Mice carrying genetic mutations are used with great frequency to determine the role of a specific gene or mutation in skeletal physiology and pathology. The three main models most frequently employed are engineered mutants (knock-outs, transgenics, knock-ins, etc), chemically induced mutations, and spontaneously occurring mutations. These models are largely made or de novo discovered (depending on the type) in inbred strains of mice as opposed to outbred lines. However, for several reasons, it is often necessary to change the background strain of an allele of interest. The most common rationale is that the background strain is undesirable due to some characteristic of the strain, such as breeding performance. Other reasons include experiments where it is necessary to compare two mutations to each other, or to study animals with two alleles present in one animal. In these latter cases, it is necessary to have the mutation(s) on the same genetic background to separate the effects of the allele(s) of interest from the differences caused by the genetic dissimilarities between any two backgrounds.
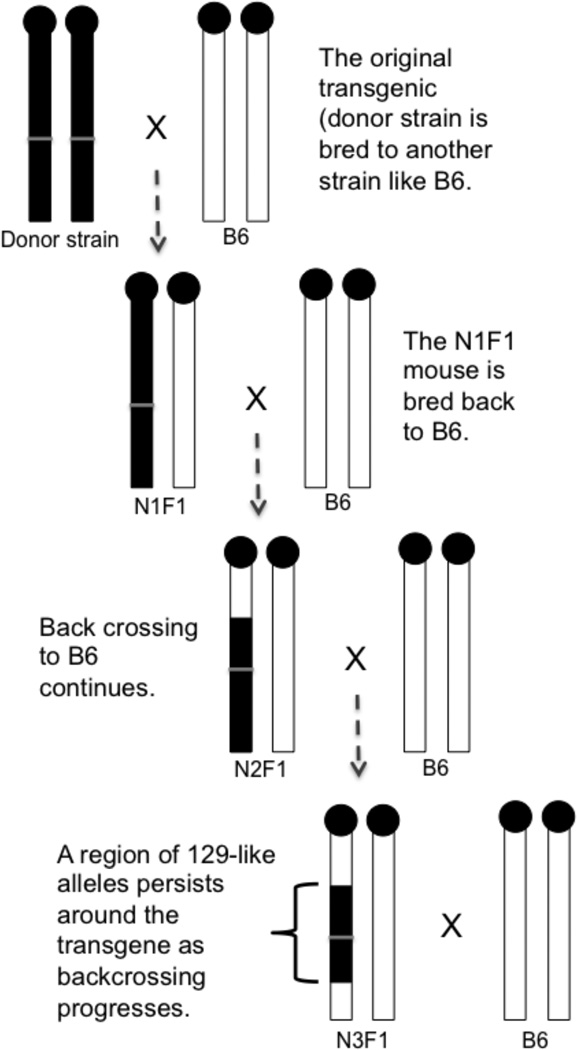
While there are now more choices available with regards to the strain background of the embryonic stem (ES) cells used to make a transgenic such as a traditional knockout made via homologous recombination, this was not always the case. As a result, many transgenic mice were made on a 129 or FVB background, as it is comparatively easy to make transgenic mice on these backgrounds (1, 2). However, 129 mice can be difficult to breed (3), and FVB are undesirable as knockouts as this background is less common, making head to head comparisons between two mutants difficult. Spontaneous mutations are rare random events and could appear at any time, on any background. Thus, it is customary to move a mutation or transgene from the original background onto a “standard” C57BL/6 (B6) strain using a backcross approach (Figure 1). In short, the mutant mouse (donor strain) is bred to a B6 mouse and the offspring carrying the mutation of interest are bred again to B6 mice. These offspring are genotyped and the carriers are bred again to B6 mice, and so on. This process is ideally repeated for 10 generations, as statistically ~99.8% of the strain is now B6-like and there is only contamination of 0.2% from the donor 129 strain (4). This is called a congenic strain and the background history of such a congenic is often reflected in the strain name. For example, the official strain name for the commercially available Sox9 floxed allele is B6.129S7-Sox9tm2Crm/J (www.jax.org) reflecting that this strain was made using ES cells from a 129SvEv strain (5) and that the floxed allele was moved to a B6 background by backcrossing at least 9 times.
Figure 1. Location of passenger genes when making a congenic mouse.
