Abstract
Introduction
Elevated vancomycin MICs in S. aureus have been associated with worse clinical outcomes in adults. For invasive MRSA infections in adults, the IDSA recommends targeting vancomycin serum trough concentrations between 15–20 μg/ml. We evaluated trends in vancomycin MICs from healthcare-associated S. aureus bacteremia isolates in children in addition to correlating vancomycin serum trough levels with clinical outcomes.
Methods
Patients and isolates were identified from a prospective S. aureus surveillance study at Texas Children's Hospital (TCH). Healthcare-associated S. aureus bacteremia isolates from 2003–2013 were selected. Vancomycin MICs by E-test were determined and medical records were reviewed. Acute kidney injury (AKI) was defined as doubling of the baseline serum creatinine.
Results
341 isolates met inclusion criteria. We observed a reverse vancomycin creep among MRSA isolates in the study period with a decline in the proportion of isolates with vancomycin MIC ≥ 2 μg/ml (from 32.7% to 5.6%, p<0.001). However, the proportion of MSSA isolates with MIC ≥ 2 μg/ml increased (from 2.9% to 9%, p=0.04). Among patients who had vancomycin troughs performed, there was no difference in duration of bacteremia or fever with vancomycin trough >15 μg/ml vs. < 15 μg/ml. A vancomycin trough > 15 μg/ml was, however, an independent risk factor for AKI.
Conclusions
Vancomycin MICs are shifting among healthcare-associated S. aureus bacteremia isolates with significant differences between MRSA and MSSA at TCH. Higher vancomycin troughs did not improve outcomes in pediatric healthcare-associated S. aureus bacteremia but were associated with increased nephrotoxicity. Further studies are needed to better understand optimal management of children with S. aureus bacteremia.
Keywords: Vancomycin, creep, S. aureus, bacteremia, children
Vancomycin remains the principal agent for the management of severe MRSA infections in children.1 Studies in adults with bacteremia and endocarditis have shown improved clinical outcomes when vancomycin troughs are over 15 μg/ml.2,3 For severe MRSA infections in adults, the Infectious Diseases Society of America (IDSA) recommends targeting vancomycin serum trough concentrations between 15–20 μg/ml.1 While acknowledging that data are lacking, the IDSA guidelines state that goal vancomycin serum troughs of 15–20 μg/ml should be “considered” in children with severe MRSA infection.
Achieving such high trough levels in children is often challenging. Studies at Le Bonheur Children's Hospital have shown that only 16.5% of children receiving vancomycin achieved a trough level 15–20 μg/ml.4 Furthermore, acute kidney injury (AKI) developed in 33% of children with vancomycin troughs > 15 μg/ml.5 In addition, recent pharmacokinetic modeling studies suggest that sufficient drug exposure may be achieved with vancomycin trough levels of 7–10 μg/ml in most children.6 To date, no studies have examined the correlation between vancomycin serum trough levels and outcomes of S. aureus bacteremia in children.
Optimal vancomycin dosing is complicated in that studies have shown that vancomycin minimum inhibitory concentrations (MIC) by E-test have subtly increased over time, a phenomenon dubbed vancomycin creep.7–9 Studies among adults have shown that bloodstream infections due to MRSA isolates with vancomycin MICs above 1.5 μg/ml are associated with higher rates of vancomycin treatment failure10 as well as endocarditis and metastatic infection.11 Studies describing vancomycin creep in pediatric subjects have been somewhat conflicting with some centers finding temporal increases in MIC while others have not.12,13
We sought to evaluate trends in vancomycin E-test MICs among healthcare-associated S. aureus bacteremia isolates at Texas Children's Hospital and to correlate the vancomycin MICs with clinical course. In addition, we sought to compare vancomycin serum trough levels with clinical outcomes.
Methods
Patients and isolates were identified from a prospective S. aureus surveillance study ongoing at Texas Children's Hospital (TCH).14 Isolates are identified during the routine course of care by the TCH clinical microbiology laboratory; isolates are sub-cultured, stored in horse blood at −80 °C in the Infectious Diseases Research Laboratory and basic clinical data are recorded. Healthcare-associated S. aureus bacteremia isolates from 2003–2013 were selected. For purposes of this study, healthcare associated infections included nosocomial and community-onset healthcare-associated (CO-HCA) infections. Patients with primary bacteremia, central-line associated bloodstream infection (CLA-BSI) and infectious endocarditis (IE) were included; to minimize the impact that source had on treatment outcomes, patients with CNS disease or localized purulent collections were excluded. Patients with end-stage renal disease (ESRD) were also excluded to minimize the impact that pre-existing renal disease may have had on vancomycin associated nephrotoxicity and trough levels. Medical records for all patients were reviewed. The highest vancomycin trough obtained during the first 96 hours of therapy was recorded and used in analyses. Vancomcyin troughs were obtained at the discretion of the treating physicians; protocols do not exist to regularly obtain vancomycin troughs on patients receiving vancomycin at our hospital.
