Abstract
Purpose
The aim of the study was to examine the relationship between sleep apnea, retinal vascular caliber and retinopathy, and their impact on cardiovascular disease (CVD) risk.
Methods
A multi-ethnic cohort of 5,803 participants was examined based on standardized grading of retinal vascular caliber and retinopathy from digital fundus photographs, self-reported physician-diagnosed sleep apnea (PDSA), and incident cardiovascular events.
Results
In women, PDSA was associated with narrower arterioles (regression coefficient [β] −5.76; 95 % confidence Interval [CI] −8.51, −3.02) after adjusting for cardio-metabolic risk factors. The incident rate ratio (IRR) of CVD was also associated with narrower arterioles (IRR for highest versus lowest tertile 1.91; 95 % CI 1.08, 3.38). In men, PDSA was not associated with arteriolar caliber. However, incident CVD was associated with narrower arterioles (IRR 1.67; 95 % CI 1.10, 2.52), wider venules (IRR 1.71; 95% CI 1.13, 2.59) and PDSA (IRR 2.03, 95 % CI 1.17, 3.51). The IRR of CVD in men with PDSA increased minimally to 2.06 (95 % CI 1.18, 3.56) after adjustment for retinal arteriolar and venular caliber. Combining women and men, the IRR of CVD was 3.41 (95 % CI 1.79, 6.50) in those with both PDSA and narrower retinal arterioles.
Conclusions
Sleep apnea was associated with narrower retinal arterioles in women but not in men. However, sleep apnea was also associated with incident CVD in men. These suggest potential gender differences in susceptibility to microvascular disease in association with sleep apnea.
Keywords: Cardiovascular disease, Retinopathy, Retinal vascular caliber, Retinal imaging, Microcirculation, Sleep apnea
Introduction
Sleep apnea is common and has been shown to have a deleterious impact on health. Hypertension, diabetes, cardiovascular disease (CVD), stroke, and mortality are among its serious complications [1–3]. Pathophysiological studies have indicated that sleep apnea and intermittent hypoxia are associated with increased levels of inflammatory markers and oxidative stress, which can result in endothelial dysfunction and atherosclerosis [4]. While the link between sleep apnea and large-vessel disease has been well documented, the potential adverse effect of sleep apnea on the microvasculature is less clear. Impaired vasoreactivity and vasoregulatory mechanisms, which could affect not only large but also small vessels, have been implicated in the pathogenesis of CVD among people with sleep apnea [5].
The human retina provides a unique non-invasive model to study small vessel disease. With the use of computer-based retinal image analysis, subtle retinal vascular changes, such as variations in retinal vascular caliber or retinopathy signs, can be studied and measured in a reliable and quantitative manner [6]. There is good evidence to suggest that these retinal vascular changes are associated with both subclinical and clinical CVD [7–9]. However, data on the possible effects of sleep apnea on retinal vasculature are limited. A few studies have shown associations between sleep apnea and changes to retinal vascular caliber or retinopathy, but the results have not been consistent [10–12].
In this study, we examined the relationships between a history of sleep apnea, retinal vascular changes, and CVD in the Multi-Ethnic Study of Atherosclerosis (MESA), which recently reported an association between sleep apnea and increased risk of CVD events [3]. We aimed to clarify the effect of sleep apnea on the retinal microvasculature and to determine if microvasculature changes mediated the effect of sleep apnea on the risk of CVD events. Furthermore, we explored gender differences in these associations given prior reports of increased susceptibility of women to microvascular disease [13, 14], and differences in associations between sleep apnea and CVD [15].
Methods
Study population
MESA is a prospective cohort study designed to investigate the characteristics and progression of subclinical CVD in individuals aged 45–84 years and their relationship to demographic and clinical risk factors. Details of the study design have been previously described [16]. In brief, participants from four ethnic groups (Whites 38 %, African-Americans 28 %, Hispanics 23 %, and Asian Chinese 11 %) without any clinically overt CVD at baseline were recruited at six US Field centers from July 2000 to July 2002.