In this cartoon, the alleles of the donor strain, 129 in this case, are shown in black and the alleles for the new background strain, C57BL/6 (B6) are shown in white. The grey bar denotes the location of the mutation of interest. Homozygous donor mice are bred to B6 mice to make the N1F1 generation animals. These mice are heterozygous for all alleles on the chromosome. The N1F1 mice are backcrossed to the B6 mice and the resulting N2F1 mice are partly B6 like on the chromosome carrying the mutation of interest, but the block around the mutation of interest remains 129-like. With each generation of backcrossing, the 129-like region gets smaller around the mutation of interest, but is never completely eliminated. It is here in this block around the mutation of interest that passenger genes are found.
It is unfortunate that there is often little or no discussion as to where this 0.2% contamination by the donor strain may be found relative to the transgene or allele of interest. This first point is the topic of the paper Passenger Mutations Confound Interpretation of All Genetically Modified Congenic Mice by Vanden Berghe and colleagues, which discusses the conceptual framework of “Passenger Genes,” and why these genes matter for interpreting a congenic phenotype. The paper also provides a simple tool that can be used to determine if this might be an issue when examining your congenic transgenic mouse (6). In simple terms, the “passenger genes effect” refers to the phenomenon that no matter how much backcrossing one does when moving a mutant allele from one background to another, some alleles from the donor strain will also be transferred and these “contaminating alleles” will be juxtaposed to the mutant allele of interest (3) (Figure 1).
Mendel’s second law of genetic inheritance states that the segregation of alleles for one gene is independent of the alleles of any other locus. However, this is not quite true as genes are arranged in chromosomes, not as free-floating units. During meiosis there is crossover of genetic material from one copy of a chromosome to the other, allowing for the segregation of alleles on a single chromosome. The truth of Mendel’s second law is therefore a function of the location of two genes in the genome relative to each other. Let us consider two mutant genes, where the mouse is heterozygous for both mutant alleles. If those genes are on different chromosomes, the inheritance of the mutant alleles for each of the two genes in the offspring cohort should be truly independent and Mendel’s second law holds true. If the two mutations are within genes on the same chromosome, the independence of inheritance is contingent on how close those two genes are on that chromosome. Genetic distance is measured in terms of centimorgans (cM). By definition, genetic recombination will occur 1% of the time between two loci that are 1 cM apart. As such, two genes on opposite ends of a single chromosome (>75 cM apart) will segregate independently of each other, as the probability of a genetic recombination event happening between them is very high. Two mutations located 1 cM apart will usually be inherited together as they are physically linked on the chromosome and the odds of a genetic recombination happening between then is one in a hundred (4). This means that, when making a congenic, the odds of bringing the alleles of the donor strain along with the mutant allele of interest is exceedingly high for the genes immediately adjacent to the mutant allele (3). In principal, the odds of bringing any given donor allele decreases the further one moves from the mutation of interest, and with each backcross generation completed. However, this is confounded by the observation that genetic recombination is not a random event. Rather, there are hot spots in the genome where recombination is much more likely to occur (7), altering the odds that recombination will happen between any two given genes. The odds that there will be at least 1 cM of donor flanking region around the mutation of interest in a congenic, even after 10 generations of backcrossing, is 0.91 (3). To put this into context of physical genetic space, there is on average 2 Mb of physical distance per 1 cM of genetic distance, and assuming universal distribution of genes in the genome, there are on average 10 genes per Mb (8). These alleles from the donor strain around the mutation of interest are referred to as passenger genes (3) and by these calculations alone, there are a minimum, on average, of 20 passenger genes per congenic strain after 10 generations of backcrossing.
While the concept of passenger genes is not a new one, the implications have been largely ignored. Notwithstanding, the main thesis underlying the paper by Vanden Berghe et al (6) is that passenger genes represent a major confounding variable that must be considered during phenotypic interpretation. And although this paper is not in the current issue of JBMR, the findings from this work are highly relevant to all investigators working with genetically manipulated mouse models.
