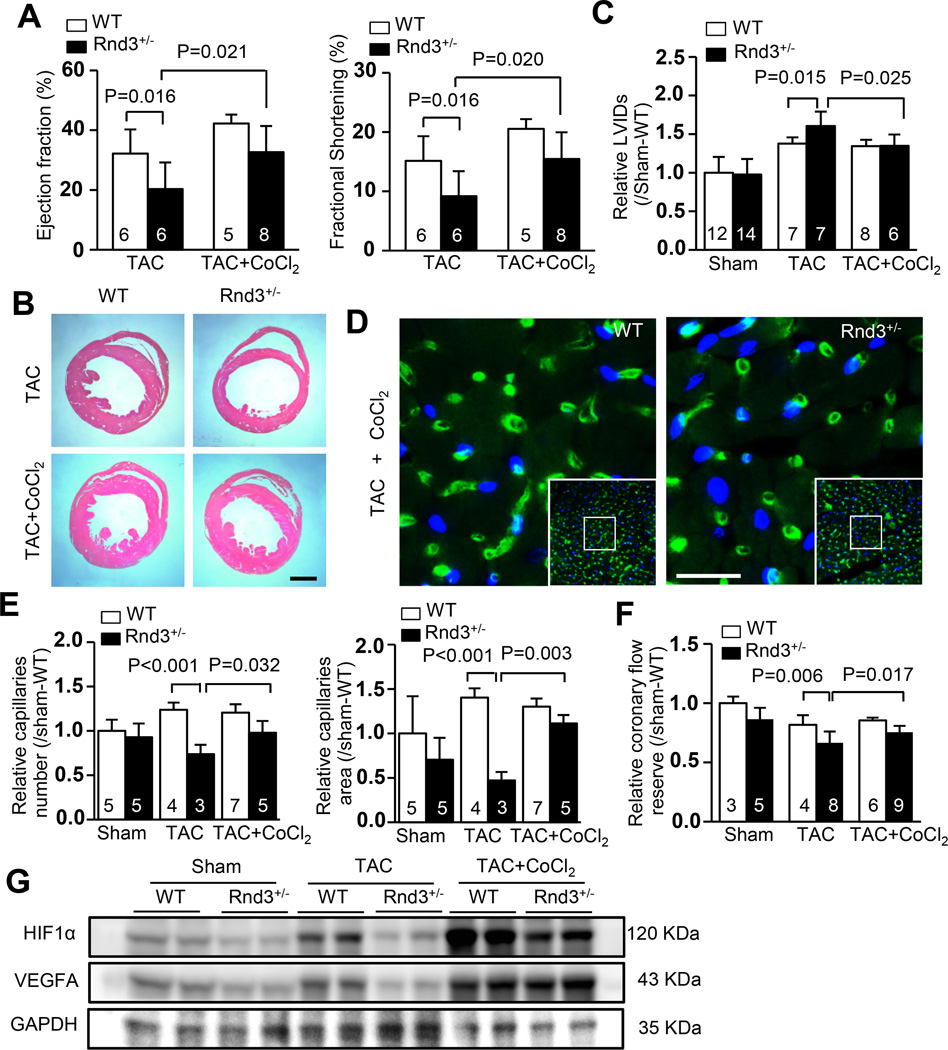
CoCl2 treatment rescued Rnd3 deficiency-mediated angiogenesis defects and dilated cardiomyopathy in Rnd3 haploinsufficient mice after hemodynamic stress
CoCl2 is an inhibitor of prolyl hydroxylase domain-containing (PHD) protein, which is responsible for the hydroxylation of HIF1α, promoting its eventual degradation. Application of CoCl2 prevents HIF1α protein from degradation. To test if HIF1α downregulation was responsible for the Rnd3 deficiency-induced cardiac dysfunction and angiogenic defects, we treated mice with CoCl2. Improvement in cardiac function was observed in Rnd3+/− mice after the administration of CoCl2. Echocardiographic assessment revealed partial recoveries in the ejection fractions and fractional shortenings of Rnd3+/− mice with CoCl2 treatment compared to the mice without CoCl2 treatment (Fig. 5a). Consistent with the improved cardiac function, H&E staining (Fig. 5b) and cardiac echo analysis (Fig. 5c) both displayed less dilation in Rnd3+/− hearts with CoCl2 treatment compared to the non-treated group of animal hearts. The post-TAC survival rate of Rnd3+/− mice was also significantly improved by CoCl2 treatment (supplemental figure S4).
