Abstract
Angiotensin II (Ang II) is a major determinant of inward remodeling and hypertrophy in pial arterioles that may have an important role in stroke during chronic hypertension. Previously we found that epidermal growth factor receptor (EGFR) is critical in Ang II-mediated hypertrophy that may involve caveolin-1 (Cav-1). In this study, we examined the effects of Cav-1 and matrix metalloproteinase-9 (MMP9) on Ang II-mediated structural changes in pial arterioles. Cav-1-deficient (Cav-1−/−), MMP9-deficient (MMP9−/−) and wild-type (WT) mice were infused with either Ang II (1000 ng/kg/min) or saline via osmotic minipumps for 28 days (n=6–8 per group). Systolic arterial pressure was measured by a tail-cuff method. Pressure and diameter of pial arterioles were measured through an open cranial window in anesthetized mice. Cross-sectional area of the wall was determined histologically in pressurized fixed pial arterioles. Expression of Cav-1, MMP9, phosphorylated-EGFR and Akt was determined by western blotting and immunohistochemistry. Deficiency of Cav-1 or MMP9 did not affect Ang II-induced hypertension. Ang II increased expression of Cav-1, pEGFR and Akt in WT mice that was attenuated in Cav-1−/− mice. Ang II-induced hypertrophy, inward remodeling and increased MMP9 expression in pial arterioles was prevented in Cav-1−/− mice. Ang II-mediated increases in MMP9 expression and inward remodeling, but not hypertrophy, was prevented in MMP9−/− mice. In conclusion, Cav-1 is essential in Ang II-mediated inward remodeling and hypertrophy in pial arterioles. Cav-1 induced MMP9 is exclusively involved in inward remodeling, not hypertrophy. Further studies are needed to determine the role of Akt in Ang II-mediated hypertrophy.
Keywords: angiotensin II, caveolin-1, cerebrovascular, hypertrophy, remodeling, MMP9
Introduction
Chronic hypertension causes structural alterations of the vascular wall, including hypertrophy (increases in wall cross-sectional area, CSA) and inward remodeling (reduction of lumen external diameter, ED).1 These changes in the cerebral circulation may become maladaptive, adversely affect local blood flow control and therefore contribute to the increased risk of stroke.2 Despite decades of research efforts, the molecular mechanisms associated with structural changes of cerebral vasculature in chronic hypertension have not been fully elucidated.
One of the hallmark effects of angiotensin II (Ang II) on cerebral pial arterioles is hypertrophy.3 Recently, we found that Ang II induces hypertrophy in pial arterioles through signaling events that involve epidermal growth factor receptor (EGFR) activation and other signaling molecules such as caveolin-1 (Cav-1) and c-Src.4 These results lead to the current study in which we examined the possible roles of Cav-1 and caveolae-compartmentalized-specific signaling cascade in Ang II-mediated hypertrophy in pial arterioles. Caveolae are plasma membrane microdomains (lipid rafts) that serve as a signaling platform to facilitate the spatial localization of signal transduction events stimulated by Ang II.5 Cav-1 is the major structural, as well as signaling, protein component in vascular caveolae.6, 7 In vascular smooth muscle cells (VSMCs), Ang II promotes the association of Ang II type 1 receptor (AT1R) with Cav-1, which in turn enables trafficking of AT1R into caveolin-enriched lipid rafts.6 This AT1R trafficking requires other signaling molecules, including reactive oxygen species and c-Src.6,8 AT1R transactivation of EGFR appears to take place in Cav-1-enriched caveolae, followed by activation of downstream EGFR-dependent signaling events for hypertrophy, including Akt/protein kinase B.9,10 These findings, however, have been performed in cell culture settings and have yet to be proven in cerebral vasculature in vivo. Therefore the first goal of this study was to examine the role of Cav-1 in Ang II-mediated hypertrophy in pial arterioles using Cav-1 knockout mice, which exhibit complete loss of Cav-1 within blood vessels.11 We also further investigated the possible involvement of the downstream signaling molecule Akt, which has been shown to have a role in hypertrophy.9
Ang II also plays a critical role in inward remodeling of pial arterioles.12 Many studies have demonstrated that the molecular mechanism responsible for Ang II-induced inward remodeling differs in important aspects from that of hypertrophy. However, we previously found that redox-sensitive signaling is involved in both Ang II-mediated hypertrophy and inward remodeling in pial arterioles,13 suggesting a certain overlapping of mechanisms between the two. Because Cav-1 is also redox-sensitive and can be activated by reactive oxygen species derived from nicotinamide adenine dinucleotide phosphate (NADPH) oxidase,6, 8 our second goal was to determine if Cav-1 is involved in inward remodeling. In addition, matrix metalloproteinases (MMPs), a family of zinc-dependent extracellular proteinase involved in the degradation of basal lamina and extracellular matrix, have been implicated in vascular remodeling in hypertension.14, 15 In particular, several studies suggest that activation of MMP9 in small vessels is also redox sensitive and Ang II-dependent.16, 17 Therefore, our third goal was to determine if MMP9 is involved in Ang II-induced and Cav-1-mediated inward remodeling in pial arterioles.