Definitions
Nosocomial infections were those in which signs and symptoms of infection developed at ≥72 hours of hospitalization.15 CO-HCA infections were those that developed in the outpatient setting in patients with underlying conditions predisposing them to frequent hospitalizations or encounters with the healthcare system.16 Primary bacteremia was considered if patients had positive blood cultures for S. aureus without a focus on physical or radiologic examination and who did not have a central venous catheter in situ. For the purposes of this study, the diagnosis of CLA-BSI describes those patients who had ≥1 positive blood culture obtained via central venous catheter plus signs/symptoms of infection and without an alternative source of bacteremia;17,18 this definition was used as paired central and peripheral blood cultures were not obtained uniformly during the study period. For patients with CLA-BSI, time to catheter removal was considered the number of calendar days between the first positive blood culture and the date of catheter removal. IE was defined using the Modified Duke Criteria.19 Duration of bacteremia was regarded as the number of calendar days between the first positive blood culture and the first date of at least two consecutive negative blood cultures. The frequency of collection of blood cultures was at the discretion of the physician of record; a common practice in our institution, however, is to obtain daily blood cultures in patients with bacteremia. All patients who survived their S. aureus infection had follow up blood cultures. AKI was regarded as a doubling of the serum creatinine over baseline that was sustained on at least two measurements20; baseline creatinine was regarded as the last measured creatinine prior to onset of signs/symptoms of infection. Antistaphylococcal β-lactam antibiotics were considered nafcillin, oxacillin, dicloxacillin, first-generation cephalosporins and piperacillin-tazobactam.
Antimicrobial susceptibility testing
Susceptibility to oxacillin and vancomycin were performed by the clinical microbiology laboratory in the routine course of clinical care. All isolates were susceptible to vancomycin by routine automated laboratory methods. In addition, all isolates had MIC to vancomycin determined with E-test micro-method21 in the Infectious Diseases Research Laboratory. E-tests were performed as previous data in adults have shown worse outcomes for S. aureus bloodstream infection when vancomycin E-test MICs are elevated.22,23 All E-tests were performed prior to review of medical records; corresponding clinical outcomes were unknown to personnel at the time MICs were determined.
Molecular Studies
In order to correlate vancomycin MIC with strain types, a convenience sample of 100 isolates was selected for molecular investigations with equal distribution between early (2003–2007) and late study periods (2008–2013) and between MRSA and MSSA. Isolates were analyzed by pulsed field gel electrophoresis as has been previously described24 and agr-groups were determined with a PCR-based assay.25
Statistical Analyses
Dichotomous variables were compared with Fisher's exact and χ2 tests; continuous variables were compared with Kruskal-Wallis and Wilcoxon Rank Sum and two-tailed p-values < 0.05 were considered statistically significant. Variables with p-values <0.1 in univariate analyses of AKI were included in a multivariate logistic regression model for risk of AKI. All analyses were performed with the use of STATA ver. 13 (STATA Corp, College Station, TX).
Results
From the database, 411 bacteremia episodes were initially identified with 341 (107 MRSA isolates and 234 MSSA isolates) meeting inclusion criteria. Reasons for subject exclusion included ESRD (n=26), isolates missing and unavailable for E-test (n=9), positive blood cultures being considered “contaminants” by the physician of record and not treated (n=8), soft tissue infection (n=6), pneumonia (n=5), osteomyelitis (n=3), database duplication (n=3), missing medical records (n=3), bacteremia due to organisms other than S. aureus (n=3), community-acquisition (n=2) and surgical site infection/hardware infection (n=2). The median age of patients included was 20.4 months (Table 1) and the most common underlying condition was prematurity (24%). Among nosocomial infections, 62 (34.8%) occurred in the neonatal intensive care unit (NICU).
Table 1.