Figure 5. Rnd3 deficiency-mediated angiogenesis defect and dilated cardiomyopathy were attenuated by CoCl2 admission.
(a) The repressed ejection fractions and fractional shortenings of the Rnd3+/− mice after TAC were significantly improved by CoCl2 treatment assessed by echocardiography. Along with the improved cardiac function, the enlarged ventricle chamber phenotype was lessened; displayed by the heart sections with H&E staining (b), and confirmed by echocardiography analysis (c). Scale bar represents 1 mm. (d) Isolectin staining showed an increased number of capillaries (green) in the post-TAC Rnd3+/− heart after CoCl2 treatment. Scale bar represents 12.5 µm. (e) The improvement of capillary density was quantified (LAS V4.0 software, Germany) and presented as capillary number and capillary area. Nuclear DAPI staining was shown in blue. (f) The reduced coronary flow reserve in the Rnd3+/− mice after TAC was recovered by CoCl2 administration. (g) Immunoblots revealed increased HIF1α and VEGFA protein expression levels after CoCl2 treatment in both Rnd3+/− and WT mouse hearts. LVIDs: left ventricular internal dimension in systole. The numbers in the columns represented the number of mice in each group.
Finally, we analyzed protein expression levels of HIF1α and VEGFA, along with cardiac capillaries and cardiac CFR without CoCl2 treatment in both WT and Rnd3+/− mouse hearts. As expected, TAC stress induced the upregulation of HIF1α and VEGFA in WT animal hearts; however, the Rnd3 haploinsufficient mice failed to respond to pressure overload and displayed no increase in HIF1α and VEGFA protein levels. The treatment of CoCl2 partially recovered the adaptive response to TAC in the Rnd3+/− animal with obvious increases in HIF1α and VEGFA protein levels (Fig. 5g). Isolectin staining showed improved capillary densities after CoCl2 applications (Fig. 5d–5e). The impaired coronary flow reserve after TAC was recovered by 13.6% due to CoCl2 administration (Fig. 5f).
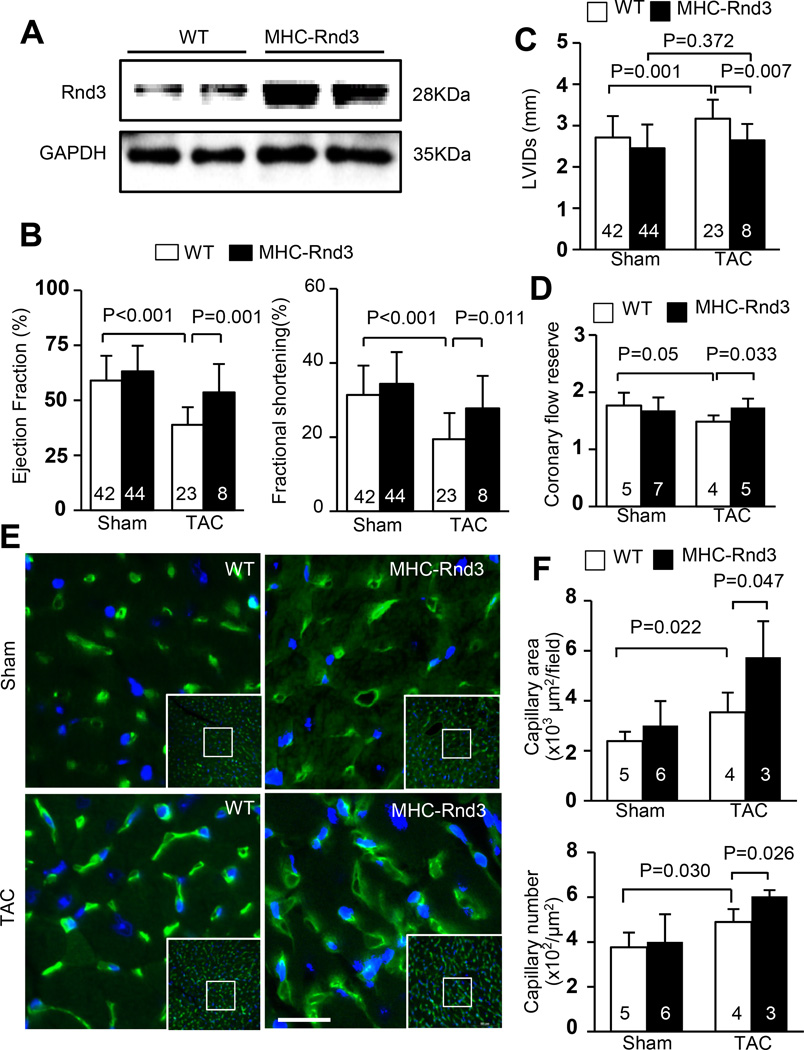
Rnd3 overexpression attenuated the transition to heart failure by preserving responsive angiogenesis