Materials and Methods
Animals
Caveolin-1 deficient mice (Cav-1−/−, 12–14 weeks old, n=60) and background- and age-matched wild type (WT, B6129SF2/J) mice (n=60) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Male-to-female ratio in each experiment and animal group was 50 %. Cav-1−/− and WT mice were derived from heterozygous Cav-1+/− mice in Jackson Laboratories (Strain: STOCK Cav1tm1/Mls/J; Stock #004585). Male MMP9 deficient mice (MMP9−/−, Strain: B6.FVB(Cg)-MMP9tm1Tvu/J; Stock #007084; 12–14 weeks old, n=40) and background- and age-matched WT (C57BL6/J) mice (n=40) were purchased from Jackson Laboratory. Animals were housed in pathogen-free facility at 24°C, exposed to 12 hours of light and allowed free access to standard chow and water. All procedures were approved by Institutional Animal Care and Use Committee of the University of Iowa and in agreement with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Statistical analysis
All data are presented as mean ± SEM. Differences were analyzed by one-way analysis of variance and post hoc test for 3 or more groups or t-test for 2 groups using Graph Pad prism 6 (Graph Pad Software, Inc., San Diego, CA, USA). Difference were considered significant when P<0.05.
For the detailed description of experimental procedures please refer to Materials and Methods in the online-only Data Supplement.
Results
Ang II increases Cav-1 Expression in Cerebral Pial Vasculature
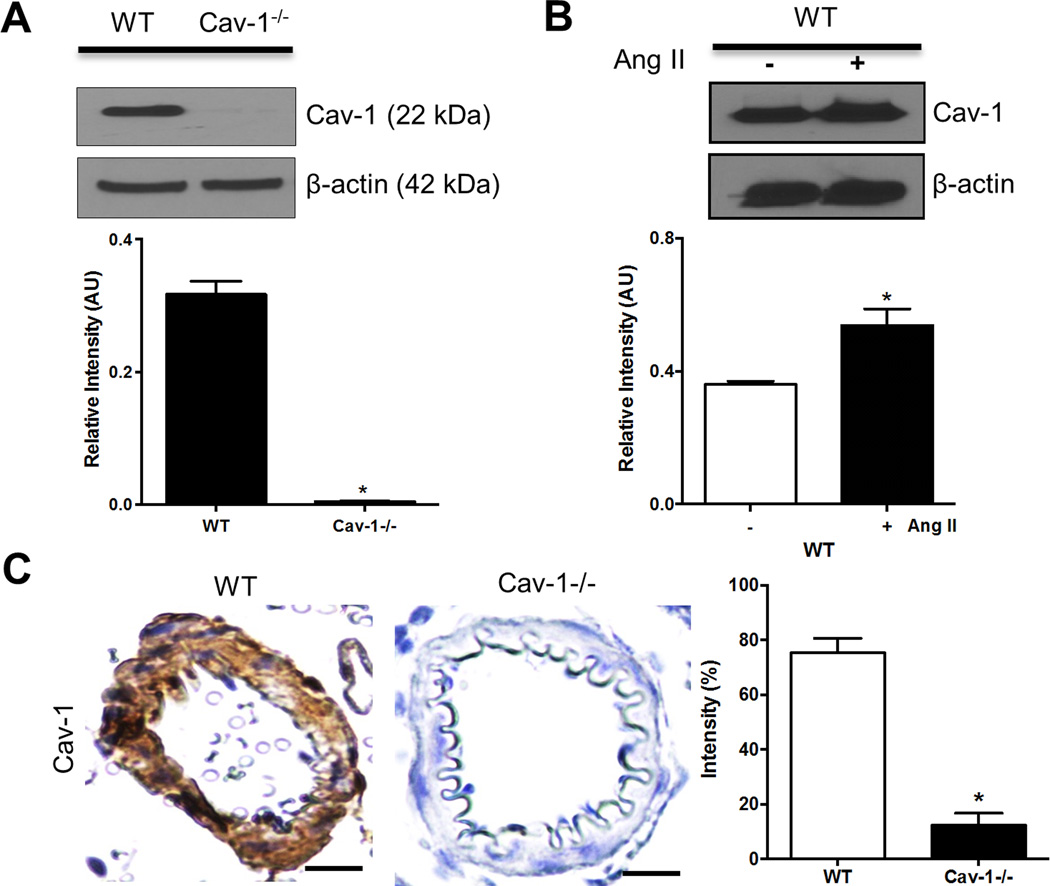
Western blotting of Cav-1 was performed to determine if Ang II activates Cav-1 in pial arteries. Cav-1 protein expression was present in pial arteries from wild-type (WT) mice, but nearly undetectable in Cav-1−/− mice (Figure 1A). In WT mice, Ang II significantly increased protein expression of Cav-1 in pial arteries compared to saline controls (Figure 1B). Furthermore, immunohistochemistry showed that Ang II increased Cav-1 expression in pial arterioles from WT, but not in Cav-1−/− mice (Figure 1C). These results suggest that Ang II activates Cav-1 in cerebral vasculature that was attenuated in Cav-1−/− mice.
Figure 1.
Ang II increases Cav-1 expression in cerebral pial arteries in WT, but not in Cav-1−/− mice. Representative western blot illustrates the presence of Cav-1 in pial arteries from WT but not in Cav-1−/− mice (A); representative images and densitometry of Cav-1 western blotting in pial arteries from Ang II-treated WT mice (B); and representative photographs and staining density of Cav-1 immunohistochemistry in pial arterioles from Ang II-treated WT and Cav-1−/− mice (C). Results are mean ± SEM of 6–8 mice. *P<0.05 vs corresponding controls. Scale bar, 10 µm.
It has been previously shown in VSMC culture studies that the association of AT1R with Cav-1 is critical for the transactivation of EGFR in caveolae.10 In this study, we showed that Ang II-induced increases in expression of pEGFR are significantly reduced in pial arterioles in Cav-1−/− compared to WT mice (Figure S1). This result confirms our previous finding that Cav-1 is involved in Ang II-mediated activation of EGFR in pial arterioles.