Selected Clinical Features of Patients with Healthcare-Associated S. aureus Bacteremia
| N=341 (%) | |
|---|---|
| Median Age, Months (IQR) | 20.4 (1.2–115.7) |
| Nosocomial Acquisition | 178 (52.2) |
| CVL in situ at Time of Infection | 233 (68.3) |
| History of Prematurity | 82 (24) |
| History of Malignancy | 69 (20.2) |
| History of Congenital Heart Disease | 44 (12.9) |
| Infectious Diagnoses | |
| Primary Bacteremia | 110 (32.3) |
| CLA-BSI | 192 (56.3) |
| Infectious Endocarditis | 39 (11.4) |
| Median Duration of Bacteremia, Days (IQR) | 2 (1–4) |
| Median Duration of Fever, Days (IQR) | 2 (2–4) |
| S. aureus Attributable Mortality | 16 (4.7) |
Vancomycin MIC Trends
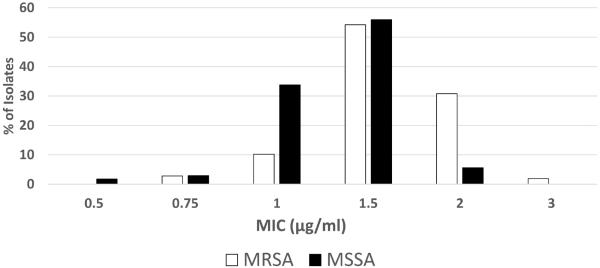
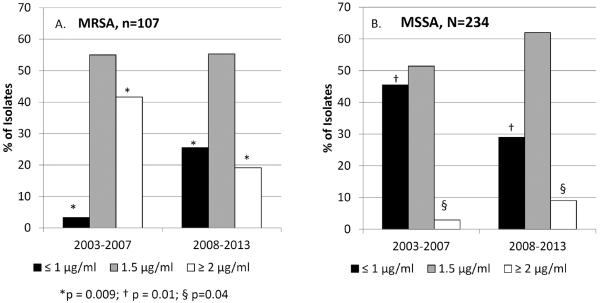
All 341 isolates were viable for E-test MIC determination. The modal vancomycin MIC for both MRSA and MSSA isolates was 1.5 μg/ml (Figure 1). A greater proportion of MRSA isolates had a vancomycin MIC ≥ 2 μg/ml than MSSA isolates (35/107, 32.7% vs. 13/234, 5.6%, p <0.001). During the study period, however, the proportion of MRSA isolates with MIC ≥ 2 μg/ml declined from 41.6% in the years 2003–2007 to 19.1% in 2008–2013 (p=0.03 Figure 2). In contrast, among MSSA in the same time period, the proportion of isolates with MIC ≤ 1 μg/ml decreased (45.5% to 29%, p=0.01) and the proportion of isolates with MIC ≥ 2 μg/ml increased (2.9% to 9%, p=0.04).
Figure 1.
Distribution of vancomycin E-test MICs.
Figure 2.
Temporal Trend in Vancomcyin E-test MICs. Panel A. Trends in MRSA E-test over time. Panel B. Trends in MSSA E-test over time.
Molecular Studies
The most common PFGE type was USA300 accounting for 35%; USA300 was more common among MRSA (29/50, 58%) than MSSA (7/50, 14%, p<0.001). USA300 isolates more often had a vancomycin MIC ≥ 2 μg/ml than non-USA300 isolates (15/36, 41.6%, vs. 16/64, 25%, p=0.06). The proportion of isolates with specific agr-groups is as follows: group I=54%, group II = 40%, group III = 4%, group IV = 2%. There were no statistically significant differences in vancomycin MIC among groups.
MRSA Bacteremia and Vancomycin MIC
MRSA bacteremia episodes with vancomycin MIC ≥ 2 μg/ml were compared with those with MIC ≤ 1.5 μg/ml (Supplementary Digital Content 1). Infections with higher vancomycin E-test MICs were associated with a slightly longer duration of bacteremia (3 vs. 2 days, p= 0.02); this difference was even more pronounced when patients with CLA-BSI were excluded (4.1 vs. 2.3 days, p=0.03). There was no difference in infectious diagnosis, underlying condition or vancomycin trough between the groups.