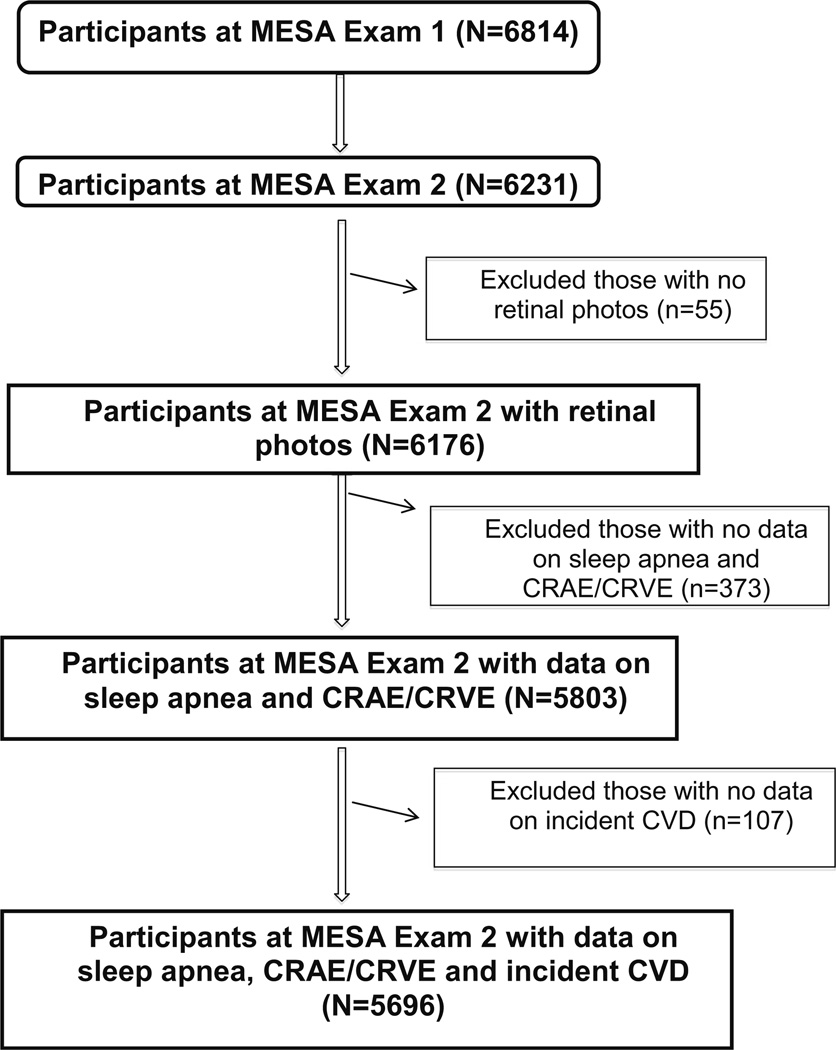
The first part of the analysis was carried out on 5,803 participants in MESA Exam 2 who had retinal photographs suitable for retinal vascular caliber measurements and information on sleep apnea diagnosis available (Fig. 1). We further excluded participants without incident CVD data and performed the second part of the analysis on 5,696 participants. The tenets of the Declaration of Helsinki were observed, and Institutional Review Board approval was granted at each study site. Written informed consent was also obtained from all participants.
Fig. 1.
Participants who were included/excluded from the MESA cohort for this study
Measurement of retinal vascular caliber and assessment of retinopathy
Fundus photography was performed according to a standardized protocol [6]. Participants had both eyes photographed with a 45°, 6.3 megapixel digital nonmydriatic camera in a darkened room. Two photographic fields were taken of each eye: the first centered on the optic disc (field 1) and the second centered on the fovea (field 2). Analysis was based on the right eye, and left eye if there was no suitable right eye image.
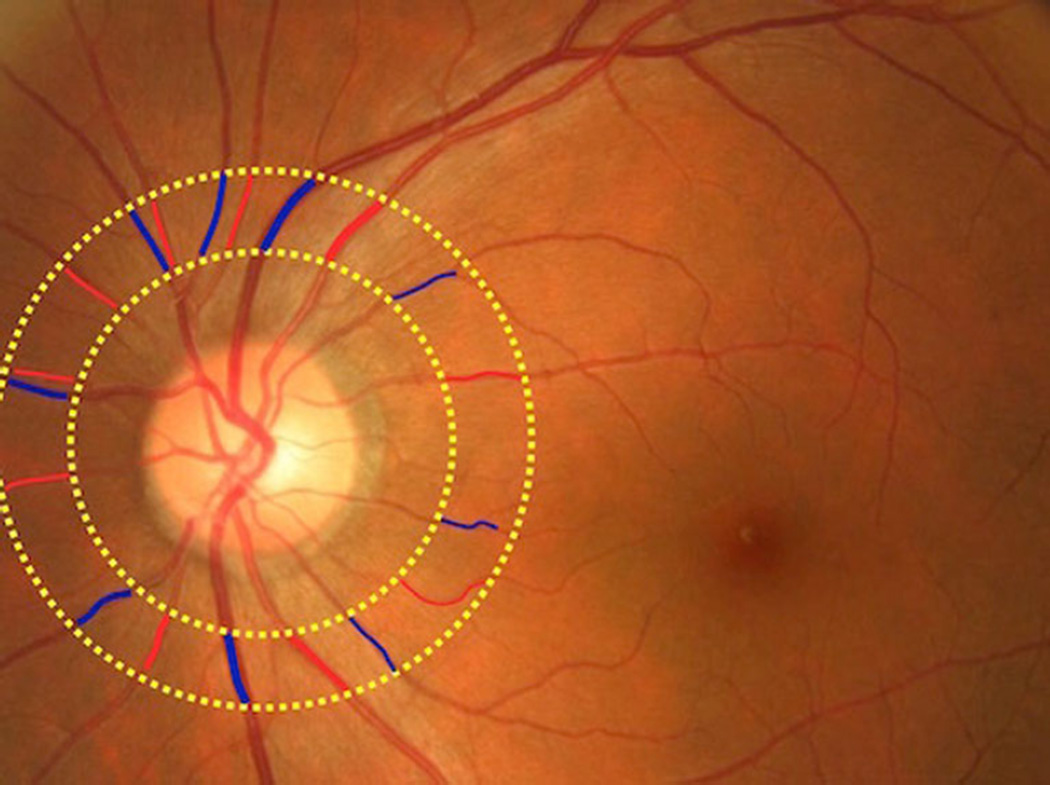
Retinal vascular caliber was measured with a computer-based program (IVAN, University of Wisconsin, Madison), based on a detailed protocol [6]. Trained graders who were masked to participant characteristics carried out these measurements. For each photograph, all arterioles and venules coursing through an area 0.5 to 1 disc diameter from the optic disc margin were measured (Fig. 2) and summarized as the central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE) [17, 18]. The equivalents represent the average projected calibers for the central retinal vessels, measured away from the optic disc. Reproducibility of retinal vascular measurements has been reported, with intra- and intergrader intraclass correlation coefficients in the range between 0.78 and 0.99 [6, 17].