Cav-1 Deficiency Prevents Ang II-Mediated Hypertrophy and Inward remodeling of Pial Arterioles
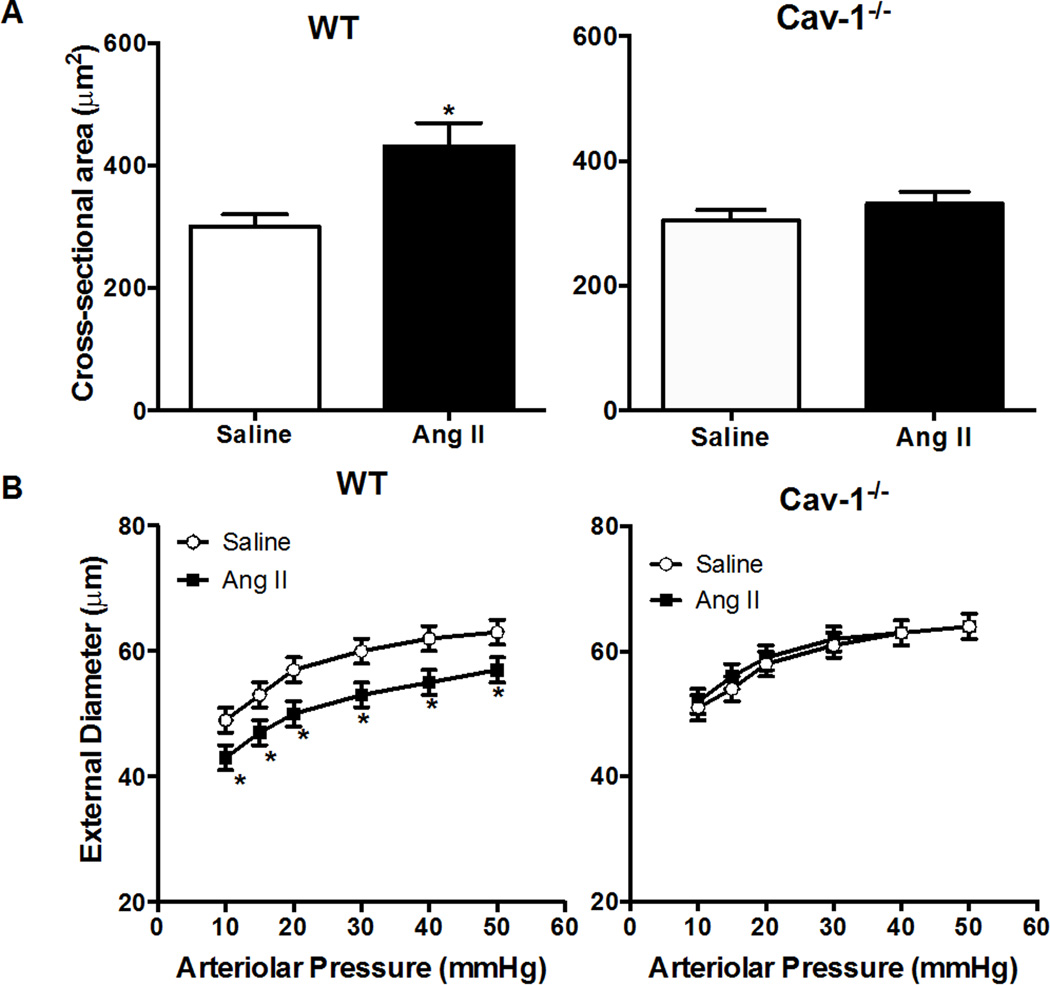
Our findings in a previous study suggested that Cav-1 may be involved in Ang II-mediated hypertrophy in pial arterioles.4 In the present study, we found that cross sectional area of the pial arteriolar wall was significantly increased by Ang II in WT (411±38 versus 300±20 µm2 in saline), but not in Cav-1−/− mice (331±24 versus 305±16 µm2) (Figure 2A). Moreover, Ang II significantly decreased external diameter in WT mice (ED: 57±2 versus 63±2 µm), but not in Cav-1−/− mice (ED: 64±2 versus 64±2 µm) (Figure 2B). Cav-1 deficiency did not affect baseline and Ang II-induced increase in blood pressure (Table 1). These findings indicate a critical role of Cav-1 in Ang II-induced pial arteriolar hypertrophy, as well as inward remodeling that appears to be independent of pressure.
Figure 2.
Deficiency of Cav-1 prevents Ang II-mediated hypertrophy and inward remodeling in pial arterioles. Cross-sectional area of pial arteriolar wall in WT and Cav-1−/− mice treated with saline or Ang II (A); Cross-sectional area of pial arteriolar wall in WT and MMP9−/− mice treated with saline or Ang II. (B): Pressure–diameter relationship of maximally dilated pial arterioles in wild-type (WT) and MMP9−/− mice treated with saline or Ang II.
Table 1.