MSSA Bacteremia and Vancomycin MIC
As only thirteen MSSA isolates had MIC ≥ 2 μg/ml, comparisons were made between those with MIC ≥ 1.5 μg/ml and < 1.5 μg/ml. Among patients with MSSA bacteremia, patients with isolates with MIC ≥ 1.5 μg/ml more often had a CVL in situ at the time of infection than those patients with isolates with lower MICs (116/144, 80.5% vs 47/90, 52%, p < 0.001). There were no differences in age, underlying characteristics or clinical outcomes by vancomycin E-test MIC among MSSA bacteremia cases as a whole. When cases of CLA-BSI are excluded, there was a greater degree of mortality directly attributable to MSSA among patients with a vancomycin MIC ≥ 1.5 μg/ml compared to those with MIC ≤1 μg/ml (8/59, 13.5% vs. 1/38, 2.7%, p=0.07). Thirty patients (12.8%) with healthcare-associated MSSA bacteremia received an antistaphylococcal β-lactam on the first day of illness. There was no difference in the proportion of cases with empiric use of an antistaphylococcal β-lactam or time to initiation of a β-lactam between the infections with high and low vancomycin MICs.
Vancomycin Troughs and Outcomes of Staphylococcus aureus Bacteremia
Overall, 127 patients (37.2%) had a vancomycin trough performed in the first 96 hours of therapy. Fifty-five patients had vancomycin troughs ≤10 μg/ml (43.3%), 33 with troughs 10-≤15 μg/ml (26%) and 39 with troughs > 15 μg/ml (30.7%). There was no difference in age among patients who did and did not have troughs performed; patients with endocarditis were more likely to have vancomycin troughs performed than were other diagnoses (21/39, 53.8%, vs. 106/302, 35.1%, p=0.03). There was no statistically significant difference in duration of bacteremia or fever among patients with and without vancomycin troughs performed. Among those patients with serum troughs performed, there was no observed decrease in duration of bacteremia or fever for patients with higher serum troughs (Table 2). There was a significant increase in the proportion of cases who experienced acute kidney injury (Trough < 10 μg/ml: 9/55, 16.2%; 10-≤15 μg/ml: 11/33, 33.3%; > 15 μg/ml: 24/39, 61.5%, p<0.001) and S. aureus attributable mortality (2/55, 3.6% vs. 3/33, 9.1% vs. 8/39, 20.5%, p=0.02) among those with higher vancomycin troughs. Similar results were seen when analyses were performed excluding infants < 12 months of age.
Table 2.
Outcomes of S. aureus bacteremia (MRSA and MSSA) by Vancomycin Trough
| Trough ≤ 10 μg/ml (n=55) | Trough 10–≤15 μg/ml (n=33) | Trough > 15 μg/ml (n=39) | P value | |
|---|---|---|---|---|
| Median Age, Months (IQR) | 15.6 (1.2–96) | 2.4 (0.1–10.3) | 9.4 (0.3–169.2) | 0.3 |
| Infectious Diagnosis of Endocarditis | 7 (12.7) | 10 (30.3) | 4 (10.3) | 0.07 |
| Median Duration of Bacteremia, Days (IQR) | 2 (1–4) | 2.5 (1–5) | 2 (1–4) | 0.4 |
| Median Time to Resolution of Fever, Days (IQR) | 2 (1–4) | 3 (2–4) | 2 (1–5) | 0.2 |
| Median Length of Hospital Stay, Days (IQR) | 17.5 (10–44) | 47.5 (24–104) | 43.5 (25–89) | 0.01 |
| AKI | 9 (16.2) | 11 (33.3) | 24 (61.5) | <0.001 |
| S. aureus Attributable Mortality | 2 (3.6) | 3 (9.1) | 8 (20.5) | 0.02 |
Outcomes were examined separately for MRSA bacteremia. Among those with serum troughs performed, higher vancomycin troughs did not correlate with a decrease in either duration of bacteremia or time to clinical resolution (Supplemental Digital Content 2). There was however, an increased risk of AKI with increasing vancomycin troughs (p<0.001). Only 36/54 (66.7%) MRSA patients with vancomycin troughs available received scheduled weight-based dosing. Similar proportions of patients with vancomycin troughs below and above 15 μg/ml received vancomycin doses in excess of 60 mg/kg/day (6/28, 21.4%, vs 2/8, 25%).
Thirty patients (12.8%) with healthcare-associated MSSA bacteremia received an antistaphylococcal β-lactam on the first day of illness. There was no difference in duration of fever or bacteremia, need for vasopressors, AKI or mortality between patients who received a β-lactam empirically and those who did not.