We generated the transgenic Rnd3 overexpression mice (MHC-Rnd3) to further demonstrate the critical role of Rnd3 in angiogenesis and the hypoxic response of the heart. Elevated cardiac Rnd3 protein levels in the MHC-Rnd3 mice were confirmed by Western blot analysis (Fig. 6a). MHC-Rnd3 mice exhibited reserved cardiac function and were more resistant to the development of heart failure after TAC stress compared to the WT mice. In comparison to the WT mice from the Sham group, MHC-Rnd3 mice showed only slight decreases in the ejection fraction and fractional shortening percentages after TAC (Fig. 6b). Furthermore, the MHC-Rnd3 mice displayed a conserved LVID in systole detected by echocardiography compared to the WT mice after TAC surgery, indicating a resistance to the development of DCM due to Rnd3 overexpression (Fig. 6c). Higher coronary flow reserves were measured in the MHC-Rnd3 mice compared to the WT mice after TAC and showed no significant differences compared to the measurements from the WT Sham group, demonstrating preserved capillary function (Fig. 6d). In addition, more cardiac capillaries and larger capillary areas were visualized in the MHC-Rnd3 mice after TAC compared to the WT controls (Fig. 6e–6f). These findings suggested that Rnd3 upregulation can prevent the transition to heart failure, and Rnd3 overexpression had multiple beneficial effects under cardiac hypoxic conditions.
Figure 6. Cardiac specific overexpression of Rnd3 protected the heart from the transition to heart failure by preserving responsive angiogenesis.
(a) Representative Rnd3 protein expression levels in the hearts of WT and Rnd3 transgenic (MHC-Rnd3) mice by Western blot analysis. (b) MHC-Rnd3 mice have reserved cardiac function and developed less severe heart failure after TAC. Only slight decreases in the cardiac ejection fractions and fractional shortenings were detected in MHC-Rnd3 mice after TAC compared to WT mice from the Sham group. (c) No significant increase in the LVID in systole was detected by echocardiography in MHC-Rnd3 mice after TAC compared to the Sham WT mice. (d) MHC-Rnd3 mice have preserved capillary function after TAC compared to WT mice. (e) More capillaries were observed in the post-TAC MHC-Rnd3 mouse heart by isolectin staining (green). (f) Larger capillary areas and more capillary numbers were observed in the MHC-Rnd3 mouse heart compared to the WT control after TAC. Cardiac capillaries were quantified by LAS V4.0 software (Germany). The numbers in the columns represented the number of mice in each group.