Physiological data for wild-type (WT) and Cav-1-deficient mice treated with angiotensin II (Ang II) (1000ng/kg/min) or saline for 4 weeks. Systolic arterial pressure was measured by tail cuff method in conscious mice at week 4 of Ang II treatment. Pial arteriolar pressure was measured under anesthetized condition.
| Parameters | WT Saline |
WT Ang II |
Cav-1−/− Saline |
Cav-1−/− Ang II |
|---|---|---|---|---|
| Systolic Arterial Pressure (mmHg) | ||||
| Week 4 | 128 ± 3 | 175 ± 6* | 127 ± 2 | 178 ± 4* |
| Pial arteriolar pressure (mmHg) | ||||
| Systolic | 47 ± 1 | 47 ± 3 | 39 ± 2 | 40 ± 3 |
| Diastolic | 35 ± 2 | 37 ± 3 | 32 ± 2 | 30 ± 3 |
| Mean | 39 ± 1 | 40 ± 3 | 34 ± 2 | 33 ± 3 |
| Pulse | 12 ± 1 | 11 ± 1 | 8 ± 1 | 10 ± 1 |
| Arterial blood gases | ||||
| pH | 7.34 ± 0.02 | 7.35 ± 0.03 | 7.37 ± 0.04 | 7.39 ± 0.02 |
| PCO2 | 30 ± 2 | 32 ± 4 | 28 ± 2 | 29 ± 3 |
| PO2 | 113 ± 4 | 107 ± 5 | 117 ± 7 | 109 ± 8 |
| Age (week) | 14.3 ± 0.3 | 17.8 ± 0.8 | 13.4 ± 0.7 | 18.0 ± 0.5 |
| Weight (g) | 27.4 ± 0.9 | 24.2 ± 1.2 | 23.0 ± 1.0 | 21.0 ± 1.3 |
| N | 8 | 8 | 8 | 8 |
Values are mean±SEM.
P<0.05 versus corresponding saline controls.
Ang II Increases Expression of MMP9
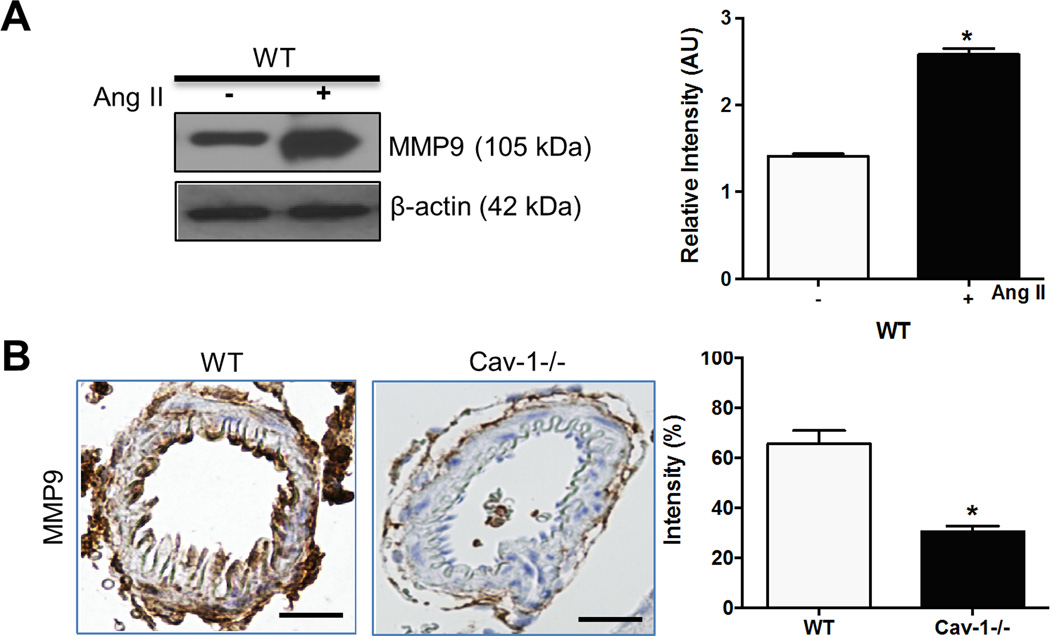
Ang II promotes VSMC migration through MMP9-dependent mechanisms.17 To investigate whether Ang II induces MMP9 expression in pial arteries, western blotting of MMP9 was performed in saline or Ang II-treated WT mice. Baseline (saline-treated) MMP9 expression was low in WT mice (Figure S2). Ang II increased MMP9 levels in pial arteries in WT mice (Figure 3A). Furthermore, immunohistochemical staining showed that Ang II increased MMP9 expression in pial arterioles in WT mice and the increase was attenuated in Cav-1−/− mice (Figure 3B). These results indicate that Cav-1 is essential in Ang II-induced elevation of MMP9 expression in pial arterioles.
Figure 3.
Ang II increases MMP9 expression in pial arteries in WT, but not in Cav-1−/− mice. Representative photograph and densitometry of MMP9 western blotting in pial arteries from saline or Ang II-treated WT mice (A); representative images and staining density of MMP9 immunohistochemistry in pial arterioles from WT and Cav-1−/− mice treated with Ang II (B). Results are mean ± SEM of 6 mice. *P<0.05 vs corresponding controls. Scale bar, 10 µm.