Acute Kidney Injury and Vancomycin
Overall, 51 patients (14.9%) developed acute kidney injury. Patients with AKI were more often <12 months old, had a history of prematurity or congenital heart disease, nosocomial acquisition, concomitant vasopressor and/or aminoglycoside use and more often had a vancomycin serum trough > 15 μg/ml (Supplemental Digital Content 3). When these variables were included in a multivariate logistic regression model, vancomycin trough > 15 μg/ml as well as vasopressor and aminoglycoside use were independently associated with AKI. Among patients with AKI 12/51 (23.5%) died of S. aureus compared to 4/290 patients without documented AKI (1.4%, p< 0.001). One patient who developed AKI ultimately required hemodialysis; all other patients with vancomycin associated AKI who survived their infection recovered renal function by the time of hospital discharge.
AKI in MRSA bacteremia was examined separately. Overall, thirteen patients with healthcare-associated MRSA bacteremia experienced AKI (13/107, 12.1%). AKI was positively associated with a vancomycin trough > 15 μg/ml (7/12, 58.3% vs. 9/42, 20.9%, p=0.02) and concomitant aminoglycoside (9/13, 69.2% vs. 33/94, 35.1%, p=0.03) and vasopressor use (4/13, 30.8% vs. 5/94, 5.3%, p=0.01, Table 3). These variables along with vancomycin E-test MIC ≥ 2 μg/ml were included in a multivariate model and serum vancomycin trough > 15 μg/ml remained independently associated with risk of AKI (p=0.02).
Table 3.
Features of Acute Kidney Injury in MRSA Bacteremia
| Patients with AKI, n=13 | Patients without AKI, n=94 | P value | Multivariate P value | |
|---|---|---|---|---|
| Median Age, Months (IQR) | 20.5 (2–206) | 22.5 (3.9–143.7) | 0.8 | |
| Vancomycin MIC ≥ 2 μg/ml | 7 (53.8) | 28 (29.8) | 0.1 | 0.6 |
| Nosocomial Acquisition | 7 (53.8) | 54 (57.4) | 1 | |
| CVL in situ | 9 (69.2) | 62 (60) | 1 | |
| History of Prematurity | 3 (23.1) | 19 (20.1) | 0.7 | |
| History of Malignancy | 2 (15.4) | 11 (11.7) | 0.6 | |
| Concomitant Aminoglycoside Administration | 9 (69.2) | 33 (35.1) | 0.03 | 0.3 |
| Any Concomitant β-lactam administration | 5 (38.4) | 27 (28.7) | 0.5 | |
| Vancomycin | 12 (92.3) | 43 (45.7) | 0.002 | |
| Trough Performed | ||||
| Vancomycin trough > 15 μg/ml | 7 (58.3) | 9 (20.9) | 0.02 | 0.02 |
| Vancomycin Trough > 10 μg/ml | 12 (100) | 19 (44.1) | 0.001 | |
| Concomitant Vasopressor Administration | 4 (30.8) | 5 (5.3) | 0.01 | 0.2 |
Discussion
Optimal vancomycin dosing remains challenging in critically ill children. We investigated 341 episodes of healthcare-associated S. aureus bacteremia at a large children's hospital. Work at TCH from a previous time period (2001–2008) showed a gradual increase in vancomycin MIC among S. aureus isolates.12 While we observed a temporal increase in vancomycin MIC among MSSA isolates, there was a decline in the proportion of MRSA isolates with MIC ≥2 μg/ml or “reverse creep.” The reasons for this discrepancy between MRSA and MSSA are unclear. Others have noted that high vancomycin MICs are associated with specific strains of MSSA, in particular agr-II as well as those with agr-dysfunction.26,27 In contrast, we were unable to show a relationship with vancomycin MIC and agr-group; there was, however, a trend for higher vancomycin MICs among USA300 isolates. One could hypothesize that the increase in MSSA isolates with higher vancomycin MICs are related to an increase in the USA300 background among MSSA isolates, as has been previously demonstrated.28
Changes in vancomycin MIC are of clinical significance in that a higher vancomycin MIC can be associated with poor response to vancomycin22,29 as well as more invasive infections11 in adults. Among MRSA cases in the present study, there was a modest but statistically significant increased duration of bacteremia associated with higher vancomycin MICs and this was particularly the case for non-CLABSI bacteremia. Holmes et al.23 observed higher mortality in MSSA isolates with MIC > 1.5 μg/ml regardless of antibiotic choice. Similarly, we observed a trend for greater S. aureus attributable mortality among MSSA isolates with a vancomycin MIC ≥ 1.5 μg/ml. While β-lactams are the drugs of choice for MSSA, these findings still have clinical import. We found that the time to initiation of antistaphylococcal β-lactam antibiotics between MSSA isolates with high and low vancomycin MICs was no different, suggesting that an elevated vancomycin E-test MIC may be a marker for virulence even in MSSA. Given that the vancomycin MICs in the population served at TCH are shifting, these trends will need to be followed in order to understand what impact this will have on therapy.