Fig. 2.
Fundus photograph with the identification of arterioles and venules in an area 0.5 to 1 disc diameter from the optic disc margin
The assessment of retinopathy was based on the identification of any characteristic lesion (microaneurysms, hemorrhages, cotton wool spots, hard exudates, intraretinal microvascular abnormalities, venous beading, and new vessels) defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) and a severity score assigned to each eye using the modified Airlie House Classification system. Any retinopathy was defined as having a score higher than or equal to levels 14. Focal arteriolar narrowing and arteriovenous nicking was also identified with the use of similar standardized protocols [19].
Assessment of sleep disordered breathing status
Sleep disordered breathing (SDB) consisting of sleep apnea and snoring was assessed during the second MESA examination with a self-administered sleep history questionnaire [3]. Physician-diagnosed sleep apnea (PDSA) was determined with the question “Have you ever been told by a doctor that you had sleep apnea (a condition in which breathing stops briefly during sleep)?”. Participants were provided with thee potential responses: “yes”, “no,” and “don’t know.” Participants with “don’t know” responses were excluded from the analysis. Snoring was also assessed using the questions “Have you ever snored (now or at any time in the past)?” and “How often do you snore now?” (Responses included [a] do not snore anymore, [b] up to 2 nights/week, [c] 3–5 nights/week, [d] 6–7 nights/week, and [e] don’t know). “Habitual snorers (HS)” were defined as participants who had a current snoring frequency greater than or equal to 3–5 nights/week and did not have PDSA [3]. Participants who were not classified as PDSA or HS were classified as having “No SDB” [3].
Assessment of cardiovascular and other risk factors
Detailed interview questionnaires were used to obtain information about past medical conditions, physical activity, tobacco usage, alcohol consumption, and medication use [16].
Height and weight were measured, and the body mass index (BMI) was calculated as kilograms per square meter. Systolic and diastolic blood pressure was measured at rest, and hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medications. Diabetes mellitus was defined as fasting glucose ≥7.0 mmol/L (126 mg/dL) or use of insulin or oral hypoglycemic medication.
Fasting (>8 h) blood samples were drawn from participants according to standardized protocols [16]. The following were then analyzed in the blood samples: serum glucose, plasma total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, plasma triglycerides, and serum high-sensitivity C-reactive protein (hsCRP). LDL cholesterol was calculated with the Friedewald equation.
Cardiovascular events
Cardiovascular events were ascertained through follow-up telephone interviews, clinic visits, participant call-ins, medical record abstractions, and obituaries. These were further verified with next-of-kin interviews and by obtaining hospital records. The information was reviewed by two paired physicians for independent classification of end-points and then to a full review committee if disagreements arose. Participants with known CVD were excluded from the baseline visit. Incident CVD was defined as any incident myocardial infarction, resuscitated cardiac arrest, definite angina, probable angina (if followed by revascularization), stroke, stroke death, coronary heart disease death, other atherosclerotic death, or other CVD death after the baseline visit as defined by the MESA protocol.
Statistical analysis
Demographic characteristic of participants were described based on their sleep status: No SDB, HS, or PDSA. Frequencies and proportions were calculated for categorical variables, and either means with standard deviation (SD) or medians with interquartile range (IQR) were calculated for continuous variables. Comparison of continuous variables and categorical variables were tested with the analysis of variance and Chi-squared test, respectively; Kruskal-Wallis rank test was used for comparing variables with skewed distribution.