Deficiency of MMP9 Prevents Ang II-Mediated Inward Remodeling But Not Hypertrophy
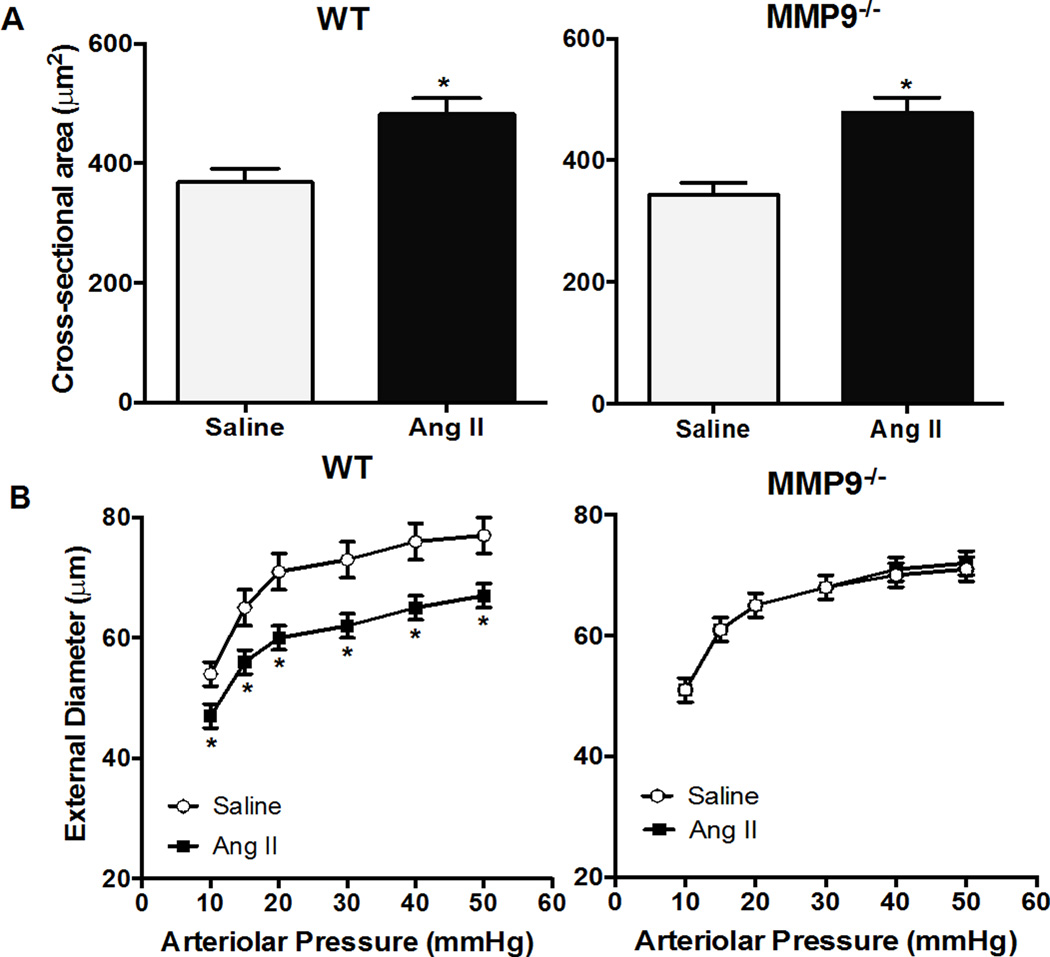
MMP9−/− mice were used to determine whether MMP9 is involved in Ang II-mediated inward remodeling. Baseline (saline-treated) MMP9 expression was low in WT mice (Figure S3A). Ang II-induced expression of MMP9 in pial arterioles from WT mice was significantly attenuated in MMP9−/− mice (Figure S3B). Ang II significantly decreased external diameter (ED: 65±2 versus 75±4 µm) in WT, but not in MMP9−/− mice (ED: 68±4 versus 68±2 µm in saline) (Figure 4B). In addition, cross sectional area of the pial arteriolar wall was significantly increased by Ang II in WT (482±35 versus 369±56 µm2 in saline) as well as in MMP9−/− mice (478±45 versus 343±51 µm2 in saline) (Figure 4A). MMP9 deficiency did not significantly impact baseline and Ang II-induced increased blood pressure (Table 2). These findings suggest that MMP9 is involved in Ang II-induced inward remodeling, but not hypertrophy, in pial arterioles.
Figure 4.
Deficiency of MMP9 prevents Ang II-mediated inward remodeling but not hypertrophy. Pressure-diameter relationship of maximally dilated pial arterioles in WT and MMP9−/− mice treated with saline or Ang II (A); cross-sectional area of pial arteriolar wall in WT and MMP9−/− mice treated with saline or Ang II. (B). Pressure�diameter relationship of maximally dilated pial arterioles in wild-type (WT) and MMP9−/− mice treated with saline or Ang II.
Table 2.
Physiological data for wild-type (WT) and MMP9-deficient mice treated with angiotensin II (Ang II) (1000ng/kg/min) or saline for 4 weeks. Systolic arterial pressure was measured by tail cuff method in conscious mice at week 4 of Ang II treatment. Pial arteriolar pressure was measured under anesthetized condition.
| Parameters | WT Saline |
WT Ang II |
MMP9−/− Saline |
MMP9−/− Ang II |
|---|---|---|---|---|
| Systolic Arterial Pressure (mmHg) | ||||
| Week 4 | 114 ± 5 | 154 ± 6* | 117 ± 4 | 157 ± 4* |
| Pial arteriolar pressure (mmHg) | ||||
| Systolic | 48 ± 4 | 41 ± 5 | 51 ± 4 | 46 ± 2 |
| Diastolic | 34 ± 4 | 30 ± 4 | 38 ± 2 | 32 ± 2 |
| Mean | 38 ± 4 | 34 ± 4 | 42 ± 3 | 37 ± 2 |
| Pulse | 14 ± 1 | 11 ± 2 | 13 ± 2 | 13 ± 1 |
| Arterial blood gases | ||||
| pH | 7.49 ± 0.02 | 7.30 ± 0.03 | 7.30 ± 0.04 | 7.47 ± 0.02 |
| PCO2 | 22 ± 2 | 44 ± 4 | 35 ± 2 | 28 ± 5 |
| PO2 | 168 ± 4 | 97 ± 5 | 122 ± 2 | 136 ± 6 |
| Age (week) | 13 ± 0.3 | 17 0.8 | 12 ± 0.4 | 18 ± 0.5 |
| Weight (g) | 25.0 ± 0.9 | 24.5 ± 1.4 | 26.6 ± 1.2 | 23.9 ± 1.1 |
| N | 6 | 6 | 6 | 6 |
Values are mean±SEM.