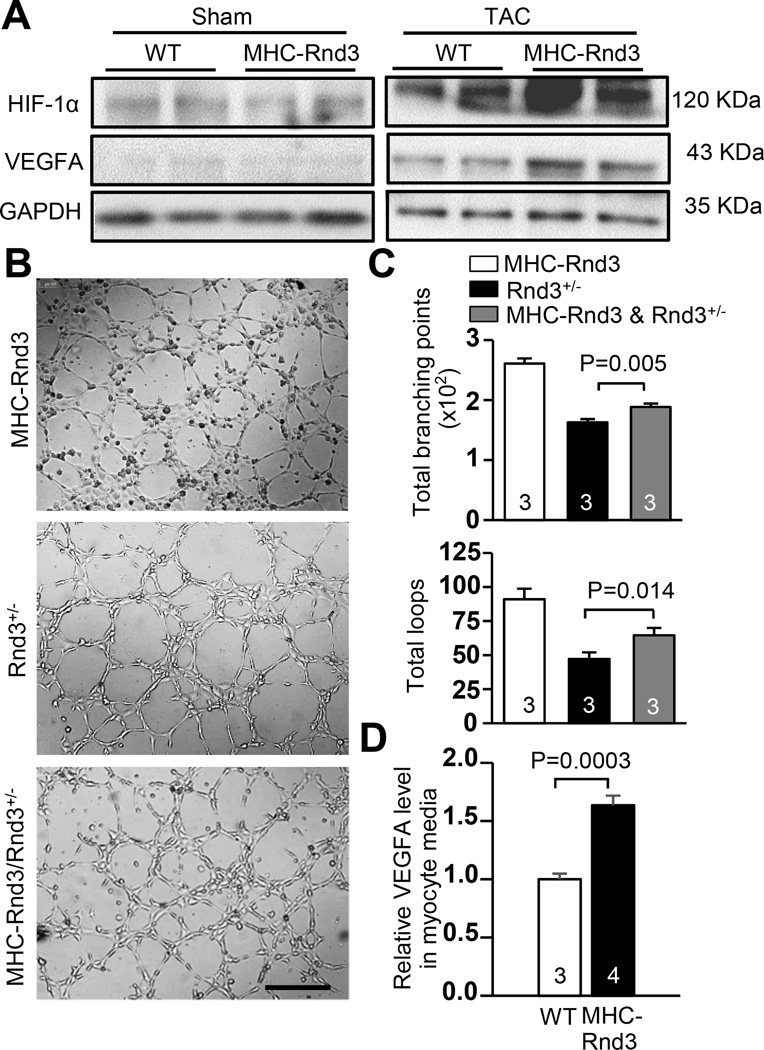
MHC-Rnd3 cardiomyocytes exhibited HIF1α-VEGFA pathway stimulation
Elevated HIF1α and VEGFA protein levels were observed, as expected, in the control WT mouse heart in response to TAC by Western blot. However, further increases in these two proteins were detected in the MHC-Rnd3 transgenic mouse heart (Fig. 7a). In order to determine the angiogenic effect of Rnd3 overexpression in the animal cardiomyocytes, HUVEC cells were treated with the media from Rnd3+/− and MHC-Rnd3 cardiomyocyte cultures. A significant promotion of HUVEC cell tube formation was observed in the culture treated with media from the MHC-Rnd3 cardiomyocytes. In addition, the previously observed tube formation deficiency of the cells cultured with Rnd3+/− cardiomyocyte media was partially rescued (Fig. 7b). The cell tube formation was quantified and analyzed by WimTube software, revealing more loops and branching points in the MHC-Rnd3 culture. Significant improvement in the total number of loops and branching points in the MHC-Rnd3/Rnd3+/− co-culture was also observed (Fig. 7c). Lastly, VEGFA levels in the media from MHC-Rnd3 and WT cardiomyocyte cultures were measured by ELISA. MHC-Rnd3 cardiomyocyte media revealed significantly higher VEGFA levels compared to the WT control (Fig. 7d). Taken together, cardiomyocytes from the MHC-Rnd3 mouse heart released more VEGFA compared to the WT control, indicating increased VEGFA paracrine activity and augmented angiogenesis.
Figure 7. HIF1α-VEGFA signaling was enhanced in MHC-Rnd3 cardiomyocytes.
(a) Upregulation of HIF1α and VEGFA protein levels in the MHC-Rnd3 mouse heart in response to TAC was shown by Western blot analysis. (b) HUVEC cell tube formation was highly promoted by the media from the MHC-Rnd3 cardiomyocytes. The resulting tube formation deficiency from culturing with the Rnd3+/− cardiomyocyte media was partially rescued when co-cultured with the MHC-Rnd3 cardiomyocyte media. Scale bar represents 500 µm. (c) The tube formation was quantified by WimTube software. (d) Cardiomyocytes from the MHC-Rnd3 heart released more VEGFA compared to the control WT cardiomyocytes, indicating strong VEGFA paracrine activity. The numbers in the columns represented the number of mice in each group.
A schematic illustration of the molecular mechanism involving Rnd3 and HIF1α-VEGFA signaling was depicted in supplemental figure S5. Rnd3 prevented HIF1α from hydroxylation by stabilizing it through direct interaction, which then led to increased activation of the VEGF gene. Without the presence of Rnd3, increased HIF1α hydroxylation occurred, which facilitated HIF1α protein degradation through UPS and resulted in impaired angiogenesis.