We used multiple linear regression models to explore the association between CRAE, CRVE, and SDB. Models were adjusted for risk factors that were significantly associated (p<0.05) with SDB in univariate analysis. Multivariable logistic regression models were used for the association between any retinopathy and SDB. Multicollinearity among risk factors was checked for each model using the variance inflation factor and interaction was tested for by entering product terms into the regression models.
We compared absolute event rates of CVD by gender and estimated the incidence rate ratio (IRR) using multiple Poisson regression models for the presence of retinal microvascular signs or comparison between tertiles of retinal vessel caliber (i.e., CRAE and CRVE) and SDB. Retinal arteriolar and venular calibers were included together in one model [20]. Lastly, we examined the joint associations of CRAE and PDSA with the IRR.
All statistical analysis was performed using Stata version 11.2 (StataCorp, College Station, Texas). p value <0.05 was considered as statistically significant.
Results
Social demographics and clinical characteristics
Among the participants who were included in this study, 205 had a history of PDSA, 1,301 were HS, and 4,297 of them had neither. Demographic and participant characteristics according to SDB status: normal, HS, or PDSA are compared in Table 1. In general, participants with PDSA and HS were more likely to be younger, male, a current smoker, more obese, have diabetes, have higher diastolic blood pressure, triglycerides, C-reactive protein, and glucose levels, and have lower cholesterol levels than persons with no SDB. Participant characteristics according to retinal microvascular signs are also provided (Appendix: Table 1.1). Left eye data were used for retinal vascular caliber in 300 (5.2 %) and for retinopathy in 255 (4.7 %) of participants. There was significant interaction between gender and SDB status. Separate analyses were therefore performed for men and women.
Table 1.
Characteristics of participants by sleep disordered breathing (SDB) status (n=5,803)
| No SDB n=4,297 n (%) |
HS n=1,301 n (%) |
PDSA n=205 n (%) |
p value | |
|---|---|---|---|---|
| Categorical variables | ||||
| Women | 2,426 (56.5) | 537 (41.3) | 70 (34.2) | <0.001 |
| Menopause, yes | 1,958 (80.7) | 427 (79.5) | 58 (82.9) | 0.725 |
| Race | ||||
| Caucasian | 1,785 (41.5) | 443 (34.1) | 94 (45.9) | <0.001 |
| African-Americans | 1,205 (28.0) | 297 (22.8) | 64 (31.2) | |
| Hispanic | 830 (19.3) | 370 (28.4) | 38 (18.5) | |
| Chinese Americans | 477 (11.1) | 191 (14.7) | 9 (4.4) | |
| Diabetes mellitus | 568 (13.3) | 219 (16.9) | 42 (20.5) | 0.004 |
| Diabetic retinopathy | 461 (10.7) | 135 (10.4) | 25 (12.2) | 0.756 |
| Current smoker | 469 (11.0) | 159 (12.3) | 29 (14.1) | 0.001 |
| Arteriovenous nicking | 175 (4.2) | 57 (4.5) | 8 (4.1) | 0.910 |
| Focal arteriolar narrowing | 41 (1.0) | 10 (0.8) | 4 (2.1) | 0.239 |
| Statin use, yes | 613 (14.3) | 1,118 (14.0) | 50 (24.4) | 0.002 |
| Continuous variables | ||||
| Agea, years | 64.0 (17.0) | 60.0 (14.0) | 62.0 (14.0) | 0.001 |
| Physical activitiesa, min/week | 3,600 (4,680) | 3,713 (5,156) | 3,630.0 (509) | 0.970 |
| Systolic blood pressurea, mmHg | 123.9 (21.2) | 124.4 (19.5) | 123.1 (18.6) | 0.675 |
| Diastolic blood pressure, mmHg | 70.1 (10.0) | 71.9 (10.0) | 71.4 (10.0) | <0.001 |
| Body Mass Index, kg/m2 | 27.8 (5.2) | 29.8 (5.8) | 32.1 (6.0) | <0.001 |
| Glucosea, (mg/dL) | 103.7 (28.9) | 109.3 (35.8) | 109.3 (26.9) | <0.001 |
| Cholesterol, (mg/dL) | 191.6 (35.9) | 192.1 (35.4) | 182.4 (33.4) | 0.001 |
| LDL, (mg/dL) | 113.4 (32.2) | 115.6 (31.6) | 107.1 (31.6) | 0.001 |
| HDL, (mg/dL) | 53.1 (15.6) | 48.3 (13.2) | 47.7 (12.8) | <0.001 |
| Triglyceridesa, (mg/dL) | 108.0 (77.0) | 123.0 (91.0) | 115.0 (86.5) | <0.001 |
| Serum hsCRPa, (mg/dL) | 1.80 (3.27) | 2.02 (3.56) | 2.36 (4.51) | 0.036 |
| CRAE, (µm) | 144.4 (13.9) | 143.9 (14.3) | 142.3 (13.9) | 0.074 |
| CRVE, (µm) | 213.7 (22.1) | 216.0 (21.4) | 213.8 (23.6) | 0.004 |
Characteristics were expressed as the mean (standard deviation) for continuous variables and the number (%) for dichotomous variables
CRAE central retinal artery equivalent, CRVE central retinal vein equivalent, LDL low-density lipoprotein, HDL high-density lipoprotein, hsCRP high-sensitivity C-reactive protein, HS habitual snorers, PDSA physician-diagnosed sleep apnea
Numbers set in bold indicate p<0.05
Median (interquartile range [IQR]) is shown, and Kruskal-Wallis rank test was used for comparison
There was a significant difference (p=0.008) in CRAE between women with (mean = 141.3 µm, Standard deviation [SD] 12.4 µm) and without (mean = 145.8 µm, SD = 13.9 µm) PDSA. In men, there was no difference in CRAE between participants with PDSA (mean = 142.8 µm, SD = 14.0 µm) and those without PDSA (mean = 142.6 µm, SD = 14.6 µm).