P<0.05 versus corresponding saline controls.
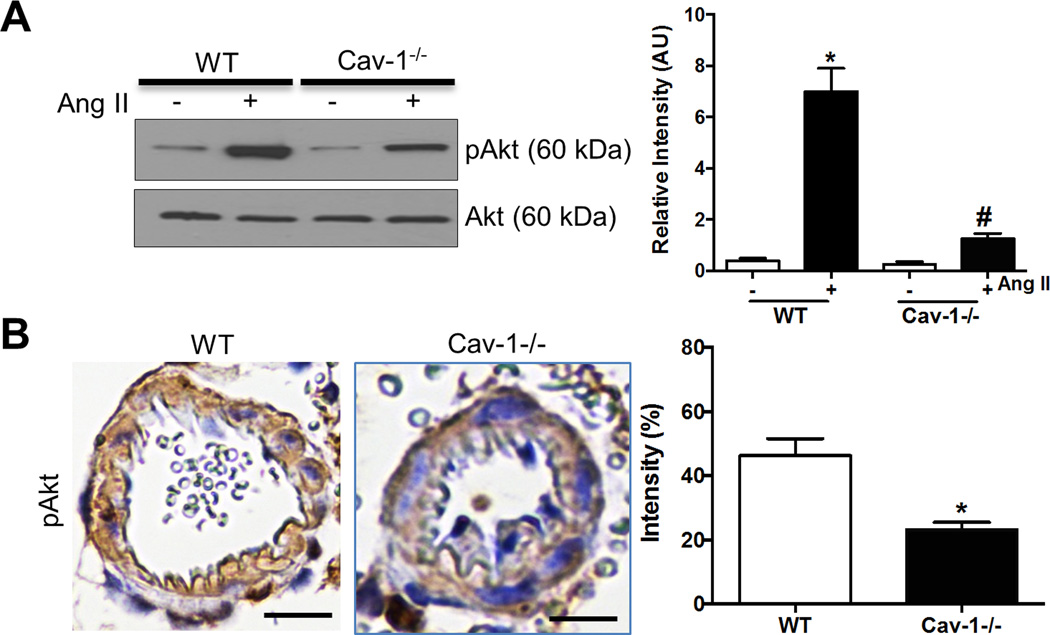
Ang II activation of Akt is Cav-1-dependent
Serine-threonine kinase Akt is known to be activated by EGFR and plays an important role in protein synthesis in the hypertrophic response.9,18 Thus we examined effects of Cav-1 deficiency on expression of Akt in cerebral vasculature. Ang II increased protein expression of pAkt in pial arteries in WT mice that was significantly reduced in Cav-1−/− mice (Figure 5A). Moreover, immunohistochemical staining showed that pAkt was present in pial arterioles in Ang II-treated WT and significantly reduced in Cav-1−/− mice (Figure 5B). These results suggest that Ang II activation of Akt is dependent on Cav-1.
Figure 5.
Ang II increases pAkt expression in WT, but not in Cav-1−/− mice. Representative images and densitometry of phosphorylated Akt western blotting in pial arteries from saline- or Ang II-treated WT and Cav-1−/− mice (A); and representative photographs and straining density of phosphorylated Akt immunohistochemistry in pial arterioles from WT and Cav-1−/− mice with Ang II (B). Results are mean ± SEM of 6–8 mice. *P<0.05 vs WT control; #P<0.05 vs WT with Ang II. Scale bar, 10 µm.
Discussion
Chronic hypertension is one of the most important modifiable risk factors for stroke.2 Therefore, it is important to understand hypertension-induced changes in cerebral small vessels, which are believed to be critical in local blood flow control. The present study builds on our previous findings that EGFR-dependent signaling is critical in Ang II-mediated hypertrophy in pial arterioles4 and, in addition, demonstrates that Cav-1 and MMP9 play an important role in Ang II-induced inward remodeling. There are several novel findings; first, we demonstrated that Cav-1 is critical in Ang II-induced hypertrophy as well as inward remodeling in pial arterioles. Both effects of Cav-1 appear to be pressure-independent. Second, we showed that Ang II-induced expression of MMP9 is Cav-1-dependent. Importantly, MMP9 is a mediator of Ang II-induced inward remodeling, but not hypertrophy, in pial arterioles. Third, Akt activation may be a downstream signaling event of Ang II-induced, Cav-1-mediated hypertrophy in pial arterioles. These findings, for the first time, suggest that Ang II mediates hypertrophy and inward remodeling in pial arterioles via two distinctively different Cav-1-dependent signaling pathways.
Chronic activation of renin-angiotensin system, in particular Ang II, plays an important role in regulating cerebral vascular structural alterations, including hypertrophy and inward remodeling. However, the molecular mechanisms of these changes are not completely understood. Previously we showed that EGFR transactivation by Ang II is critical for hypertrophy in pial arterioles that may also involve Cav-1 signaling.4 This study aimed at further understanding the role of Cav-1 in this process. Surprisingly, Cav-1 deficiency not only inhibits Ang II-mediated hypertrophy, but also inward remodeling, which is independent of EGFR. This finding suggests that Cav-1 mediates inward remodeling in pial arterioles by activating downstream signaling cascade that is independent of EGFR. Apart from that, Cav-1 is also involved in hypertrophy in pial arterioles mediated by EGFR, as supported by our data that Cav-1 deficiency reduced activation of EGFR. These interesting findings led us to further study the role of Cav-1 on inward remodeling and hypertrophy in pial arterioles separately.