Vanden Berghe et al began their analysis using data from the Mouse Genome Informatics database (http://www.informatics.jax.org). They estimated that approximately 8000 transgenic, or ~80%, of all of the genetically modified mice made and published by January of 2015 were made using a 129 ES cell. They then asked how likely it was that the passenger genes in these congenics could confound interpretation. As most congenics involved 129 mice, all of their work is based on the analysis of the 129 alleles. Using complete genome sequences available for 129-like strains from The Wellcome Trust Sanger Institute (9), these authors determined that there were 949 insertion/deletion mutations (so called indels), and 446 single nucleotide polymorphisms that could have a functional consequence for that gene when comparing 129 strains to B6 mice. In total, these mutations impacted 1084 genes. A startling 188 of these mutations resulted in a predicted gain or loss of a STOP codon and 875 resulted in a frameshift mutation. Accounting for only indels and polymorphisms with functional consequences in their calculations, Vanden Berghe et al. estimated the impact of this large number of 129 mutations on the existing collection of knockout mice. Assuming that the interval containing donor alleles is 10 cM wide around the transgene, 99.5% of all B6.129 congenic trangenics carry at least 20 genes with a putative 129-specific functional mutation. A 5 cM interval would have a 97% chance of having 11 functionally mutated passenger genes and a 1 cM interval has a 71% chance of having at least 2 mutated passenger genes. Given that the odds are 91% that the donor interval is at least 1 cM in breadth, this means that the majority of all congenic transgenics have at least one putatively functional passenger gene from this analysis alone. What the authors did not consider was that, as we know from forward genetics, most polymorphisms that underlie genetic loci and modulate a phenotype of interest are not likely to be in the coding region of a gene (10). This would imply that a significant proportion of all genes are putatively different functionally when comparing the 129 strains to C57BL/6J and that the number could be well larger than the 1084 genes they highlight. Thus it is likely that all B6.129 congenic lines carry functional passenger genes.
To take this concept further and prove that this passenger gene phenomenon could matter in vivo, the authors used a well-studied model of lipopolysaccharide (LPS) challenge. In short, B6 mice will succumb rapidly to a large injection of LPS. Part of the reason for this is that Casp11 will drive the synthesis of a fatal amount of interleukin-1β by infected macrophages. Many 129 strains have a frameshift mutation in the Casp11 gene, making them functional nulls for Casp11 and therefore resistant to LPS challenge (6). Mice lacking Mmp13 have been reported to be resistant to LPS challenge (11), but this gene is less than 1 cM from Casp11. The authors showed that B6.129-congenic mice that are 129-like for Casp11 and null for Mmp13 (a widely used congenic transgenic), are resistant to LPS, but when this congenic is backcrossed even further to B6 and they are rendered B6-like for Casp11the Mmp13 null mice are susceptible to LPS challenge. This shows that Mmp13 loss was not protective for LPS challenge but rather the Casp11 passenger gene mutation was the effective entity (6).
Given the number of transgenic and mutant mice that are congenic, the passenger gene effect is widespread in the research community. It is unclear what this might mean though for the huge body of literature in existence utilizing this type of mouse model. To help researchers determine if their mouse model might be impacted by a mutated passenger gene, Vanden Berghe and colleagues (6) developed a simple-to-use tool that allows you to determine if and what possible mutated passenger genes might be affecting it (htpp:// http://me-pamufind-it.org/). This tool asks for the name of your gene and the number of times your congenic has been backcrossed to B6. It then provides you with a list of possible mutated passenger genes around your transgene. This instrument is an excellent starting tool for raising awareness of an under-considered issue and provides a first pass for what to look for that might affect your model. The strength of this resource is that it considers three different 129 strains as possible sources of mutations, as not all 129 strains are the same (9), nor was a single line of 129 ES cells used to make all the transgenic lines (12). The limitations of the analysis provided is that it is restricted to 129 mutations, which is an issue for bone biologists as many models are B6.FVB congenics. In its current iteration, this type of analysis is unable to account for backcrossing to anything other than B6. Despite the limitations, the paper nicely summarizes the pervasiveness of the problem and highlights the need to take the time to precisely know the genetic background of your mice.