Table 2 shows that PDSA was significantly associated with narrower arterioles in women after multivariate analysis in both model 1 (β [regression coefficient] −5.32; 95 % confidence interval [CI] −8.13, −2.51) and model 2 (β 5.76; 95 % CI −8.51, −3.02). No associations were observed between PDSA and CRAE in men, or between PDSA and CRVE in either men or women. HS was also not associated with either CRAE or CRVE. There was also no significant association between SDB status and retinopathy or focal retinal arteriolar abnormalities (data not shown).
Table 2.
Risk associations of retinal vessel calibers and sleep disordered breathing (SDB) status by gender
| SDB status |
CRAE | CRVE | ||
|---|---|---|---|---|
| Model 1: β (95 % CI) | Model 2: β (95 % CI) | Model 1: β (95 % CI) | Model 2: β (95 % CI) | |
| Female | ||||
| No SDB | 0 | 0 | 0 | 0 |
| HS | −0.92 (−2.23, 0.38) | −0.38 (−1.69, 0.93) | 1.04 (−0.87, 2.96) | 0.30 (−1.69, 2.29) |
| PDSA | −5.32 (−8.13, −2.51) | −5.76 (−8.51, −3.02) | −1.66 (−6.82, 3.50) | −4.94 (−10.25, 0.36) |
| Male | ||||
| No SDB | 0 | 0 | 0 | 0 |
| HS | −0.69 (−1.93, 0.55) | −0.42 (−1.67, 0.83) | 0.27 (−1.51, 2.05) | −1.12 (−2.95, 0.70) |
| PDSA | −1.16 (−3.56, 1.24) | −1.53 (−3.83, 0.78) | −0.75 (−4.53, 3.02) | −2.84 (−6.57, 0.88) |
Model 1: Adjusted for age, gender, study site, race/ethnicity, and the interaction between sleep disorder and age if needed. Model 2: Adjusted for variables in Model 1 plus cholesterol, high-density lipoprotein, diastolic blood pressure, antihypertensive medication use, body mass index, diabetes, smoking, high-sensitivity C-reactive protein, statin use, and the interaction between sleep disorder and age as needed
95 % CI 95 % confidence interval, β regression coefficient, CRAE central retinal artery equivalent (retinal arteriolar caliber), CRVE central retinal vein equivalent (retinal venular caliber), SDB Sleep disordered breathing, PDSA physician-diagnosed sleep apnea, HS habitual snorers
Numbers set in bold indicate p<0.05
In Table 3, we examined the relationship between retinal vascular caliber, SDB, and incident CVD. After adjusting for cardiovascular risk factors in separate models, incident CVD was significantly associated with the lowest tertile of CRAE (IRR 1.91; 95 % CI 1.08, 3.38) in women, and the lowest tertile of CRAE (IRR 1.67; 95 % CI 1.10, 2.52) and the highest tertile of CRVE (IRR 1.71; 95 % CI 1.13, 2.59) in men. PDSA was also associated with incident CVD, but only in men (IRR 2.03; 95 % CI 1.17, 3.51) and not in women. In supplementary analyses confined to women only, all results were similar after additional adjustment for menopause status. The IRR for the association between PDSA and incident CVD in men changed minimally to 2.06 (95 % CI 1.18, 3.56) when CRAE and CRVE were included in the same model.
Table 3.