Studies in hypertensive patients and animal models suggest that MMP9 activity plays a major role in arterial remodeling.19, 20 MMP9 digests extra-cellular matrix and allows migration of VSMC, which is an early event of the remodeling process in vascular wall.14, 21 Importantly, Ang II has been shown to induce MMP9 in an NADPH oxidase dependent manner.22 Therefore, we examined the role of MMP9 in Ang II-induced inward remodeling. Our results show that MMP9 is only involved in Cav-1 mediated inward remodeling and not hypertrophy. This agrees with the idea of MMP9-mediated VSMC migration rather than proliferation.23 These results further suggest that although Cav-1 has pro-proliferation properties24, 25, it stimulates hypertrophy in pial arterioles through a mechanism that does not involve MMP9. In addition, the results strengthen the concept that the mechanism involved in inward remodeling differs from that of hypertrophy.
Recently we have shown that EGFR transactivation by Ang II is critical for hypertrophy in pial arterioles.4 VSMC culture experiments show that transactivation of EGFR requires Cav-1 and takes place in Cav-1-enriched lipid rafts called caveolae.6, 26 The results of our present study support these findings and provide strong in vivo evidence that Cav-1 is an important signaling molecule in pial arteriolar hypertrophy. We then investigate downstream signaling events. The serine-threonine kinase Akt is a critical enzyme in VSMC hypertrophy.27 Importantly, Cav-1 may have a role in EGFR-mediated activation of Akt.10, 28, 29 In the present study, Ang II-induced phosphorylation of Akt was significantly attenuated in pial arterioles from Cav-1−/− mice, which suggests that Akt may be an important downstream link in pial arteriolar hypertrophy mediated by Cav-1-dependent Ang II-induced transactivation of EGFR. Further studies, however, are needed to verify this role of Akt. Previous studies showed that Cav-1−/− deficiency protects against various disease states. For example, Cav-1 deficiency inhibits development of atherosclerosis and reduces early brain injury after experimental intracerebral hemorrhage.30, 31 The findings of our present study agree with these previous studies that Cav-1 deficiency inhibits proatherogenic stimuli that normally act through caveolae and thereby prevents Ang II-induced cerebral arterial hypertrophy and remodeling.
The impact of Cav-1 and MMP9 deficiency on Ang II-induced hypertrophy and remodeling appeared to be pressure-independent, based on our tail-cuff results that the knockout animals developed similar level of hypertension by Ang II when compared to their WT counterparts. It is interesting to note that the WT control of Cav-1−/− mice had slightly higher baseline blood pressure than that of the WT control of MMP9−/− mice, possibly due to their different genetic background. The same reason may also be applied to explain that Cav-1−/− mice were more sensitive to the pressor effect of Ang II than that of MMP9−/− mice. Despite that, Cav-1−/− mice appeared to be more sensitive to the blood pressure lowering effect of ketamine/xylazine because they had slightly lower cerebral arteriolar pressure than that of MMP9−/− mice measured in anesthetized state.
A few limitations in this study are noteworthy. First, we did not develop a causal relationship between Cav-1 and MMP9 in remodeling of pial arterioles. This would be better determined in double Cav-1 and MMP9 knockout mice that were, unfortunately, not available to us. However, we did develop a compelling case for the notion that Cav-1 is essential in Ang II-mediated increase in MMP9 expression. Second, the use of western blotting to determine protein expression of Cav-1, pEGFR, Akt and MMP9 was limited to larger pial arteries because pial arterioles contain insufficient amounts of protein. This has been partly resolved by immunohistochemistry. Third, although we showed, in separate experiments that Akt can be activated by Ang II and EGFR, further studies will be necessary to determine if Akt is an important downstream signaling molecule that is involved in Ang II-Cav-1-EGFR-mediated hypertrophy in pial arterioles.
Perspectives
Ang II, the effector molecule of the renin-angiotensin system, is a major determinant of hypertrophy and remodeling in cerebral pial arterioles. However, the underlying molecular mechanisms are incompletely understood. In this study, we showed that Cav-1 is essential in Ang II-EGFR-mediated hypertrophy. Cav-1 is also an important signaling molecule in inward remodeling, but appears to work through a different mechanism in MMP9. This study once again agrees with the concept that hypertrophy and inward remodeling of pial arterioles work through distinctly different mechanisms. Further in-depth studies are needed to understand how MMP9 affects inward remodeling and how potentially Akt signaling mediates hypertrophy in pial arterioles.
Supplementary Material
Novelty and Significance.
What is new
Cav-1 is essential in both Ang II-induced hypertrophy and inward remodeling in cerebral pial arterioles. MMP9 is critical in Cav-1-mediated inward remodeling, but not hypertrophy. The EGFR-Cav-1 signaling cascade is critical in Ang II-induced hypertrophy that may involve downstream signaling molecule Akt.
What is relevant
Ang II-induced pial arteriolar hypertrophy and inward remodeling are not completely understood and thought to be distinctive and independent. This study reveals that independent of EGFR, Cav-1 critically contributes to Ang II-mediated MMP9 up-regulation that only facilitates inward remodeling and not hypertrophy. Cav-1 also is involved in Ang II-induced hypertrophy in an EGFR-dependent manner that may involve Akt.