While this paper suggests that all congenics have a passenger gene effect, this does not mean that these mice are unusable and that data from them are false. Rather this paper shows that while backcrossing is expensive and time consuming, there is long-term value gained from investing in this effort. Determining how large the real donor region is for any congenic is very simple and can be quickly and cheaply done using PCR based genotyping of microsatellites. There are literally hundreds of pre-designed microsatellite marker assays, such as the MIT markers, available that can be used to differentiate 129-like alleles from B6 ones (http://www.informatics.jax.org). Similarly B6 vs FVB markers are also available. Thus, it becomes a simple task to genotype a congenic, determine the size of the true donor region by PCR and use this tool by Vanden Berghe to find out if any of the 129-like passenger genes could have a functional consequence. If this is found to be the case, in the best case, some time should be taken to breed them out by conducting more back crossing and in the worst case, it may be necessary to make a new genetic model or obtain one of the new transgenics being made by the international knockout mouse consortium (http://www.mousephenotype.org/). If this latter route is employed, it must be noted that these mice, including the ones made by KOMP, were made using a C57BL/6N strain background, which is not the same strain as the usual C57BL/6 (13). Lastly, it should be mentioned that there are many B6x129 congenic mice available. An allele complementation test (4) can be time consuming, but may be a valuable way to separate the effects of a transgenic allele from a background passenger gene allele.
In summary, the existence of passenger genes has not received the attention that it deserves. Reproducibility in pre-clinical studies is a serious issue and one that is receiving increased attention as of late. Indeed, new policies and practices are being implemented by the NIH specifically to address reproducibility and rigor (14). A lament commonly heard is one wherein a transgenic mouse is obtained from a vendor or a collaborator, but after a couple of months, the phenotype of interest has “disappeared”, or was not the same as published. In many cases, a careful interrogation of the breeding history of the current generation of mice uncovers the answer. Often, a vendor will breed mice back to B6 and passenger genes or other alleles that interact with the transgene are lost during backcrossing or some other strain background change has happened along the way. Thus, the published finding may have been misattributed to a transgene, or is as result of some sort of complicated gene-gene interaction event. As this paper by Vanden Berghe and colleagues shows with the Casp11 example, careful attention to the genetic background of all of our mouse models is needed for truthful interpretation of results and is key for to meeting the new NIH reproducibility standards. This includes recognition of the existence of passenger genes and elimination of them when appropriate.
Acknowledgments
Work in both author's laboratories is supported by a grants from the NIH/NIAMS (CLAB: AR060234 and AR064790, CJR: AR061164).
References
- 1.Limaye A, Hall B, Kulkarni AB. Manipulation of mouse embryonic stem cells for knockout mouse production. Current protocols in cell biology / editorial board, Juan S Bonifacino [et al] 2009 Sep;:1–24. doi: 10.1002/0471143030.cb1913s44. Chapter 19:Unit 19 3 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, et al. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(6):2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lusis AJ, Yu J, Wang SS. The problem of passenger genes in transgenic mice. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(10):2100–2103. doi: 10.1161/ATVBAHA.107.147918. [DOI] [PubMed] [Google Scholar]
- 4.Silver LM. Mouse Genetics: Concepts and Applications. Oxford University Press; 1995. [Google Scholar]
- 5.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes & development. 2002;16(21):2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanden Berghe T, Hulpiau P, Martens L, Vandenbroucke RE, Van Wonterghem E, Perry SW, et al. Passenger Mutations Confound Interpretation of All Genetically Modified Congenic Mice. Immunity. 2015;43(1):200–209. doi: 10.1016/j.immuni.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paigen K, Szatkiewicz JP, Sawyer K, Leahy N, Parvanov ED, Ng SH, et al. The recombinational anatomy of a mouse chromosome. PLoS genetics. 2008;4(7):e1000119. doi: 10.1371/journal.pgen.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridgway WM, Healy B, Smink LJ, Rainbow D, Wicker LS. New tools for defining the 'genetic background' of inbred mouse strains. Nature immunology. 2007;8(7):669–673. doi: 10.1038/ni0707-669. [DOI] [PubMed] [Google Scholar]
- 9.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477(7364):289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nature reviews Genetics. 2005;6(4):271–286. doi: 10.1038/nrg1576. [DOI] [PubMed] [Google Scholar]
- 11.Vandenbroucke RE, Dejonckheere E, Van Hauwermeiren F, Lodens S, De Rycke R, Van Wonterghem E, et al. Matrix metalloproteinase 13 modulates intestinal epithelial barrier integrity in inflammatory diseases by activating TNF. EMBO molecular medicine. 2013 Jul;5(7):932–948. doi: 10.1002/emmm.201202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nature genetics. 1997 May;16(1):19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 13.Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14(7):R82. doi: 10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014 Jan 30;505(7485):612–613. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]