Discussion
The HIF1α-VEGFA signaling pathway is the best studied molecular mechanism in the regulation of hypoxia/ischemia-induced angiogenesis.18 VEGFA, mainly secreted by cardiomyocytes in the heart,16 is the major driving force promoting endothelial cell proliferation during angiogenesis. HIF1 works as an αβ-heterodimer that induces transcriptional activation by binding to hypoxia response elements (HREs) in response to hypoxia. HIF1β is a constitutive nuclear protein while HIF1α is dynamically regulated. In normoxic conditions, HIF1α can be hydroxylated by PHD, and then quickly degraded by von Hippel-Lindau E3 ubiquitin ligase complex (pVHL). In the lack of oxygen, the hydroxylation of HIF1α is blocked, which prevents HIF1α from pVHL-mediated proteolysis and results in an accumulation of HIF1α protein. The latter translocates into the nuclei to form a transcriptional complex with HIF1β, p300, and CREB binding protein (CBP), and initiates the transcription of a number of genes including the VEGF family of genes (Figure S5).18 In this study, we demonstrated that Rnd3 functioned as a new mediator in the angiogenesis process by interacting with and stabilizing HIF1α, adding a new dimension to the regulation of the HIF complex function, which secured HIF1α-VEGFA-mediated angiogenesis progression during stress (Figure S5). Deficiency of Rnd3 compromised the HIF1α-VEGFA signaling and impaired the responsive angiogenesis. Application of CoCl2, a chemical that inhibits PHD and stabilizes HIF1α,19 partially rescued the Rnd3 haploinsufficient mouse phenotype and resulted in improvement of cardiac functions and CFR (Figure S5).
Since drugs can have pleiotropic effects in vivo, we verified the critical role of Rnd3 in the heart through the use of the transgenic Rnd3 overexpression mice. Forced-expression of Rnd3 partially rescued the Rnd3+/− angiogenic defect both in vitro and in vivo. Interestingly, a recent study showed Rnd3 as a transcriptional target of HIF1α in gastric cancer cells.20 If the same regulation existed in the heart, there would be a forward feedback mechanism in which Rnd3 would stabilize HIF1α and the latter would enhance Rnd3 gene transcription.
Pathological stimuli such as hypertension, ischemia, or chronic neurohormonal hyperactivation are known to promote capillary growth within the myocardium.21–23 However, the compensatory enhancement of angiogenesis in response to these pathological stimuli appears to be insufficient, and this insufficiency is one of the critical factors responsible for the transition of the heart from compensated hypertrophy to heart failure.1–4
In this study, we revealed a critical role of Rnd3 in the responsive angiogenesis process in the heart. We detected a 57.9% reduction of Rnd3 protein levels in end-stage human failing hearts. While the etiologic meaning of Rnd3 downregulation in heart failure patients remains unknown, understanding the pathological consequence of Rnd3 downregulation is clearly important and has clinical implications. The Rnd3 haploinsufficient mouse recapitulated the situation observed in heart failure patients, and is a patient-relevant mouse model. Importantly, the mutant mice displayed impaired adaptive coronary angiogenesis that has not previously been linked to Rnd3 function. Given the critical role of HIF1α in pro-angiogenic signaling, identification of its endogenous stabilizer, Rnd3, offers a new positive regulator toward to the HIF1α-VEGFA signaling pathway. The findings have basic and clinical significance. The Rnd3 mutant mice provide a patient-relevant animal model for further mechanistic investigation.