Summary
Although Ang II stimulated both hypertrophy and inward remodeling in cerebral pial arterioles via Cav-1-dependent manner, it appears that the signaling pathways for hypertrophy and remodeling downstream of Cav-1 differ in important ways. Cav-1-dependent MMP9, independent of EGFR, is exclusively responsible for inward remodeling of pial arterioles, while Cav-1-dependent activation of EGFR is responsible for hypertrophy that may also involve downstream signaling of Akt. This, once again, suggests that Ang II induce hypertrophy and inward remodeling through different mechanisms.
Acknowledgments
We thank Tom Gerhold for excellent technical assistance on open cranial window experiments and genotyping of genetic knockout mice.
Sources of Funding
This work was supported by Department of Pathology, The University of Iowa, National Institute of Health Grant NS72628 and American Heart Association (Midwest Affiliate) Grant-in-Aid Award 09GRNT2250310. Dr. S.L. Chan was recipient of American Heart Association Post-doctoral Fellowship 0725668Z.
Footnotes
Conflict of Interest/Disclosure
None.
References
- 1.Touyz RM. The role of angiotensin II in regulating vascular structural and functional changes in hypertension. Curr Hypertens Rep. 2003;5:155–164. doi: 10.1007/s11906-003-0073-2. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Wolf PA, Gorelick PB. Risk factors and their management for stroke prevention: Outlook for 1999 and beyond. Neurology. 1999;53:S15–S24. [PubMed] [Google Scholar]
- 3.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- 4.Chan SL, Umesalma S, Baumbach GL. Epidermal growth factor receptor is critical for angiotensin II-mediated hypertrophy in cerebral arterioles. Hypertension. 2015;65:806–812. doi: 10.1161/HYPERTENSIONAHA.114.04794. [DOI] [PubMed] [Google Scholar]
- 5.Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett. 2004;9:195–220. [PubMed] [Google Scholar]
- 6.Ushio-Fukai M, Alexander RW. Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension. 2006;48:797–803. doi: 10.1161/01.HYP.0000242907.70697.5d. [DOI] [PubMed] [Google Scholar]
- 7.Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizaka N, Griendling KK, Lassegue B, Alexander RW. Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension. 1998;32:459–466. doi: 10.1161/01.hyp.32.3.459. [DOI] [PubMed] [Google Scholar]
- 9.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 10.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, Marumo F, Hirata Y, Inagami T. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem. 1999;274:36843–36851. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 11.Adebiyi A, Zhao G, Cheranov SY, Ahmed A, Jaggar JH. Caveolin-1 abolishment attenuates the myogenic response in murine cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1584–H1592. doi: 10.1152/ajpheart.00584.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- 13.Chan SL, Baumbach GL. Deficiency of Nox2 prevents angiotensin II-induced inward remodeling in cerebral arterioles. Front Physiol. 2013;4:133. doi: 10.3389/fphys.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 16.Martinez-Lemus LA, Zhao G, Galinanes EL, Boone M. Inward remodeling of resistance arteries requires reactive oxygen species-dependent activation of matrix metalloproteinases. Am J Physiol Heart Circ Physiol. 2011;300:H2005–H2015. doi: 10.1152/ajpheart.01066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang H, Chen W, Gao Y, Shen Y, Luo M. Molecular mechanisms associated with Angiotensin-converting enzyme-inhibitory peptide activity on vascular extracellular matrix remodeling. Cardiology. 2014;127:247–255. doi: 10.1159/000356951. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Hitomi H, Hosomi N, Shibayama Y, Nakano D, Kiyomoto H, Ma H, Yamaji Y, Kohno M, Ichihara A, Itoh H, Nishiyama A. Prorenin induces vascular smooth muscle cell proliferation and hypertrophy via epidermal growth factor receptor-mediated extracellular signal-regulated kinase and Akt activation pathway. J Hypertens. 2011;29:696–705. doi: 10.1097/HJH.0b013e328343c62b. [DOI] [PubMed] [Google Scholar]
- 19.Friese RS, Rao F, Khandrika S, Thomas B, Ziegler MG, Schmid-Schonbein GW, O'Connor DT. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31:521–533. doi: 10.3109/10641960802668730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran ED, DeLano FA, Schmid-Schonbein GW. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res. 2010;47:423–431. doi: 10.1159/000281582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 22.Browatzki M1, Larsen D, Pfeiffer CA, Gehrke SG, Schmidt J, Kranzhofer A, Katus HA, Kranzhofer R. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005;42:415–423. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- 23.Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 24.Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 25.Sedding DG, Braun-Dullaeus RC. Caveolin-1: dual role for proliferation of vascular smooth muscle cells. Trends Cardiovasc Med. 2006;16:50–55. doi: 10.1016/j.tcm.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, Alexander RW. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- 27.Hixon ML, Muro-Cacho C, Wagner MW, Obejero-Paz C, Millie E, Fujio Y, Kureishi Y, Hassold T, Walsh K, Gualberto A. Akt/PKB upregulation leads to vascular smooth muscle cell hypertrophy and polyploidization. J Clin Invest. 2000;106:1011–1020. doi: 10.1172/JCI8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H, Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res. 2005;96:635–642. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Han HJ. Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. Am J Physiol Cell Physiol. 2009;297:C935–C944. doi: 10.1152/ajpcell.00121.2009. [DOI] [PubMed] [Google Scholar]
- 30.Chang CF, Chen SF, Lee TS, Lee HF, Chen SF, Shyue SK. Caveolin-1 deletion reduces early brain injury after experimental intracerebral hemorrhage. Am J Pathol. 2011;178:1749–1761. doi: 10.1016/j.ajpath.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.