Risk associations of Retinal Vessel Calibers, Sleep Disordered Breathing (SDB) and incident Cardiovascular Disease (CVD) (n=5,696)
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Incident rate (‰) | IRR (95 % CI) | p value | IRR (95 % CI) | p value | |
| Female | |||||
| CRAE | |||||
| Tertile 3, >150 µm | 4.5 (3.1, 6.5) | 1 | 1 | ||
| Tertile 2, 139–150 µm | 4.8 (3.3, 7.1) | 1.09 (0.62, 1.91) | 0.774 | 1.09 (0.62, 1.92) | 0.759 |
| Tertile 1, <139 µm | 9.2 (6.8, 12.2) | 1.91 (1.08, 3.38) | 0.026 | 1.91 (1.08, 3.38) | 0.027 |
| CRVE | |||||
| Tertile 1, ≤204 µm | 6.9 (5.0, 9.3) | 1 | 1 | ||
| Tertile 2, 204–223 µm | 4.7 (3.2, 6.9) | 0.99 (0.57, 1.71) | 0.964 | 0.99 (0.57, 1.71) | 0.975 |
| Tertile 3, ≥223 µm | 6.4 (4.6, 8.9) | 1.67 (0.94, 2.99) | 0.082 | 1.69 (0.95, 3.03) | 0.076 |
| SDB status | |||||
| No SDB | 6.1 (4.9, 7.5) | 1 | 1 | ||
| HS | 5.3 (3.3, 8.7) | 0.96 (0.54, 1.72) | 0.896 | 0.95 (0.53, 1.69) | 0.850 |
| PDSA | 8.4 (2.7, 25.9) | 1.80 (0.55, 5.88) | 0.329 | 1.76 (0.54, 5.74) | 0.350 |
| Male | |||||
| CRAE | |||||
| Tertile 3, >150 µm | 10.1 (7.5, 13.6) | 1 | 1 | ||
| Tertile 2, 139–150 µm | 11.2 (8.8, 14.6) | 1.21 (0.80, 1.83) | 0.365 | 1.23 (0.82, 1.87) | 0.319 |
| Tertile 1, <139 µm | 16.3 (13.3, 20.1) | 1.67 (1.10, 2.52) | 0.016 | 1.74 (1.15, 2.66) | 0.010 |
| CRVE | |||||
| Tertile 1, ≤204 µm | 11.8 (9.1, 15.3) | 1 | 1 | ||
| Tertile 2, 204–223 µm | 12.6 (9.9, 16.1) | 1.38 (0.94, 2.03) | 0.103 | 1.35 (0.92, 1.99) | 0.130 |
| Tertile 3, ≥223 µm | 14.0 (11.1, 17.7) | 1.71 (1.13, 2.59) | 0.012 | 1.67 (1.10, 2.55) | 0.016 |
| SDB status | |||||
| No SDB | 13.2 (11.1, 15.6) | 1 | 1 | ||
| HS | 10.2 (7.6, 13.8) | 0.99 (0.68, 1.42) | 0.942 | 0.99 (0.69, 1.43) | 0.962 |
| PDSA | 23.4 (14.3, 38.2) | 2.03 (1.17, 3.51) | <0.001 | 2.06 (1.18, 3.56) | 0.010 |
Model 1: Separate models for CRAE and CRVE in one model and SDB status in another model. Adjusted for age, race/ethnicity, BMI, cigarette smoking, diabetes mellitus, total cholesterol, HDL, triglycerides, diastolic blood pressure, antihypertensive medication use, statin use, benzodiazepine use, and current alcohol use. Model 2: CRAE, CRVE, and SDB status analyzed simultaneously in a single model and adjusted for variables in Model 1
IRR incidence rate ratios, 95 % CI 95 % confidence interval, CRAE central retinal artery equivalent, CRVE central retinal vein equivalent, SDB Sleep disordered breathing, PDSA physician-diagnosed sleep apnea, HS habitual snorers
Numbers set in bold indicate p<0.05
We also examined the joint associations of CRAE and PDSA with incident CVD in Table 4. There was a notable increase in the incident rate ratio of CVD from 1.53 (95 % CI 1.17, 2.00) for those with either PDSA or narrower retinal arterioles, to 3.41 (95 % CI 1.79, 6.50) for those who had both PDSA as well as narrower retinal arterioles.
Table 4.
Joint associations of CRAE and PDSA with incident CVD
| Group | Person year | Number of CVD cases | Incident rate | IRR (95 % CI) | p value |
|---|---|---|---|---|---|
| 1 | 20,681 | 149 | 7.2 (6.1, 8.5) | 1 | |
| 2/3 | 10,758 | 133 | 12.4 (10.4, 14.6) | 1.53 (1.17, 2.00) | 0.002 |
| 4 | 392 | 11 | 2.8 (1.6, 50.7) | 3.41 (1.79, 6.50) | <0.001 |
Group 1: No PDSA and two higher tertiles of CRAE (n=3,682); Group 2: No PDSA and lowest tertile of CRAE (n=1,817); Group 3: PDSA and two higher tertiles of CRAE (n=121); Group 4: PDSA and lowest tertile of CRAE (n=76). Model: Adjusted for age, gender, race/ethnicity, BMI, cigarette smoking, diabetes mellitus, total cholesterol, HDL, triglycerides, diastolic blood pressure, antihypertensive medication use, statin use, benzodiazepine use, current alcohol use, and CRVE
IRR incidence rate ratios, 95 % CI 95 % confidence interval, CRAE central retinal artery equivalent, PDSA physician-diagnosed sleep apnea, CVD cardiovascular disease
Numbers set in bold indicate p<0.05
Discussion
In this multi-ethnic cohort study, our data showed that women with PDSA had narrower retinal arteriolar caliber. This cross-sectional association could not be explained by traditional cardiovascular risk factors. In addition, we showed that men but not women with PDSA were at an increased risk of CVD and that retinal vascular caliber did not seem to mediate this relationship. Those who had both PDSA and narrower retinal arterioles also had a greater incident rate ratio of CVD as compared to those who had only one of either PDSA or narrower retinal arterioles.