Studies of adults with MRSA pneumonia have reported that a vancomycin area under the curve (AUC)/MIC ratio ≥ 400 was associated with an improved clinical and microbiologic outcome.30 Work in adults has revealed that a vancomycin trough concentration of 15–20 μg/ml will achieve an AUC/MIC >400 in most patients infected with an organism with an MIC ≤ 1 μg/ml.31 Kullar et al. reported greater degrees of vancomycin success when troughs were between 15–20 μg/ml compared to lower troughs among adults with MRSA bacteremia.2 Aggressive dosing strategies have been advocated by national guidelines for adult patients with severe MRSA infections to achieve these trough goals.1,3
To the best of our knowledge, this work represents the first attempt to correlate vancomycin troughs with outcomes of S. aureus bacteremia in children. While only 37.2% of patients with S. aureus bacteremia had vancomycin serum troughs performed, we were unable to show a clinical benefit in patients with a serum vancomycin trough > 15 μg/ml. This contrast from adult studies may result from the small number of children with troughs performed. In addition, previous studies in adults have shown that the mean duration of vancomycin-susceptible MRSA bacteremia is 6 days;32 the far shorter duration of bacteremia in children (median 2 days) makes illustration of clinical benefit in our pediatric study extremely challenging. There was a trend for a longer duration of bacteremia, as well as greater mortality, in children with higher vancomycin troughs; this finding was likely an artifact, however, in that children who were perceived as being more ill may have been dosed more aggressively. Beyond these concerns, the modal vancomycin MIC = 1.5 μg/ml in the isolates studied would suggest that obtaining optimal AUC/MIC ratio is more challenging than previously believed.
While we were unable to illustrate a benefit to higher vancomycin troughs in this patient population, there was a clear risk of increasing AKI with higher troughs which was independent from other concomitant nephrotoxic medications. Sinclair et al.33 found that children receiving vancomycin who experienced AKI had higher vancomycin troughs than those who did not have AKI. In our retrospective study, it is difficult to know if high vancomycin levels caused AKI rather than that AKI was related to hemodynamic instability, other medications or sepsis leading to a delayed clearance of vancomycin and higher troughs. Nevertheless, the absence of a clear benefit calls into question the wisdom of pursuing aggressive trough levels in children with S. aureus bacteremia. It is important to note, however, that the inclusion of only children with healthcare-associated bacteremia who often have had numerous previous hospitalizations and underlying conditions, likely contributed to the high rate of AKI in this population. Furthermore, the study design only assessed for the co-administration of nephrotoxic medications and does not account for the impact of medication administration in previous hospitalizations and the possible cumulative impact of nephrotoxic agents. Similarly, only children with ESRD were excluded and not those with a history of other mild renal disease or AKI in previous hospitalizations. In addition, more children with AKI had vancomycin troughs performed and this may have influenced our findings.
Studies in adults have demonstrated the superiority of antistaphylococcal β-lactams to vancomycin for the management of MSSA bacteremia.34,35 Many experts in the field recommend empiric initiation of vancomycin along with an antistaphylococcal β-lactam for patients with suspected severe staphylococcal infection followed by de-escalation once susceptibilities are known.36 While scientific rationale for such a practice exists, we were unable to show a benefit to empiric combination therapy for MSSA bacteremia. However, the number of patients who received combination therapy was small (12.8%) and likely hampered the ability to detect a clinical benefit.
A number of additional limitations to our study are acknowledged. First, these data are from a single center and may not reflect a wider experience. In addition, the inclusion of very young children in this study group likely influenced the relative proportion of cases with low troughs. Furthermore, the inclusion of children who did not receive weight based dosing hinders our ability to draw conclusions regarding dosing and outcomes of healthcare-associated staphylococcal bacteremia. Providers may have been using a “goal trough” as a threshold to provide an additional dose of vancomycin in children not receiving scheduled weight based dosing. The use of a doubling of the serum creatinine alone to define AKI rather than a combination of creatinine clearance and urine output (such as with the pediatric Risk for renal dysfunction, Injury to kidney, Failure of kidney, Loss of kidney function and End-stage renal disease (pRIFLE) criteria) may have led to errors in determination of AKI rates. The doubling of the serum creatinine, however, has been used by other investigators in studies of drug toxicity.20,37 Furthermore, changes from baseline creatinine have been previously been shown to better correlate with negative outcomes than decreases in urine output in critically ill children.38 Finally, given that our study only included patients with primary bloodstream infection, this data cannot necessarily be applied to those patients with secondary bacteremia or those who have a pyogenic focus secondary to S. aureus.