Rnd3 was originally identified as a repressor of Rho signaling by either directly binding to Rho-associated coiled-coil protein kinase 1 (ROCK1) or indirectly targeting RhoA through p190-B RhoGAP.24–27 The importance of Rho kinase ROCK 1 in the heart has been investigated by our lab as well as by other groups.28–32 We demonstrated that ROCK1-null mice were resistant to pressure overload-induced injury and fibrotic response.30 In human patients, we first found that ROCK1 was a caspase-3 target. The cleavage of ROCK1 by caspase-3 generated a constitutively active isoform of Rho kinase fragment that was accumulated in human failing hearts.28 The transgenic mice with forced expression of this cleaved ROCK1 isoform led to fibrotic cardiomyopathy. Treatment with Rho kinase inhibitor reversed the cardiac phenotype and improved cardiac functions.29 Therefore, it wa logical to assume that the Rnd3 deficiency-mediated cardiac dysfunction was through the augmentation of ROCK1 activity. However, by taking the approaches of genetic deletion of ROCK1 and the utilization of a Rho kinase inhibitor, we recently found that Rho kinase activation was partially, but not entirely, responsible for Rnd3 deficiency-induced cardiomyopathy, suggesting that there was a Rho kinase-independent mechanism regulated by Rnd3.15
Perspectives
This study extended the previous observation and uncovered a new function of Rnd3, in which Rnd3 participated in the regulation of responsive angiogenesis. The downregulation of Rnd3 resulted in an angiogenic response defect in the animal heart, contributing to the transition to heart failure. The associated molecular mechanism was conceived that Rnd3 bound to HIF1α, and stabilized HIF1α through the inhibition of its hydroxylation and prevented the protein from degradation. Downregulation of Rnd3 resulted in an increase in HIF1α hydroxylation and a subsequent decrease in its protein levels; which in turn impaired HIF-VEGF signaling and attenuated responsive angiogenesis progression. The findings uncovered a new function of this small GTPase in the refinement of the HIF1α-VEGFA regulatory pathway, and provided a potentially new target of pharmacological manipulation for HIF1α-VEGF signaling. The assessment of Rnd3 expression levels in patients could be a new reference biomarker for human heart failure.
Supplementary Material
Novelty and Significance.
1) What is New?
This study uncovered previously undescribed function of Rnd3 and revealed the associated molecular mechanism, which Rnd3 was as a novel pro-angiogenic factor involved in cardiac responsive angiogenesis through HIF1α-VEGFA signaling promotion. A significant decrease level of Rnd3 protein was detected in human failing myocardia. Genetic deletion of Rnd3 in mice impaired angiogenic response in heart in response to pressure overload.
2) What is Relevant?
Neovascularization is a compensatory mechanism in heart in response to hypertension. The insufficiency of compensatory angiogenesis in heart in hypertension patients contributes to the transition to heart failure.
3) Summary
This study demonstrated a pro-angiogenic role of Rnd3 through the regulation of the HIF1α-VEGFA signaling pathway. The decrease in Rnd3 expression levels contributed to the insufficiency of adaptive coronary angiogenesis in the mouse heart in response to pressure overload. The finding of the significant downregulation of Rnd3 in myocardia of heart failure patients implicated the translational and clinical significance of this discovery.
Acknowledgments
Sources of Funding
This study was supported by the following funding sources: the China Scholarship Council (X. Yue); the American Heart Association Postdoctoral Fellowship 13POST17260043 (X.Yang.); the National Natural Science Foundation of China 81460070 (T.L.), 1160020 and 81460042 (J.G.); the American Heart Association Grant-in-Aid 205000033, the NIH-NHLBI R01HL102314, the NIH-NHLBI R01HL123953, and the NIH-K02HL098956 (J.C.).
Footnotes
Disclosure
None.
References
- 1.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorn GW., 2nd Myocardial angiogenesis: Its absence makes the growing heart founder. Cell Metab. 2007;5:326–327. doi: 10.1016/j.cmet.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. P53-induced inhibition of hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 4.Chintalgattu V, Ai D, Langley RR, Zhang J, Bankson JA, Shih TL, Reddy AK, Coombes KR, Daher IN, Pati S, Patel SS, Pocius JS, Taffet GE, Buja LM, Entman ML, Khakoo AY. Cardiomyocyte pdgfr-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest. 2010;120:472–484. doi: 10.1172/JCI39434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 6.Simons M. Angiogenesis: Where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 7.Dor Y, Djonov V, Abramovitch R, Itin A, Fishman GI, Carmeliet P, Goelman G, Keshet E. Conditional switching of vegf provides new insights into adult neovascularization and pro-angiogenic therapy. Embo J. 2002;21:1939–1947. doi: 10.1093/emboj/21.8.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harada K, Friedman M, Lopez JJ, Wang SY, Li J, Prasad PV, Pearlman JD, Edelman ER, Sellke FW, Simons M. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am J Physiol. 1996;270:H1791–H1802. doi: 10.1152/ajpheart.1996.270.5.H1791. [DOI] [PubMed] [Google Scholar]