Our finding that women with PDSA had narrower retinal arterioles is consistent with data from the Sleep Heart Health Study [11], which demonstrated a significant association between reduced arteriovenous ratio (AVR) and increased respiratory disturbance index (RDI), most consistent in the range where RDI was less than 10. On the other hand, there was no association between PDSA and narrower retinal arterioles among men in our study. This could be explained in part by the difference in severity of PDSA between men and women. In practice, there is a lower index of suspicion for sleep apnea in women and this could lead to underdiagnosis of the condition but with more severe cases being detected among them. Alternatively, women may be more susceptible than men to microvascular injury from cardio-metabolic effects [13]. This gender difference is supported by evidence of greater endothelial dysfunction in women compared to men with sleep apnea [21], as well as a greater impact of diabetes on heart disease mortality in women as compared to men [22]. Furthermore, population studies have showed an association between retinal arteriolar narrowing and coronary heart disease in women but not men [23, 24], and may represent the greater role of microvascular dysfunction and remodeling in CVD in women.
In contrast to our findings, Shankar et al. showed that more severe sleep apnea, quantified using the apnea-hypopnea index, was associated with retinal venular widening but not retinal arteriolar narrowing [12]. This suggested that inflammation and metabolic irregularities, which are associated with retinal venular widening, may have played a role in mediating the effects of sleep apnea [25]. The reason for the discrepancy is not clear, but could have arisen from methodological or racial differences between the two studies. While our cohort is multiethnic, the report of Shankar et al. was based on a predominantly White cohort. It has been shown that racial differences do exist in the interplay of adiposity and inflammation [26].
There was also no apparent association between PDSA and retinopathy signs in our study. Sleep apnea was reportedly associated with retinal microaneurysms in the Sleep Heart Health Study, and similarly, a few other studies have associated sleep apnea with the presence of retinopathy among people with diabetes [11, 10]. Our study population consisted of generally healthy adults free of CVD and may have had limited power to detect differences in retinopathy.
Before testing the mediating effects of retinal vascular calibers in the relationship between PDSA and incident CVD, we first confirmed the association between retinal vascular calibers and incident CVD. Our results showed that retinal arteriolar narrowing and retinal venular widening in men and retinal arteriolar narrowing in women were associated with an increased risk of incident CVD in the MESA cohort. Retinal venular narrowing in women showed a trend toward increased CVD risk but did not reach statistical significance (p=0.082). This finding is fairly consistent with previous prospective population-based studies [27, 23]. Recent meta-analyses of 6 large cohort studies also showed that the risk of coronary heart disease is increased in women with narrower retinal arterioles and wider venules, and that increased stroke risk is associated with wider retinal venular caliber [24, 28].
With the similar influence of sleep apnea on the incidence of CVD as reported previously in the MESA [3], we performed further analysis to determine whether retinal arteriolar narrowing or retinal venular widening might explain, in part, the association between sleep apnea and the risk of incident CVD. However, the beta coefficients for the association between PDSA and incident CVD, before and after additional adjustment for retinal vascular calibers remained relatively unchanged. This suggests that PDSA increases the risk for CVD through pathways that do not involve changes to microvascular caliber or traditional cardiovascular risk factors. We further showed that having a combination of both PDSA and narrowed retinal arterioles resulted in an even greater risk for incident CVD, suggesting additive influences. Further studies are still needed to verify the role of microvascular disease in the link between sleep apnea and CVD risk.
Strengths of our study include its relatively large multiethnic population-based cohort, comprehensive and standardized collection of data on potential confounders, measurements of retinal vascular caliber and grading of retinopathy according to standardized protocols with good reliability, and thorough adjudication process for CVD events. Limitations should also be noted. First, the problems with a cross-sectional analysis for the relationship between sleep apnea and retinal vascular caliber are its inability to establish temporality and possible survival bias However, given the pathophysiological pathways described above and our present knowledge on the development of sleep apnea, it seems more likely that sleep apnea leads to the variations in retinal vascular caliber than the converse. Second, the diagnosis of sleep apnea was based on self-reported information, which may be influenced by recall bias and is likely a specific but not sensitive indicator of sleep apnea. The 3.5 % prevalence of PDSA in this sample is approximately 3-fold lower than what has been reported in population studies, indicating the likely underestimation of this disorder [29, 30]. Third, there were no objective measures of sleep apnea severity such as measures of intermittent hypoxemia and we did not have information on subjects who might have been treated for their sleep apnea. Fourth, although we had a large population, there may have been limited power to detect meaningful associations because of low event rates, particularly among relationships that involve retinopathy, focal narrowing, AV nicking, incident CVD in women or in Group 4 of the subgroup analysis that explored joint associations (Table 4). Finally, despite the adjustment for many risk factors, the unexplained variance in our statistical models also suggests that there might still be unmeasured residual confounders (e.g., long-term effect of hypertension) that we have not accounted for.