In conclusion, many questions remain regarding the management of healthcare-associated S. aureus bacteremia in children. Among the population at TCH there was reverse vancomycin creep observed for MRSA isolates while the MICs for MSSA isolates have increased. Elevated vancomycin MICs were associated with worse clinical outcomes for both MRSA and MSSA. A clear benefit to elevated vancomycin troughs was not seen in children, although the data set was limited. However, nephrotoxicity is definitely associated with vancomycin serum troughs > 15 μg/ml. Further work is needed to better understand optimal vancomycin dosing in critically ill children.
Supplementary Material
Acknowledgments
Funding Source
This study was supported in part by the National Institutes of Health (NIAID K23AI099159 to JCM)
Conflicts of Interest Disclosure
The S. aureus surveillance study was funded in part by Pfizer (investigator initiated grant to SLK); this sponsor had no role in the conduct of the study or review of data. SLK also receives funding for unrelated investigator initiated studies from Forest Labs and Pfizer.
References
- 1.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 2.Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–81. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 3.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29:1275–9. doi: 10.1592/phco.29.11.1275. [DOI] [PubMed] [Google Scholar]
- 4.Chhim RFAS, Lee KR. Vancomycin dosing practices, trough concentrations, and predicted area under the curve in children wiht suspected invasive staphylococcal infection. J Pediatr Infect Dis Soc. 2013;2:259–62. doi: 10.1093/jpids/pis083. [DOI] [PubMed] [Google Scholar]
- 5.CA K, NIchols K, Lyon K, Veverka MM, Wilson A. Are elevated vancomycin serum trough concentrations achieved within the first 7 days of therapy associated with acute kidny injury in children? J Pediatr Infect Dis Soc. 2014;3:127–31. doi: 10.1093/jpids/pit076. [DOI] [PubMed] [Google Scholar]
- 6.Frymoyer A, Guglielmo BJ, Hersh AL. Desired vancomycin trough serum concentration for treating invasive methicillin-resistant Staphylococcal infections. Pediatr Infect Dis J. 2013;32:1077–9. doi: 10.1097/INF.0b013e318299f75c. [DOI] [PubMed] [Google Scholar]
- 7.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883–6. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother. 2007;60:788–94. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- 9.Hawser SP, Bouchillon SK, Hoban DJ, Dowzicky M, Babinchak T. Rising incidence of Staphylococcus aureus with reduced susceptibility to vancomycin and susceptibility to antibiotics: a global analysis 2004–2009. Int J Antimicrob Agents. 2011;37:219–24. doi: 10.1016/j.ijantimicag.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Hsu DI, Hidayat LK, Quist R, et al. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of meticillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents. 2008;32:378–85. doi: 10.1016/j.ijantimicag.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Miller CE, Batra R, Cooper BS, et al. An association between bacterial genotype combined with a high-vancomycin minimum inhibitory concentration and risk of endocarditis in methicillin-resistant Staphylococcus aureus bloodstream infection. Clin Infect Dis. 2012;54:591–600. doi: 10.1093/cid/cir858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason EO, Lamberth LB, Hammerman WA, Hulten KG, Versalovic J, Kaplan SL. Vancomycin MICs for Staphylococcus aureus vary by detection method and have subtly increased in a pediatric population since 2005. J Clin Microbiol. 2009;47:1628–30. doi: 10.1128/JCM.00407-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman JL, Harrison CJ, Myers AL, Jackson MA, Selvarangan R. No evidence of vancomycin minimal inhibitory concentration creep or heteroresistance identified in pediatric Staphylococcus aureus blood isolates. Pediatr Infect Dis J. 2014;33:216–8. doi: 10.1097/01.inf.0000436281.18687.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Inf Dis. 2005;40:1785–91. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 15.Hulten KG, Kaplan SL, Lamberth LB, et al. Hospital-acquired Staphylococcus aureus infections at Texas Children's Hospital, 2001–2007. Infec Control Hosp Epidemiol. 2010;31:183–90. doi: 10.1086/649793. [DOI] [PubMed] [Google Scholar]