- 9.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of pdgf-bb and fgf-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 10.Post MJ, Laham R, Sellke FW, Simons M. Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc Res. 2001;49:522–531. doi: 10.1016/s0008-6363(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 11.Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C, Goldberg MA, Akita GY, Gregory RJ, Isner JM. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an hif-1alpha/vp16 hybrid transcription factor. Circulation. 2000;102:2255–2261. doi: 10.1161/01.cir.102.18.2255. [DOI] [PubMed] [Google Scholar]
- 12.Heineke J, Auger-Messier M, Xu J, Oka T, Sargent MA, York A, Klevitsky R, Vaikunth S, Duncan SA, Aronow BJ, Robbins J, Crombleholme TM, Molkentin JD. Cardiomyocyte gata4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochain C, Channon KM, Silvestre JS. Angiogenesis in the infarcted myocardium. Antioxid Redox Signal. 2013;18:1100–1113. doi: 10.1089/ars.2012.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, Laham RJ, Li W, Pike M, Sellke FW, Stegmann TJ, Udelson JE, Rosengart TK. Clinical trials in coronary angiogenesis: Issues, problems, consensus: An expert panel summary. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 15.Yue X, Yang X, Lin X, Yang T, Yi X, Dai Y, Guo J, Li T, Shi J, Wei L, Fan GC, Chen C, Chang J. Rnd3 haploinsufficient mice are predisposed to hemodynamic stress and develop apoptotic cardiomyopathy with heart failure. Cell Death Dis. 2014;5:e1284. doi: 10.1038/cddis.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, Gu Y, Nath AK, Huang Y, Hickey R, Dalton N, Peterson KL, Ross J, Jr, Chien KR, Ferrara N. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. Proc Natl Acad Sci U S A. 2001;98:5780–5785. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedito R, Hellstrom M. Notch as a hub for signaling in angiogenesis. Exp Cell Res. 2013;319:1281–1288. doi: 10.1016/j.yexcr.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the hif system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 19.Xi L, Taher M, Yin C, Salloum F, Kukreja RC. Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of hif-1alpha and ap-1 and inos signaling. Am J Physiol Heart Circ Physiol. 2004;287:H2369–H2375. doi: 10.1152/ajpheart.00422.2004. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Zhou H, Li Q, Qiu M, Li Z, Tang Q, Liu M, Zhu Y, Huang J, Lang N, Liu Z, Deng Y, Zhang S, Bi F. Epigenetic modification of rhoe expression in gastric cancer cells. Oncol Rep. 2011;25:173–180. [PubMed] [Google Scholar]
- 21.Tomanek RJ, Torry RJ. Growth of the coronary vasculature in hypertrophy: Mechanisms and model dependence. Cellular & molecular biology research. 1994;40:129–136. [PubMed] [Google Scholar]
- 22.Matsunaga T, Warltier DC, Weihrauch DW, Moniz M, Tessmer J, Chilian WM. Ischemia-induced coronary collateral growth is dependent on vascular endothelial growth factor and nitric oxide. Circulation. 2000;102:3098–3103. doi: 10.1161/01.cir.102.25.3098. [DOI] [PubMed] [Google Scholar]
- 23.Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR, Jr, Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol. 2000;35:1654–1660. doi: 10.1016/s0735-1097(00)00594-5. [DOI] [PubMed] [Google Scholar]
- 24.Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. Rhoe binds to rock i and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA, Lee SW. Rhoe is a pro-survival p53 target gene that inhibits rock i-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16:2466–2472. doi: 10.1016/j.cub.2006.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Wennerberg K, Forget MA, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, Der CJ, Hansen SH. Rnd proteins function as rhoa antagonists by activating p190 rhogap. Curr Biol. 2003;13:1106–1115. doi: 10.1016/s0960-9822(03)00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riento K, Villalonga P, Garg R, Ridley A. Function and regulation of rhoe. Biochem Soc Trans. 2005;33:649–651. doi: 10.1042/BST0330649. [DOI] [PubMed] [Google Scholar]
- 28.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of rho-associated coiled-coil protein kinase 1 (rock-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci U S A. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Li Q, Lin X, Ma Y, Yue X, Tao Z, Wang F, McKeehan WL, Wei L, Schwartz RJ, Chang J. Mechanism of fibrotic cardiomyopathy in mice expressing truncated rho-associated coiled-coil protein kinase 1. Faseb J. 2012;26:2105–2116. doi: 10.1096/fj.11-201319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, Wei L. Targeted deletion of rock1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 2006;20:916–925. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
- 31.Del Re DP, Miyamoto S, Brown JH. Rhoa/rho kinase up-regulate bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069–8078. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 32.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in rock1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.