In conclusion, our data demonstrated an association between PDSA and narrower retinal arterioles in women. There was also an increased risk of CVD in men and women with narrower retinal arterioles, and men with wider retinal venules and PDSA. The increased risk of CVD in men with PDSA was not mediated by variations in retinal vascular caliber, and having both narrower retinal arterioles and PDSA further increased the risk of CVD, suggesting that they increase CVD risk through different pathophysiological mechanisms. Additional research utilizing quantitative measures of SDB and assessing both the micro- and macro-vascular are needed to further investigate the pathophysiological processes associated with SDB-associated CVD.
Supplementary Material
Acknowledgments
The authors would like to thank the investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding: This research was supported by contracts, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95167, and a grant NHLBI T32 HL076132 from the National Heart, Lung and Blood Institute. Additional support was provided by National Institute of Health grant HL69979-03 (Klein R, Wong TY).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11325-015-1177-z) contains supplementary material, which is available to authorized users.
Disclosures None of the authors have any conflicts of interest.
References
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeboah J, Redline S, Johnson C, Tracy R, Ouyang P, Blumenthal RS, Burke GL, Herrington DM. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis. 2011;219:963–968. doi: 10.1016/j.atherosclerosis.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111:1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 7.Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. 2008;27:161–176. doi: 10.1016/j.preteyeres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Cheung N, Bluemke DA, Klein R, Sharrett AR, Islam FM, Cotch MF, Klein BE, Criqui MH, Wong TY. Retinal arteriolar narrowing and left ventricular remodeling: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2007;50:48–55. doi: 10.1016/j.jacc.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R, Coker LH, Knopman DS, Shibata DK, Catellier D, Wong TY. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain. 2010;133:1987–1993. doi: 10.1093/brain/awq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West SD, Groves DC, Lipinski HJ, Nicoll DJ, Mason RH, Scanlon PH, Stradling JR. The prevalence of retinopathy in men with Type 2 diabetes and obstructive sleep apnoea. Diabet Med. 2010;27:423–430. doi: 10.1111/j.1464-5491.2010.02962.x. [DOI] [PubMed] [Google Scholar]
- 11.Boland LL, Shahar E, Wong TY, Klein R, Punjabi N, Robbins JA, Newman AB. Sleep-disordered breathing is not associated with the presence of retinal microvascular abnormalities: the Sleep Heart Health Study. Sleep. 2004;27:467–473. doi: 10.1093/sleep/27.3.467. [DOI] [PubMed] [Google Scholar]
- 12.Shankar A, Peppard PE, Young T, Klein BE, Klein R, Nieto FJ. Sleep-disordered breathing and retinal microvascular diameter. Atherosclerosis. 2013;226:124–128. doi: 10.1016/j.atherosclerosis.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res. 2011;90:9–17. doi: 10.1093/cvr/cvq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 18.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki R, Cheung N, Wang JJ, Klein R, Klein BE, Cotch MF, Sharrett AR, Shea S, Islam FA, Wong TY. Retinal vessel diameters and risk of hypertension: the Multiethnic Study of Atherosclerosis. J Hypertens. 2009;27:2386–2393. doi: 10.1097/HJH.0b013e3283310f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liew G, Sharrett AR, Kronmal R, Klein R, Wong TY, Mitchell P, Kifley A, Wang JJ. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48:52–57. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- 21.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27:1113–1120. doi: 10.1093/sleep/27.6.1113. [DOI] [PubMed] [Google Scholar]
- 22.Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA. 1988;260:3456–3460. [PubMed] [Google Scholar]
- 23.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 24.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, Dejong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151:404–413. doi: 10.7326/0003-4819-151-6-200909150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, de Jong PT. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 26.Negi SI, Pankow JS, Fernstrom K, Hoogeveen RC, Zhu N, Couper D, Schmidt MI, Duncan BB, Ballantyne CM. Racial differences in association of elevated interleukin-18 levels with type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2012;35:1513–1518. doi: 10.2337/dc11-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RG, Prince CT, Klein R, Orchard TJ. Retinal vessel diameter and the incidence of coronary artery disease in type 1 diabetes. Am J Ophthalmol. 2009;147:653–660. doi: 10.1016/j.ajo.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, de Jong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–1332. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 30.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.