- 16.Hulten KG, Kaplan SL, Gonzalez BE, et al. Three-year surveillance of community onset health care-associated staphylococcus aureus infections in children. Pediatr Infec Dis J. 2006;25:349–53. doi: 10.1097/01.inf.0000207404.50143.1e. [DOI] [PubMed] [Google Scholar]
- 17.Carrillo-Marquez MA, Hulten KG, Mason EO, Kaplan SL. Clinical and molecular epidemiology of Staphylococcus aureus catheter-related bacteremia in children. Pediatr Infect Dis J. 2010;29:410–4. doi: 10.1097/INF.0b013e3181c767b6. [DOI] [PubMed] [Google Scholar]
- 18.Edwards JR, Peterson KD, Andrus ML, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infec Control. 2007;35:290–301. doi: 10.1016/j.ajic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 20.Moffett BS, Hilvers PS, Dinh K, Arikan AA, Checchia P, Bronicki R. Vancomycin-associated acute kidney injury in pediatric cardiac intensive care patients. Congenit Heart Dis. 2015;10:E6–10. doi: 10.1111/chd.12187. [DOI] [PubMed] [Google Scholar]
- 21.CLSI . Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. In: Institute CLS, editor. CLSI document M100-S20-U. CLSI; Wayne, PA: 2010. [Google Scholar]
- 22.Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52:3315–20. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes NE, Turnidge JD, Munckhof WJ, et al. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis. 2011;204:340–7. doi: 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 24.Hulten KGSLK, Gonzalez BE, Hammerman WA, Lamberth LB, Versalovic J, Mason EO. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2006;25:349–53. doi: 10.1097/01.inf.0000207404.50143.1e. [DOI] [PubMed] [Google Scholar]
- 25.Moore PC, Lindsay JA. Genetic variation among hospital isolates of methicillin-sensitive Staphylococcus aureus: evidence for horizontal transfer of virulence genes. J Clin Microbiol. 2001;39:2760–7. doi: 10.1128/JCM.39.8.2760-2767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viedma E, Sanz F, Orellana MA, et al. Relationship between agr dysfunction and reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus causing bacteraemia. J Antimicrob Chemother. 2014;69:51–8. doi: 10.1093/jac/dkt337. [DOI] [PubMed] [Google Scholar]
- 27.Holmes NE, Turnidge JD, Munckhof WJ, et al. Genetic and molecular predictors of high vancomycin MIC in Staphylococcus aureus bacteremia isolates. J Clin Microbiol. 2014;52:3384–93. doi: 10.1128/JCM.01320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCaskill ML, Mason EO, Jr., Kaplan SL, Hammerman W, Lamberth LB, Hulten KG. Increase of the USA300 clone among community-acquired methicillin-susceptible Staphylococcus aureus causing invasive infections. Pediatr Infect Dis J. 2007;26:1122–7. doi: 10.1097/INF.0b013e31814536e0. [DOI] [PubMed] [Google Scholar]
- 29.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 30.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–42. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 31.Mohr JF, Murray BE. Point: Vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2007;44:1536–42. doi: 10.1086/518451. [DOI] [PubMed] [Google Scholar]
- 32.Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38:448–51. doi: 10.1086/381093. [DOI] [PubMed] [Google Scholar]
- 33.Sinclair EA, Yenokyan G, McMunn A, Fadrowski JJ, Milstone AM, Lee CK. Factors associated with acute kidney injury in children receiving vancomycin. Ann Pharmacother. 2014;48:1555–62. doi: 10.1177/1060028014549185. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Kim KH, Kim HB, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52:192–7. doi: 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodise TP, Jr., McKinnon PS, Levine DP, Rybak MJ. Impact of empirical-therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:3731–3. doi: 10.1128/AAC.00101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McConeghy KW, Bleasdale SC, Rodvold KA. The empirical combination of vancomycin and a beta-lactam for Staphylococcal bacteremia. Clin Infect Dis. 2013;57:1760–5. doi: 10.1093/cid/cit560. [DOI] [PubMed] [Google Scholar]
- 37.Totapally BR, Machado J, Lee H, Paredes A, Raszynski A. Acute kidney injury during vancomycin therapy in critically ill children. Pharmacother. 2013;33:598–602. doi: 10.1002/phar.1259. [DOI] [PubMed] [Google Scholar]
- 38.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.